Abstract
The Notch signaling pathway plays a substantial role in human NK cell development. However, the role of Notch on KIR upregulation and acquisition of effector function has not been explored. To evaluate how Notch influences terminal differentiation, cord blood-derived NK cells or sorted peripheral blood NK cells were cultured with IL-15 for 7 days with inhibitory or activating Notch signals. Inhibition of Notch signaling significantly decreased KIR expression while activation enhanced it. Overexpression of activated Notch on cord blood-derived NK cells resulted in a 2-fold increase in KIR expression indicating that Notch signaling plays a direct, cell intrinsic, role in KIR regulation. Moreover, Notch mediated KIR expression on NK cells is regulated through cis-inhibition by delta-like ligand 1 (DLL1). Notch signaling also enhances CD16 upregulation that precedes KIR expression. Concomitant with the upregulation of KIR and CD16, Notch signaling induces increased cytolytic effector capacity and cytokine secretion, even in post-transplant samples where NK cell function is inherently defective. Given these attributes of Notch signaling, we propose that Notch agonists may enhance NK cell maturation and tumor killing in a post-transplant setting.
Introduction
Natural Killer (NK) cells are a critical component of the immune system where they play a role in viral responses and tumor control. Human NK cells develop from CD34+ hematopoietic stem cells (HSCs) and must traverse through a number of developmental stages prior to acquisition of CD56 expression and functional competence (1). NK cell commitment is marked by CD56 expression that can be divided further into two populations of NK cells based on CD56 intensity, with the CD56bright NK cells preceding, and giving rise to the CD56dim NK cells (2, 3). CD56dim NK cells are thought to be more functionally mature with greater cytotoxic capacity and cytokine production after target cell recognition (4–6). Acquisition of inhibitory killer immunoglobulin-like receptor (KIR) expression occurs progressively during development within the CD56dim NK cell subset and results in increased function, driving NK cell education or licensing (7–10). Although some of the components necessary for KIR expression on NK cells have been elucidated (11–13), many questions remain concerning which signaling pathways are involved in KIR expression and functional maturation of NK cells.
The Notch signaling pathway has been shown to have a role in the development and function of the innate and adaptive immune system (14). Early mouse experiments showed that Jagged2, a Notch ligand, was capable of inducing NK cell development in vitro from murine HSCs (15). However, subsequent murine studies indicated that in vivo NK cells develop independently of Notch signaling (16). In humans, our group and others have demonstrated that activation of the Notch pathway at early points in NK cell development leads to accelerated NK cell appearance in the cultures but also results in a developmental block at the CD56bright stage, thus preventing NK cells from achieving KIR expression and full maturation (17–19). Notch activation early in development abrogates the need for stroma or IL-15 to drive NK cell commitment (acquisition of CD56). More importantly, ablation of Notch signaling early on through use of γ-secretase inhibitor (gSI) or Notch-blocking antibodies resulted in almost complete loss of NK cell development, indicating that Notch signals critically influence NK cell development in humans.
Little is known about the role of Notch at later stages of NK cell maturation. One study showed that Notch activation itself can enhance IFN-γ secretion by decidual and peripheral blood NK (PBNK) cells, suggesting that Notch signaling may influence function on mature NK cells (20). Our group has demonstrated that a pair of microRNAs (miR-181a/b) that target a negative regulator of Notch signaling, nemo-like kinase (NLK), are expressed at their highest levels in the more mature CD56+ NK cells, illustrating the potential need for Notch signaling at later stages of NK cell development (21). Taken together, the data imply that Notch signaling in humans has a prominent role during early NK cell differentiation, but might also play a separate role for more mature NK cells. The present study shows that Notch signaling at later stages of NK cell development results in enhanced KIR expression, CD16 expression, and NK cell functionality. Additionally, we provide a mechanism for regulation of Notch-mediated KIR expression.
Materials and Methods
Cell Culture
Peripheral blood NK (PBNK) cells were magnetically isolated from peripheral blood through negative selection (StemCell Technologies) while umbilical cord blood (UCB) CD34-derived NK cells were differentiated from CD34+ hematopoietic progenitor cells (HPCs) isolated from umbilical cord blood by double-column positive selection using anti-CD34 microbeads (Miltenyi Biotec). Prior to magnetic separation, a Histopaque gradient (Sigma-Aldrich) was utilized to obtain mononuclear cells. Where noted, PBNK cells were further sorted into CD56+KIR−, CD56brightKIR−, or CD56dimKIR− NK cells using a FACSAria II cell sorter (BD Biosciences) and used for cell culture or processed for RNA or protein. Depending upon the experiment, UCB CD34-derived NK cells were differentiated for 21 or 28 days in culture as previously described (22). For co-culture experiments, OP9 cells (bearing different ligands or none) were maintained and plated as described prior to co-culture (23) after irradiation with 2,000 rads. All studies utilized the following media with or without γ-secretase inhibitor (Calbiochem) where noted: complete DMEM (Cellgro) with 10 ng/ml IL-15 (R&D), supplemented with 10% human AB serum (Valley Biomedical), 30% Ham F-12 medium (Cellgro), 100 U/mL of penicillin (Invitrogen), 100 U/mL of streptomycin (Invitrogen), 24µM 2-β–mercaptoethanol, 50µM ethanolamine, 20 mg/L of ascorbic acid, and 50 µg/L of sodium selenate.
Patient Samples
Transplant patient samples utilized for functional studies have been described previously (24). Briefly, 28 days post-transplant samples were harvested and cryopreserved from acute myelogenous leukemia patients that received adult donor HLA-partially matched T cell–depleted (CD34+-selected) grafts with no post-transplant immunosuppression. Cells were then incubated with the human erythroleukemia cell line K562 (2:1 (E:T) ratio) for 5 hours and NK cells were analyzed for function. Samples were obtained after informed consent and approval from the University of Minnesota Institutional Review Board in compliance with the declaration of Helsinki.
KIR-ligand-Typing
HLA-C group dimorphism is characterized by polymorphism at codons 77 (AGC vs AAC) and codon 80 (AAC vs AAA). A custom Taqman® SNP genotyping assay (Life Technologies, Carlsbad, CA) for codon 77 was tested using a LightCycler 480 instrument (Roche). HLA-B genotyping was performed in two amplification steps followed by pyrosequencing. Initial amplification step (PCRI) was as described by Pozzi et.al (25). This HLA-B specific PCR I product was then used for a second amplification step, as described by Yun et.al (26). HLA-C1, C2 or Bw4 ligands were assigned based on this sequence data.
Antibodies and Flow Cytometry
The antibodies used in this study were CD56 PE-Cy7 and APC-Cy7, CD158a/CD158b/CD158e1 PE (used in experiments were KIR were pooled), CD158e1 BV421, TNF-α AF647, IFN-γ Pacific blue, DLL1 and DLL4 PE, purified mouse IgM isotype control (BioLegend), CD158b FITC, CD107a FITC, purified mouse anti-human CD16 (BD Bioscience), CD3 ECD, CD158b APC (Beckman Coulter), and CD158a PE-Cy7 (eBioscience). For CD16 activation studies, cells were cultured with anti-CD16 or isotype control for 30 min and then crosslinked with goat-anti mouse IgG for 5 hours. Staining, acquisition, and analysis were performed as previously described (27). Finally, cells were run on an LSRII flow cytometer (BD Biosciences) and data were analyzed with FlowJo software (TreeStar).
Quantitative RT-PCR and Western Blot Analysis
For evaluation of transcripts and protein, NK cells were enriched magnetically and (where noted) further separated magnetically into KIR− and KIR+ populations. RNA was processed and qRT-PCR on Notch1 (AGTGCGTTCTGAGCCCGTGC and TTGTGACACGGGTTGGGCCG), c-MYC (ACCACCAGCAGCGACTCT and CTCTGACCTTTTGCCAGGAG), DLL1 (CGGAGGGAGCTGCACGGATCT and CGTCCGCACAGGTCATGGCA), DLL4 (CCCTGTGCCAACGGGGGACA and GTGGGCGCAAGGGTTACGGG), and GAPDH (TGTCTCCTGCGACTTCAACAGC and TGTAGGCCATGAGGTCCACCAC) was carried out with SYBR Green reagent (Applied Biosystems). For immunoblots, all procedures were done as previously described (21, 28). Notch1 antibody was purchased from Cell Signaling.
Transfection Studies
Day 28 UCB CD34-derived NK cells were placed in media with IL-15 (1 ng/mL) for 24 hours and transduced with the Amaxa Human Macrophage Nucleofector Kit (Lonza) in a Nucleofector II (Amaxa Biosystems). DNA minicircles containing GFP coding region or ICN coding region were generated and 2.7 ug (total) were transduced per transfection. For knockdown studies, we utilized 300 picomoles (total) of FlexiTube siRNA’s specific for DLL1 and the Alexa Fluor-488 labeled ALLStars Negative Control siRNA (QIAGEN). Five hours post transduction, the cells were re-plated with media plus IL-15 (10 ng/mL) and cultured in this medium alone (for minicircles) or co-cultured with in this medium with OP9 cells (for siRNA knockdowns) for 7 days.
Statistical Analysis
Paired t tests used for comparisons maintained internal pairing within each donor sample. For multiple comparisons a repeated measures ANOVA was carried out, adjusted by using Tukey’s post test. Statistical significance is indicated as NS: P>0.05; *P<p0.05; **P<0.01; and ***P<0.001. On all graphs, bars represent the mean ± SEM (29). Statistical analyses were carried out with Prism software (GraphPad Software, Inc.).
Results
Notch signaling at later stages of NK cell development
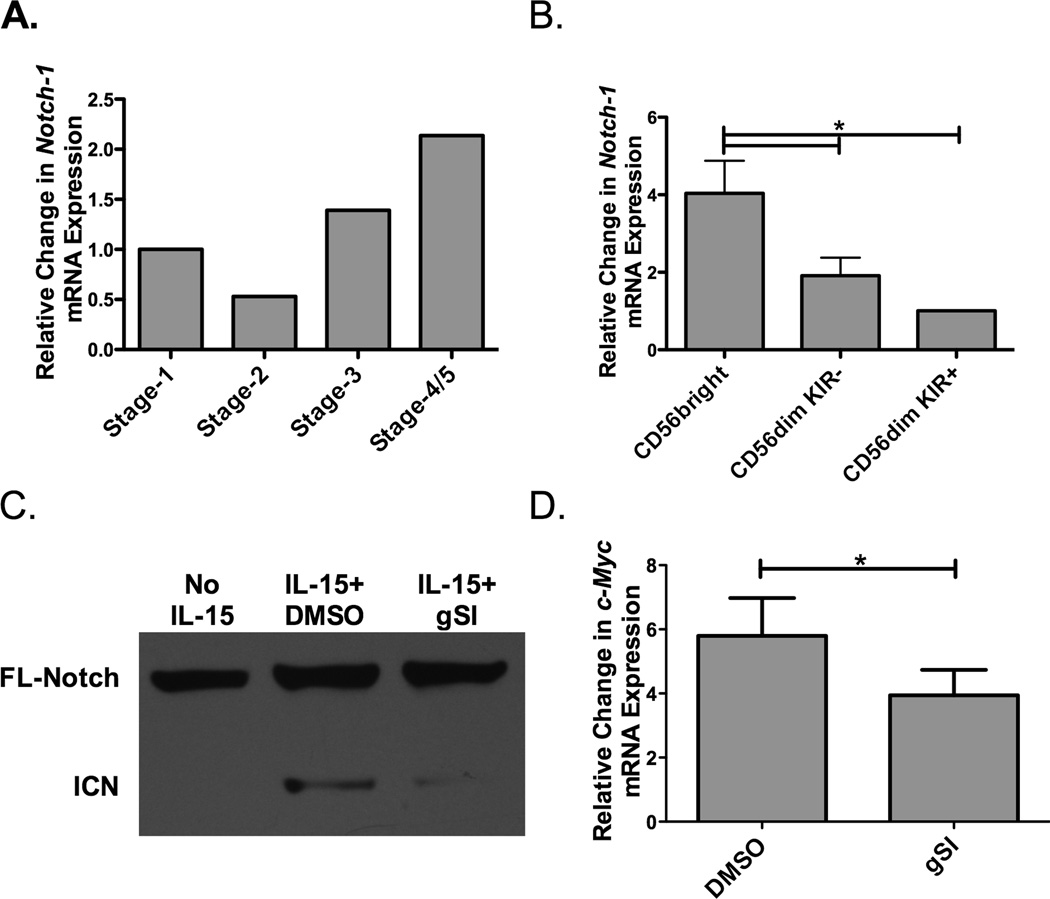
Our group and others have described a role for Notch signaling in early NK cell development (17–19). However, the role of Notch at later stages of development remains unclear. To begin to address this question, Notch-1 expression at different stages of NK cell development was assessed (Figure 1A). Cells were pooled from 5 different cord blood donors and sorted into 4 developmental groups (stage 1-CD34+CD117−CD56−, stage 2-CD34+CD117+CD56−, stage 3-CD34−CD117+CD56−CD94/CD16−, and stage 4/5-CD34−CD117−CD56+CD94/CD16+). Notch-1 transcripts were then evaluated. The data indicated that Notch-1 seems to be expressed on stage 4/5 NK cells at higher levels than on immature NK cells. To explore Notch-1 expression further in peripheral blood at later stages of development (stage 4 and 5), NK cells were sorted into CD56bright (stage 4), CD56dimKIR− (early stage 5), and CD56dimKIR+ (late stage 5) subsets (Figure 1B). Notch-1 is expressed at high levels on CD56bright NK cells, a stage that precedes CD16 and KIR expression, followed by CD56dimKIR− and CD56dimKIR+ NK cells. This expression pattern suggests that Notch signaling plays a role after NK cell commitment, at a stage where NK cell education and acquisition of function occurs.
Figure 1. Regulation of Notch expression and signaling on NK cells.
A) Pooled umbilical cord blood (UCB, n=5) was used as a source of hematopoietic progenitors and Notch-1 transcript expression (normalized to GAPDH) was determined at different stages of NK cell development (Stage 1-CD34+CD117−CD56−, Stage 2-CD34+CD117+CD56−, Stage 3-CD34−CD117+CD56−CD94/CD16−, and Stage 4/5-CD34−CD117−CD56+CD94/CD16+). (B) Peripheral blood NK cells were first enriched utilizing magnetic beads and then sorted based on CD56 expression and presence of KIR. Notch-1 transcript expression (normalized to GAPDH) was determined in CD56brightKIR−, CD56dimKIR−, and CD56dimKIR+ NK cells (n = 4). (C) Representative Western Blot of full length (FL) and intercellular Notch-1 (ICN) protein in enriched adult NK cells after overnight culture with no cytokine or 10 ng/ml of IL-15 in the presence of either DMSO or 20 µM γ-secretase inhibitor (gSI) (n = 6). (D) c-Myc transcript expression (normalized to 18s) in adult NK cells after 3 days of stimulation with 10 ng/ml of IL-15 in the presence of either DMSO or 20 µM gSI (n=6).
Our group has previously shown a direct link between c-Myc and KIR expression, mediated through IL-15 signaling (13). To test if Notch signaling is also modulated downstream of IL-15, peripheral blood NK (PBNK) cells were treated with IL-15 overnight in the presence or absence of γ-secretase inhibitor (gSI), which is known to prevent Notch cleavage and activation. Notch activation was evaluated by cleavage of Notch protein by Western blotting (Figure 1C). IL-15 induced Notch cleavage, as noted by presence of the intracellular Notch (ICN) band in the IL-15 and DMSO (the control for gSI) group vs. the unstimulated NK cells (No IL-15). Treatment with gSI resulted in diminished Notch cleavage indicating that IL-15 specifically induces Notch signaling on NK cells. As c-Myc is downstream of Notch, we asked whether IL-15 induced Notch signaling can amplify c-Myc. NK cells were treated with IL-15 in the presence or absence of gSI for three days and c-Myc transcripts were evaluated (Figure 1D). Blocking Notch cleavage reduced the c-Myc upregulation, indicating that Notch signals downstream of IL-15 are needed for optimal c-Myc expression, and perhaps also for subsequent KIR expression.
Notch signaling modulates KIR expression
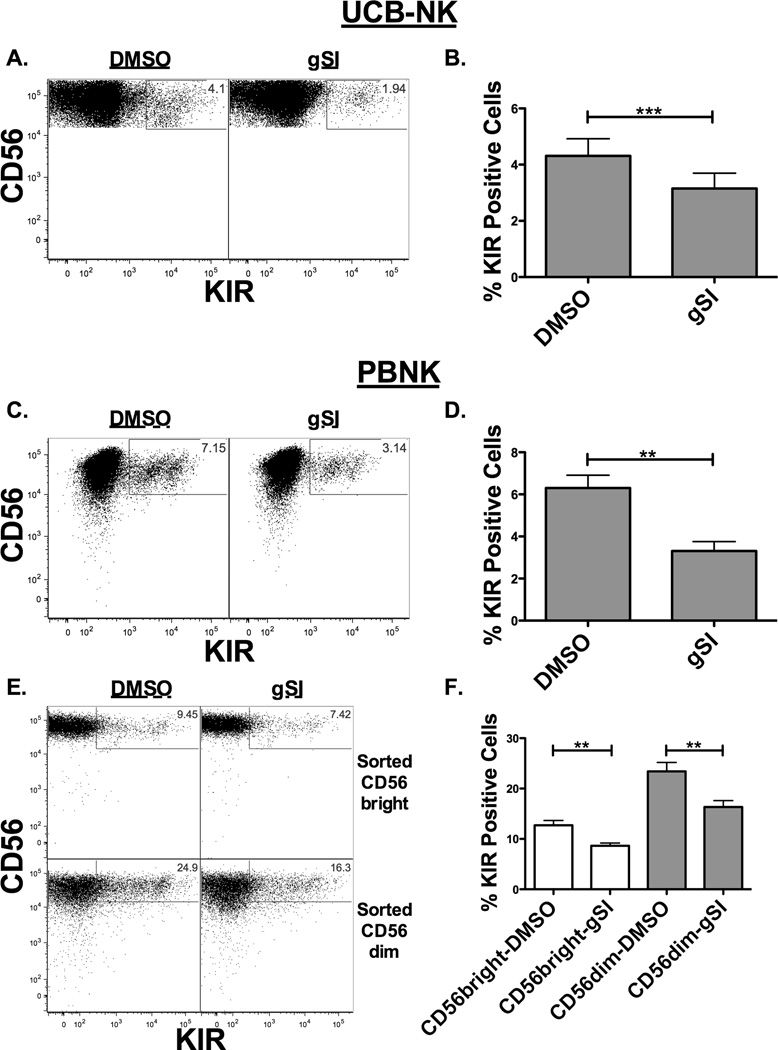
To determine whether Notch signaling can influence KIR expression at stage 4/5 of development, CD34+ cells were differentiated into NK cells for 21 days in culture to avoid the effects of Notch early during development, and then treated with or without gSI and IL-15 for 7 days (Figure 2A and 2B). Blocking Notch signaling late in development consistently resulted in decreased induction of KIR expression. These results were confirmed on sorted CD56+KIR− PBNKs that had been treated with IL-15 with or without gSI for 7 days (Figure 2C and 2D). In all conditions, blockade of Notch signaling similarly reduced KIR induction on NK cells. In additional studies, PBNK cells were further sorted into either CD56brightKIR− or CD56dimKIR− NK cells to investigate whether Notch signaling in both NK cell subsets is capable of enhancing KIR expression (Figure 2E and 2F). Interruption of Notch signaling in CD56bright and CD56dim NK cells resulted in decreased KIR expression, which has been associated with decreased function (24).
Figure 2. Notch signaling blockade results in reduced KIR expression.
KIR expression was evaluated on developing NK cells or adult NK cells after inhibition of the Notch pathway. (A–B) UCB progenitors were differentiated into NK cells by co-culture with EL08-1D2 stroma and cytokines for 21 days, harvested and cultured for an additional 7 days in 10 ng/ml IL-15 with DMSO or 20 uM gSI. Flow plots (A) and aggregate data (B) showing KIR expression on CD56+CD3− NK cells (n = 14). (C) Representative dot plot and (D) aggregate data of fresh sorted adult donor CD56+KIR− NK cells cultured for 7 days in 10 ng/ml IL-15 with DMSO or 20 uM gSI showing KIR expression on CD56+CD3− NK cells (n = 4). (E) Representative dot plot and (F) aggregate data of sorted CD56brightKIR− (top panels and open bars) or CD56dimKIR− (bottom panels and filled bars) NK cells cultured for 7 days in 10 ng/ml IL-15 with DMSO or 20 uM gSI showing KIR expression on CD56+CD3− NK cells (n = 5). On panel (F) CD56bright and CD56dim paired comparison were done separately as these represent different experiments.
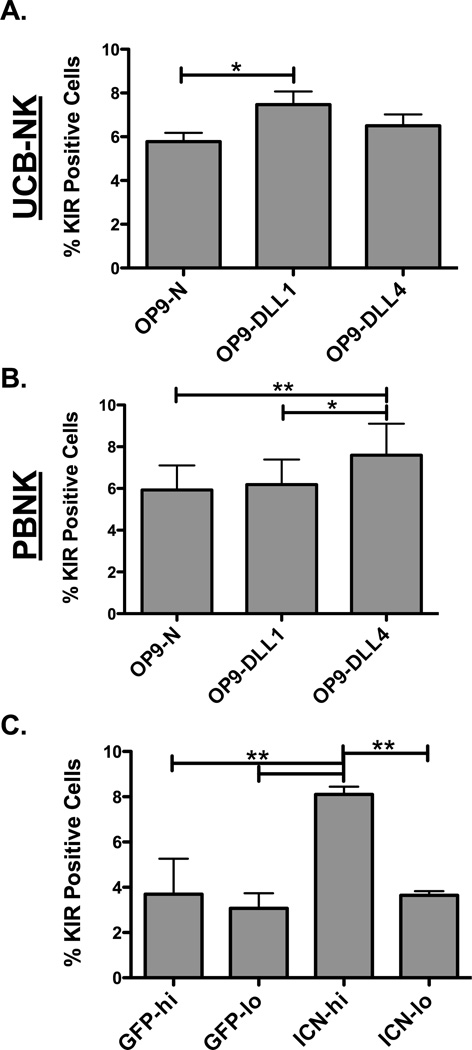
Although blockade of Notch signaling decreased induction of KIR expression, complimentary and more direct approaches were investigated to definitively show the linkage between Notch and KIR expression. Notch activation was triggered on day 21 CD34-derived NK cells (predominantly stage 4/5) by co-culture for 7 days with control OP9 cells (OP9-N) or OP9 cells expressing Notch ligands, Delta-Like 1 (OP9-DLL1) or Delta-Like 4 (OP9-DLL4), in the presence of IL-15 (Figure 3A). Co-culture with OP9 cells containing Notch ligands resulted in an increased proportion of KIR positive cells. However, the magnitude of the increase was likely impaired by rapid NK cell-directed cytotoxicity towards the stroma, which limited the sustained delivery of Notch signaling. When the same experiment was repeated with PBNKs (Figure 3B), co-culture with OP9-DLL4 cells, but not with OP9-DLL1 cells, resulted in an increased proportion of KIR positive cells. While this finding is intriguing, subtle differences have been noted in the past between DLL1 and DLL4 signaling, perhaps explaining why PBNKs and UCB CD34-derived NKs display distinctive sensitivities to the DLLs (30). To test whether the effect of Notch on KIR expression is cell intrinsic, day 28 CD34-derived NK cells were transduced with the active intracellular portion of Notch (ICN) and GFP (to track transduced cells) or with GFP alone (Supplementary Figure 1A). Cells were then cultured for 7 days with IL-15 and KIR expression was evaluated on the GFP low (GFP-lo) and GFP high (GFP-hi) populations or ICN low (ICN-lo) and ICN high (ICN-hi) populations based on the level of GFP expression in either group (Figure 3C). No differences in KIR expression were seen in the control group that had been transduced with just a GFP expressing minicircle based on either the high or low GFP expression. However, co-transduction with ICN and GFP DNA minicircles resulted in a significant induction of KIR expression in cells that showed high transduction (as evidenced by high GFP expression) compared with cells that showed low-level transduction, where levels of KIR did not differ from controls. Taken together, these data indicate that Notch signaling modulates KIR expression, and it does so in a cell-intrinsic manner.
Figure 3. Notch signaling activation leads to enhanced KIR expression.
(A) Day 21 UCB CD34-derived NK cells or (B) CD56+CD3−KIR− NK cells sorted from PBMCs were co-cultured for 7 days with 10 ng/ml IL-15 and OP9 Native (OP9-N) cells or OP9 cells expressing either delta-like 1 (OP9-DLL1) or delta-like 4 (OP9-DLL4) notch ligands. Cells were then harvested and KIR expression was assessed on CD56+CD3− NK cells (n = 4 and n = 7 respectively). (C) UCB CD34-derived NK cells were harvested at day 28, transduced with minicircles expressing GFP alone or GFP plus the active intracellular portion of Notch (ICN), and cultured for 7 days with 10 ng/ml IL-15. Cells were then gated on CD56+CD3− NK cells and high transduction (hi) or low transduction (lo) based on GFP expression. KIR proportions were assessed on the GFP control (GFP) group or the GFP plus ICN (ICN) transduced cells (n = 4).
A role for Notch signaling in NK cell education mediated by KIR interactions with cognate ligand (self-KIR) was evaluated next. Donors were KIR-ligand typed and self-KIR acquisition on CD56dim sorted NK cells (Supplementary Figure 1B) and cord blood derived NK cells (data not shown) was measured after 7 days of culture with IL-15 and gSI or DMSO control. Although more single self-KIR NK cells were present after 7 days of culture, interruption of Notch signaling by gSI resulted in a similar decrease in KIR irrespective of the presence or absence of cognate HLA ligand (Supplementary Figure 1B left panel). As NKG2A can also mediate NK cell education, we analyzed the data by excluding NKG2A (Supplementary Figure 1B center panel) and found no differences either. In aggregate, Notch signaling had no effect on overall or individual KIR expression patterns when cognate ligand was included in the analysis (Supplementary Figure 1B right panel).
Notch signaling enhances CD16 expression prior to KIR upregulation
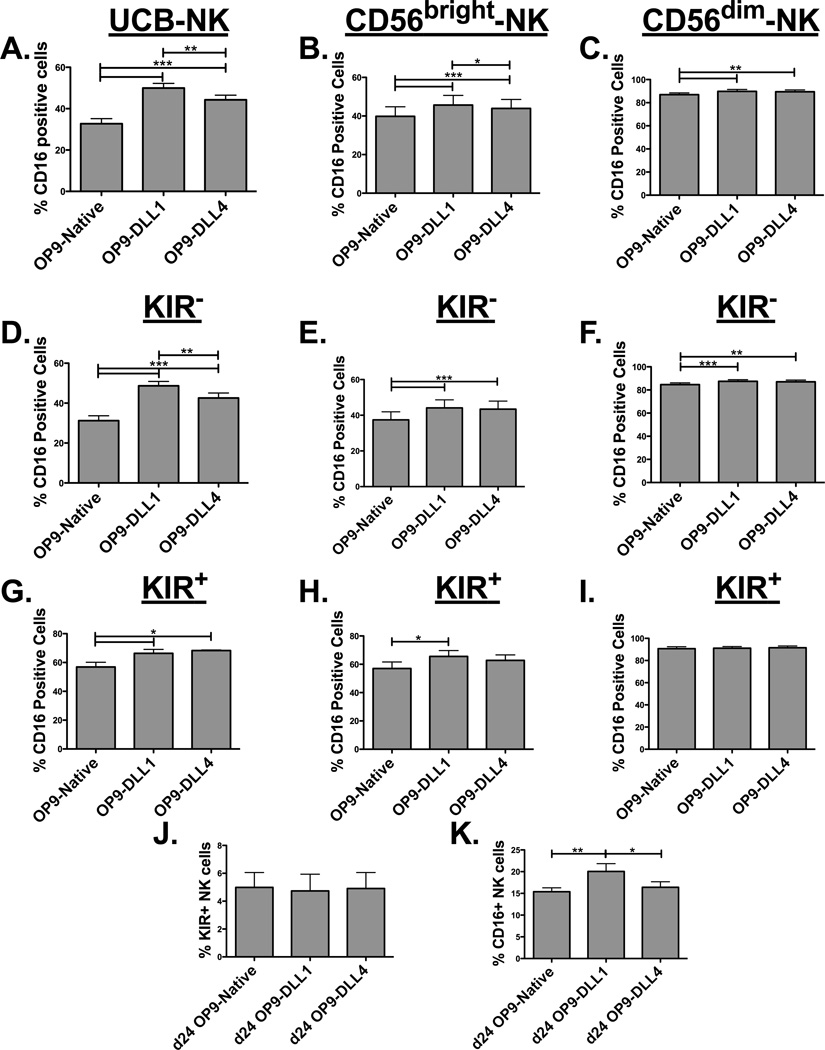
Expression of CD16 on NK cells occurs after the CD56bright stage of development as NK cells become CD56dim (1). To investigate whether Notch signals can influence CD16 expression, day 21 differentiated CD34-derived NK cells were cultured with OP9 cells expressing or lacking Notch ligands. Notch signaling at later intervals of development resulted in an increased proportion of NK cells expressing CD16 (Figure 4A). Although less so, Notch ligands also enhanced CD16 expression on CD56bright (Figure 4B) and had a minimal effect on CD56dim (Figure 4C) NK cells sorted from peripheral blood. Notch signaling increased CD16 expression (MFI) on a per cell basis on the CD34-derived UCB NK and the sorted CD56dim subsets (Supplementary Figure 1C). We next analyzed CD16 expression on NK cells without or with KIR expression. This analysis showed that Notch-mediated CD16 upregulation was more significant in the less mature KIR− NK cells (Figure 4D) than for KIR+ NK cells (Figure 4G). The differences were reduced on sorted CD56bright NK cells (Figures 4E and 4H) and further decreased on the CD56dim (Figures 4F and 4I) KIR negative peripheral blood NK cells as maturation progressed, consistent with the Notch-1 expression seen in these subsets (Figure 1B). Taken together, these data indicate that Notch signaling after NK cell commitment promotes CD16 expression at a stage prior to KIR expression. To further explore this sequencing, CD34-derived UCB NK cells were incubated with OP9 cells containing Notch ligands at day 21, but here they were harvested earlier (after 3 days [at day 24] compared to the 7 days used in other experiments) to better separate the kinetics of Notch mediated KIR versus CD16 upregulation. At day 24 Notch has not yet induced KIR expression (Figure 4J) but DLL1 has already enhanced CD16 upregulation (Figure 4K). This kinetic analysis supports the premise that Notch signals upregulate CD16 prior to KIR expression.
Figure 4. Notch signaling enhances CD16 expression prior to KIR expression.
(A) Day 21 UCB CD34-derived (n = 4), (B) PBMC sorted CD56brightCD3−KIR− (n = 8), and (C) PBMC sorted CD56dimCD3−KIR− (n = 8) NK cells were co-cultured with 10 ng/ml IL-15 and OP9-Native, OP9-DLL1, or OP9-DLL4 cells for 7 days and harvested to assess CD16 expression. (D and G) CD16 expression on KIR− or KIR+ NK cell populations from UCB NK (n =4), (E and H) CD56bright NK (n = 8), and (F and I) CD56dim NK (n = 8) in the same setting described above. (J) KIR and (K) CD16 expression was tested at day 24 (3 days after start of co-culture) of UCB NK culture (instead of day 28) to assess the role of Notch signaling on expression of these molecules at an earlier time point; co-incubation with OP9 cells was started at day 21 as previously described.
Notch signaling pushes NK cells to terminal differentiation
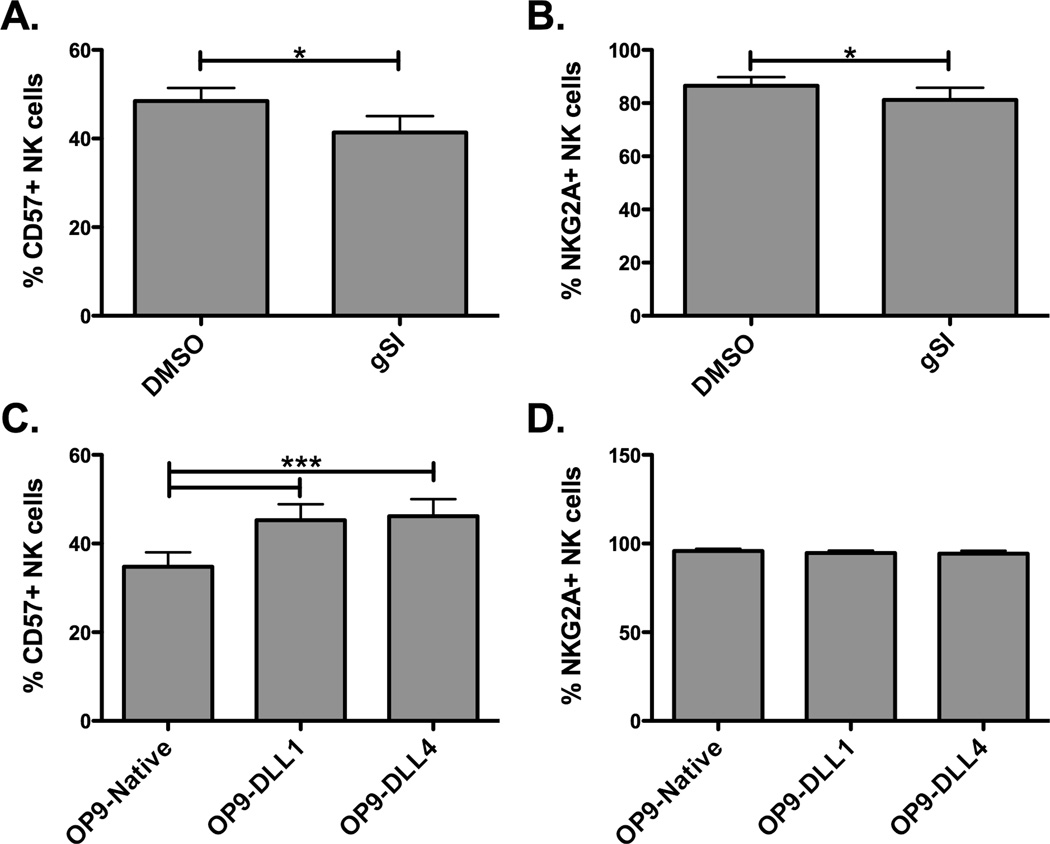
Since Notch signaling mediates KIR and CD16 expression, we next tested its effect on NKG2A and CD57, which are well characterized in expression as NK cells terminally differentiate. NKG2A is expressed at high levels on CD56bright NK cells, intermediate levels on the CD56dim NK cells, and is further decreased with KIR expression while CD57 is expressed in an opposite pattern (1, 7). To address the effect of Notch signaling on expression of these markers, CD56dimKIR− NK cells were sorted from PBMCs and placed in culture with IL-15 and Notch inhibitors or with Notch-ligand bearing cells. Inhibition of Notch signaling with gSI lowered the proportion of NK cells expressing CD57 with little effect on NKG2A (Figure 5A and 5B). Conversely, induction of Notch signaling through co-culture with OP9 cells containing Notch ligands increased the proportion of NK cells expressing CD57 by 25%, but did not have an effect on NKG2A expression (Figure 5C and 5D). These data indicate that Notch signaling is involved in either maintenance or induction CD57+ NK cells, known to be a marker of terminally differentiated and functional competent NK cells.
Figure 5. Notch signaling can also alter expression of other differentiation markers.
PBMC sorted CD56dimCD3−KIR− NK cells were co-cultured with 10 ng/ml IL-15 and DMSO or 20 uM gSI (A–B) or OP9-Native, OP9-DLL1, or OP9-DLL4 cells (C–D) for 7 days and harvested to asses CD57 (A and C) and NKG2A (B and D) expression (n = 7).
Notch signaling at later stages of development results in enhanced NK cell function
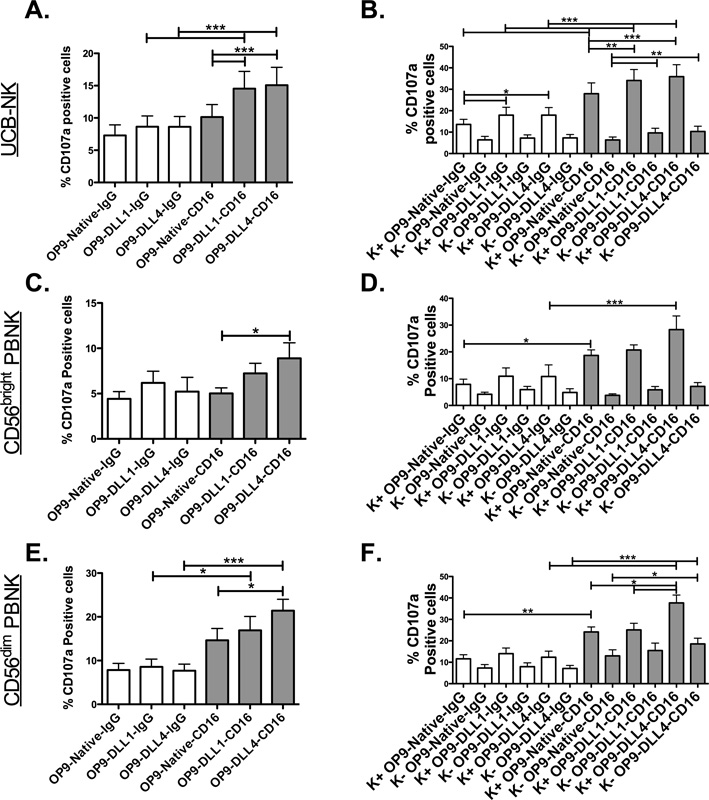
Notch-mediated induction of maturation markers (KIR, CD16, and CD57) suggests a phenotype of enhanced function. To test this hypothesis, CD34-derived NK cells or sorted PBNK cells (CD56brightKIR− or CD56dimKIR−) were co-cultured with IL-15 and OP9 cells for 7 days and then activated through CD16 crosslinking to assess NK cell activation (Figures 6A, 6C, and 6E respectively). Basal levels of CD107a expression, possibly mediated through the killing of the OP9 cells during co-culture, could be seen in the IgG control but this was not changed by Notch ligands. In contrast, CD16 crosslinking was enhanced by Notch signaling and resulted in increased CD107a (Figure 6), IFNγ and TNFα (Supplementary Figure 1D). Given that Notch signaling increases KIR expression and KIR expression is linked to NK cell function, functional readouts were stratified based on KIR expression (Figure 6B, 6D, and 6F). KIR+ NK cells were more functional than KIR− NK cells, especially after CD16 crosslinking, with the greatest fold differences found in the more immature CD34-derived NK cells and CD56bright NK cells compared to the CD56dim NK cells. In PBNK cells, DLL4 yielded significantly enhanced function, whereas in CD34-derived NK cells both ligands resulted in similar functional increases. These data indicate that Notch signaling results in enhanced function both by increasing CD16 mediated signals and by increasing the proportion of more functional KIR+ NK cells.
Figure 6. Notch signaling can enhance NK cell function through CD16 crosslinking.
UCB CD34-derived NK cells (A–B, n = 15), sorted CD56brightKIR− PBNK cells (C–D, n = 7), or sorted CD56dimKIR− PBNK cells (E–F, n = 11) were cultured with 10 ng/ml IL-15 and OP9-N, OP9-DL1, or OP9-DL4 cells for 7 days. Cells were then cross-linked with (control) mouse anti-human IgG (open bars) or mouse anti-human CD16 (filled bars). (A, C, and E) CD107a expression on total CD56+CD3− NK cells or (B, D, and F) subpopulations based on KIR expression are shown. Repeated measures ANOVA with Tukey’s multiple comparison test was utilized for statistical analysis. For KIR break up panels separate tests were carried out for KIR+ and KIR− groups to diminish statistical clutter.
Notch signaling can rescue the function of defective NK cells after transplantation
The data presented above indicate that Notch signaling at later points of NK cell development can result in enhanced function. We next choose to explore an in vivo setting of developing hematopoiesis where NK cell function is defective, as we have seen early after T cell-depleted allogeneic transplantation (24). To investigate whether Notch signaling can rescue these defects, cells were collected at day 28 after transplant, cryopreserved, and thawed PBMCs were cultured for one week with IL-15 and OP9 cells with or without Notch ligands and then activated with K562 target cells. A modest but consistent increase in degranulation was noted when the NK cells were co-cultured with Notch ligands and activated by targets (Figure 7A). A more significant increase was seen in IFNγ (Figure 7B) and TNFα expression (Figure 7C) upon target-mediated activation of effectors that had received Notch signals. At this time point after transplant, CD16 was low consistent with increased CD56bright NK cells seen early after transplantation (31). CD16 was even lower when NK cells were cultured with targets but this is explained by ADAM17 clipping induced by NK cell activation (32). Notch signaling increased basal CD16 and countered some of the downmodulation seen by target cell exposure (Figure 7D). Collectively, these findings indicate that inducing Notch signaling increases NK cell function and CD16 expression post-transplant, which could be important for inducing an NK cell graft versus tumor effect alone or in combination with antibody mediated cellular cytotoxicity through CD16.
Figure 7. Notch signaling can enhance function of NK cells early after hematopoietic cell transplantation.
Frozen PBMCs from adult donor T cell depleted grafts (d28 post transplant) were thawed, rested overnight, and placed in culture with 10 ng/ml IL-15 and OP9-N, OP9-DL1, or OP9-DL4 cells for 7 days. NK cell effectors alone (open bars/Eff) or after 5 hour co-culture with K562 target cells (filled bars/Eff+Targ) were assessed for (A) CD107a, (B) IFNγ, and (C) TNFα (n = 10). (D) CD16 was also measured on these cells to determine if Notch modulates expression on clinical samples (n = 10). Given the stark differences between controls (Eff) and activation group (Eff+Targ) repeated measures ANOVA with Tukey’s multiple comparison test was carried out individually within those groups.
Notch mediated KIR expression on NK cells is limited by delta-like ligand 1 cis-inhibition
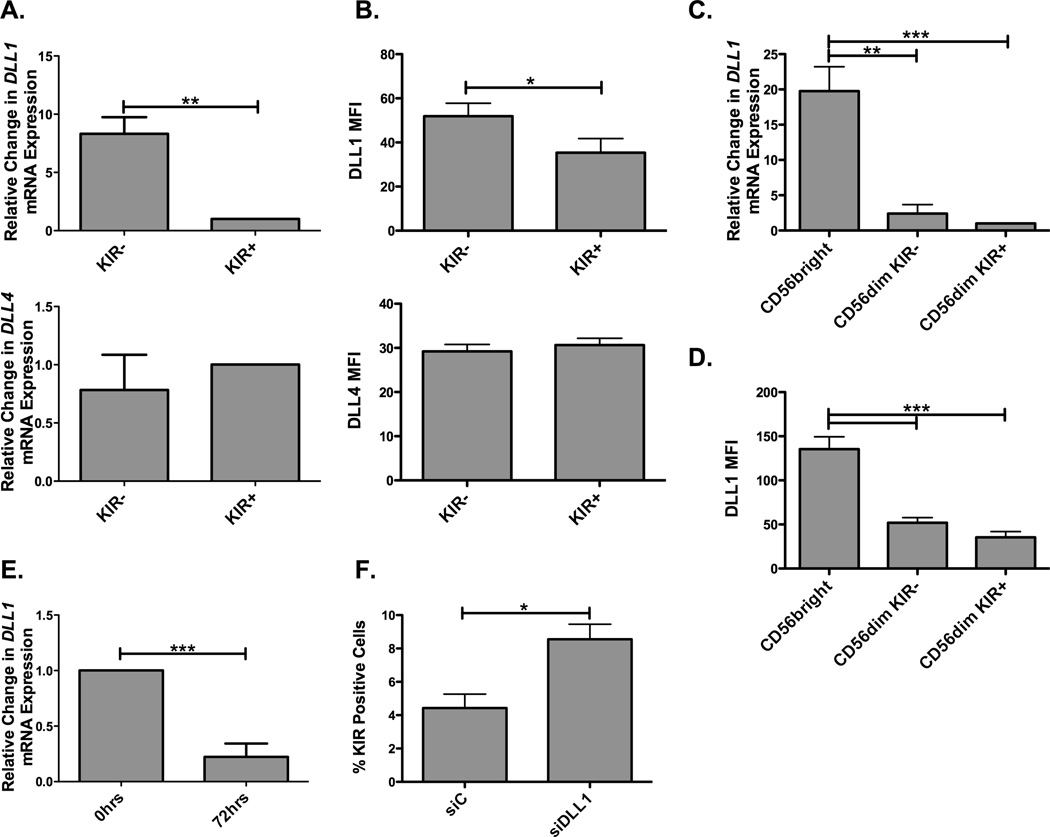
Thus far this study has focused on the role of Notch signaling in late stages of NK cell maturation and how it impacts NK cell function. However, the underlying factors that regulate Notch signaling on NK cells remain unknown. While DLL expression in trans results in Notch cleavage and activation, Delta expression in cis has been shown to inhibit Notch activation in different cell systems (33). Given these findings, since Notch signaling influences KIR expression, Delta-like ligand expression was studied in magnetically isolated KIR+ and KIR− NK cells. DLL1 transcript and protein were observed to be higher on KIR− NK cells (Figure 8A and 8B) while there were no differences in DLL4 transcript or protein expression. These results are consistent with the possibility that DLL1 cis-inhibition might be responsible for control of KIR expression on NK cells. To explore this further, NK cells were sorted into subsets and analyzed for delta-like ligand expression. DLL1 transcripts and protein (Figure 8C and 8D) were found to be highly expressed in CD56bright NK cells and expressed at the lowest levels in the most mature CD56dimKIR+ NK cell subset. No significant changes were seen in DLL4 expression (data not shown). This supports the hypothesis that cis inhibition might be strongest in the CD56bright developmental stage, which precedes expression of CD16 and KIR. Therefore, down modulation of DLL1 expression would be needed to generate CD16+ and KIR+ NK cells. We have previously shown that IL-15 can mediate KIR expression through c-Myc (13). To investigate whether exposure to this cytokine could be responsible for DLL1 downmodulation, CD56bright NK cells, which show maximal levels of DLL1, were cultured with IL-15 and DLL1 transcript expression was evaluated after 72 hours (Figure 8E). DLL1 expression was decreased 4 fold after 72 hours of IL-15 treatment, indicating that IL-15 signaling is involved in downmodulation of DLL1. This finding suggests that, amongst other mechanisms, IL-15 induced KIR expression occurs through downregulation of inhibitory cis-DLL1. To test directly whether cis-inhibition is responsible for controlling KIR expression, we transduced UCB derived NK cells with a siRNA specific for DLL1 (to knockdown DLL1 in cis) and then co-cultured the cells with OP9-DLL1 cells to provide DLL in trans (Figure 8F). Knockdown of DLL1 in cis nearly doubled the expression of KIR (compared to the siC) when the cells were exposed to delta-like ligands in trans, indicating that cis expression of DLL1 is partially responsible for restricting KIR expression on CD56bright NK cells.
Figure 8. Cis-Delta-Like 1 expression on NK cells limits Notch signaling and acquisition of KIR.
(A) A two-step magnetic enrichment was used to obtain CD56+CD3− KIR+ or KIR− NK cells and delta-like 1 (DLL1/top) and delta-like 4 (DLL4/bottom) transcripts were assessed and normalized to 18s (n = 6). (B) DLL1 (top) and DLL4 (bottom) protein expression was assessed (in terms of MFI) on CD56dimCD3− NK cells from PBMCs of healthy human donors (n = 4). (C) DLL1 transcript (normalized to GAPDH) and (D) protein (in terms of MFI) expression were analyzed from CD56bright, CD56dimKIR− and CD56dimKIR+ NK cells sorted from PBMCs (n = 4). (E) CD56bright NK cells were sorted from PBMCs and treated for 0 hrs (no treatment) or 72 hrs with 10 ng/ml IL-15. Cells were then harvested and assessed for DLL1 transcript expression and normalized to GAPDH (n = 4). (F) d28 CD34 derived NK cells were harvested and transduced with siRNA controls (siC) or siRNAs specific for DLL1 (siDLL1) and then rested for one day. Cells were next cultured for 6 days with 10 ng/ml IL-15 and OP9-DLL1 cells to provide notch signals in trans and harvested at day 35. KIR expression was then assessed on the CD56+CD3− NK cells (n = 4).
Discussion
In combination with previous data, our results suggest a temporally specific need for Notch signaling during NK cell development. Blockade of Notch signaling at an early stage in human NK cell differentiation cultures stops emergence of NK cells, indicating that Notch signaling is needed early for HSC differentiation to the NK lineage (18). Conversely, this and other studies show that activation of Notch signaling early results in accelerated NK cell development, even in the absence of stroma or IL-15, demonstrating that Notch signals comprise one of the key elements for early NK cell differentiation (17, 19). However, constitutive activation of the Notch pathway early on through transduction with the active portion of Notch results in a developmental and functional block at the CD56bright stage, thus suggesting that even though Notch signaling is necessary for NK cell commitment, it becomes detrimental at later stages of development by inhibiting full maturation. Furthermore, a stage-specific modular presence of microRNAs that control expression of NLK, a negative regulator of Notch signaling, can be seen during NK cell development, thereby demonstrating a need for tuning of Notch signals during differentiation (21). Our present study shows that Notch also signals at later stages of development to induce NK cell terminal maturation by increasing CD16 and KIR expression and that this enhances NK cell function. Taken together, these data form a developmental human NK cell model in which, to attain full functional maturation, Notch signals are required initially, detrimental during intermediate stages and important during final stages including acquisition of function.
It is important to understand the mechanisms by which Notch signaling is regulated and modulates NK cell maturation. As might be expected, IL-15, which has been shown to have a role in the maturation of NK cells and on KIR expression (11–13, 34), also has a role in this process. We show that IL-15 induces downregulation of DLL1 on CD56bright NK cells, which express high levels of DLL1, by releasing them from cis-inhibition by delta-like ligands, similar to what has been described in other cell systems (33), thus facilitating functional Notch signaling in trans. The DLL signals in trans-result in Notch-mediated cell-intrinsic upregulation of KIR, as least in part through c-Myc transcription. c-Myc transcription is downstream of both IL-15 and Notch and has been shown to bind to the distal KIR promoter and enhance its transcription (13). Our findings indicate that the effect is additive as inhibition of Notch signaling through gSI or Notch blocking antibodies (data not shown) culminated in a decrease, but not complete ablation, of KIR expression in the cultures when IL-15 was present. Of importance, Notch signaling was capable of inducing KIR, CD16, and CD57 expression on mature PBNK cells, showing that terminal NK cell maturation can be enhanced in the periphery given the proper stimulus. IL-15 is produced by several cell types, including monocytes, macrophages, dendritic cells, keratinocytes, muscle cells, renal epithelial cells, and endothelial cells (35–38), and can be upregulated through granulocyte-macrophage colony-stimulating factor (GM-CSF), toll-like receptor (TLR) agonists, and type I IFNs (37, 39). On this basis, one could surmise that pathogenic infections might be capable of inducing NK cell maturation through Notch signaling downstream of IL-15 in the periphery. In fact, our group has previously shown that CMV reactivation post-transplant enhances KIR expression and functional maturity on NK cells (27, 40), perhaps in part through this Notch-mediated mechanism. Besides possibly driving Notch signaling downstream of IL-15, pathogenic infections can also drive expression of Notch ligands on dendritic cells (DCs) (41–44), perhaps creating a more direct source for Notch signaling-mediated functional maturation of NK cells since not all Notch ligand-driven DC effects are controlled directly by T helper differentiation (45, 46).
NK cell based immunotherapies are currently being tested in the clinic (47–49). Accordingly, the possibility of enhancing NK cell maturation and function is of great translational interest. As previously noted, CMV infection induces NK cell maturation and is associated with less myeloid leukemia relapse post HSC transplant (50–52). However, the mortality rate associated with CMV infection might outweigh the tumor control benefits it provides (50–52). Therefore, understanding the mechanisms by which this particular infection drives NK cell functional maturation is critical to development of novel therapeutic strategies. Our data indicate that Notch signaling might provide one of these mechanisms. Notch signaling induces functional maturation of NK cells through both KIR and CD16 expression. We have shown that KIR+ NK cells have higher cytotoxic capabilities than KIR− NK cells, likely due to their ability to undergo education or licensing (8–10). Although we did not see a direct role for Notch signaling in specifically inducing expression of KIR for self-ligands, increasing the proportion of KIR+ NK cells (through Notch) increases the total functional potential of the NK cell population by generating a larger pool of cells capable of becoming educated and function. Our data indicate that Notch signaling also induces CD16 expression prior to KIR upregulation, possibly making NK cells more responsive to Fc-mediated stimuli independent of KIR expression. It should be noted that our co-culture system does not provide continuous Notch signaling as the NK cells kill the OP9 cells quickly, necessitating transfer of NK cells onto new OP9 cells days after initiation of the culture to maintain Notch signals. Despite these limitations, we were still able to demonstrate Notch-mediated rescue of cytotoxicity, cytokine production and CD16 expression on patient samples 28 days after transplantation. These results suggest that increasing Notch signals may be an approach to increasing NK cell maturation and function after transplantation to enhance clearance of minimal residual disease. However, Notch signals have also been shown to result in detrimental effects due to increased GVHD, so ex-vivo activation of Notch signaling might me the safer route to induce NK cell maturation (53–55). An added benefit of targeting the Notch pathway in the AML setting is that Notch activation has recently been shown to directly inhibit AML growth and survival (56). Therefore, future use of clinical grade Notch agonists might enhance clearance of tumors in at least two ways, including direct inhibition of tumor growth and survival and by increasing the immunotherapeutic value of the NK cells that target the tumors and prevent relapse.
Supplementary Material
Acknowledgements
We thank the Zúñiga-Pflücker lab for the kind gift of the OP9-DLL1 and OP9-DLL4 cells.
This project has been funded with federal funds from the NIH, under contract 2T32HL007062-36, CA65493, and CA111412.
Footnotes
The authors declare no competing financial interests.
References
- 1.Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends in immunology. 2013 doi: 10.1016/j.it.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moretta L. Dissecting CD56dim human NK cells. Blood. 2010;116:3689–3691. doi: 10.1182/blood-2010-09-303057. [DOI] [PubMed] [Google Scholar]
- 3.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 4.Juelke K, Killig M, Luetke-Eversloh M, Parente E, Gruen J, Morandi B, Ferlazzo G, Thiel A, Schmitt-Knosalla I, Romagnani C. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood. 2010;116:1299–1307. doi: 10.1182/blood-2009-11-253286. [DOI] [PubMed] [Google Scholar]
- 5.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Bjorklund AT, Flodstrom-Tullberg M, Michaelsson J, Rottenberg ME, Guzman CA, Ljunggren HG, Malmberg KJ. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116:3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 8.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–2441. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 11.de Rham C, Ferrari-Lacraz S, Jendly S, Schneiter G, Dayer JM, Villard J. The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors. Arthritis research & therapy. 2007;9:R125. doi: 10.1186/ar2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romagnani C, Juelke K, Falco M, Morandi B, D'Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, Conte R, Strowig T, Moretta A, Munz C, Thiel A, Moretta L, Ferlazzo G. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 13.Cichocki F, Hanson RJ, Lenvik T, Pitt M, McCullar V, Li H, Anderson SK, Miller JS. The transcription factor c-Myc enhances KIR gene transcription through direct binding to an upstream distal promoter element. Blood. 2009;113:3245–3253. doi: 10.1182/blood-2008-07-166389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nature reviews. Immunology. 2013;13:427–437. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 15.DeHart SL, Heikens MJ, Tsai S. Jagged2 promotes the development of natural killer cells and the establishment of functional natural killer cell lines. Blood. 2005;105:3521–3527. doi: 10.1182/blood-2004-11-4237. [DOI] [PubMed] [Google Scholar]
- 16.Nozad Charoudeh H, Tang Y, Cheng M, Cilio CM, Jacobsen SE, Sitnicka E. Identification of an NK/T cell-restricted progenitor in adult bone marrow contributing to bone marrow- and thymic-dependent NK cells. Blood. 2010;116:183–192. doi: 10.1182/blood-2009-10-247130. [DOI] [PubMed] [Google Scholar]
- 17.Bachanova V, McCullar V, Lenvik T, Wangen R, Peterson KA, Ankarlo DE, Panoskaltsis-Mortari A, Wagner JE, Miller JS. Activated notch supports development of cytokine producing NK cells which are hyporesponsive and fail to acquire NK cell effector functions. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:183–194. doi: 10.1016/j.bbmt.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haraguchi K, Suzuki T, Koyama N, Kumano K, Nakahara F, Matsumoto A, Yokoyama Y, Sakata-Yanagimoto M, Masuda S, Takahashi T, Kamijo A, Takahashi K, Takanashi M, Okuyama Y, Yasutomo K, Sakano S, Yagita H, Kurokawa M, Ogawa S, Chiba S. Notch activation induces the generation of functional NK cells from human cord blood CD34-positive cells devoid of IL-15. J Immunol. 2009;182:6168–6178. doi: 10.4049/jimmunol.0803036. [DOI] [PubMed] [Google Scholar]
- 19.Beck RC, Padival M, Yeh D, Ralston J, Cooke KR, Lowe JB. The Notch ligands Jagged2, Delta1, and Delta4 induce differentiation and expansion of functional human NK cells from CD34+ cord blood hematopoietic progenitor cells. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:1026–1037. doi: 10.1016/j.bbmt.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manaster I, Gazit R, Goldman-Wohl D, Stern-Ginossar N, Mizrahi S, Yagel S, Mandelboim O. Notch activation enhances IFNgamma secretion by human peripheral blood and decidual NK cells. Journal of reproductive immunology. 2010;84:1–7. doi: 10.1016/j.jri.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Cichocki F, Felices M, McCullar V, Presnell SR, Al-Attar A, Lutz CT, Miller JS. Cutting edge: microRNA-181 promotes human NK cell development by regulating Notch signaling. J Immunol. 2011;187:6171–6175. doi: 10.4049/jimmunol.1100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cichocki F, Miller JS. In vitro development of human Killer-Immunoglobulin Receptor-positive NK cells. Methods Mol Biol. 2010;612:15–26. doi: 10.1007/978-1-60761-362-6_2. [DOI] [PubMed] [Google Scholar]
- 23.Holmes R, Zuniga-Pflucker JC. The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harbor protocols. 2009;2009 doi: 10.1101/pdb.prot5156. pdb prot5156. [DOI] [PubMed] [Google Scholar]
- 24.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Weisdorf DJ, Miller JS. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118:2784–2792. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pozzi S, Longo A, Ferrara GB. HLA-B locus sequence-based typing. Tissue antigens. 1999;53:275–281. doi: 10.1034/j.1399-0039.1999.530308.x. [DOI] [PubMed] [Google Scholar]
- 26.Yun G, Tolar J, Yerich AK, Marsh SG, Robinson J, Noreen H, Blazar BR, Miller JS. A novel method for KIR-ligand typing by pyrosequencing to predict NK cell alloreactivity. Clin Immunol. 2007;123:272–280. doi: 10.1016/j.clim.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gleason MK, Lenvik TR, McCullar V, Felices M, O'Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, Niki T, Hirashima M, Blazar BR, Miller JS. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119:3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman DG, Bland JM. Standard deviations and standard errors. BMJ. 2005;331:903. doi: 10.1136/bmj.331.7521.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohtashami M, Shah DK, Nakase H, Kianizad K, Petrie HT, Zuniga-Pflucker JC. Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J Immunol. 2010;185:867–876. doi: 10.4049/jimmunol.1000782. [DOI] [PubMed] [Google Scholar]
- 31.Cooley S, Xiao F, Pitt M, Gleason M, McCullar V, Bergemann TL, McQueen KL, Guethlein LA, Parham P, Miller JS. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110:578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, Luo X, Cooley S, Verneris M, Walcheck B, Miller J. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 2013;121:3599–3608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.del Alamo D, Rouault H, Schweisguth F. Mechanism and significance of cis-inhibition in Notch signalling. Current biology : CB. 2011;21:R40–R47. doi: 10.1016/j.cub.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 34.Yu H, Fehniger TA, Fuchshuber P, Thiel KS, Vivier E, Carson WE, Caligiuri MA. Flt3 ligand promotes the generation of a distinct CD34(+) human natural killer cell progenitor that responds to interleukin-15. Blood. 1998;92:3647–3657. [PubMed] [Google Scholar]
- 35.Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, Grabstein K, Caligiuri MA. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. The Journal of clinical investigation. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- 37.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 38.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. The EMBO journal. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neely GG, Robbins SM, Amankwah EK, Epelman S, Wong H, Spurrell JC, Jandu KK, Zhu W, Fogg DK, Brown CB, Mody CH. Lipopolysaccharide-stimulated or granulocyte-macrophage colony-stimulating factor-stimulated monocytes rapidly express biologically active IL-15 on their cell surface independent of new protein synthesis. J Immunol. 2001;167:5011–5017. doi: 10.4049/jimmunol.167.9.5011. [DOI] [PubMed] [Google Scholar]
- 40.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 42.Skokos D, Nussenzweig MC. CD8- DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. The Journal of experimental medicine. 2007;204:1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J, Krawczyk CJ, Pearce EJ. Suppression of Th2 cell development by Notch ligands Delta1 and Delta4. J Immunol. 2008;180:1655–1661. doi: 10.4049/jimmunol.180.3.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sauma D, Espejo P, Ramirez A, Fierro A, Rosemblatt M, Bono MR. Differential regulation of Notch ligands in dendritic cells upon interaction with T helper cells. Scandinavian journal of immunology. 2011;74:62–70. doi: 10.1111/j.1365-3083.2011.02541.x. [DOI] [PubMed] [Google Scholar]
- 45.Ong CT, Sedy JR, Murphy KM, Kopan R. Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PloS one. 2008;3:e2823. doi: 10.1371/journal.pone.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worsley AG, LeibundGut-Landmann S, Slack E, Phng LK, Gerhardt H, Reis e Sousa C, MacDonald AS. Dendritic cell expression of the Notch ligand jagged2 is not essential for Th2 response induction in vivo. European journal of immunology. 2008;38:1043–1049. doi: 10.1002/eji.200737335. [DOI] [PubMed] [Google Scholar]
- 47.Murphy WJ, Parham P, Miller JS. NK cells--from bench to clinic. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:S2–S7. doi: 10.1016/j.bbmt.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thielens A, Vivier E, Romagne F. NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Current opinion in immunology. 2012;24:239–245. doi: 10.1016/j.coi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Martner A, Thoren FB, Aurelius J, Hellstrand K. Immunotherapeutic strategies for relapse control in acute myeloid leukemia. Blood reviews. 2013;27:209–216. doi: 10.1016/j.blre.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, Christoph S, Gromke T, Kordelas L, Ottinger HD, Ross RS, Horn PA, Schnittger S, Beelen DW. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 51.Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, Riddell SR, Boeckh M. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito S, Pophali P, Co W, Koklanaris EK, Superata J, Fahle GA, Childs R, Battiwalla M, Barrett AJ. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone marrow transplantation. 2013 doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Sandy AR, Wang J, Radojcic V, Shan GT, Tran IT, Friedman A, Kato K, He S, Cui S, Hexner E, Frank DM, Emerson SG, Pear WS, Maillard I. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117:299–308. doi: 10.1182/blood-2010-03-271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandy AR, Chung J, Toubai T, Shan GT, Tran IT, Friedman A, Blackwell TS, Reddy P, King PD, Maillard I. T cell-specific notch inhibition blocks graft-versus-host disease by inducing a hyporesponsive program in alloreactive CD4+ and CD8+ T cells. J Immunol. 2013;190:5818–5828. doi: 10.4049/jimmunol.1203452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tran IT, Sandy AR, Carulli AJ, Ebens C, Chung J, Shan GT, Radojcic V, Friedman A, Gridley T, Shelton A, Reddy P, Samuelson LC, Yan M, Siebel CW, Maillard I. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. The Journal of clinical investigation. 2013;123:1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kannan S, Sutphin RM, Hall MG, Golfman LS, Fang W, Nolo RM, Akers LJ, Hammitt RA, McMurray JS, Kornblau SM, Melnick AM, Figueroa ME, Zweidler-McKay PA. Notch activation inhibits AML growth and survival: a potential therapeutic approach. The Journal of experimental medicine. 2013;210:321–337. doi: 10.1084/jem.20121527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.