Abstract
Background
Deficits in executive function, including measures of working memory, inhibition and cognitive flexibility, have been documented in preschoolers born very low birth weight (VLBW) compared with preschoolers born with normal birth weight (NBW). Maternal verbal scaffolding has been associated with positive outcomes for both at-risk and typically developing preschoolers.
Aims
The purpose of this study was to examine associations between maternal verbal scaffolding, Verbal IQ (VIQ) and executive function measures in preschoolers born VLBW.
Subjects
A total of 64 VLBW and 40 NBW preschoolers ranging in age from 3 ½ to 4 years participated in the study.
Outcome Measures
VIQ was measured with the Wechsler Preschool and Primary Scale of Intelligence – 3rd edition. Executive function tests included the Bear Dragon, Gift Delay Peek, Reverse Categorization and Dimensional Change Card Sort-Separated Dimensions.
Study Design
Maternal verbal scaffolding was coded during a videotaped play session. Associations between maternal verbal scaffolding and preschoolers’ measures of VIQ and executive function were compared. Covariates included test age, maternal education, and gender.
Results
Preschoolers born VLBW performed significantly worse on VIQ and all executive function measures compared to those born NBW. Maternal verbal scaffolding was associated with VIQ for VLBW preschoolers and Gift Delay Peek for the NBW group. Girls born VLBW outperformed boys born VLBW on VIQ and Bear Dragon.
Conclusion
Integrating scaffolding skills training as part of parent-focused intervention may be both feasible and valuable for early verbal reasoning and EF development.
Keywords: Language, executive function, parenting, child, preschool, infant, very low birth weight
1. INTRODUTION
Technological advancements in Neonatal-Perinatal medicine have resulted in improved survival rates of infants born preterm and very low birth weight (VLBW). The long-term sequelae of prematurity are great, as these children remain at very high risk for brain injury and neurodevelopmental deficits across a range of developmental domains [1] and [2]. Intelligence quotient (IQ) is most commonly used to assess neurodevelopmental outcomes in school-aged children born VLBW. Though mean IQ scores of children born VLBW have been shown to be up to 10 points below those of matched full-term peers [3], most fall within the average range when major disabilities are excluded [4], [5], [6] and [7].
A construct that may be more sensitive to deficits found among children born VLBW is executive function (EF), which refers to the cognitive processes that underlie flexible, goal directed responses to novel situations [8] including working memory, inhibitory control, and cognitive flexibility [9], [10] and [11]. The development of EF is rapid over the first years of life and is thought to be critical in the development of self-regulation in the preschool period [12]. In typically developing preschool populations, there is some debate about whether EF includes distinct dimensions related to inhibition and cognitive flexibility, as it does in older children, or whether EF represents an undifferentiated and unitary concept [13] and [14]. Supporting the dimensional perspective, Carlson [15] analyzed preschoolers’ (aged two through five) performance on a variety of EF tests and determined that different EF dimensions emerge at different ages. Further, each dimension impacts overall EF performance to a differing extent. They are therefore best measured by specific tasks tapping into each dimension. Further, Garon, Bryson and Smith [16] emphasized the hierarchical nature of the development of EF in early childhood, finding that the development of basic components required for EF in the first 3 years of life is uneven compared to that of children between 3 to 5 years. Best and Miller [17] also found that inhibition was shown to greatly improve during the preschool years in contrast to working memory’s more gradual linear development over the preschool period. Additionally, 5 ½ year old children born VLBW have difficulty with the specific EF dimensions of impulse control and working memory [18]; these specific EF areas may be particularly important to address when investigating developmental outcomes in early childhood (e.g., ages 3–4). Thus, though there is some debate, numerous studies support the dimensional EF perspective for young children.
Recent studies examining EF among school-aged children have found significant deficits in preterm children compared to those born full-term [19], [20] and [21]. These deficits persist even after taking IQ differences into account [20]. However, few studies have investigated EF abilities among preterm and VLBW children aged 3 years and younger. This is important, as the rudiments of EF are emerging as early as the first year of life [17], and an improved understanding of the developmental trajectory of early EF development may inform intervention strategies. In one of the few EF studies of toddlers born VLBW, Woodward and colleagues [11] found that 2 year olds born VLBW had more difficulty encoding new information into working memory compared with normal birth weight (NBW) infants.
Studies examining the neural mechanisms contributing to the EF deficits found in preterm populations have highlighted the potential role that subcortical white matter abnormalities, particularly the white matter circuits connecting frontal, striatal, and thalamic regions, may play in explaining the EF differences found in preterm compared with full-term samples [22]and [23]. Using prospective longitudinal data from 110 very preterm and 113 full term children, Woodward and colleagues [11], found that white matter abnormalites on neonatal magnetic resonance imaging (MRI) predicted deficits in executive functioning skills at four years of age [10]. Many have theorized that the presence of such white matter abnormalities impacts subsequent cortical development and neural connectivity, particularly within prefrontal circuits, which may subsequently have cascading effects on executive functioning skills [24] and [25].
The quality of early parent-child interactions has been positively associated with EF development. In fact, maternal verbal scaffolding, autonomy support, sensitivity, and contingent responsiveness have been positively associated with the development of EF in full-term children [26 and [27], with maternal verbal scaffolding in particular gaining support as a parenting practice that is likely to improve EF development [28] and [29]. Maternal verbal scaffolding refers to maternal verbal input that provides information about objects and actions and offers children age-appropriate problem-solving strategies [30].
In NBW children, maternal verbal scaffolding has been shown to influence children’s development of verbal abilities. For instance, maternal verbal scaffolding used at age 2 has been shown to positively affect EF at age 4 and verbal abilities assessed at 3 years among children born NBW [30] and [31]. Though there is a paucity of research describing verbal scaffolding in VLBW children, Landry and colleagues also found that the use of verbal scaffolding at 2 and 3 years predicted greater verbal reasoning ability at 6 years [32]. They suggest that maternal verbal input during the early years when children are rapidly developing language may support later executive processing skills. Dilworth-Bart and colleagues [33] found that scaffolding was related to verbal working memory among preschoolers born VLBW. However, despite studies such as these, there remains little data regarding the association between maternal verbal scaffolding, early verbal reasoning skills and EF abilities in preschoolers born VLBW.
Maternal scaffolding may play a particularly important role in the neurodevelopmental outcomes of children born VLBW given their increased risk for neurodevelopmental deficits. According to the differential susceptibility hypothesis, infants with certain vulnerability factors may be differentially susceptible to both positive and negative parenting [34]. For instance, compared to infants with less reactive temperaments, infants with more difficult temperaments have been shown to be more influenced by both positive and negative care, showing better outcomes with positive parenting and more negative outcomes with less optimal parenting. Applying this hypothesis to children born preterm, Poehlmann and colleagues [35] argued that, since infants born VLBW display early difficult temperamental characteristics, they are likely to show similar differential vulnerability to positive and negative care. Their findings supported this by showing that infants born preterm, who were temperamentally more prone to distress and who experienced less optimal parenting (i.e., more critical and angry parenting) at nine months exhibited the most externalizing behaviors at 24 months, whereas easily distressed infants who experienced more optimal parenting at 9 months showed the least externalizing difficulties. Similar findings were also reported regarding internalizing difficulties, indicating that more optimal parenting was associated with less internalizing difficulties and less optimal parenting was related to more internalizing difficulties in temperamentally difficult preterm children. In line with this, maternal scaffolding behaviors may also show differential association with executive functioning outcomes in VLBW and NBW children given the added risk characteristics associated with prematurity.
The purpose of the current study was to examine the association between maternal verbal scaffolding, verbal performance and EF in preschoolers born VLBW and NBW. We hypothesized that: 1) preschoolers born VLBW would have lower scores on tests of verbal ability (WPPSI-III Verbal IQ) and EF skills (Bear Dragon, Reverse Categorization Test, Dimensional Change Card Sort-Separated Dimensions and Gift Delay Peek) compared with preschoolers born NBW; and 2) both preschoolers born VLBW and NBW whose mothers utilized more verbal scaffolding would have higher Verbal IQ and EF measure scores.
2. METHODS
2.1. Study Population
A total of 104 preschoolers were included in the current study, VLBW (n=64) and NBW (n=40). Inclusion criteria for the VLBW cohort included birth weight ≤1500 grams. Inclusion criteria for the normal birth weight (NBW) cohort included: appropriate birth weight for gestational age and gestational age ≥36 weeks. Exclusion criteria for the VLBW group included: prenatal exposure to illicit drugs, vision/hearing impairment, known genetic abnormality, and multiple gestation [36]. Exclusion criteria for the NBW cohort included: medical complications at or subsequent to birth which included exposure to illicit drugs, genetic abnormalities, vision or hearing impairment. Children were between 3 years 1 month and 4 years 10 months of age at the time of testing, which took place between April 2004 and August 2009 (Table 1).
Table 1.
Demographic Characteristics and Test Scores of VLBW and NBW groups
| VLBW n= 64 | NBW n= 40 | ||
|---|---|---|---|
| Factor | Mean (SD) | Mean (SD) | P value |
| Test Age (months) | 46.5 (5.0) | 44.5 (4.1) | P=0.04 |
| Birth weight (grams) | 1195.9 (272.0) | 3213.0 (429.1) | P<0.001 |
| Gestational Age (wks) | 29.2 (2.1) | 38.8 (1.6) | P<0.001 |
| Days on Ventilation | 9.8 (15.8) | 0 (0) | NA |
| IVH ≥3 or PVL | 5 (8%) | 0 (0) | NA |
| Small Gestation Age | 12 (21%) | 0 (0) | NA |
| Child Ethnicity | |||
| White | 19 (30%) | 15 (37.5%) | |
| Native American | 10 (16%) | 6 (15%) | |
| Hispanic/Latino | 33 (51%) | 18 (45%) | |
| other | 2 (3%) | 1 (2.5%) | |
| ♦Maternal Education | 1.7 (1.5) | 2.1 (2.0) | P<0.001 |
| Maternal Age | 32.6 (7.6) | 33.3 (8.1) | P=0.69 |
| Verbal IQ | 93.3 (13.9) | 107.8 (12.0) | P<0.001 |
| Performance IQ | 91.9 (13.2) | 102.6 (10.8) | P<0.001 |
| Full Scale IQ | 90.7 (13.1) | 106.0 (11.3) | P<0.001 |
| Gift Peek | 34.0 (23.2) | 48.0 (16.2) | P<0.005 |
| Bear Dragon | 15.7 (12.9) | 29.3 (7.8) | P<0.001 |
| ^Reverse Cat. % pass | 100 [0,100] | 100 [100,100] | P<0.001 |
| ^ΔDCCSS % pass | 83 [0,100] | 100 [100,100] | P<0.001 |
| ^Maternal Scaffolding | 2.2 (1.7) | 2.9 (2.1) | P=0.06 |
IVH - interventricular hemorrhage; PVL - periventricular hemorrhage
0- <H.S., 1- H.S. graduate; 2- HS+ 1 yr college; 3-Associate degree; 4-Bachelor degree; 5- Some graduate school, 6- Masters degree +
DCCSS - Dimensional Change Card Sort-Separated Dimensions
Values reported are Median [Q1, Q3] for highly skewed variables where Q1 and Q3 are the first and third quartiles.
The University of New Mexico’s Human Research Review Committee provided review and approval for this study, which was in compliance with institutional research standards for human research and was completed in accordance with the Helsinki Declaration. Recruitment was conducted by University of New Mexico Hospital (UNMH) Clinical and Translational Science Center (CTSC) pediatric research nurses. In order to recruit preschoolers born VLBW, CTSC pediatric research nurses reviewed prior admission lists from the University of New Mexico Hospital Newborn Intensive Care (UNMH-NICU) to identify infants eligible for inclusion. A University of New Mexico graduate student called the parents of the eligible preschoolers who were within the enrollment age window and provided a brief description of the study. If the parents expressed interest, an appointment for the study was scheduled. To recruit preschoolers born NBW, flyers with a brief study description and contact phone number were posted in public places in the Albuquerque, New Mexico area (e.g., local swimming pools, libraries, pediatric clinics). Parents interested in participation were provided a description of the study; if they expressed a desire to participate, they were scheduled for an appointment.
The testing took place between April 2004 and August 2009. Of the 300 VLBW children who were eligible to participate through the duration of data collection, 75 (25 %) mothers could be reached. Of the 75 eligible mothers reached, 64 (85%) agreed to participate and completed the study. Based on the limited demographic information (i.e., maternal and child ethnicity, maternal relationship status, maternal age) available at recruitment, mothers who could not be contacted and mothers who declined participation appear comparable to those who completed the study. In addition, participants (i.e., those that completed the study) and non-participants (i.e., those that could not be contacted, that refused to participate, or that failed to keep their scheduled appointment) appeared commensurate with the larger UNMH population.
2.2. Study Design
All parents provided informed consent prior to the testing or videotaping. Once informed consent was obtained, an evaluation of the maternal-child interaction, as well as the preschoolers’ cognitive and EF abilities, was conducted by two authors (PM, SD). Forty percent (n = 26) of the evaluations among the VLBW subsample were conducted at the participant’s home and the remainder at the UNMH Pediatric Clinic. All the evaluations of the NBW group were conducted in the Pediatric Clinic. The testing took approximately two hours to complete. Perinatal medical information was obtained through hospital record review and descriptive information is included in Table 1.
2.3. Assessment Measures
2.3.1. Maternal Verbal Scaffolding
The Verbal Scaffolding Scale [30] and [37] was used to assess parenting behaviors. Child and mother dyads were videotaped for 8 to10 minutes with a standard set of toys including pretend food, a cash register and blocks. The first five minutes of the videotaped mother-child interaction were coded when the mother and child were actively engaged in play and the research assistant was not present. Verbal scaffolding behaviors, defined using the Verbal Scaffolding Manual [30] and [37], were coded. A maternal statement was considered to be a verbal scaffolding statement if it helped the child to make associations or provided strategies to help the child solve a problem. Maternal verbal scaffolding statements included those that involved associating an object with a specific location, using ‘like that’ comparisons, describing objects (e.g., “apples are red”), defining the uniqueness or features of an object, or identifying the specific function of an object. Other categories of maternal verbal scaffolding included describing cause and effect, emotions, senses, contrasts, categories of objects, and linking nouns with nouns. The total number of verbal scaffolding episodes was determined. A more detailed description of the scoring is provided in the Verbal Scaffolding Manual [30] and [37]. All coding was done in strict adherence to the detailed Verbal Scaffolding Manual. A master coder (PM) initially trained the two coders, ensuring that they reached 90% reliability with her coding. Once reliability was established, every videotape was coded by both coders, who maintained 90% consistency for coding. If differences of greater than 90% were found for coding of videotape, the master coder re-coded the tape and consensus coding was reached among the 3 coders.
2.3.2. Cognitive Assessment
The Wechsler Preschool and Primary Scale of Intelligence-Third Edition (WPPSI-III) [38] was used as a measure of verbal and performance abilities. The WPPSI-III is a standardized and structured cognitive assessment, administered by a certified tester, for use in children aged 2:6 (2 years, 6 months old) to 7:3. The scale involves activities such as pointing at pictures, naming pictures, answering questions about day-to-day information, building with blocks, and assembling puzzles. The WPPSI-III generates Verbal IQ, Performance IQ, and Full Scale IQ scores; the Verbal IQ score was used in the current study.
2.3.3. Executive Function Assessment
The Bear Dragon task [39], [40] and [41] was used primarily as a measure of inhibition. In addition to inhibitory control, this task also has some early working memory demands. In this visually mediated Go-No-Go task, children are instructed to inhibit certain responses to commands. The examiner introduces children to a “nice” bear puppet (using a soft, high-pitched voice) and a “grumpy” dragon puppet (using a gruff, low-pitched voice). It is then explained that in this game, “we will listen to the nice bear and do what he asks us to do” (e.g., touch your nose), but “we will not listen to what the grumpy dragon tells us, so we will not do what he asks us to do”. Practice trials are administered in which the bear gives a command in a nice voice (“touch your nose”) and the dragon gives a command in a gruff voice (“touch your tummy”). The child passes the practice trial if they comply with the bear and do not comply with the command given by the dragon. Up to six practice trials are given, with verbal rule checks after each trial until the child passes one command by each puppet. After practicing, there are 10 test trials with the bear and dragon commands in alternating order. A rule reminder is provided halfway through the testing, regardless of performance. Children are seated at a table throughout the task. To score this task, each response is assigned a score from 0 to 3 (full correct, i.e. no movements on dragon or correct move on bear = 3; partial correct, e.g., moves hand near nose on bear trial, nonrelated movement on dragon trial = 2; wrong move, e.g., moves hand near nose on dragon trial, nonrelated movement on bear trial = 1; fail item, i.e., no move on bear trial, full move on dragon trial = 0). Children who are unable to pass the practice trials are given a score of 0. Points are added to obtain a total score out of 33 possible points (3 points for each of the 10 test trials plus 3 points for correctly completing at least one of the practice trial items) [19] and [42].
The Gift Delay Peek task [19] was used as a measure of inhibition. The child is told that they have earned a present, but the examiner has forgotten to wrap it. The child is turned 180 degrees away from the examiner and is told not to peek or turn towards the present until the examiner tells the child they are ready. The examiner then loudly wraps the present for up to one minute. No reminders are given. The task is discontinued and the child receives the gift at one minute or when the child peeks at the gift. A score is provided for the number of seconds prior to the child peeking at the examiner.
The Reverse Categorization task [43] was used as a measure of working memory and rule use and the Dimensional Change Card Sort-Separated Dimensions task (DCCSS) [44] was used as a measure of cognitive flexibility and working memory. These tasks consist of sorting cards into boxes based on various dimensions (e.g. category, shape, color) while the rules are varied (i.e., sort by color, sort by shape).
During the Reverse Categorization task, the child is introduced to two buckets and asked to help the experimenter sort mommy animals into a “Mommy” bucket and baby animals into a “Baby” bucket [43]. Then the examiner suggests that they play a “silly game” and reverse the rules, with baby animals going in the “Mommy” bucket and mommy animals going in the “Baby” bucket. Percent correct out of ten trials is calculated for the task.
For the DCCSS, target cards are sorting cards are used according to procedures outlined by Diamond and colleagues [44]. Target cards include a picture of a baby mounted on a blue background and a picture of a mommy mounted on a yellow background. The sorting cards consist of pictures of babies mounted on yellow backgrounds and pictures of mommies mounted on blue backgrounds. After a practice trial is given to assure knowledge of the dimensions and colors used in the task, children are told that they should play the “color game” in which the blue cards go in the blue box (marked with blue baby) and the yellow cards go in the yellow box (marked with a yellow mommy). The children are given the sorting cards (e.g. yellow baby, blue mommy) and the experimenter labels the color, “Here is a blue one, where does it go?” Each sorting card matches one target card on one dimension (color) and matches a second target card on the other dimension (shape). The child is first asked to sort six cards by color (this is referred to as the pre-switch phase), and then the child is asked to switch dimensions and sort six cards by shape (this is referred to as the post-switch phase). This requires the child to inhibit the previous sorting rule (color) and only pay attention to the current relevant dimension (shape). Knowledge questions (e.g. “Where do the blue/baby ones go?”) and rule reminders (e.g. “Remember, blue/baby ones go here (point) and yellow/mommy ones go here (point)”) are given on alternating trials. The percent correct score is calculated based on performance on the six post-switch trials.
2.4. Socio-demographic Variables
Maternal and toddler race and ethnicity were obtained by parent report. Maternal education was categorized on an ordinal 0–6 scale as follows: less than high school graduate (0), high school graduate (1), partial college (2), associate degree (3), college graduate (4), some graduate school (5), and advanced graduate degree (6). Perinatal medical variables were collected through medical record review [45].
2.5. Statistical Analysis
This study included preschoolers born either VLBW (N=64) or NBW (N=40) and their mothers. Due to the non-Gaussian distributions for some variables, Mann-Whitney-Wilcoxon two-sample tests were used to test for differences between the VLBW and NBW groups for socio-demographic variables. Two sample t-tests and Mann-Whitney-Wilcoxon tests were used to measure the differences between the groups for tests of VIQ and EF. Analysis of covariance (ANCOVA) was used to compare associations between maternal verbal scaffolding and VIQ and EF measures among VLBW and NBW preschoolers, adjusting for socio-demographic covariates. Because IQ has been determined to be an inappropriate covariate when investigating neurodevelopmental outcomes among developmentally delayed populations [46], IQ was not included as a covariate. A two sample t-test and a Mann-Whitney-Wilcoxon exact test were used to compare girls born VLBW and boys born VLBW on VIQ and EF measures, respectively. Spearman partial rank correlations (ρ) were computed, controlling for test age, to measure associations between maternal verbal scaffolding scores, VIQ and EF test measures for the VLBW and NBW groups separately. Spearman partial rank correlations, controlling for test age, were used to measure association between VIQ and EF test measures. ANCOVA was used to assess the gender X maternal scaffolding interaction in the association between maternal scaffolding and verbal IQ.
3. RESULTS
3.1. Comparison of Test Measures
The VLBW and NBW groups were not significantly different in gender or maternal age. Twenty six of the VLBW preschoolers (40%) were tested in their home; none of the NBW preschoolers were tested in home. Using two sample t-tests, no differences were found between the VLBW preschoolers tested at home or in the clinic in maternal verbal scaffolding scores or VIQ or any of the EF test measures.
Using Mann-Whitney-Wilcoxon exact tests, significant differences were found between the VLBW and NBW preschoolers on maternal education, gestational age, birth weight, and test age (See Table 1). Mann-Whitney-Wilcoxon exact tests indicated that the preschoolers born VLBW scored significantly lower than children born NBW on VIQ and all EF test measures (Table 1). These differences remained significant even after adjusting for maternal education using Analysis of Covariance (ANCOVA).
3.2 Preliminary Correlational Analyses
Using Spearman partial rank correlations, there was a significant positive association between maternal education and Verbal IQ score in the NBW and VLBW groups (ρ=0.72, p<0.0001and ρ =0.30, p=.02, respectively). Maternal education was not found to be associated with any of the EF test measures in the VLBW or NBW groups.
To investigate the associations between VIQ and EF measures, we used Spearman partial rank correlations, controlling for test age. There was a significant positive association between Verbal IQ and Bear Dragon among both the VLBW and NBW groups (ρ=0.44, p=0.001and ρ =0.34, p=.04, respectively). Verbal IQ was also significantly associated with Reverse Categorization scores in both the VLBW and NBW groups (ρ=0.40, p=0.002and ρ =0.62, p<0.0001, respectively).
3.2. Maternal Verbal Scaffolding Associations
Mann-Whitney-Wilcoxon exact testing revealed that there were no significant differences in the amount of maternal verbal scaffolding used between the mothers of VLBW and NBW preschoolers. Among the VLBW preschoolers, Spearman correlations showed a significant positive association between maternal verbal scaffolding and maternal education (ρ=0.38, p=0.004). There were no significant associations between maternal verbal scaffolding and maternal education or any other socio-demographic variable for the NBW group.
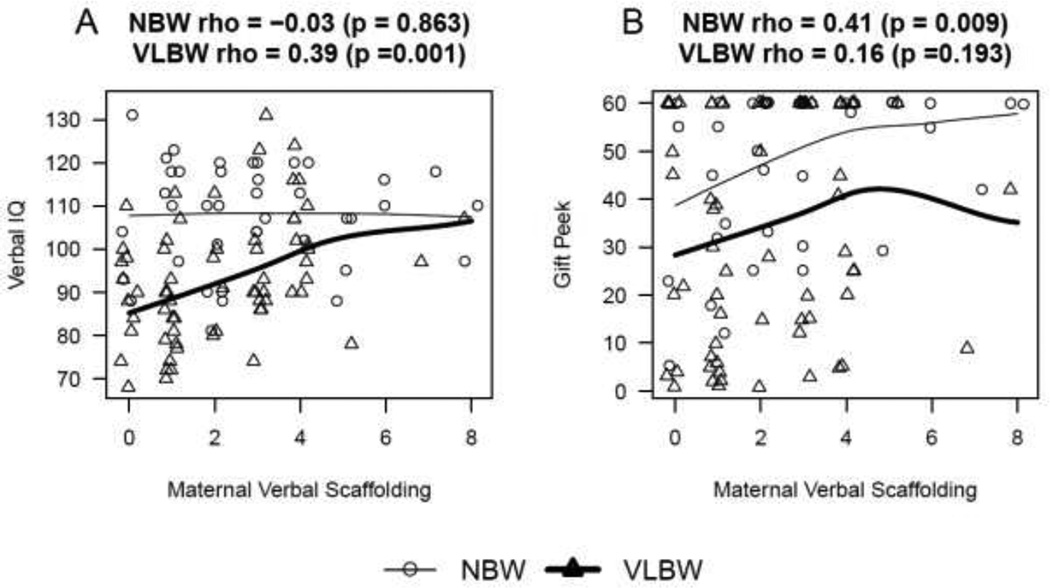
The VLBW preschoolers evidenced a significant association between maternal verbal scaffolding and Verbal IQ (ρ=0.37, p=0.004). The preschoolers born NBW had a significant positive association between maternal verbal scaffolding and gift peek score (ρ=0.41, p=0.009; see figure 1).
Figure 1.
Plot illustrating the association of Maternal Verbal Scaffolding score vs EF measures for VLBW and NBW groups. Panel A shows the relationship for Verbal IQ score, which is significant (as measured by Spearman correlation) for the VLBW group. Panel B shows the relationship for Gift Delay score, which is significant (as measured by Spearman correlation) for the NBW group.
3.3. Post-Hoc Analysis: Gender Associations
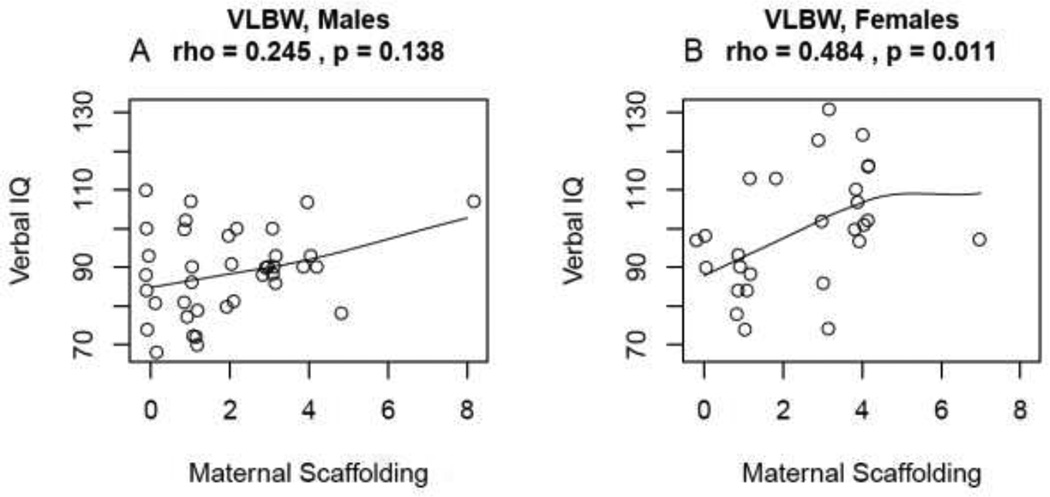
To determine if gender differences explained some of the differential relationships, post-hoc analyses were performed. Using ANCOVA with adjustment for test age, and comparing the groups by gender, significant differences were found only within the VLBW group for VIQ (F1,62=10.90, p=0.002) and Bear Dragon (F1,60=10.75, p=0.002. The VLBW girls had significantly higher mean scores on all of the above test measures. Gender differences in the associations between maternal verbal scaffolding and VIQ and EF measures were also explored. A positive association was found between maternal verbal scaffolding and Verbal IQ scores for VLBW girls only (ρ=0.50, p=0.01; see Figure 2). A formal test for gender X maternal verbal scaffolding interaction for this association using ANCOVA was not significant (F1,62=3.24, p=0.08).
Figure 2.
Plot illustrating the association of Maternal Verbal Scaffolding score vs. Verbal IQ score conditional on gender for the VLBW group. The relationship is significant for females (as measured by Spearman correlation) in panel B but not for males in panel A.
4. DISCUSSION
The purpose of this study was to compare the associations between maternal verbal scaffolding and measures of cognition and EF in preschoolers born VLBW and NBW. As expected, preliminary analyses found that children born VLBW had significantly lower scores on Verbal IQ and all EF measures. These findings are consistent with findings from previous studies that show poorer neurodevelopmental outcomes in children born VLBW compared to children born full-term [47], [48] and [49]. We found no significant differences in the amount of maternal verbal scaffolding used by mothers of VLBW or NBW children. This was contrary to what was expected, because studies comparing preterm and full-term samples have repeatedly found parenting differences, particularly in the first year of the child’s life. In particular, mothers of preterm children are consistently rated as more intrusive and less responsive in the literature [50] and [51]. These studies, however, have mostly focused on emotionally responsive parenting behaviors (e.g., maternal responsiveness to child emotional cues and emotional attunement) rather than parenting behaviors that facilitate children’s cognitive development, such as maternal verbal scaffolding. Although the importance of emotionally supportive parenting behaviors cannot be understated with regard to their impact on child socio-emotional outcomes, examining parenting behaviors that are primarily focused on children’s cognitive development may deepen our understanding of the parenting behaviors needed to support learning [32].
Maternal verbal scaffolding, termed “cognitively supportive behavior” by Landry and colleagues [32], is one such behavior. In a previous study of maternal verbal scaffolding in mothers of 18 month old children born preterm and full-term, we found that full-term mothers demonstrated more complex verbal scaffolding behaviors [27]. Although we expected to replicate this finding in the current study, it is possible that the variability in the samples’ age ranges account for the differences found. It may be that 18 month old children born preterm may differ from their full-term counterparts to a greater degree than three year old children born preterm, leading mothers of 18 month old children born preterm versus full-term to respond accordingly. Studies have shown that children born preterm are more different than their full-term counterparts early in life, due to their medical vulnerability [51]. Since parenting is a bidirectional relationship whereby the parent and child are both active participants in creating interactions, the child’s needs at different time points influence what behaviors a parent displays [52], [53] and [54]. It is also possible that use of non-verbal scaffolding strategies such as structuring activities in addition to verbal scaffolding contributes to the benefit of this type of play strategy at different ages, as was found by Hammond and associates [31]. To fully understand this, however, future studies must examine parenting differences longitudinally to explore how parenting practices, such as maternal verbal scaffolding behaviors, change over time in relation to child interactive behavior.
We hypothesized that among both groups, maternal verbal scaffolding would be associated with increased Verbal IQ scores and EF measures. We found that among the VLBW group, more maternal verbal scaffolding was associated with higher Verbal IQ; however this relationship seems to be driven by the association among the female children born VLBW. Previous studies have found that maternal verbal scaffolding strategies are positively associated with a number of neurocognitive outcomes, both concurrently and longitudinally, including verbal and nonverbal problem-solving skills [30] and EF skills [29] and [31]. Compared to our findings, however, previous studies regarding verbal scaffolding and outcomes have not been gender-specific but have found overall gender differences. Studies that specifically looked at the developmental outcome of toddlers born VLBW found girls scored higher on measures of cognition [55]. Similar to these finding, girls born VLBW in our study scored higher on a test of Verbal IQ than the boys born VLBW. One hypothesis requiring empirical investigation is that perhaps the effects of maternal verbal scaffolding efforts are enhanced for girls to the extent that girls are more responsive to this type of an interaction.
Gender differences were found for the VLBW group only, with VLBW girls scoring significantly higher on tests of VIQ and Bear Dragon compared to VLBW boys. These findings are consistent with previous studies demonstrating that girls born VLBW have better neurocognitive outcomes [55] [56]. Others have found that full-term girls also score higher on tests of self-regulation [57] than full-term boys, though we did not find this in our study. This may be due to the smaller sample size of the NBW group, limiting the power necessary to find differences. Further investigation of early development of EF skills in preschool age boys and girls born NBW and VLBW groups is important as we develop better assessment tools in this area.
Within the NBW group, maternal verbal scaffolding was significantly associated with decreased impulsivity (Gift Delay Peek). This finding is also consistent with the literature, which has identified associations between both VLBW and full term groups with measures of EF and maternal verbal scaffolding [30] and [31]. The association between maternal verbal scaffolding and Gift Delay Peek was not significant for the VLBW preschoolers. Because the VLBW preschoolers scored significantly lower on the Gift Delay Peek task than the NBW group, it is possible that the level of disinhibition demonstrated by the VLBW group was associated with a decreased ability to benefit from maternal verbal scaffolding efforts more generally. In particular, verbal scaffolding may be a more effective intervention technique for those preschoolers who have better self-regulation, including increased inhibition, although this is an area that warrants further empirical investigation.
Also consistent with the literature, we found that maternal verbal scaffolding was significantly associated with maternal education in the VLBW group. In our previous study examining maternal scaffolding behaviors in a different group of 18 month olds born VLBW and NBW, mothers of toddlers born VLBW who had obtained higher education used more maternal verbal scaffolding behaviors [27]. In both our previous and current studies, we found this association between education and scaffolding for the VLBW subsamples only, suggesting that mothers of toddlers and preschoolers born NBW may use similar amounts of scaffolding, regardless of education. These findings require further investigation, as one would expect a similarly strong association among mothers of children born NBW. In fact, Carr and Pike [58] found that maternal education was significantly associated with maternal verbal scaffolding practices among mothers of children born full term, with higher education being associated with more maternal verbal scaffolding. Hammond and associates [31] also found maternal education was significantly associated with the use of maternal verbal scaffolding on a structured task and subsequently with tests of EF. Parental education level may not only be directly associated with the use of more adaptive parenting practices, but may also represent wider social and economic influences that positively affect child outcomes [58], [59] and [60] and future research should include additional socio-demographic measures.
A key limitation of the current study is that only maternal verbal scaffolding behaviors were assessed; correlates of non-verbal scaffolding behaviors in parent-child interactions were not explored. Since communication can occur nonverbally, understanding how nonverbal parenting strategies scaffold children’s play will be vital to providing a more comprehensive understanding of the correlates of maternal scaffolding behaviors. Some studies suggest that parents of children born VLBW are more intrusive and less responsive compared with parents of children born NBW [50] [51]. Whether they use more non-verbal scaffolding methods to foster cognitive development in their children, and whether children born VLBW elicit more of these strategies from their parents compared to NBW children, is unknown but worthy of further exploration.
Although studying maternal verbal scaffolding in isolation of other parenting behaviors provides only a partial picture of the overall strategies employed by parents, it does help further our understanding of what behaviors could be the focus of early intervention. Longitudinal investigations could provide greater insight into the developmental sequencing of maternal behavior and child outcomes. Despite these limitations, the differential associations found between verbal scaffolding behaviors and VIQ and EF measures may inform future studies as the presence of verbal scaffolding may be only part of the clinical picture. Further investigation into the synchrony, timing and appropriateness of these scaffolding statements is important to address in future research to help us to better understand the ways in which parents structure their interactions with their young children to support children’s developing competencies. In addition investigating the combination of maternal sensitivity and scaffolding as predictors of EF would be valuable.
In conclusion, preschoolers born VLBW had significantly lower Verbal IQ and EF scores compared with preschoolers born NBW, and different VIQ and EF tests were related to maternal verbal scaffolding in VLBW and NBW preschoolers. Maternal verbal scaffolding was associated with VIQ within the VLBW group, and a test of inhibition (Gift Delay Peek) in the NBW group. Maternal verbal scaffolding is a relatively simple behavior that is observed during naturally occurring play interactions, thus integrating scaffolding skills training as part of a parent-focused intervention may be both feasible and inexpensive.
Highlights.
Maternal verbal scaffolding was associated with increased verbal abilities in preschoolers born very low birth weight (VLBW).
Preschoolers born normal birth weight (NBW) had better scores on a test of inhibition when their mothers used more maternal verbal scaffolding.
Maternal verbal scaffolding was associated with higher maternal education level only for the preschoolers born VLBW.
Maternal verbal scaffolding used during free play is an easy intervention that benefited preschoolers born VLBW and NBW.
ACKNOWLEDGEMENTS
Statistical support for this grant was provided by Ronald Schrader Ph.D. through the University of New Mexico Clinical and Translational Science Center, (UL1RR031977-01). University of New Mexico School of Medicine Pediatric Department provided a grant to help with the research. We thank Joy Van Meter, BS for her assistance with coding of the tapes and the many families who participated in this study. We thank the many families that participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflict interest to report relevant to this manuscript.
REFERENCES
- 1.Litt J, Taylor HG, Klein N, Hack M. Learning disabilities in children with very low birth weight: prevalence, neuropsychological correlates, and educational interventions. J Learn Disabil. 2005;38:130–141. doi: 10.1177/00222194050380020301. [DOI] [PubMed] [Google Scholar]
- 2.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephens BE, Vohr BR. Neurodevelopmental outcome of the premature infant. Pediatr Clin North Am. 2009;56:631–646. doi: 10.1016/j.pcl.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Aylward GP. Cognitive and neuropsychological outcomes: more than IQ scores. Ment Retard Dev Disabil Res Rev. 2002;8:234–240. doi: 10.1002/mrdd.10043. [DOI] [PubMed] [Google Scholar]
- 5.McGrath M, Sullivan M. Birth weight, neonatal morbidities, and school age outcomes in full-term and preterm infants. Issues Compr Pediatr Nurs. 2002;25:231–254. doi: 10.1080/01460860290042611. [DOI] [PubMed] [Google Scholar]
- 6.McGrath MM, Sullivan M, Devin J, Fontes-Murphy M, Barcelos S, DePalma JL, Faraone S. Early precursors of low attention and hyperactivity in a preterm sample at age four. Issues Compr Pediatr Nurs. 2005;28:1–15. doi: 10.1080/01460860590913945. [DOI] [PubMed] [Google Scholar]
- 7.Weindrich D, Jennen-Steinmetz C, Laucht M, Schmidt MH. Late sequelae of low birthweight: mediators of poor school performance at 11 years. Dev Med Child Neurol. 2003;45:463–469. doi: 10.1017/s0012162203000860. [DOI] [PubMed] [Google Scholar]
- 8.Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sokol B, Muller U, Carpendale J, Young A, Iarocci G. Self- and social-regulation: The development of social interaction, social understanding, and executive functions. New York, NY: Oxford University Press; 2010. [Google Scholar]
- 10.Woodward LJ, Clark CA, Pritchard VE, Anderson PJ, Inder TE. Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev Neuropsychol. 2011;36:22–41. doi: 10.1080/87565641.2011.540530. [DOI] [PubMed] [Google Scholar]
- 11.Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- 12.Blair C, Zelazo PD, Greenber MT. The measurement of executive function in early childhood. Dev Neuropsychol. 2005;28(2):561–571. doi: 10.1207/s15326942dn2802_1. [DOI] [PubMed] [Google Scholar]
- 13.Wiebe SA, Espy KA, Charak D. Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Dev Psychol. 2008;44(2):575. doi: 10.1037/0012-1649.44.2.575. [DOI] [PubMed] [Google Scholar]
- 14.Wiebe SA, Sheffield T, Nelson JM, Clark CA, Chevalier N, Espy KA. The structure of executive function in 3-year-olds. J Exp Child Psychol. 2001;108(3):436–452. doi: 10.1016/j.jecp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson SM. Developmentally sensitive measures of executive function in preschool children. Dev Neuropsychol. 2005;28(2):595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- 16.Garon N, Bryson SE, Smith IM. Executive function in preschoolers: a review using an integrative framework. Psychol Bull. 2008;134(1):31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- 17.Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81(6):1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohm B, Smedler AC, Forssberg H. Impulse control, working memory and other executive functions in preterm children when starting school. Acta Paediatr. 2004;93:1363–1371. doi: 10.1080/08035250410021379. [DOI] [PubMed] [Google Scholar]
- 19.Anderson PJ, Doyle LW. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114:50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- 20.Bayless S, Stevenson J. Executive functions in school-age children born very prematurely. Early Hum Dev. 2007;83:247–254. doi: 10.1016/j.earlhumdev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Curtis WJ, Lindeke LL, Georgieff MK, Nelson CA. Neurobehavioural functioning in neonatal intensive care unit graduates in late childhood and early adolescence. Brain. 2002;125:1646–1659. doi: 10.1093/brain/awf159. [DOI] [PubMed] [Google Scholar]
- 22.Edgin JO, Inder TE, Anderson PJ, Hood KM, Clark C, Woodward LJ. Executive functioning in preschool children born very preterm: relationship with early white matter pathology. J Int Neuropsychol Soc. 2008;14:90–101. doi: 10.1017/S1355617708080053. [DOI] [PubMed] [Google Scholar]
- 23.Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, Chitnis X, Williams SC, Murray RM. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131:205–217. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- 24.Luciana M. Cognitive development in children born preterm: Implications for theories of brain plasticity following early injury. Development and Psychopathology. 2004;15:1017–1047. doi: 10.1017/s095457940300049x. (2003). [DOI] [PubMed] [Google Scholar]
- 25.Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PloS one. 2012;7(12):e51879. doi: 10.1371/journal.pone.0051879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: early parenting precursors of young children’s executive functioning. Child Dev. 2010;81(1):326–339. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- 27.Lowe J, Erickson SJ, MacLean P, Schrader R, Fuller J. Association of maternal scaffolding to maternal education and cognition in toddlers born preterm and NBW. Acta Paediatr. 2013;102(1):72–77. doi: 10.1111/apa.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson SM. Executive function in context: development, measurement, theory, and experience. Monogr Soc Res Child Dev. 2003;68(3):138–151. [PubMed] [Google Scholar]
- 29.Bibok MB, Carpendale JI, Müller U. Parental scaffolding and the development of executive function. New Dir Child Adolesc Dev. 2009;123:17–34. doi: 10.1002/cd.233. [DOI] [PubMed] [Google Scholar]
- 30.Landry SH, Miller-Loncar CL, Smith KE, Swank PR. The role of early parenting in children’s development of executive processes. Dev Neuropsychol. 2002;21(1):15–41. doi: 10.1207/S15326942DN2101_2. [DOI] [PubMed] [Google Scholar]
- 31.Hammond SI, Müller U, Carpendale JI, Bibok MB, Liebermann-Finestone DP. The effects of parental scaffolding on preschoolers’ executive function. Dev Psychol. 2012;48(1):271. doi: 10.1037/a0025519. [DOI] [PubMed] [Google Scholar]
- 32.Landry SH, Smith KE, Swank PR, Guttentag C. A responsive parenting intervention: The optimal timing across early childhood for impacting maternal behaviors and child outcomes. Dev Psychol. 2008;44(5):1335–1353. doi: 10.1037/a0013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dilworth-Bart J, Poehlmann J, Hilgendorf AE, Miller K, Lambert H. Maternal scaffolding and preterm toddlers’ visual-spatial processing and emerging working memory. J Pediatr Psychol. 2010;35(2):209–220. doi: 10.1093/jpepsy/jsp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pluess M, Belsky J. Vantage sensitivity: Individual differences in response to positive experiences. Psychological Bulletin. 2013;139:901–916. doi: 10.1037/a0030196. [DOI] [PubMed] [Google Scholar]
- 35.Poehlmann J, Schwichtenberg AJ, Shlafer RJ, Hahn E, Bianchi JP, Warner R. Emerging self-regulation in toddlers born preterm or low birth weight: Differential susceptibility to parenting? Development and psychopathology. 2011;23(01):177–193. doi: 10.1017/S0954579410000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basso O, Wilcox A. Mortality risk among preterm babies: immaturity vs. underlying pathology. Epidemiology. 2010;21(4):521–527. doi: 10.1097/EDE.0b013e3181debe5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith KE, Landry SH, Swank PR. Does the content of mothers’ verbal stimulation explain differences in children’s development of verbal and nonverbal cognitive skills? J Sch Psychol. 2000;38:27–49. [Google Scholar]
- 38.Wechsler D. The Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III) San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- 39.Kochanska G, Murray KT, Jacques TY, Koenig AL, Vandegeest KA. Inhibitory control in young children and its role in emerging internalization. Child Dev. 1996;67:490–507. [PubMed] [Google Scholar]
- 40.Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: continuity and change, antecedents, and implications for social development. Dev Psychol. 2003;36:220–232. [PubMed] [Google Scholar]
- 41.Reed MA, Pien DL, Rothbart MK. Inhibitory self-control in preschool children. Merrill Palmer Quart. 1984;30(2):131–147. [Google Scholar]
- 42.Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s theory of mind. Child Dev. 2001;72(4):1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- 43.Carlson SM, Mandell DJ, Williams L. Executive function and theory of mind: stability and prediction from ages 2 to 3. Dev Psychol. 2004;40(6):1105. doi: 10.1037/0012-1649.40.6.1105. [DOI] [PubMed] [Google Scholar]
- 44.Diamond A, Carlson S, Beck D. Preschool children’s performance in task switching on the Dimensional Change Card Sort task: separating the dimensions aids the ability to switch. Dev Neuropsychol. 2005;28(2):689–729. doi: 10.1207/s15326942dn2802_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, Fanaroff AA. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146(6):798–804. doi: 10.1016/j.jpeds.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 46.Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes AM, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aarnoudse-Moens CS, Smidts DP, Oosterlaan J, Duivenvoorden HJ, Weisglas-Kuperus N. Executive function in very preterm children at early school age. J Abn Child Psychol. 2009;37(7):981–993. doi: 10.1007/s10802-009-9327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cserjesi R, Van Braeckel KN, Butcher PR, Kerstjens JM, Reijneveld SA, Bouma A, Bos AF. Functioning of 7-year-old children born at 32 to 35 weeks gestational age. Pediatrics. 2012;130(4):e838–e846. doi: 10.1542/peds.2011-2079. [DOI] [PubMed] [Google Scholar]
- 49.Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34(4):393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- 50.Bozzette M. A review of research on premature infant-mother interaction. Newborn Infant Nurs Rev. 2007;7(1):49–55. [Google Scholar]
- 51.Goldberg S, DiVitto B. Parenting children born preterm. In: Bornstein MH, editor. Handbook of parenting. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2002. pp. 329–354. [Google Scholar]
- 52.Belsky J. The determinants of parenting: a process model. Child Dev. 1984;55:83–96. doi: 10.1111/j.1467-8624.1984.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 53.Sameroff AJ. Early influences on development: fact or fancy? Merrill Palmer Quart. 1975;21:267–294. [Google Scholar]
- 54.Sameroff AJ, Chandler M. Reproductive risk and the continuum of caretaking casualty. In: Horowitz FD, Hetherington EM, Scarr-Salapatek S, Siegel G, editors. Review of child development research. Chicago: University of Chicago Press; 1975. pp. 187–244. [Google Scholar]
- 55.Hintz SR, Kendrick DE, Vohr BR, Kenneth Poole W, Higgins RD. NICHD Neonatal Research Network. Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birth weight infants. Acta Paediatr. 2006;95(10):1239–1248. doi: 10.1080/08035250600599727. [DOI] [PubMed] [Google Scholar]
- 56.Lowe JL, Duncan AF, Bann CM, Fuller J, Hintz SR, Das A, Higgins RD, Watterberg KL. Early working memory as a racially and ethnically neutral measure of outcome in extremely preterm children at 18–22 months. Eary Hum Dev. 2013;89:1055–1061. doi: 10.1016/j.earlhumdev.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kochanska G, Philibert RA, Barry RA. Interplay of genes and early mother-child relationship in the development of self-regulation from toddler to preschool age. J Child Psychol Psychiatry. 2009;50(11):1331–1338. doi: 10.1111/j.1469-7610.2008.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carr A, Pike A. Maternal scaffolding behavior: links with parenting style and maternal education. Dev Psychol. 2012;48.2:543. doi: 10.1037/a0025888. [DOI] [PubMed] [Google Scholar]
- 59.Brown L, Iyengar S. Parenting styles: the impact on student achievement. Marriage Fam Rev. 2008;43:14–38. [Google Scholar]
- 60.Pettit GS, Tianyi Y, Dodge K, Bates JE. A developmental process analysis of cross-generational continuity in educational attainment. Merrill Palmer Quart. 2009;55(3):250. doi: 10.1353/mpq.0.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]