Abstract
Background & Aims
Continuous stimulation of pattern recognition receptors (PRRs), including nucleotide-binding oligomerization domain-2 (NOD2) (variants in NOD2 have been associated with Crohn's disease), alters the phenotype of myeloid-derived cells, reducing production of inflammatory cytokines and increasing clearance of microbes. We investigated the mechanisms by which microbial clearance increases in macrophages under these conditions.
METHODS
Monocytes were purified from human peripheral blood mononuclear cells and differentiated to monocyte-derived macrophages (MDMs). We also isolated human intestinal macrophages. Bacterial clearance by MDMs was assessed in gentamicin protection assays. Effects of intracellular zinc and autophagy were measured by flow cytometry, immunoblot, reverse transcription PCR, and microscopy experiments. Small interfering RNAs were used to knock down specific proteins in MDMs. NOD2–/– and C57BL/6J mice, maintained in a specific pathogen-free facility, were given antibiotics, muramyl dipeptide (to stimulate NOD2), or dextran sodium sulfate; intestinal lamina propria cells were collected and analyzed.
RESULTS
Chronic stimulation of human MDMs through NOD2 upregulated the expression of multiple genes encoding metallothioneins, which bind and regulate levels of intracellular zinc. Intestinal myeloid-derived cells are continually stimulated through PRRs; metallothionein expression was upregulated in human and mouse intestinal myeloid-derived cells. Continuous stimulation of NOD2 increased levels of intracellular zinc, thereby increasing autophagy and bacterial clearance. The metal-regulatory transcription factor-1 (MTF-1) was required for regulation of metallothionein genes in human MDMs. Knockdown of MTF-1 did not affect baseline clearance of bacteria by MDMs. However, the increase in intracellular zinc, autophagy, and bacterial clearance observed with continuous NOD2 stimulation was impaired in MDMs upon MTF-1 knockdown. Addition of zinc or induction of autophagy restored bacterial clearance to MDMs following metallothionein knockdown. NOD2 synergized with the PRRs TLR5 and TLR9 to increase the effects of metallothioneins in MDMs. In mice, the intestinal microbiota contributed to the regulation in expression of metallothioneins, levels of zinc, autophagy, and bacterial clearance by intestinal macrophages.
CONCLUSIONS
In studies of human MDMs and in mice, continuous stimulation of PRRs induces expression of metallothioneins. This leads to increased levels of intracellular zinc and enhanced clearance of bacteria via autophagy in macrophages.
Keywords: innate immunity, inflammatory bowel disease, signal transduction, intestinal inflammation
Introduction
Loss-of-function polymorphisms in NOD2 confer the highest genetic risk for developing Crohn's disease (CD)1. Muramyl dipeptide (MDP) is the minimal component of peptidoglycan that stimulates the cytoplasmic bacterial sensor NOD22,3. Acute NOD2 stimulation with MDP induces inflammatory cytokines4. As peripheral monocytes migrate into the intestinal lamina propria, they are chronically exposed to microbial products, including MDP5. With chronic NOD2 stimulation in myeloid-derived cells, cytokine secretion is decreased6-8 while microbial clearance is enhanced9. These dual outcomes parallel the phenotype observed in intestinal macrophages11, and likely contribute to microbial clearance in a manner that minimizes tissue injury. Moreover, human peripheral macrophages derived from CD-associated homozygote or compound heterozygote NOD2 variants do not demonstrate either of these outcomes upon chronic NOD2 stimulation6,9,10. As the pro-inflammatory cytokines that contribute to mechanisms mediating microbial clearance are significantly attenuated after chronic NOD2 stimulation, the goal of this study was to determine cytokine-independent mechanisms contributing to enhanced microbial clearance upon chronic NOD2 stimulation. Interestingly, upon chronic LPS stimulation of mouse macrophages, pro-inflammatory transcripts are downregulated, while select transcripts contributing to microbial clearance are upregulated12. Similarly, in a microarray examining LPS-induced endotoxin tolerant human mononuclear cells, proinflammatory mediators were downregulated, whereas multiple metallothionein genes were upregulated13. The specific contributions of the upregulated transcripts, including metallothioneins, to the unique phenotype of chronic PRR stimulated cells, in particular to putative increased microbial clearance, were not investigated in these studies.
Metallothioneins (MTs) are intracellular cysteine-rich proteins that maintain intracellular zinc homeostasis. A single report suggested reduced bactericidal activity in peritoneal macrophages from MT−/− mice14. Additionally, zinc can improve intestinal inflammation in both mouse and human studies15,16-18. However, mechanisms for these beneficial intestinal effects are not well understood. There is increasing recognition for the role of micronutrients such as zinc in modulating immune function and immune-mediated diseases, which points to the importance of dissecting the mechanisms through which zinc mediates its effects. Given the relationship between zinc and MTs, we hypothesized that the upregulated MT genes would contribute to the enhanced microbial clearance observed under chronic PRR stimulation in human macrophages.
We found that MTs were highly upregulated in human macrophages upon chronic NOD2 stimulation, as well as in ex vivo human and mouse intestinal myeloid-derived cells. NFκB and caspase-1 regulated MT induction in chronic NOD2-stimulated MDMs. We found that metal-regulatory transcription factor-1 (MTF-1) was a critical transcription factor regulating the expression of MT genes in human macrophages. Through targeting MTF-1, we identified that MT upregulation led to increased intracellular zinc and subsequent zinc-induced autophagy, which was critical for the enhanced intracellular bacterial clearance observed after chronic NOD2 stimulation of MDMs. Interestingly, the MTs did not contribute to baseline microbial clearance in macrophages, but rather selectively contributed to the enhanced microbial clearance observed with chronic NOD2 stimulation. Furthermore, NOD2 synergized with other PRRs, such as TLR5 and TLR9, to enhance MT-mediated outcomes. Reduction in intestinal microbiota in mice in vivo decreased, whereas enhanced intestinal microbial exposure increased, MT expression, intracellular zinc, autophagy and microbial clearance in intestinal macrophages. These studies demonstrate a novel mechanism through which microbial clearance is enhanced under the chronic PRR stimulation conditions observed in the intestine, and highlight zinc regulation as a potentially important therapeutic target in intestinal inflammation.
Materials and Methods
Recruitment and genotyping
Informed consent was obtained per protocol approved by Yale University IRB. We performed genotyping by TaqMan SNP genotyping (Applied Biosystems, Foster City, CA) or Sequenom platform (Sequenom Inc., San Diego, CA). We utilized cells from healthy individuals not homozygous for R702W, G908R or Leu1007insC NOD2 mutations.
Primary MDMs and intestinal mononuclear isolation
Monocytes were purified from human peripheral blood mononuclear cells by positive CD14 selection (Miltenyi Biotec, Auburn, CA) and cultured with 10 ng/ml M-CSF (Shenandoah Biotechnology, Warwick, PA) for MDMs differentiation. Human intestinal macrophages were isolated as described6.
Mice
NOD2−/− mice (C57BL/6J) (Jackson Laboratory, Bar Harbor, ME) were maintained in a specific pathogen-free facility and used between 2-4 months of age. Experiments were per agreement with the Yale University Institutional Animal Care and Use Committee and according to National Institutes of Health guidelines.
MDM stimulation
Cultured MDMs were treated with 100 μg/ml MDP (Bachem, King of Prussia, PA). Zinc chloride, rapamycin (Sigma-Aldrich, St. Louis, MO), BAY-117082 (Calbiochem, La Jolla, CA), JNK inhibitor II, PD98059, SB203580 (Invivogen), YVAD-cmk (Bachem), DMSO or IL-1 receptor antagonist (IL-1Ra) (GenScript, Piscataway, NJ) were used as indicated.
Transfection of small interfering RNAs (siRNAs)
SMARTpool siRNA against MTF-1, NEMO, NOD2 or caspase-1 (four pooled siRNA for each gene, Dharmacon, Lafayette, CO) or scrambled siRNA were transfected into MDMs (Amaxa, San Diego, CA).
RNA isolation and RT-PCR
RNA was isolated, reverse transcribed and quantitative PCR performed as in19 on the ABI 7500 (Applied Biosystems). Sample were run in duplicate and normalized to GAPDH. Primer sequences are available on request.
Protein expression analysis
Western blot was performed as per6 using anti-MTF-1, anti-MT-1/2 (Abcam, Cambridge, MA), anti-MT-1A (Sigma-Aldrich), anti-MT-1B/H/X (Santa Cruz Biotechnology, Dallas, TX), anti-LC3-II (Cell Signaling, Danvers, MA), or anti-GAPDH antibodies (EMD Millipore).
Intracellular zinc measurement
Intracellular zinc was measured with 1 μM FluoZin-3 AM (Invitrogen, Carlsbad, CA) according to manufacturer instructions. TPEN (8 μM; Sigma-Aldrich) was utilized to chelate intracellular zinc.
Intracellular bacterial killing
Cells were infected with Salmonella typhimurium (S. typhimurium) (10:1), adherent invasive E. coli (AIEC) strain LF82 (10:1) (generous gift from Dr. Emiko Mizoguchi) or Staphylococcus aureus (S. aureus) (1:1) for a total of 2h. Gentamicin protection assay was performed as in9 beginning 1h after bacterial infection.
Microscopy
Nuclear staining was performed with TOPRO-3 (Invitrogen). Image capture was done with a confocal laser-scanning microscope (LSM 510 META inverted Axiovert 200; Carl Zeiss).
In vivo studies
In some cases mice were first provided with a 4 week oral antibiotic regimen consisting of vancomycin hydrochloride (500 mg/L; Hospira, Inc, Lake Forest, IL), ampicillin (1g/L; DAVA Pharmaceuticals, Inc, Fort Lee, NJ), metronidazole (1g/L; Teva Pharmaceuticals, Sellersville, PA, 18960) and neomycin sulfate (1g/L; MP Biomedicals, Solon, Ohio) as per20 (96.5±1.1% reduction in fecal bacterial 16s rRNA levels). A subset of mice was treated with PBS or 100 μg MDP i.p. × 3 sequential days prior to harvest. In other cases, mice were given 1% (wt/vol) dextran sodium sulfate (DSS; MP Biomedicals, Santa Ana, CA) in their drinking water.
Intestinal lamina propria cell isolation
Small intestinal lamina propria cells were isolated as previously described21. CD11b+ cells were then isolated utilizing anti-CD11b beads ((Miltenyi Biotech). CD11b purity was >90%.
Generation of BMM
Bone marrow single-cell suspensions from mice were cultured in RPMI 1640 10% L929-conditioned medium to generate bone marrow-derived macrophages (BMM). Cultures were fed fresh medium every 3d and used at 6-8d.
Statistical analysis
Significance was assessed using two-tailed Student t test or ANOVA when analyzing multiple comparisons. p<0.05 was considered significant.
Results
Metallothioneins are upregulated after chronic NOD2 stimulation of primary human MDMs and in intestinal myeloid-derived cells
We first questioned if MTs are induced after prolonged NOD2 stimulation in primary human MDMs. We previously found 48h of NOD2 stimulation to be optimal for downregulating cytokine secretion upon restimulation through NOD2 or other PRRs6,10 and for upregulating intracellular bacterial clearance9. Human MT-1 consists of multiple isoforms, denoted by A, B, E, F, G, H, M, X22. MT transcripts were mildly increased upon acute NOD2 stimulation (4h), further increased after chronic stimulation (48h) and yet further increased in some cases upon restimulation of NOD2 (Fig 1A). The upregulation ranged from 5- to 15-fold upon restimulation relative to untreated cells for multiple MT-1 isoforms and for MT-2A (Fig 1A), and was consistently observed for each individual (Supplementary Fig 1A). In contrast, cytokine transcripts increased acutely, but decreased after chronic stimulation (Fig 1B). Consistent with the mRNA findings, MT-1 and -2 protein expression increased after prolonged NOD2 stimulation (Fig 1C-D). Consistent with their chronic exposure to microbial products, MTs were expressed at higher levels in ex vivo human intestinal myeloid-derived cells relative to unstimulated peripheral-derived macrophages (Fig 1E). Taken together, MTs are upregulated upon chronic NOD2 stimulation in human MDMs and in myeloid cells in the intestinal environment where there is ongoing PRR stimulation.
Figure 1. Chronic NOD2 stimulation upregulates MT isoforms in MDM and intestinal macrophages.
(A) MDMs were treated with 100 μg/ml MDP for 4h, or pre-treated for 48h, washed, and left untreated or restimulated for an additional 4h with MDP. Fold change MT isoform mRNA+SEM (n=8; each subject served as their own control). (B) Fold change IL-6, IL-8 and TNF mRNA+SEM. Representative western blot for (C) total MT-1/2 (n=4), and (D) (top) MT-1A and (bottom) MT-1B/H/X (n=4) in MDMs treated with 100 μg/ml for the indicated times. (E) MT-1G, MT-1M and MT-1X mRNA expression was assessed in untreated human ileal myeloid-derived cells (n=8) and peripheral MDMs (n=8). Pretx, pre-treatment; Tx, treatment; M, MDP. Significance between treated compared to untreated or select conditions as indicated. *, p<0.05; **, p<0.01; ***, p<0.001.
Chronic NOD2 stimulation increases intracellular zinc and zinc-mediated bacterial clearance in MDMs
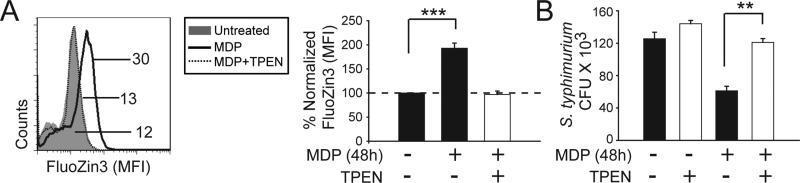
Metallothioneins play a crucial role in regulating intracellular zinc homeostasis. Consistent with the increased MT expression, chronic MDP treatment (48h) increased intracellular zinc levels compared to unstimulated cells (Fig. 2A). To determine if increased intracellular zinc contributes to the enhanced intracellular bacterial clearance after chronic NOD2 stimulation in human MDMs, we utilized a cell-permeable, high affinity zinc chelator, TPEN (N,N,N′,N′-tetrakis-(2-pyridylmethyl) ethylenediamine) to decrease intracellular zinc levels (Fig 2A). Levels of calcium (Supplementary Fig 1B) and iron (Supplementary Fig 1C) were unchanged under these TPEN-treated conditions. As previously observed9, upon chronic NOD2 stimulation MDMs demonstrated increased S. typhimurium clearance (Fig 2B). In contrast, reducing the increased intracellular zinc observed after chronic NOD2 stimulation reversed the enhanced S. typhimurium clearance (Fig. 2B). Importantly, chelating intracellular zinc did not significantly impair the ability of unstimulated MDMs to clear S. typhimurium (Fig. 2B). A similar reversal in enhanced bacterial clearance with chronic NOD2 stimulation was observed upon binding intracellular zinc through a second approach utilizing NBD-TPEA (Supplementary Fig 1D). Therefore, the increased MT expression observed upon chronic NOD2 stimulation is associated with increased intracellular zinc; this increased intracellular zinc contributes to the increased bacterial clearance observed after chronic NOD2 stimulation.
Figure 2. Chronic NOD2 stimulation increases intracellular zinc and zinc is necessary for NOD2-enhanced bacterial clearance.
(A) MDMs were treated with MDP for 48h ± TPEN for 1h. Intracellular zinc was measured by flow cytometry utilizing the zinc-detecting dye FluoZin-3. (Left) Representative flow cytometry plot with MFI values. (Right) Fold intracellular zinc induction after MDP treatment normalized to untreated cells (n=7)+SEM. (B) MDMs (n=6) were stimulated with MDP for 48h, incubated with TPEN for 1h and intracellular S. typhimurium clearance is shown as CFU + SEM.**,p<0.01; ***, p<0.001.
MTF-1 regulates MT expression in human macrophages
We next sought to determine if the upregulated MT expression after chronic NOD2 stimulation in MDMs directly regulates the increased intracellular zinc and enhanced bactericidal activity observed in these cells. As MT-1M was one of the most upregulated MTs after chronic NOD2 stimulation, we utilized siRNA to reduce MT-1M (Supplementary Fig. 2A). Upon reduction of MT-1M expression, we observed a significant, but only partial reversal of the enhanced bacterial killing observed after chronic NOD2 stimulation (Supplementary Fig. 2B). Consistently, intracellular zinc levels were only partially decreased with reduced MT-1M expression (Supplementary Fig. 2C).
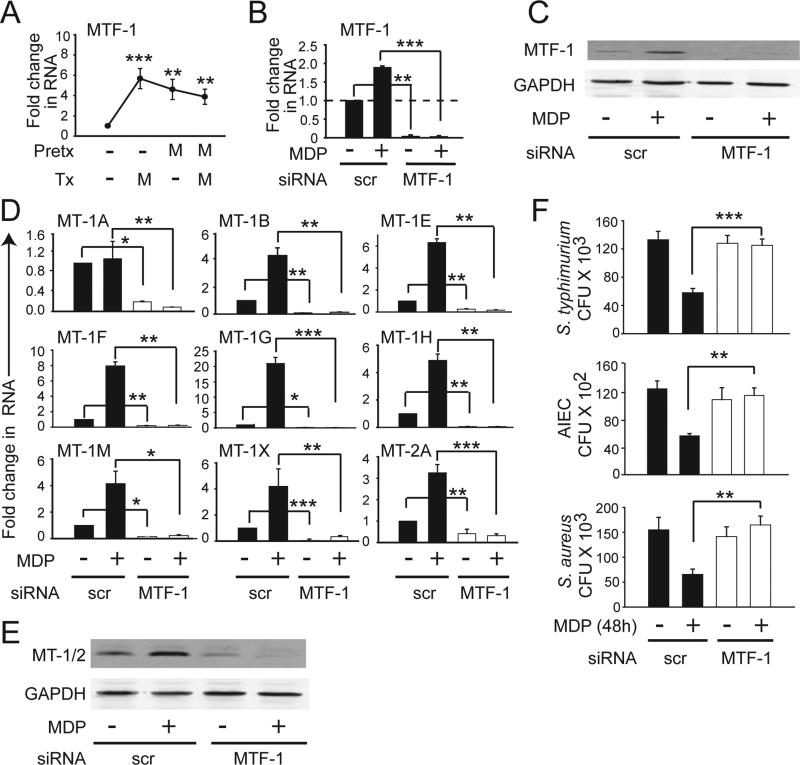
We hypothesized that the modest reversal of NOD2-enhanced bacterial killing upon MT-1M knockdown was due to compensation by the other upregulated MTs. We therefore sought to reduce expression of multiple MT isoforms simultaneously. Previous reports found that the transcription factor MTF-1 regulates endogenous MT genes in mouse systems23. Whether MTF-1 can regulate expression of MT genes in human cells or under PRR stimulation has not been assessed. MTF-1 was upregulated after acute NOD2 stimulation (Fig. 3A). Peak MTF-1 expression (Fig. 3A) occurred prior to the peak induction of MTs after NOD2 stimulation (Fig. 1A), consistent with the potential for this transcription factor to mediate MT induction. To examine the ability of MTF-1 to regulate MT expression, we utilized siRNA to MTF-1 and observed an effective decrease in baseline and NOD2-induced MTF-1 mRNA (Fig 3B) and protein expression (Fig. 3C). Importantly, upon MTF-1 knockdown, both basal and chronic NOD2-induced mRNA (Fig 3D) and protein expression (Fig 3E) for multiple MT isoforms was reduced. In summary, MTF-1 regulates baseline and NOD2-induced expression of MT isoforms in human MDMs, thereby providing an approach to address the role of MTs in NOD2-enhanced bacterial clearance.
Figure 3. MTF-1 regulates NOD2-induced MT expression and bacterial killing.
(A) MDMs were treated with MDP as in Fig.1. Fold MTF-1 mRNA induction. (B-F) MDMs were transfected with MTF-1 siRNA for 24h and then treated with 100 μg/ml MDP for 48h. (B) MTF-1 mRNA expression. (C) Representative western blot for MTF-1 expression. (D) mRNA expression of MT isoforms. (E) Representative western blot using an antibody recognizing both MT-1 and MT-2 isoforms. (F) Clearance of intracellular S. typhimurium, S. aureus or AIEC. Mean + SEM for n=4 (A-C) or n=8 (D-F). Pretx, pre-treatment; Tx, treatment; scr, scrambled; *, p<0.05; **, p<0.01;***, p<0.001.
MTs are critical for the enhanced intracellular bacterial clearance in chronic NOD2 stimulated macrophages
We next decreased MT-1 and MT-2 isoforms by knocking down MTF-1 expression and examined bacterial clearance. Reduced MTs did not impair intracellular S. typhimurium clearance in untreated human MDMs (Fig 3F), similar to the zinc chelation studies (Fig 2B). In contrast, the enhanced bacterial clearance after chronic NOD2 stimulation was completely reversed (Fig. 3F). We observed similar results upon infection with other bacteria, including AIEC (Fig. 3F), which colonize the ileum of Crohn's disease patients with increased frequency24 and S. aureus (Fig. 3F). Of note, reduced MT expression did not affect bacterial entry at early time points (Supplementary Fig 3A) or alter the reduced cytokine secretion in chronic NOD2-stimulated MDMs (Supplementary Fig 3B). Taken together, the upregulated MTs in chronic NOD2 stimulated human MDMs play a critical and selective role in enhancing bacterial clearance.
NFκB and caspase-1 regulate MT induction in chronic NOD2-stimulated MDMs
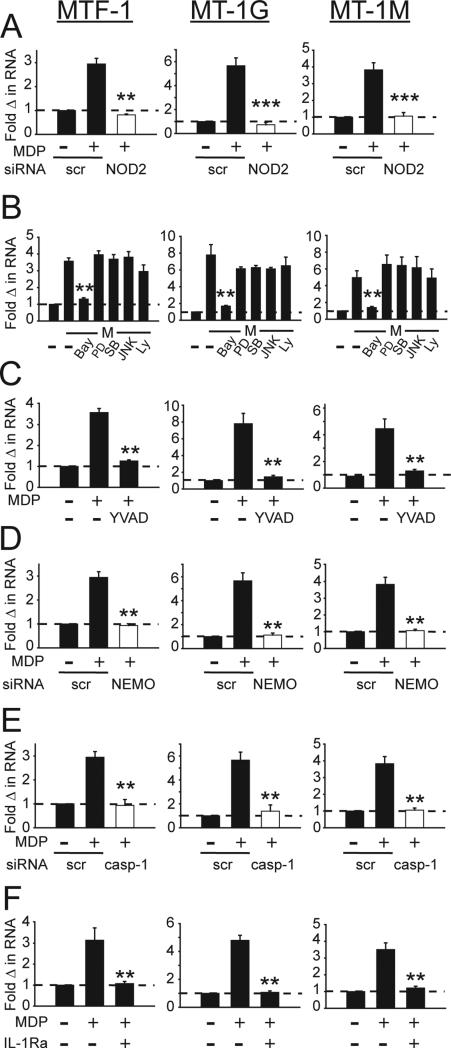
We next sought to determine the signaling pathways regulating MT induction upon chronic NOD2-stimulation. We examined MTF-1 as the overall transcription factor regulating MTs, as well as two of the more upregulated MTs, MT-1G and MT-1M. We first ensured that MTF-1 and MT induction after chronic MDP treatment was specific to NOD2-mediated signaling through NOD2 knockdown (Supplementary Fig. 4A, Fig. 4A).
Figure 4. NFκB and caspase-1 regulate MT induction upon chronic NOD2 stimulation in MDM.
(A) MDMs (n=4) were transfected with scrambled or NOD2 siRNA for 24h, and stimulated with MDP for 48h. (B&C) MDMs (n=8) were stimulated with MDP for 48h. Prior to stimulation, MDMs were incubated for 1h with (B) 0.1 μM BAY-117082 (NFκB inhibitor), PD98059 (inhibits ERK activator MEK1), SB202190 (p38 inhibitor), JNK inhibitor II, or Ly294002 (PI3K inhibitor), or (C) 0.1 μM YVAD (caspase-1 inhibitor). (D, E) MDMs (n=4) were transfected with scrambled, (D) NEMO siRNA or (E) caspase-1 (casp-1) siRNA for 24h and then treated with MDP for 48h. (F) MDMs (n=4) were treated with IL-1 receptor antagonist (IL-1Ra) for 1h prior to treatment with MDP for 48h. (A-F) Fold MTF-1, MT-1G and MT-1M mRNA induction+SEM. scr, scrambled; M, MDP. **, p<0.01; ***, p<0.001.
We next considered signaling pathways in human MDMs activated upon NOD2 stimulation, including the NFκB, MAPK and PI3K pathways25,19. We initially used pharmacological inhibitors to assess the role of these signaling pathways, and confirmed findings with independent knockdown approaches described later. Upon NFκB inhibition, chronic NOD2 stimulation failed to upregulate MTF-1, MT-1G or MT-1M, whereas inhibition of ERK, p38, JNK, or PI3K did not impair MT upregulation (Fig. 4B). To ensure these inhibitors were blocking expected downstream outcomes, we confirmed effective inhibition of NOD2-mediated TNF secretion with each of the inhibitors (Supplementary Fig. 4B). Cell viability was intact in the presence of the inhibitors. We previously found that NOD2 signaling leads to rapid caspase-1 activation, which in turn is required for subsequent select downstream responses19,25. Hence, we blocked caspase-1 signaling by YVAD and found that MDP treatment failed to upregulate MTF-1, MT-1G or MT-1M (Fig. 4C). As expected, YVAD treatment effectively decreased NOD2- mediated IL-1 secretion (Supplementary Fig. 4C). To verify the role of NFκB and caspase-1 in NOD2-induced expression of MTs through a non-pharmacological approach, we utilized siRNA to NEMO and to caspase-1 (Supplementary Fig. 4D&E), respectively. Consistent with the pharmacological inhibitor results, chronic MDP treatment failed to induce MT genes in MDMs transfected with either NEMO (Fig. 4D) or caspase-1 siRNA (Fig. 4E). Caspase-1 activation leads to cleavage of pro-IL1 and subsequent secretion of active IL-1. We previously showed in human MDMs that this caspase-1-dependent autocrine IL-1 is critical for optimal signaling and cytokine secretion upon acute NOD2 stimulation, and the enhanced bacterial clearance observed upon chronic NOD2 stimulation9,19. Through use of physiological IL1Ra to block IL-1β signaling, we found that autocrine IL-1 was critical for NOD2-mediated MTF-1 and MT induction (Fig 4F). Therefore, the NOD2-mediated induction of MT expression is dependent upon NFκB and caspase-1-mediated IL-1 secretion.
MTs regulate intracellular zinc levels
We next sought to understand the mechanism(s) through which MTs mediate enhanced bacterial killing in human MDMs after chronic NOD2 stimulation. Chronic NOD2 stimulation enhanced both expression of MTs (Fig 1) and intracellular zinc (Fig 2A), and the upregulated intracellular zinc was critical for NOD2-enhanced bacterial clearance (Fig 2B). Therefore we asked if MTs regulate the NOD2-enhanced zinc levels in human MDMs. Cells transfected with MTF-1 siRNA failed to upregulate zinc upon chronic NOD2 stimulation (Fig. 5A). Importantly, adding exogenous zinc to complement the deficient intracellular zinc levels after MTF-1 knockdown restored the enhanced bacterial clearance observed after chronic NOD2 stimulation (Fig. 5B). Taken together, upon chronic NOD2 stimulation of MDMs, expression of MTs is induced, which in turn upregulates intracellular zinc, thereby leading to enhanced intracellular bacterial clearance.
Figure 5. MT-mediated intracellular zinc induction is necessary for enhanced autophagic-mediated bacterial killing following chronic NOD2 stimulation.
(A) MDMs were transfected with scrambled or MTF-1 siRNA for 24h, then treated with MDP for 48h, and intracellular zinc was measured by FluoZin-3. (Left) Representative flow cytometry plot with MFI values. (Right) Normalized FluoZin-3+SEM (n=6). (B-E) MDMs were transfected with scrambled or MTF-1 siRNA for 24h, then treated with MDP for 48h with or without 100 μM zinc (ZnCl2). (B) Clearance intracellular S. typhimurium (n=6). (C) (Left) Representative image with nuclei stained with TOPRO-3 iodide dye (blue) and anti-LC3 antibody (red). Scale bar=10μM. (Right) Percent LC3 punctae positive cells (50 fields for each n=2 from 3 independent experiments). (D) Representative western blot and densitometric analysis (n=4) for LC3-II. (E) Intracellular S. typhimurium clearance assessed after rapamycin (1 μg/ml) treatment for 30 min. Significance comparisons are shown for select conditions. **, p<0.01; ***, p<0.001. NT, no treatment; scr, scrambled; rapa, rapamycin.
MT-mediated zinc accumulation plays a crucial role in NOD2-induced autophagy and subsequent enhanced intracellular bacterial clearance
We next sought to further define the mechanism wherein the increased MT expression and intracellular zinc levels enhance intracellular bacterial clearance after chronic NOD2 stimulation. Zinc can regulate ROS production26. We previously found that ROS increased with chronic NOD2 stimulation in MDMs due to increased expression of NADPH oxidase complex members; this increased ROS contributes to enhanced microbial clearance9. However, reducing intracellular zinc with TPEN did not impair ROS production with chronic NOD2 stimulation of MDMs (Supplementary 5A). We therefore considered modulation of other microbial clearance mechanisms. Zinc and zinc ionophores can induce autophagy in neuronal cells27,28. Acute NOD2 stimulation can also induce autophagy, which in turn, can contribute to bacterial clearance29-31. Whether autophagy is enhanced under the chronic NOD2 stimulation conditions observed at sites of ongoing microbial exposure, and whether MTs and/or zinc contribute to this process in human macrophages has not been examined. Chronic NOD2 stimulation induced autophagy as demonstrated by an increase in cells with LC3 punctae (Fig 5C) and an increase in LC3-II by western blot (Fig 5D). In contrast, autophagy was not increased after chronic NOD2 stimulation in MTF-1 siRNA-transfected cells (Fig 5C&D). We observed similar autophagic induction after zinc addition alone (Fig 5D). Moreover, addition of exogenous zinc to MTF-1 siRNA-transfected, chronic NOD2-stimulated cells was able to restore LC3-II levels (Fig 5D), consistent with the restoration of intracellular bacterial clearance with addition of exogenous zinc (Fig 5B). Cathepsin D activity in chronic NOD2-stimulated and zinc-treated macrophages was not decreased, and in fact, was increased relative to unstimulated macrophages (Supplementary Fig 5B), such that increased LC3-II was not due to lysosomal dysfunction. Moreover, with chronic NOD2 stimulation, p62 expression was decreased in the context of the increased LC3-II expression (Supplementary Fig 5C), which can serve as a measure of effective autophagy32. Finally, inducing autophagy through an independent approach utilizing rapamycin was able to completely restore the enhanced bacterial killing in MTF-1 siRNA-transfected, chronic NOD2 stimulated MDMs (Fig 5E). Rapamycin did not alter early bacterial entry (Supplementary 5D). In summary, NOD2-induced MTs and the subsequent increase in intracellular zinc are critical for inducing autophagic-mediated clearance of intracellular bacteria in human MDMs.
NOD2 synergizes with TLR5 and TLR9 to upregulate MTs, intracellular zinc, autophagy and microbial clearance
As intestinal macrophages are exposed to multiple PRRs, we next assessed if chronic stimulation of other PRR similarly upregulates MT transcript expression in human MDMs, and if these other PRRs might cooperate with NOD2 to regulate MT-mediated outcomes. We examined TLR5 and TLR9 given their relevant roles in the intestine4. MTF-1 and MT isoforms were upregulated after chronic stimulation of TLR5 (flagellin) or TLR9 (CpG); NOD2 synergized with these TLRs to further upregulate MT genes (Supplementary Fig. 6A). Moreover, NOD2 synergized with TLR5 and TLR9 to upregulate intracellular zinc, autophagy and microbial clearance (Supplementary Fig. 6B-D). The MDP-mediated synergy was dependent upon NOD2 as evidenced by NOD2 knockdown (Supplemental Fig 6). Taken together, under the chronic microbial ligand exposure that occurs in the intestinal environment, NOD2 can synergize with other PRRs to upregulate MTs and MT-mediated outcomes.
Intestinal microbiota regulate levels of MTs, intracellular zinc, autophagy and microbial clearance in intestinal macrophages in mice
Relative to peripheral macrophages, intestinal macrophages are more efficient in bacterial clearance11. Given the ability of chronic microbial ligand exposure to upregulate MTs and autophagy, we questioned if autophagy was increased in intestinal relative to peripheral macrophages in mice. We first confirmed that similar to human intestinal myeloid cells (Fig 1E), CD11b+ isolated small intestinal mouse macrophages express increased MTF-1, MT1 and MT2 relative to peripheral BMM (Fig 6A). We further found that both baseline and bacterial-induced autophagy was increased in intestinal macrophages relative to BMM (Fig 6B). Therefore, consistent with the consequences of chronic PRR stimulation in peripheral macrophages, intestinal macrophages demonstrate increased MT expression and autophagy relative to peripheral macrophages.
Figure 6. Intestinal bacteria regulate MT expression, zinc, autophagy and bacterial clearance in mouse intestinal macrophages.
(A) MTF-1, MT-1 and MT-2 mRNA expression in small intestinal (SI) compared to BM macrophages from C57BL/6 mice (n=4/group). (B) Representative western blot of LC3-II from 2 of 4 mice ± S. aureus co-culture (4h) and densitometric analysis (n=4/group) for fold increase in LC3-II+SEM. Significance is compared to uninfected BMM or as indicated. (C-F) Mice were treated with oral antibiotics (ABX) ± i.p. MDP (as per Materials & Methods) and SI lamina propria macrophages were isolated. (C) Fold change in MTF-1, MT-1 and MT-2 mRNA expression. (D) Representative flow cytometry plot for intracellular zinc and summary graph of FluoZin-3 MFI. (E) Representative LC3-II western blot and quantitative densitometry summary graph. (F) Intracellular S. typhimurium clearance. (C-F) Data is shown as mean+SEM for n=3/group. Statistical significance compared to untreated samples unless otherwise indicated. *, p<0.05, **, p<0.01; ***, p<0.001.
We next hypothesized that reducing exposure of intestinal macrophages to microbial ligands in vivo would decrease MT expression and MT-mediated outcomes. We found this to be the case, such that small intestinal macrophages harvested from mice after 4 weeks of oral antibiotic treatment demonstrated decreased expression of MTs, intracellular zinc, and LC3-II, and decreased efficacy in microbial clearance relative to mice without antibiotic treatment (Fig 6C-F). We then questioned if administration of MDP to mice treated with antibiotics could rescue the defects observed in intestinal macrophages. We selected the MDP treatment regimen used in7, as this was effective in reducing severity of experimental colitis; improved bacterial clearance might be hypothesized to be a mechanism contributing to the attenuated colitis. Three sequential days of intraperitoneal. MDP administration was able to rescue the impaired expression of MTs, intracellular zinc, LC3-II and clearance of intracellular bacteria in intestinal macrophages from antibiotic-treated mice, as well as enhance these outcomes under homeostatic conditions (Fig 6C-F).
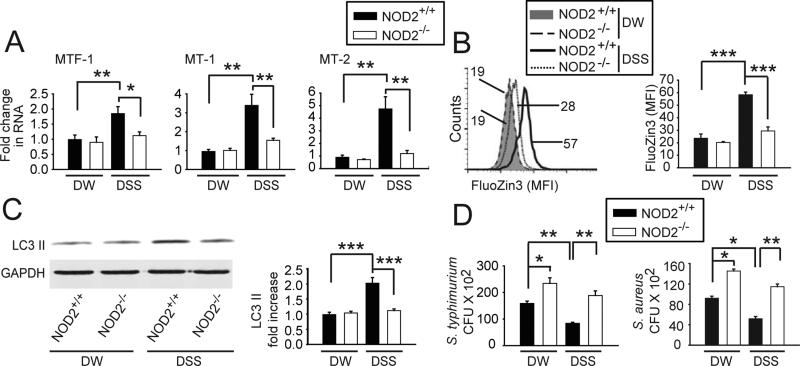
We next hypothesized that increased exposure to microbial ligands in vivo would lead to outcomes converse to those observed during antibiotic treatment. We treated mice with a short duration (2d), low concentration (1%) of DSS. Intestinal macrophages isolated from DSS-treated mice demonstrated increased expression of MTs, intracellular zinc, LC3-II and clearance of bacteria (Fig 7A-D). Although NOD2−/− mice do not develop spontaneous colitis, they are more susceptible to DSS colitis7 , such that we questioned if NOD2−/− intestinal macrophages were impaired in MT-mediated outcomes upon DSS exposure. Expression of MTs, intracellular zinc and autophagy was not impaired under homeostatic conditions in intestinal macrophages from NOD2−/− mice (Fig 7A-C). However, NOD2−/− intestinal macrophages were impaired in induction of these pathways upon DSS exposure (Fig 7A-C). Under homeostatic conditions, NOD2−/− intestinal macrophages demonstrated a defect in intracellular bacterial clearance (Fig 7D). This is consistent with multiple studies showing a role for NOD2 in bacterial clearance in peripheral macrophages4. Furthermore, NOD2−/− intestinal macrophages were unable to enhance bacterial clearance efficiency after DSS treatment to the same levels as observed in NOD2+/+ intestinal macrophages (Fig 7D). Taken together, reduced microbial exposure in vivo decreases, whereas enhanced microbial exposure increases, MT– mediated outcomes in intestinal macrophages.
Figure 7. NOD2 contributes to induction of MTs, zinc, autophagy and bacterial clearance in mouse intestinal macrophages.
NOD2+/+ or NOD2−/− mice were given 1% DSS or regular drinking water (DW) for 2 days and lamina propria macrophages were isolated. (A) Fold change in MTF-1, MT-1 and MT-2 mRNA expression compared to DW-treated NOD2+/+ mice (n=3/per group). (B) Representative flow cytometry for intracellular zinc and summary graph of FluoZin-3 (n=6/group)+SEM. (C) Representative western blot for LC3-II expression and quantitative densitometry graphs (n=5/per group)+SEM. (D) Clearance of intracellular S. typhimurium or S. aureus CFU (n=3/group; representative of 2 independent experiments)+SEM. *, p<0.05, **, p<0.01; ***, p<0.001.
Discussion
We have identified that upregulated metallothioneins serve as a critical mechanism to enhance intracellular bacterial clearance under conditions of chronic PRR stimulation in primary human MDMs. Under these chronic PRR stimulation conditions, the pro-inflammatory cytokine secretion that generally contributes to intracellular bacterial clearance is significantly attenuated, yet bactericidal activity is increased. Human intestinal macrophages demonstrate a similar phenotype in which PRR-induced cytokine secretion is decreased, while bactericidal activity is increased11, which likely contributes to enhanced bacterial clearance in a manner that minimizes tissue injury. We found that NOD2-initiated NFκB and caspase-1 activation induce the expression of a broad range of MT isoforms. These MTs upregulate intracellular zinc, which in turn, upregulate autophagy-mediated bacterial clearance after chronic NOD2 stimulation of human macrophages. Human and mouse intestinal macrophages express higher levels of MTs than peripheral macrophages, consistent with the increased bactericidal activity in intestinal macrophages. Finally, intestinal microbiota in vivo modulate MT expression and MT-mediated outcomes in intestinal macrophages in mice. It remains possible that the antibiotics and DSS used to decrease and increase microbial exposure, respectively, may also have microbiota-independent effects influencing the outcomes observed. Of note is that we utilized a low dose (1%) and duration (2d) of DSS exposure to reduce the degree of inflammation and epithelial injury. Induction of MT-mediated outcomes in intestinal macrophages during epithelial injury would provide a mechanism for improved clearance of the translocated bacteria. Therefore, we identify the metallothionein regulation of intracellular zinc as a critical mediator of enhanced bacterial clearance by macrophages under conditions of chronic PRR stimulation (Supplementary Fig. 7).
An increase in cellular MT levels can lead to an efficient increase in the intracellular available zinc pool. Our data clearly demonstrate a role for zinc in regulating autophagy-mediated bacterial clearance after chronic PRR stimulation. In addition to its crucial role in removing organelle and bacteria-containing cargo, autophagy is important in maintaining intestinal immune homeostasis. This has been highlighted by both IBD genetic studies identifying autophagy related genes33, and animal studies34,35. Moreover, autophagy can reduce inflammasome activation and cytokine secretion, thereby reducing inflammation34. Therefore, that zinc can enhance autophagy in primary human macrophages highlights zinc-mediated outcomes that can simulate phenotypic features of intestinal macrophages, wherein cytokine production is decreased and bacterial clearance is increased. Zinc deficiency exacerbates15, whereas zinc supplementation improves, the severity of experimental colitis in rodents17. In human IBD, serum zinc levels are reduced compared to the healthy individuals36. Interestingly, zinc supplementation improved intestinal permeability in a small cohort of Crohn's disease patients16. Furthermore, oral zinc supplementation is recommended for treatment of acute diarrhea in the developing world due to its ability to reduce the duration and severity of acute diarrheal symptoms18. Our studies provide insight into potential mechanisms through which zinc may be mediating its beneficial effects in these conditions. Furthermore, as we find that defects in MT-mediated bacterial killing under chronic PRR stimulated conditions are complemented by both zinc and by inducing autophagy in an independent manner, careful monitoring of zinc levels and zinc supplementation and/or additional interventions to induce autophagy may prove to be effective therapeutic strategies in diseases of intestinal inflammation, including Crohn's disease.
Supplementary Material
Acknowledgements
The authors report no conflict of interest. We thank Matija Hedl for critical reading of this manuscript. This work was supported by NIH: R01DK099097, R01DK077905, DK062422, DK-P30-34989, U19-AI082713, The Broad Foundation and the Crohn's and Colitis Foundation of America.
Abbreviations
- CD
Crohn's disease
- MDMs
monocyte-derived macrophages
- MDP
muramyl dipeptide
- MT
metallothionein
- MTF-1
metal-regulatory transcription factor
- NOD
nucleotide-binding oligomerization domain
- PRR
pattern recognition receptor
- TPEN
N,N,N′,N′-tetrakis-(2-pyridylmethyl) ethylenediamine
- BMM
bone marrow-derived macrophages
- CFU
colony forming units
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution: AL and CA contributed to study design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and obtaining funds. CA contributed to study supervision.
Authors have no conflict of interests to declare
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 3.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–12. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 4.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–37. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng S, Abraham C. NF-kappaB1 inhibits NOD2-induced cytokine secretion through ATF3-dependent mechanisms. Mol Cell Biol. 2013;33:4857–71. doi: 10.1128/MCB.00797-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci U S A. 2007;104:19440–5. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe T, Asano N, Murray PJ, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–59. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kullberg BJ, Ferwerda G, de Jong DJ, et al. Crohn's disease patients homozygous for the 3020insC NOD2 mutation have a defective NOD2/TLR4 cross-tolerance to intestinal stimuli. Immunology. 2008;123:600–605. doi: 10.1111/j.1365-2567.2007.02735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedl M, Abraham C. The NLRP1 and NLRP3 Inflammasome is Essential for Distinct Outcomes of Decreased Cytokines but Enhanced Bacterial Killing Upon Chronic Nod2 Stimulation. Am J Physiol Gastrointest Liver Physiol. 2013:583–596. doi: 10.1152/ajpgi.00297.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedl M, Abraham C. Secretory mediators regulate Nod2-induced tolerance in human macrophages. Gastroenterology. 2011;140:231–41. doi: 10.1053/j.gastro.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–8. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 13.Pena OM, Pistolic J, Raj D, et al. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol. 2011;186:7243–54. doi: 10.4049/jimmunol.1001952. [DOI] [PubMed] [Google Scholar]
- 14.Itoh N, Shibayama H, Kanekiyo M, et al. Reduced bactericidal activity and nitric oxide production in metallothionein-deficient macrophages in response to lipopolysaccharide stimulation. Toxicology. 2005;216:188–96. doi: 10.1016/j.tox.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Iwaya H, Kashiwaya M, Shinoki A, et al. Marginal zinc deficiency exacerbates experimental colitis induced by dextran sulfate sodium in rats. J Nutr. 2011;141:1077–82. doi: 10.3945/jn.111.138180. [DOI] [PubMed] [Google Scholar]
- 16.Sturniolo GC, Di Leo V, Ferronato A, et al. Zinc supplementation tightens “leaky gut” in Crohn's disease. Inflamm Bowel Dis. 2001;7:94–8. doi: 10.1097/00054725-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Sturniolo GC, Fries W, Mazzon E, et al. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. 2002;139:311–5. doi: 10.1067/mlc.2002.123624. [DOI] [PubMed] [Google Scholar]
- 18.Atia AN, Buchman AL. Oral rehydration solutions in non-cholera diarrhea: a review. Am J Gastroenterol. 2009;104:2596–604. doi: 10.1038/ajg.2009.329. [DOI] [PubMed] [Google Scholar]
- 19.Hedl M, Abraham C. Nod2-induced autocrine interleukin-1 alters signaling by ERK and p38 to differentially regulate secretion of inflammatory cytokines. Gastroenterology. 2012;143:1530–43. doi: 10.1053/j.gastro.2012.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Lahiri A, Haines GK, 3rd, et al. NOD2 Regulates CXCR3-Dependent CD8+ T Cell Accumulation in Intestinal Tissues with Acute Injury. J Immunol. 2014;192:3409–18. doi: 10.4049/jimmunol.1302436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627–47. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heuchel R, Radtke F, Georgiev O, et al. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. Embo J. 1994;13:2870–5. doi: 10.1002/j.1460-2075.1994.tb06581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412–21. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 25.Hedl M, Abraham C. Distinct roles for Nod2 protein and autocrine interleukin-1 beta in muramyl dipeptide-induced mitogen-activated protein kinase activation and cytokine secretion in human macrophages. J Biol Chem. 2011;286:26440–9. doi: 10.1074/jbc.M111.237495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John E, Laskow TC, Buchser WJ, et al. Zinc in innate and adaptive tumor immunity. J Transl Med. 2010;8:118. doi: 10.1186/1479-5876-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park MH, Lee SJ, Byun HR, et al. Clioquinol induces autophagy in cultured astrocytes and neurons by acting as a zinc ionophore. Neurobiol Dis. 2011;42:242–51. doi: 10.1016/j.nbd.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Lee SJ, Koh JY. Roles of zinc and metallothionein-3 in oxidative stress-induced lysosomal dysfunction, cell death, and autophagy in neurons and astrocytes. Mol Brain. 2010;3:1–9. doi: 10.1186/1756-6606-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travassos LH, Carneiro LA, Ramjeet M, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 30.Cooney R, Baker J, Brain O, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–7. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 31.Homer CR, Richmond AL, Rebert NA, et al. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn's disease pathogenesis. Gastroenterology. 2010;139:1630–41. 1641, e1–2. doi: 10.1053/j.gastro.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komatsu M, Waguri S, Koike M, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–63. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 33.Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955–62. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saitoh T, Fujita N, Jang MH, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–8. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 35.Cadwell K, Liu JY, Brown SL, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–63. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sikora SK, Spady D, Prosser C, El-Matary W. Trace elements and vitamins at diagnosis in pediatric-onset inflammatory bowel disease. Clin Pediatr (Phila) 2011;50:488–92. doi: 10.1177/0009922810397041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.