Abstract
A recent study reported lower anxiety in the 5xFAD transgenic mouse model of Alzheimer's disease, as measured by reduced time on the open arms of an elevated plus maze. This is important because all behaviors in experimental animals must be interpreted in light of basal anxiety and response to novel environments. We conducted a comprehensive anxiety battery in the 5xFAD transgenics and replicated the plus-maze phenotype. However, we found that it did not reflect reduced anxiety, but rather abnormal avoidance of the closed arms on the part of transgenics and within-session habituation to the closed arms on the part of wild-type controls. We noticed that the 5xFAD transgenics did not engage in the whisker-barbering behavior typical of mice of this background strain. This is suggestive of abnormal social behavior, and we suspected it might be related to their avoidance of the closed arms on the plus maze. Indeed, transgenic mice exhibited excessive home-cage social behavior and impaired social recognition, and did not permit barbering by wild-type mice when pair-housed. When their whiskers were snipped the 5xFAD transgenics no longer avoided the closed arms on the plus maze. Examination of parvalbumin (PV) staining showed a 28.9% reduction in PV+ inhibitory interneurons in the in barrel fields of 5xFAD mice, and loss of PV+ fibers in layers IV and V. This loss of vibrissal inhibition suggests a putatively aversive overstimulation that may be responsible for the transgenics’ avoidance of the closed arms in the plus maze.
Keywords: anxiety, cognition, memory, behavior, Alzheimer's disease, transgenic mice, neurodegeneration, subiculum, hippocampus, neocortex
Introduction
Alzheimer’s disease is the leading cause of dementia, characterized by progressive cognitive deterioration and changes in personality and social behaviors. Pathognomonic features of Alzheimer’s disease include aggregation of amyloid-β (Aβ) into neuritic plaques, intracellular accumulations of hyperphosphorylated tau known as neurofibrillary tangles, and widespread neurodegeneration. The inability to recapitulate cortical and hippocampal neurodegeneration, arguably the most important pathological feature of Alzheimer’s disease, is a major drawback of most mouse models. Like other transgenic lines that overexpress amyloid precursor protein (APP), the 5xFAD transgenic mouse exhibits age-related cognitive decline and development of plaques and plaque-associated pathology. However, unlike other APP-overexpressing lines, the 5xFAD transgenics also exhibit robust age-related neuronal loss in the subiculum, cortical layer V, and the medial septum/vertical limb of the diagonal band (Devi & Ohno, 2010; Eimer & Vassar, 2013; Jawhar et al., 2012; Joyashiki et al., 2011; Moon et al., 2012; Oakley et al., 2006; Ohno et al., 2007). This makes them a valuable line in which to assess novel therapeutics that attempt to prevent ongoing neurodegeneration.
Jawhar et al. (2012) recently reported an age-related anxiolytic phenotype in 5xFAD transgenics, measured by increased time on open arms in the elevated plus maze. However, the transgenics in that study spent only 10% of the time in the closed arms at the most advanced age, suggesting abnormal behavior not necessarily related to anxiety. In the plus maze, most mice will spend 70–80% of the time in closed arms, and as anxiety decreases the ratio will approach 50% (Brigman et al., 2009; Griebel et al., 2000; Wahlsten et al., 2003). This suggests that transgenic mice were actively avoiding the closed arms in that study, as if entering them was aversive. Understanding the influence of stress on experimental subjects is important for the assessment of cognition, particularly for unitary tasks in which memory or memory impairment is inferred from movement or lack of movement as opposed to choice (Harrison et al., 2009b; Mcdonald & Overmier, 1998).
To examine these issues we conducted a more comprehensive assessment of anxiety and novelty-induced stress. In doing this we discovered that the transgenic mice failed to barber the whiskers of their cage-mates, a normal behavior for many strains of group-housed mice. In the past we have seen this associated with altered social behavior (Lijam et al., 1997), and we expected that it may also be related to the transgenics’ avoidance of the closed arms on the plus maze. We report here abnormal social behavior and social recognition in the 5xFAD transgenics, but normal anxiety. Instead, the plus-maze and barbering phenotypes may be attributable to the loss of tonic inhibition in layer IV barrel cortex.
Methods
Subjects
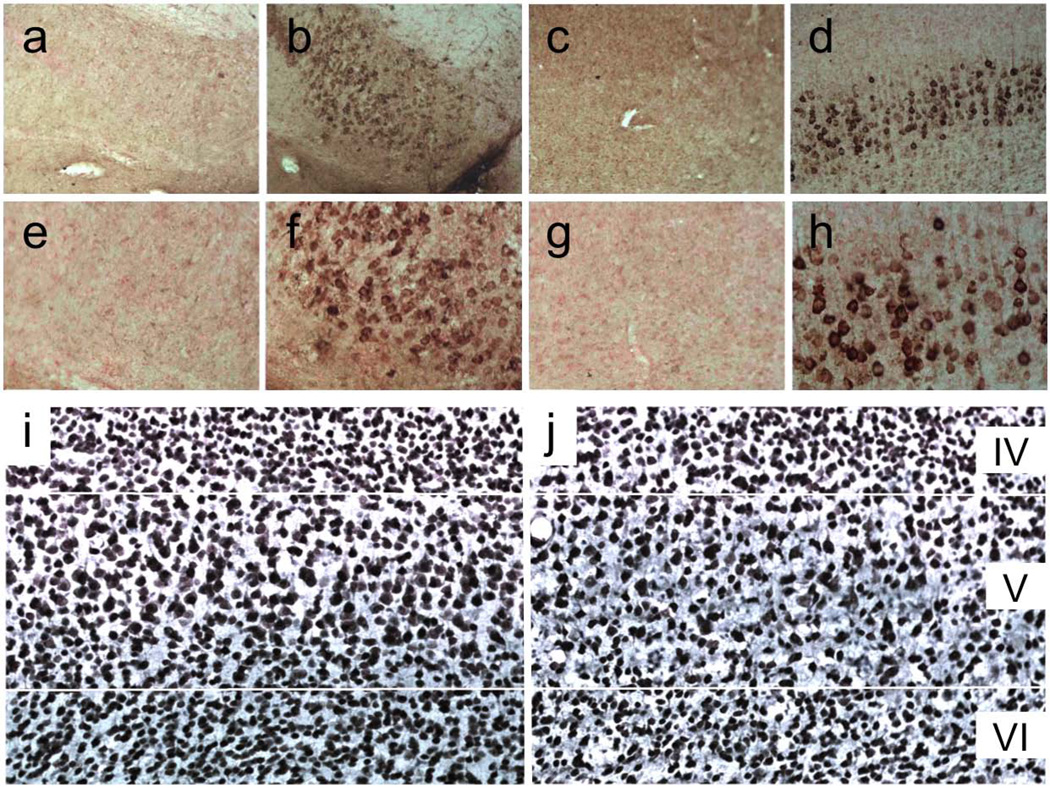
Mice were male and female 5xFAD transgenic (#006554, Jackson Labs) generated on a B6SJLF1 background (line Tg6799; Oakley et al., 2006). Mice were bred in-house with periodic back-crossing to B6SJLF1/J wild-type stock from Jackson Labs (#100012). The wild-type and 5xFAD lines carry the recessive mutant Pde6brd1 and Dysfim (dysferlin) alleles, which cause retinal degeneration and spontaneous peripheral myopathy, respectively. All mice were genotyped for both mutant alleles and any mouse homozygous for either of these mutations were excluded from the study. Most of the studies were conducted in mice 9 months of age at the start of testing and sacrificed at 14 months of age. Mice used for parvalbumin stereology were sacrificed at 12 months of age. The 5xFAD human transgenes harbor two point mutations in presenilin 1 (PSEN1; M146L & L286V), and the Florida (I716V), London (V717I), and Swedish (KM670/671NL) mutations in APP. They have detectable Aβ as early as 6 weeks of age, largely intraneuronal, with robust accumulation restricted to the subiculum and cortical layer V by 3 months (Fig. 1a–h). Interestingly, the APP levels in these two brain areas are no higher than in surrounding cortical and hippocampal regions (Oakley et al., 2006). Figure 1 shows representative images from our lab, but the Aβ accumulation and neuronal loss in the 5xFAD transgenics has been documented numerous times in the literature (Crowe & Ellis-Davies, 2013; Eimer & Vassar, 2013; Jawhar et al., 2012; Oakley et al., 2006; Ohno et al., 2007) and significant neurodegeneration has been reported as early as 2 months of age (Moon et al., 2012). Oakley et al. showed that at 2–3 months the intraneuronal staining was almost exclusively Aβ42. Mice were housed by genotype, 2–5 per cage in tub cages under standard conditions in an AALAC-approved vivarium. All mice were produced by mating a 5xFAD sire with a wild-type dam, and genotyped using PCR for both APP and PSEN1 transgenes. All 5xFAD transgenics described herein were hemizygous for both mutant transgenes. Mice had free access to food and water for the duration of the study. All experiments were conducted in the light cycle and approved by the Institutional Animal Care and Use Committee.
Figure 1. Early Aβ accumulation in the subiculum and cortical layer V in 5xFAD mice.
(a) Subicular (a,b) and layer V (c,d) Aβ accumulation in 3-month-old 5xFAD transgenics (b,d) compared to wild-type controls (a,c). Panels e–h are higher-magnification images of panels a–d, showing that Aβ accumulation at this age is largely intraneuronal. (i,j) NeuN-stained cortical sections from 14-month-old wild-type (i) and 5xFAD (j) mice. Transgenics show a considerable loss of pyramidal neurons, primarily in layer V but also evident in layer VI.
Behavior
Mice were trained on a battery of behavioral tests to measure anxiety and memory from 9 to 11 months of age. The elevated plus maze, large open field, and light-dark tests of anxiety were conducted first, followed by the first two sessions of the savings test, the water maze, and finally the last two savings sessions. Assessments of home-cage social behavior and dominance followed cognitive testing. Finally, an additional plus-maze session was given after whiskers were cut in some mice at 14 months of age, and included an additional cohort of age-matched mice with whiskers. All tasks except social cognition were conducted in the same order for all mice. Social recognition was conducted in a separate cohort of experimentally-naïve 12-month-old mice.
Sensorimotor
Locomotor activity was assessed as part of the savings test of contextual memory. Mice were placed in commercially-available activity monitors (MED-Associates, Inc., Georgia, VT) for a 10-min. session, as previously described (Dhanushkodi et al., 2013; Harrison et al., 2008, 2009a, 2010; Siesser et al., 2005; Siesser et al., 2006). The activity monitors measured 27 × 27 cm, with 16 infrared photocell beams equally spaced in the x and y axes of the horizontal plane, 1 cm from the floor of the monitor. An additional vector of 16 photobeams was situated 5 cm above the floor to track rearing.
Anxiety
Three commonly-used anxiety tests were conducted as previously described (Bernardo et al., 2009; Dhanushkodi & McDonald, 2011; Flanigan & Cook, 2011; Harrison et al., 2009b, 2009c, 2010; Reiserer et al., 2007). All three tests were conducted in the same room, under fluorescent lighting that provided illumination of 750–900 lux at the maze surfaces. Data from all tasks were collected using macros written for the public domain software NIH Image (Harrison et al., 2009b; Miyakawa et al., 2001). The elevated plus maze comprised four arms, 30 cm long × 6 cm wide, elevated 40 cm off the floor. The two “closed” arms had clear acrylic walls 15 cm high. The other two arms were “open” (without walls), but had 1-mm ridges along the edge to help prevent falling. Mice were placed gently in the central area (8 × 8 cm) at the intersection of the four arms at the beginning of the 5-min. session. An image was taken every 0.5 sec., and classified as being in open or closed arms or in the central area. The primary measure of anxiety in this task is time on closed arms as a percentage total time on arms, i.e., excluding time on the central area (Fernandes & File, 1996; File, 2001; Harrison et al., 2009b). Videos were also analyzed for risk-assessment behaviors such as stretch-attend postures and head-dipping, both inversely related to anxiety. A stretch-attend posture was scored when the mouse stretched from the central area onto an open arm of the maze, keeping its hind paws in the central area. Each stretch-attend event was classified as either "open" or "center" depending on whether the mouse subsequently entered the open arm or retreated back into the center. Head-dipping occurred when a mouse looked over the edge of an open arm with its entire head outside of and below the surface of the maze. Risk-assessment behaviors were analyzed as events per minute on the open arms, to control for significant genotype differences in the amount of time spent on open arms.
The large open field comprised a round, white tub formed from a single piece of low-density polyethylene, 92 cm in diameter with 47-cm high walls. Mice were placed in the periphery of the tub, facing the wall, and allowed to explore freely for 5 min. The field was initially divided by the software into three virtual zones of approximately equal area: an 8.4-cm-wide periphery (2,206.2 cm2), a central zone of 53.2-cm diam. (2,222.9 cm2), and an intermediate zone that comprised a 57.0-cm-wide annulus (2,218.6 cm2) between the periphery and the central zone (Dhanushkodi & Mcdonald, 2011). The activity and time spent in each zone was recorded, as well as latency to leave the periphery and enter the central area.
The light/dark test assessed the tendency of mice to explore a novel, lighted area when a dark area is available (Bazalakova et al., 2007; Bernardo et al., 2009; Dhanushkodi & Mcdonald, 2011; Harrison et al., 2009b). The apparatus was an acrylic box 30 cm wide × 40 cm deep × 30 cm high, divided into light and dark compartments 20 cm deep. Mice were placed in the dark compartment from a hinged lid at the top, which was connected to the lighted compartment by a small hole. The latency to emerge into the light and time spent in each compartment were measured.
Cognition
Three cognitive tests were used to help us understand the potential interaction of anxiety and cognition: savings, water maze, and social recognition. Savings is the tendency to learn something more quickly a second time (Ebbinghaus, 1885). For mice, the savings test takes advantage of a mouse's tendency to habituate over time in an environment. We have shown in three papers that APP/PSEN1 transgenic mice are impaired on a contextual savings task (Harrison et al., 2009a; Harrison et al., 2010; Harrison et al., 2008). In the present study, mice were placed in commercially-available activity monitors (MED Associates) for four 10-min. sessions. The first two sessions were 24 hours apart, and the last 2 were 24 hours apart 6 weeks later, each time in the same activity monitor. The mouse was left undisturbed in its home cage for the 24 hours between first two and last two sessions. Savings is calculated as the difference in activity between sessions 3 and 1 divided by the difference in activity between sessions 2 and 1.
Spatial learning and memory were assessed in a water maze, 118 cm in diameter, as previously described (Bernardo et al., 2009; Bernardo et al., 2007; Dhanushkodi & Mcdonald, 2011; Ding et al., 2008). The water ranged from 22.5–23.0° C and was made opaque using non-toxic white tempera paint. A clear acrylic platform 10 cm in diameter was submerged 0.5 cm below the surface of the water. Water maze testing was conducted in four phases: channel training, cued-platform, hidden-platform, and probe. Channel training involved four trials on each of two days in which mice were placed in one side of a channel within the pool and trained to swim straight to the platform on the other side. The channel consisted of pair of acrylic walls that spanned the diameter of the pool, with a platform on one side adjacent to the wall. The platform was 0.5 cm below the surface of the water and the channel walls were 15 cm apart. Mice were placed in one end and were given 60 s to reach the platform on the other side. The purpose of the channel training was to acclimate the mice to the water environment and training regimen involving four trials and an escape platform, as well as to measure their swim speed while swimming straight. Swim speed is typically measured during acquisition or probe trials, during which mice may have short latencies or be turning repeatedly in search of the platform. With short-latency trials mice do not reach full speed; similarly, when turning mice typically slow down. Thus these measures may not be the most accurate measure of the mouse’s swim speed. In channel training as well as all other phases, mice were placed gently in the water at the edge of the pool, facing the wall, to begin each trial. Swim speed during channel training was taken from the last three trials on the second day of training, in a 67-cm stretch of straight swimming beginning 34 cm from the starting point and ending 7 cm from the proximal edge of the platform.
Two daily sessions of cued-platform training were conducted after channel training. Four trials per session were conducted in spaced fashion, i.e., each mouse received its first trial before the first mouse received its second trial. In this phase, the platform was marked by a black acrylic ball 30 cm above the platform, atop a 0.5-cm diameter vinyl pole arising from the center of the platform. The platform and starting locations varied randomly across trials, with four possible platform locations in equivalent locations in the center of each of four virtual quadrants in the pool. Data were captured and analyzed using NIH Image macros specially-written for water maze (Harrison et al., 2009b; Miyakawa et al., 2001). Swim paths were recorded and swim distances, escape latencies, and search error were calculated from the recordings. Swim speed and the amount of time spent in the periphery (8 cm) of the pool were also recorded.
At the end of cued-platform training, hidden-platform reference-memory training was conducted as previously described (Bernardo et al., 2007, 2009; Dhanushkodi & McDonald, 2011; Harrison et al., 2008, 2009a, 2009b, 2009c, 2010). In this task, mice were trained to find the hidden platform using the visuospatial extra-maze room cues, with four massed trials per day for 11 days and a 20-sec. inter-trial interval (ITI). The platform location did not change during the course of training, but the starting location varied randomly from trial to trial. Mice not finding the platform within 60 s were placed gently on the platform for the duration of the ITI.
A probe trial to assess memory was conducted 24 hours after the last hidden-platform session, in which the platform was removed and mice were allowed to swim freely for 60 s. Swim paths were recorded and used to calculate the amount of time spent in a 40-cm diam. zone centered on the former platform location and equivalent positions in each of the other three quadrants. Platform crossings and time spent in the 40-cm target zone were used as primary measures of spatial memory. The 40-cm zone, centered in the target quadrant, is a more precise measure of spatial memory than total time in the target quadrant for several reasons. First, the area is less than half that of the quadrant as a whole. Second, the circular zone reaches nearly to the radii defining the sides of the quadrant but excludes the periphery; thus adventitious peripheral swimming is not counted toward a good memory score. Finally, with a quadrant analysis in a pool this size greater accuracy is not always associated with quadrant location. For example, in a 118-cm-diam. pool a mouse might be as far as 41 cm from the platform but still inside the target quadrant, and as near as 22 cm from the platform but in a neighboring quadrant.
Social recognition
Five 2-min. social recognition sessions were conducted for each mouse. In each session, a juvenile mouse, 30–35 days old, was placed in a clean tub cage with the adult 5xFAD or wild-type mouse. The sessions were video-recorded and scored for interaction. The same juvenile mouse was used for the initial test and recognition tests at 90 min. and 24 h. following the initial test. Immediately after each recognition test, a novel juvenile mouse was placed in the cage for 2 min. The social recognition scores were calculated as the ratio of time spent exploring a juvenile on re-exposure divided by the initial exposure, i.e., recognition/initial*100. A discrimination ratio was also calculated to determine whether mice spent more time exploring the novel juvenile. Mice spending equal time exploring both juveniles (poor memory) received a score of 100; greater exploration of the novel juvenile resulted in scores greater than 100.
Home-cage and social behaviors
As part of our normal colony management, we check for wounds, missing fur, whisker status, etc., in all mice. Male and female mice of many strains bite or “barber” each others’ whiskers, as well as the fur surrounding the whiskers. Typically, it’s the dominant mouse who barbers the whiskers of the other mice in the cage, and it is not uncommon to see mice in the colony without whiskers. All mice were housed by genotype, and we noticed that the 5xFAD mice did not engage in the home-cage whisker-barbering behaviors normally exhibited by mice of this background strain. Only one wild-type mouse in each cage had vibrissae, whereas all 5xFAD transgenics had a full complement. In the past we discovered that lack of barbering was associated with deficient social behavior (Lijam et al., 1997). Thus, after water-maze testing the home cage behaviors were recorded for a duration of 4 hours, and social interactions scored. Dominance was measured using a two-mouse tube test, as previously described (Lijam et al., 1997). Each 5xFAD transgenic was tested against eight wild-type mice of the same gender—two with full whiskers and six without whiskers. Mice were placed simultaneously at the ends of a darkened tube, 30 cm long × 2.5 cm in diameter, and tails gently pulled to encourage entry. After meeting in the middle of the tube, the mouse that backed out was deemed submissive. Following the dominance test, the cage compositions were re-arranged for many of the mice, such that one wild-type mouse without whiskers was paired in a cage with one 5xFAD transgenic with a full set of whiskers. After two weeks together the mice were re-examined for whisker status. At 14 months of age, whiskers were cut to <1 mm in length, and mice were re-assessed on the elevated plus maze. A cohort of experimentally-naïve mice of the same age, five of each genotype and all with a full set of whiskers, was assessed on the plus maze at the same time.
Histology and immunohistochemistry
Mice were perfused transcardially under brief isoflurane anesthesia, first with ice cold saline and then with 4% paraformaldehyde for 30 min. Brains were removed and fixed overnight in the same fixative, and 40-µm coronal sections were taken through the rostral-caudal extent of the hippocampus for histological analysis. Sections were stained with mouse anti-NeuN (MAB 377, 1:1000, Millipore) to illustrate neuronal loss or 4G8 mouse antibody targeting human and mouse Aβ and APP (SIG-39220, 1:500, Signet Laboratories), followed by a horse anti-mouse biotinylated secondary antibody treated with avidin-biotin complex (Vectastain Elite ABC kit, Vector Labs) and visualized using diaminobenzidine. Images were captured using a Nikon Microphot FX microscope. To visualize GABAergic interneurons in the somatosensory cortex, sections were stained with 4',6-diamidino-2-phenylindole (DAPI; D1306, Invitrogen) and/or a rabbit anti-rat antibody targeting parvalbumin (PV-25, 1:5000, Swant). Sections were visualized using 3,3'-diaminobenzidine (DAB) for stereology or an Alexa Fluor 594 donkey anti-rabbit IgG (A21207, 1:200, Invitrogen Molecular Probes) for imaging with a BioRad MRC 1024 confocal microscope.
Unbiased stereology
Stereological quantification of DAB-stained PV-positive neurons were conducted in three mice of each genotype as previously described (Dhanushkodi et al., 2013) using Stereo Investigator software (MicroBrightField). Sections were counter-stained with methylene blue to better identify barrels. For each mouse, PV+ neurons were counted in layer IV barrels and barrel-associated layer V of the left hemisphere of one-eighth of the cortical sections (10–11 sections per mouse), starting with a random section for each mouse. The contour of layer V was first delineated using Stereo Investigator's anatomical mapping tool at low power. PV+ cell bodies were then counted in frames of 25 × 25 µm in each of the selected sections, generated using Stereo Investigator's random sampling grid. The frames were selected using the systematic random sampling scheme, which provides an unbiased and efficient sampling technique. In every counting-frame location, the top of the section was identified, after which the plane of the focus was moved 4 µm deeper through the section (guard zone) to prevent counting inaccuracies due to uneven section surfaces. The resulting focal plane served as the first point of the counting process. All PV-positive cells that came into focus in the next 8-µm segment (dissector height) were counted if they were entirely within the counting frame or touching the upper or right side of the counting frame. One wild-type mouse was excluded because the identified region of interest only included 48 sampling frames and generated an unacceptably high Gunderson coefficient of error. For the remaining mice 623–1153 counting frames per mouse were sampled in layer V and 433–761 counting frames per mouse from 29 (wild-type) and 30 (5xFAD) barrels were sampled in in layer IV.
Statistical analyses
Most behavioral data were analyzed using factorial analyses of variance (ANOVA) or repeated-measures ANOVA (RMANOVA), with genotype as a between-subjects factor, followed when appropriate by orthogonal t-tests. Conceptually-similar measures were analyzed together using multivariate ANOVA (MANOVA) fitted to a sum matrix. Time-series data were analyzed using hierarchical linear modeling, with time as a balanced continuous repeated measure and subject as a random nominal factor nested within genotype. Dominance scores were analyzed using single-sample t-tests against chance (50%). Test statistics for skewness and kurtosis were calculated as skewness and excess kurtosis divided by their respective standard errors (Cramer, 1997). To protect against spurious Type I errors, follow-up analyses were conducted only after a significant omnibus effect, except with comparisons having specific a priori hypotheses. All comparisons were two-tailed with α set at 0.05.
Results
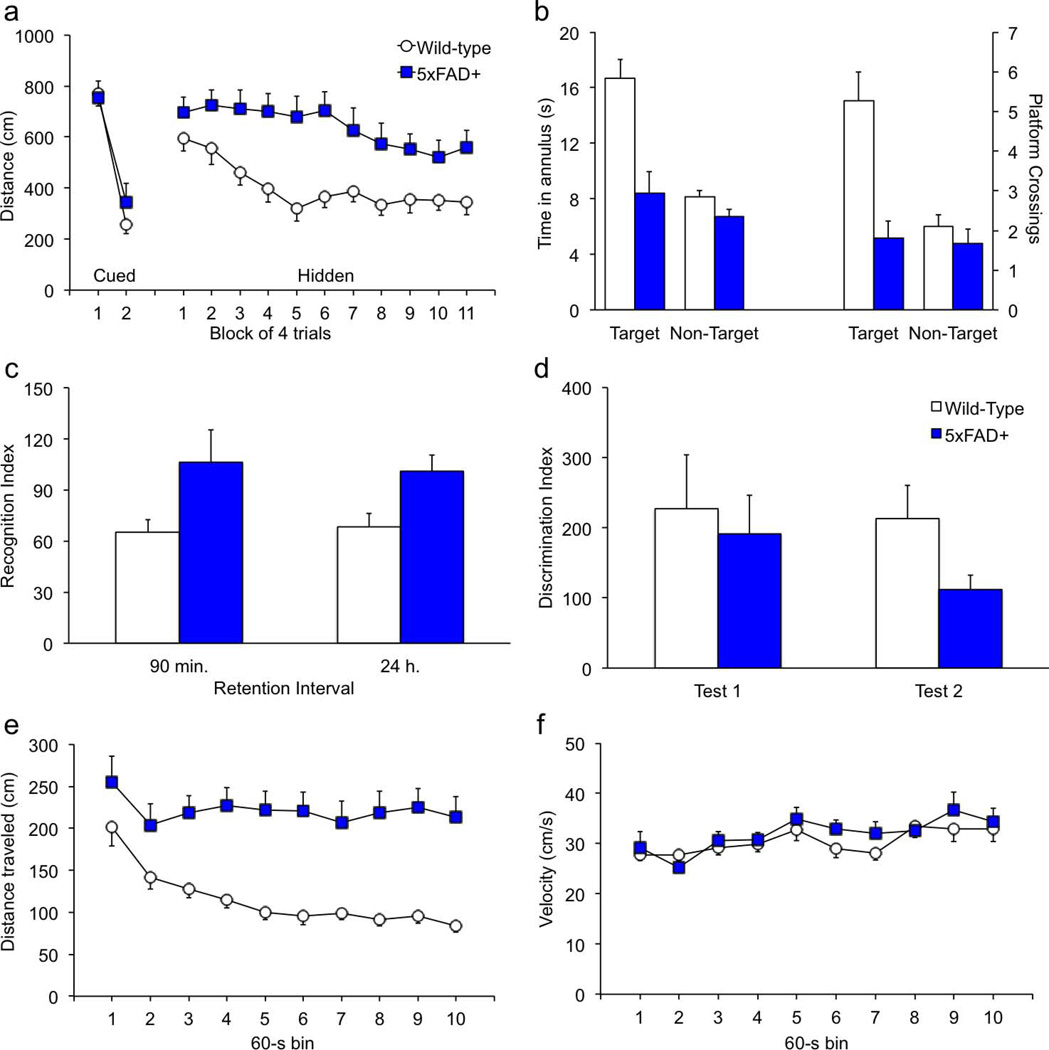
Mice of both genotypes learned to swim quickly to the platform during channel training; swim speeds did not differ between groups [data not shown; F(1,31) = 2.2; p = .149; Genotype × Session F(1,31) = 0.7, p = .393]. In addition, both groups learned to find the cued platform equally well [Fig. 2a; genotype F(1,31) = 0.3; p = .587; Genotype × Session F(1,31) = 1.5, p = .234]. In contrast, the 5xFAD transgenics had significantly longer path lengths when swimming to the hidden platform [F(1,30) = 19.7, p < .0001]. Swim speeds did not differ by genotype during acquisition of the hidden-platform task [F(1,30) = 1.4, p = .245], but 5xFAD transgenics spent significantly more time swimming in the periphery [F(1,30) = 9.1, p = .0051]. Peripheral swimming is often observed during initial sessions and may be indicative of anxiety, thigmotaxis, or an inability to make spatial associations, e.g., after hippocampal lesions (Bernardo et al., 2007; Clark et al., 2007; Inostroza et al., 2011; Wolfer et al., 1998). There was no genotype difference in peripheral swimming during cued-platform training [genotype F(1,31) = 1.7; p = .206; Genotype × Session F(1,31) < 0.1, p = .951]. Thus we can be confident that the peripheral swimming on the part of the 5xFAD mice during hidden-platform training is attributable to a spatial learning impairment and not a thigmotactic or acute stress response. Twenty-four hours after the last hidden-platform session a probe trial was conducted in the absence of a platform. Figure 2b shows that 5xFAD mice spent significantly less time than wild-type mice in the 40-cm zone surrounding the center of the platform location [F(1,27) = 11.7, p = .0020], and crossed the former platform location significantly fewer times [F(1,27) = 10.6, p = .0030]. Platform crossings and 40-cm zone time in equivalent positions in the other three quadrants did not differ between genotypes [F's < 1.6, p's > .221].
Figure 2. 5xFAD transgenics have impaired spatial and social memory.
(a) At approximately 10 months of age, mice of both genotypes learned quickly to find the cued platform in the water maze. On the hidden-platform version of the task, transgenic mice took significantly longer to find the platform. (b) On the probe trial conducted 24 hours after the last acquisition session, wild-type mice showed proficient memory by spending significantly more time in the target zone than in equivalent positions in the other three quadrants. In contrast, 5xFAD transgenics did not show selective search for the former platform location, indicative of impaired spatial memory. On the social recognition, 12-month-old mice were exposed to a novel juvenile mouse for 2 min. in a clean tub cage. Ninety min. later mice were re-exposed to the same juvenile (recognition), followed by a 2-min. exposure to a novel juvenile (discrimination). Twenty-four hours after the initial exposure, mice were re-exposed to the initial juvenile followed by exposure to a third novel juvenile. (c) 5xFAD mice showed significantly impaired recognition at both 90 min. and 24 hr. following initial exposure (no recognition = 100). (d) Discriminability was somewhat poorer in transgenic mice, although the difference was not statistically significant (no discrimination = 100). (e) In the locomotor activity assessment conducted at 9 months of age, wild-type mice habituated significantly over the course of the 10-min. session, where as activity of transgenic mice remained high. (f) When moving in the activity monitors, the velocity did not differ between genotypes.
Home-cage social behavior was assessed for 4 hours, and videos were coded for sniffing, following, mounting, tail-pulling, and grooming cage-mates. There was a significant overall increase in social behaviors among the transgenic mice [not shown; λ = 0.044, F(1,8) = 9.8, p = .0139]. Follow-up tests for specific behaviors showed that, except for grooming one another, the number of social events was significantly higher among 5xFAD transgenics than in wild-type controls for each of the social behaviors [F’s > 6.0; p’s < .0393]. Similar results were obtained for the amount of time spent engaging in each social activity [F’s > 6.1; p’s < .0389]. Bouts of self-grooming and time spent self-grooming were similar between genotypes [F's < 1.2, p's > .283], as was time sleeping [F(1,8) = 0.1, p = .890]. Neither genotype proved more dominant than the other on the dominance test, and there were no significant differences between contests against whiskered or whiskerless wild-types (data not shown; t’s <1.6, p’s > .170). When whiskerless wild-type mice were housed together in pairs with full-whiskered 5xFAD transgenics, all of the mice of both genotypes had a full set of whiskers after 2 weeks. On the social interaction tests, 5xFAD mice had significantly impaired social recognition overall [Fig. 2c; F(1,27) = 6.6, p = .0164]. Follow-up tests showed that transgenics were impaired at both the 90-min. and 24-hour retention intervals [t's > 2.0, p's < .0465]. In contrast, transgenic mice were unimpaired on the discrimination test conducted immediately following the recognition tests [Fig. 2d; genotype F(1,27) = 1.3, p = .259; Genotype × Test F(1,27) = 0.5, p = .508].
Compared to wild-type controls, 5xFAD transgenics were hyperactive in the activity monitors. In fact, they scarcely habituated during the session; thus savings could not be calculated. Figure 2e shows within-session data collapsed across the four activity sessions. 5xFAD transgenics had significantly more locomotor activity than wild-type controls [Fig. 3a; F(1,29) = 33.2, p < .0001; Genotype × Time F(1,277) = 20.1, p < .0001]. Ambulatory activity decreased significantly during the session in wild-type mice [F(1,198) = 55.1, p < .0001], but not in 5xFAD transgenics [F(1,108) = 0.5, p = .498]. There were no main or interaction effects of genotype on velocity while moving in the activity monitors [Fig. 2f; F’s < 0.9, p’s > .354].
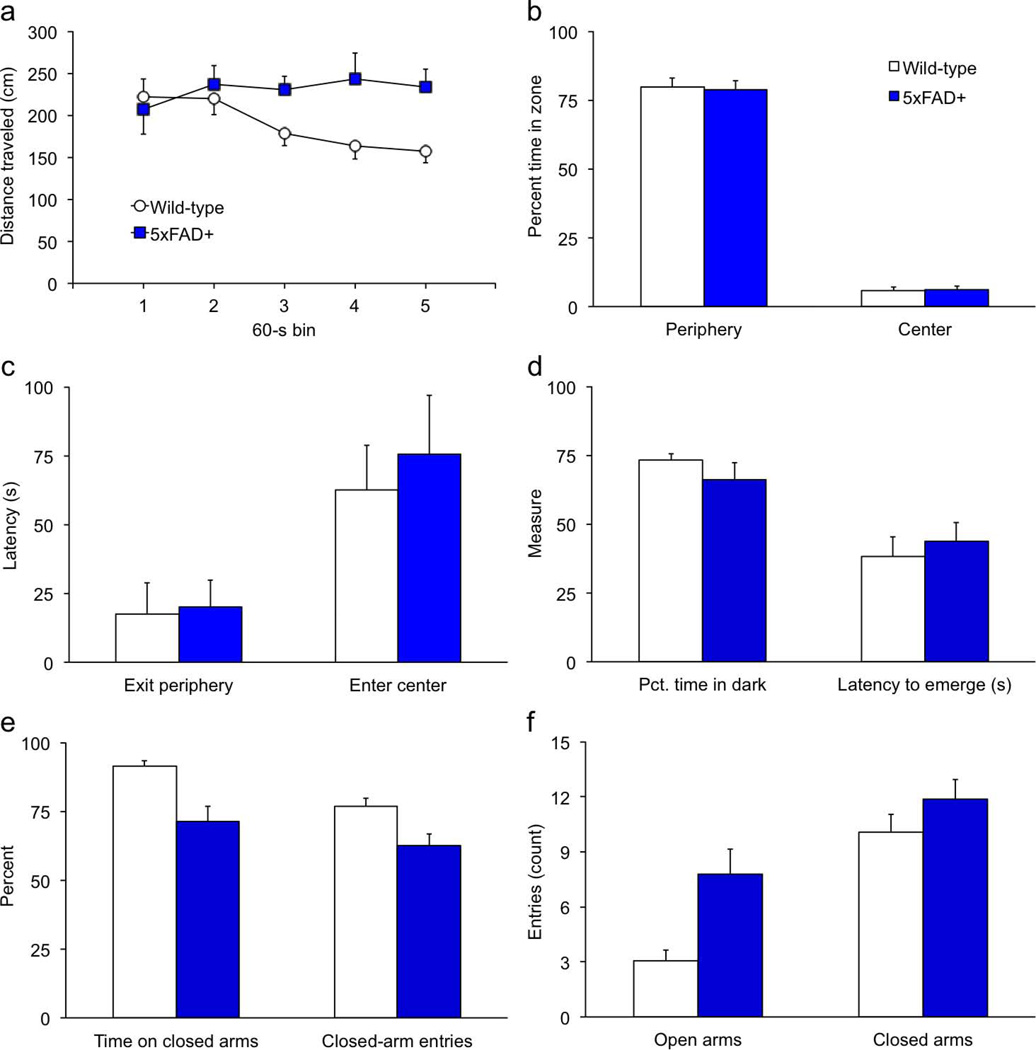
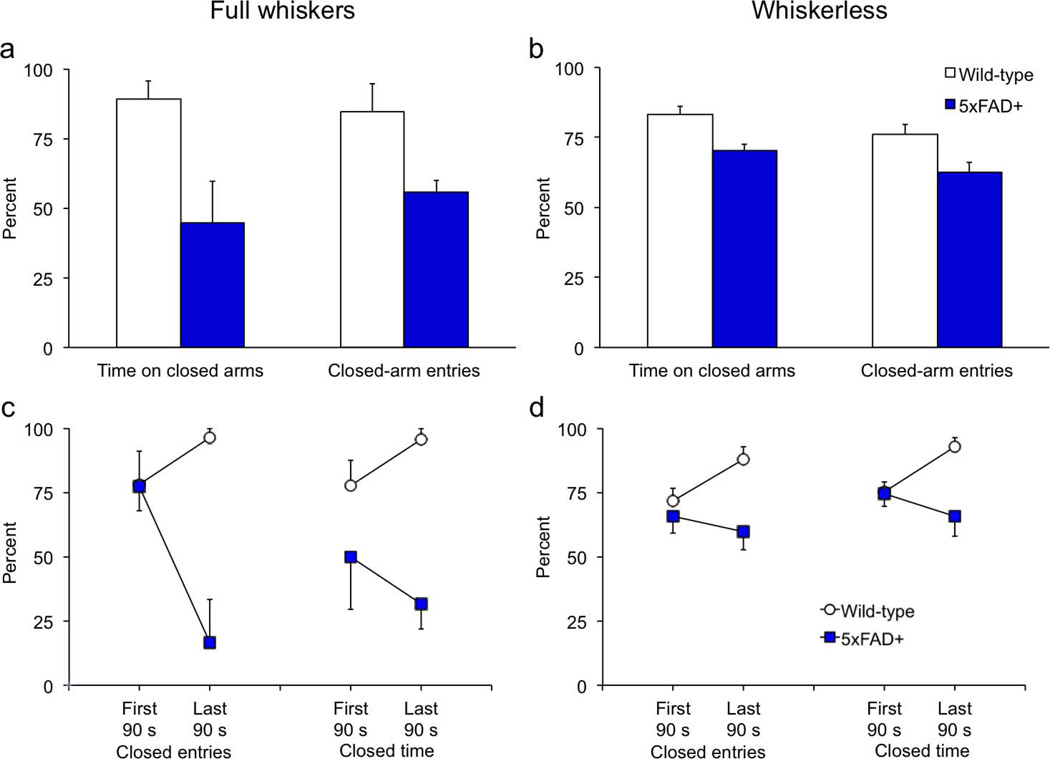
Figure 3. 5xFAD transgenics spend less time in the closed arms of the plus maze but are normal on all other indices of anxiety.
Anxiety testing was conducted when mice were 9 to 10 months of age. Mice were placed in the open field or light/dark box for a 5-min. period and allowed to explore freely. (a) As in the activity monitors, 5xFAD transgenics failed to habituate in the 92-cm diam. open field. (b–c) The percent time in the peripheral and central zones (b), and latencies to exit the periphery and enter the central area (c), were normal in transgenic mice. (d) The latency to emerge from the dark, and percent time spent in the dark half of the light/dark box did not differ between genotypes. (e) Transgenic mice spent significantly less time on the closed arms and had a lower proportion of close-arm entries in the elevated plus maze. (f) 5xFAD mice made more entries onto the open arms than wild-type controls. The number of entries onto the closed arms did not differ between genotypes.
5xFAD transgenics were also more active than wild-type controls in the open field test of anxiety (Fig. 3a). The main effect of genotype was not significant [F(1,34) = 3.6, p = .0660], but the Genotype × Block interaction [F(4,136) = 3.6, p = .0076] showed that 5xFAD transgenics did not habituate to the apparatus during the session. There were no genotype differences on any measure of anxiety in the open field, including time in periphery or center and latency to exit the periphery or enter the center [Figs. 3b,c; F’s < 0.7, p’s > .233]. Similarly, on the light/dark test wild-type and 5xFAD mice did not differ in the amount of time spent in the light and dark compartments or the latency to emerge from the dark compartment [Fig. 3d; F’s < 1.6, p’s > .221].
On the elevated plus maze, 5xFAD mice spent significantly less time in closed arms compared to wild-type mice [Fig. 3e; F(1,34) = 17.1, p = .0002]. The proportion of entries into closed arms was also significantly lower in transgenic mice [F(1,34) = 8.1, p = .0075]. Time in the central area did not differ between genotypes [data not shown; F(1,34) = 0.6, p =.429]. Risk assessment behaviors (head-dips, stretch-attend postures, and the proportion of stretch-attend postures followed by entry onto an open arm) also did not differ by genotype on the plus maze [data not shown; F’s < 0.5, p’s > .500]. There was no genotype difference in the absolute number of closed-arm entries [Fig. 3f; F(1,34) = 1.4, p =.246], but 5xFAD mice spent significantly less time per entry on the closed arms compared to wild-type controls [data not shown; F(1,34) = 5.2, p = .0288]. This replicates the phenotype reported by Jawhar et al. (2012) and may indicate reduced anxiety in the transgenics. However, we suspected that the plus-maze data in Jawhar et al. reflected more than simply acute anxiety, so we examined the time course of activity in our mice and found that the behaviors during the session changed differently in the two genotypes. An analysis of distance traveled in the plus maze showed that transgenic mice were more active and failed to habituate [Fig. 4a; distance F(1,34) = 6.6, p = .0147; Genotype × Distance F(9,306) = 2.4, p = .0117]. Like the activity monitors and large open field, activity of wild-type mice significantly diminished during the session [F(9,198) = 12.0, p = .0012], while 5xFAD transgenic mice maintained a high level of activity throughout the session [F(9,108) = 1.2, p = .287]. In terms of total arm entries, closed-arm entries, and closed arm time, Figure 4b shows that transgenic and wild-type mice performed similarly during the first 90 sec. of the 5-min. session [F’s < 2.4, p’s > .131], but were significantly different by the end [F’s > 6.0, p’s < .0200]. Analysis within genotype showed that wild-type mice increased time in closed arms by the end of the session [F(1,22) = 4.9, p = .0384], whereas closed-arm time of 5xFAD transgenics did not change significantly [F(1,12) = 0.04, p = .839]. Although time in closed arms may be indicative of high anxiety, it can be confounded with habituation within the session. Specifically, as exploratory activity wanes, mice will spend more time resting in the preferred environment of the closed arms.
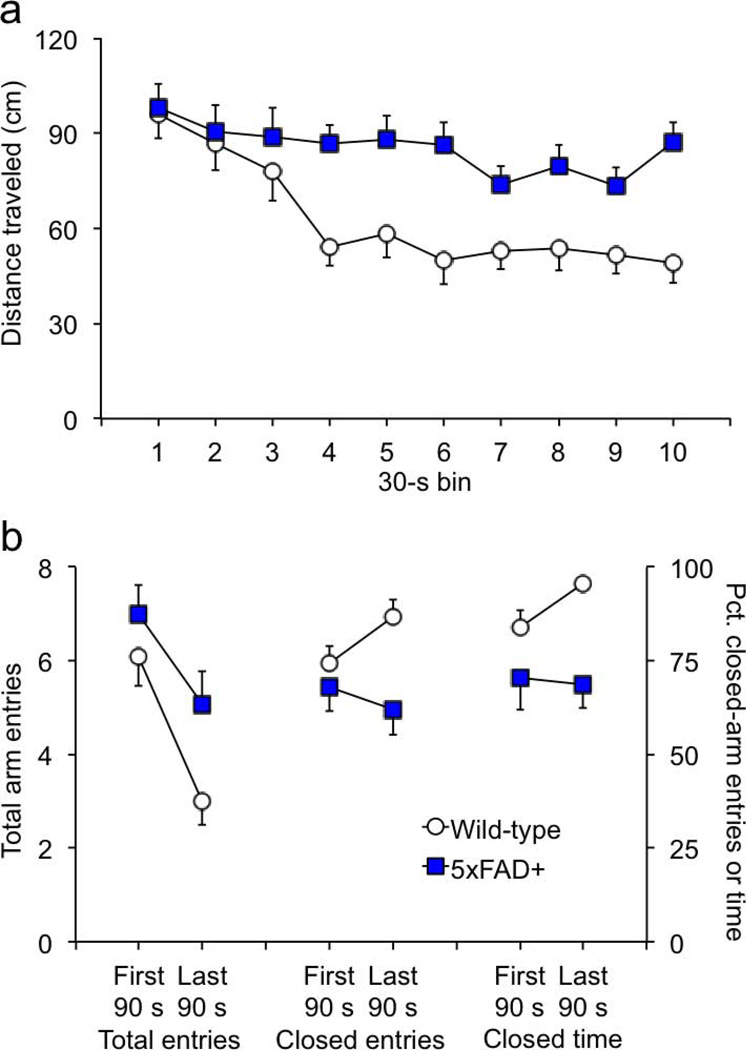
Figure 4. The differences in plus-maze behavior largely emerge after the first 90 s of the 5-min. session.
(a) The locomotor activity levels of the two genotypes was similar at the beginning of the 5-min. session. After the first 90 s, wild-type mice habituated significantly while activity of the transgenics remained high. (b) The two genotypes had similar numbers of total and closed-arm entries in the first 90 s of the session. The number of total entries decreased for both genotypes by the end of the session. However, the proportion of closed-arm entries significantly increased in wild-type mice but did not significantly change in 5xFAD transgenics. Wild-type mice spent more time in the closed arms than transgenics in the first 90 s of the session. By the end of the session, wild-type mice were spending nearly all of the time in the closed arms, whereas this proportion remained constant in 5xFAD mice. Because of these differences, data from the full 5-min. session cannot be used as a measure of acute stress in the present study.
Although differences between wild-type and transgenic mice were not statistically significant in the first 90 s of the plus-maze session, there was still a substantive difference (~16%) in time on closed arms between the genotypes (Fig. 4b, right). We suspected that this might be related to whisker sensitivity in the transgenics, so we re-tested the mice on the elevated plus maze 5 months later, at 14 months of age, after all behavioral testing was completed. The day before re-testing, we trimmed the whiskers of all mice to < 1 mm. A small cohort of experimentally-naïve mice of the same age, all with a full set of whiskers, were tested at the same time. Figure 5a shows that 14-month-old transgenics with whiskers spent even less time than 9-month-old transgenics (Fig. 3e) on the closed arms of the plus maze, replicating the age-dependent effect described by Jawhar et al. (2012). Whiskered transgenics spent less time in closed arms and made fewer closed-arm entries than wild-type controls [F's > 6.8, p's < .031]. Time in the center area did not differ [data not shown; F(1,8) < 0.1, p = .922]. Figure 5c shows that, unlike wild-type mice, 5xFAD transgenics did not habituate to the closed arms as the session progressed [F(1,4) < 1.8, p = .249]. This difference is more pronounced than the effect observed in younger mice (Fig. 4b), and confirms our assertion that much of the difference in arm distribution can be explained by a failure to habituate on the part of the mutant mice.
Figure 5. Aged, whiskerless 5xFAD transgenics behave normally in the first 90 s of the plus-maze session.
The whiskers of the 5xFAD and wild-type mice were trimmed and they were given a second session on the plus maze at 14 months of age. A small group of age-matched naïve mice (n=5/genotype) with a full complement of whiskers was used for comparison. (a) Consistent with the age-related phenotype reported in the literature, the older, whiskered 5xFAD mice spent less time on the closed arms compared to the younger, whiskered transgenics (Figs. 2e,3b). (c) Similar to the younger mice, older wild-type mice tended to habituate to the closed arms as the session progressed, whereas the transgenics persisted in avoiding the closed arms. (b) In contrast, the 14-month-old whiskerless transgenics spent about the same amount of time in the closed arms over the 5-min. session as they did when they were younger and whiskered. (d) Further analysis showed that behavior of 5xFAD transgenics in the first 90 s of the session was indistinguishable from that of wild-type mice, and genotype differences were attributable to behavior later in the session when wild-type mice habituated to the closed arms.
When whiskers were trimmed, however, the age-related decrease in closed-arm time was not evident (Fig. 5b). Although closed-arm time and closed-arm entries were both significantly lower in transgenics than wild-type mice (F's > 7.6, p's < .011), the genotype differences in the first 90 s of the session disappeared (Fig. 5d). The behavior of the whiskerless wild-type mice was largely similar to that of their full-whiskered counter parts (Fig. 5c–d), but that of the transgenic mice differed sharply. During the first 90 s of the session, closed-arm entries and closed-arm time were no different between whiskerless transgenic and wild-type mice [Fig. 5d; F's < 0.6, p's > .483]. By the end of the session however, significant genotype differences emerged [F's > 11.1, p's < .0025], reflecting habituation of wild-type but not 5xFAD mice. Repeated-measures analyses within genotypes confirmed that behavior of whiskerless wild-type mice changed significantly during the session [F's > 4.7, p's <.044], but that of their transgenic counterparts remained stable [F's < 1.0, p > .337].
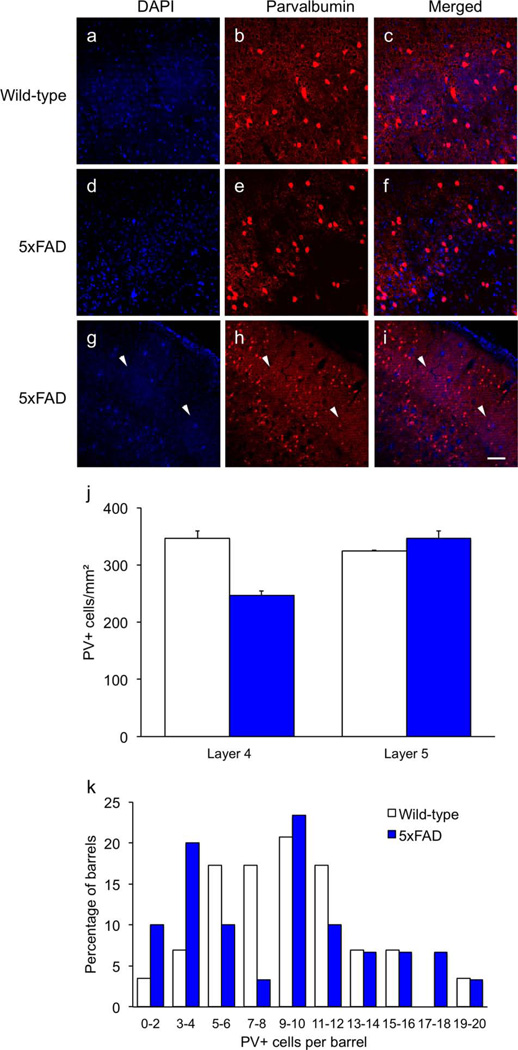
Fluorescent staining of cortical sections from 14-month-old mice showed a reduction in PV+ fibers and an apparent reduction in PV+ cell bodies in 5xFAD mice in layers V and VI, compared to wild-type controls (Fig. 6). Although it appears as if the 5xFAD transgenics have more PV+ cell bodies in layer IV barrels compared to wild-type controls in Figure 6, there was considerable variation and some barrels were nearly devoid of PV+ perikarya (Fig. 7a–i). Stereological quantification of PV+ neurons in 12-month-old mice showed that the density of PV+ neurons in layer V did not differ between genotypes [t(4) = 1.8, p = .269], but transgenics exhibited a 28.9% loss of PV+ cell bodies in layer IV {Fig. 7j; t(4) = 6.8, p = .0066]. The areas sampled did not differ between groups [t's < 1.0, p's > .426]. Figure 7k shows that the distribution of PV+ neurons in the barrels of 5xFAD transgenics was significantly skewed (2.10) and kurtotic (3.58) compared to wild-type mice (0.56 & 1.01 respectively; critical value for both test statistics = 2.0). Although many barrels in 5xFAD mice contained normal numbers of PV+ neurons, a subset appeared to have reduced numbers, creating a bimodal distribution.
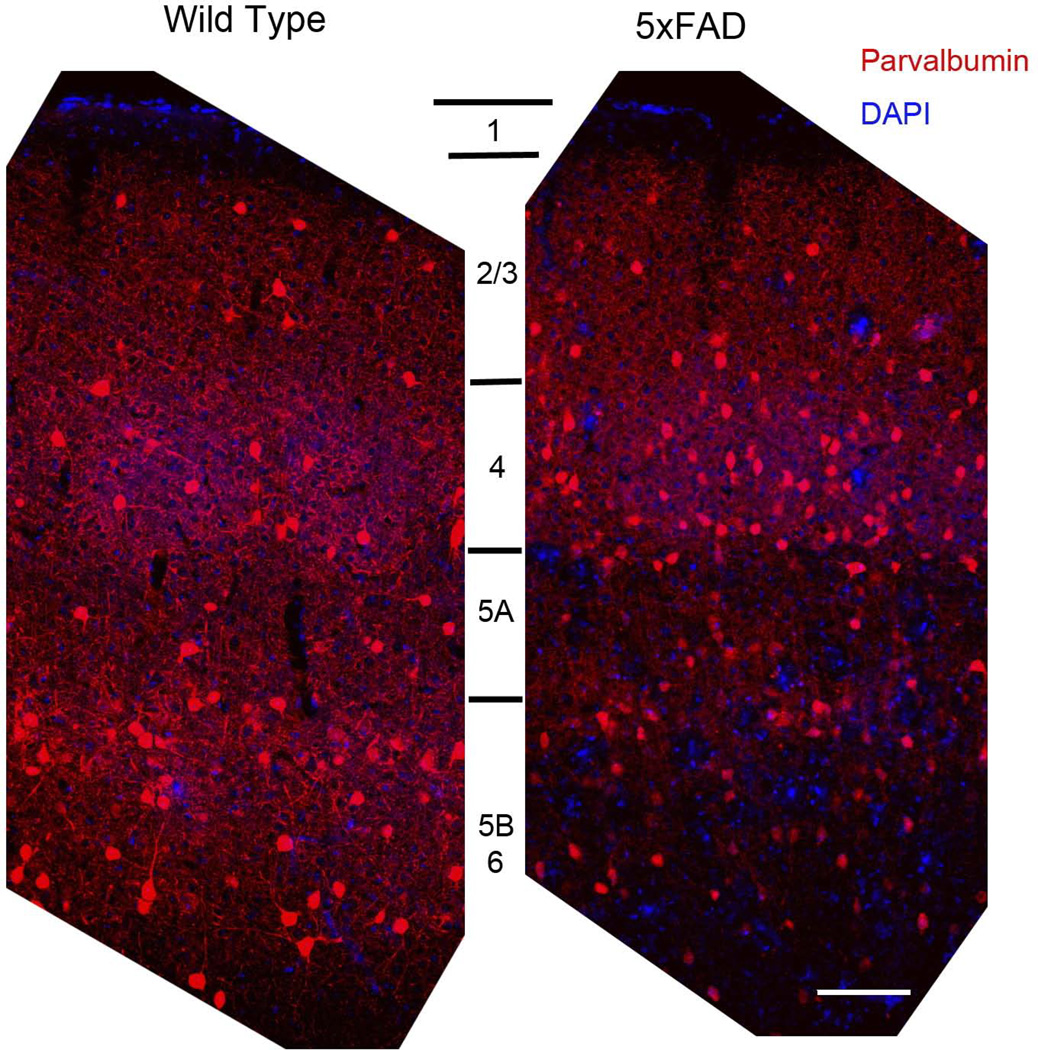
Figure 6. 5xFAD mice have reduced parvalbumin-positive fibers in layers V and VI.
Parvalbumin-positive inhibitory neurons with cell bodies in layer V project to adjacent targets in layer V as well as pyramidal neurons in layer IV. Wild-type mice (left panel) show a dense innervation throughout layers IV, V, and VI, but a marked reduction is observed in 14-month-old transgenics in layers V and VI (right panel). Scale bar = 100 µm.
Figure 7. Some barrels in 5xFAD transgenics lack parvalbumin-positive cell bodies.
Although it was possible to observe some barrels in 5xFAD transgenics with a greater number of PV-positive neurons than wild-type controls (e.g., Fig. 6), the overall pattern was mixed. As with layer V, 12-month-old transgenics exhibited a marked reduction of PV+ fibers in layer IV in many sections (a–f). Many barrels in transgenic mice were almost completely devoid of PV+ neurons in 14-month-old transgenics (g–i, arrows). Stereological quantification of DAB-stained PV+ neurons in 12-month-old mice showed a significant reduction in layer IV but not layer V (j). The distribution of PV+ neurons per barrel was bimodal in 5xFAD mice, suggesting loss of PV+ neurons in a subset of barrels (k). Scale bar = 50 µm a–f, 100 µm g–i.
Discussion
We report here normal anxiety but impaired cognition and social behavior in the 5xFAD transgenic mice. We replicated the effect reported by Jahwar et al. (2012) showing an age-dependent increase in open-arm time in the 5xFAD mice. However, we cannot attribute this phenotype to decreased anxiety; instead we show that this behavior is better explained by the transgenic mice avoiding the closed arms and failing to habituate within the session. The avoidance of the closed arms may be attributable to abnormal or excessive vibrissal sensation due to decreased tonic inhibition or impaired sensory integration after loss of inhibitory innervation. Similarly, the deficit in social recognition may be attributable to the subicular degeneration characteristic of these transgenics. Taken together, these results demonstrate functional impairments in the 5xFAD transgenics that are consistent with the discrete lesions putatively induced by brain-region-specific Aβ overexpression.
On the elevated plus maze, 5xFAD mice spent more time on open arms than wild-type controls, replicating the previously-published effect of Jahwar et al. (2012). However, a more detailed analysis showed that performance of 5xFAD transgenics was similar to that of wild-type mice in the beginning of the session, but diverged as the wild-types began to habituate after the first 90 s. Habituation in the plus maze is accompanied by a tendency to linger in closed arms as a preferred place to rest. In contrast, the performance of transgenic mice varied little over time in terms of locomotor activity or the proportion of arm choices. Specifically, the relatively normal plus-maze performance exhibited by 5xFAD transgenics in the first 90 s of the session persisted, but the behavior of wild-type mice changed. If time in the closed arms is a valid measure of acute stress response to a novel environment in the wild-type mice, it would have been highest at the beginning of the session; instead, we saw the opposite pattern: time in closed arms increased as the session elapsed. In this sense the full 5-min. plus-maze session cannot be used to accurately reflect the state of acute anxiety in the present study, and we cannot use data beyond the first 90 sec. to infer differences in anxiety between the genotypes. Given this and performance on the other anxiety tests, we conclude that the 5xFAD transgenics do not exhibit differential anxiety. This is important when measuring cognition, especially using unitary, aversively-motivated tasks such as the reference-memory version of the water maze or conditioned freezing (McDonald, 1998 #80; Harrison, 2009 #665). Both increases and decreases in anxiety can be misinterpreted as cognitive impairments in the water-maze (Abeliovich et al., 1993; Bowers et al., 2000; Grant et al., 1992; Miyakawa et al., 1994). Thus we can be confident that the spatial learning impairments exhibited by the 5xFAD transgenics in the present study are not attributable to lower anxiety.
Although habituation of wild-type mice was responsible for some of the differences in time on closed arms, it cannot explain it all. Transgenic mice spent ~16% less time in the closed arms even in the first 90 s. We suspected that this may be related to their lack of barbering behavior. We had observed this phenomenon before in the Dvl−/− mice (Lijam et al., 1997), and discovered that it was associated with submissiveness and a deficit in social behavior. However, the 5xFAD transgencis engaged in more, not less social behavior, and were not submissive, suggesting that the 5xFAD mice did not allow their cagemates to barber their whiskers. When re-tested on the plus maze with whiskers trimmed, transgenic mice were no longer reluctant to enter the closed arms and the remaining genotype differences during the plus-maze session could be explained entirely by their failure to habituate. In contrast, transgenic mice of the same age but with a full complement of whiskers avoided the closed arms at the beginning and end of the 5-min. session. These data suggest that vibrissal input may be aversive in the transgenic mice, and this putative aversiveness is driving avoidance of the closed arms.
In addition to the plus-maze phenotype, 5xFAD transgenics had abnormal social behavior and impaired social memory. Social behaviors are complex and involve many parts of the brain, including the striatum, prefrontal cortex, and amygdala. Considerable evidence also implicates the subiculum as a mediator of some social behaviors and social cognition. Gonzalez-Lima et al. (1994) showed that individually-housed mice given twice-daily opportunities for social interaction had significantly higher 2-deoxyglucose incorporation in the subiculum than socially-deprived controls, suggesting that higher subicular metabolic activity is associated with social activity. Inhibition of cannabinoid receptors in the subiculum had a profound affect on social recognition, impairing acquisition, consolidation, and retrieval (Segev & Akirav, 2011). Given its complexity, we cannot make a definitive connection between subicular pathology in the 5xFAD transgenics and their excessive home-cage social behaviors in the present study. Indeed, the social behaviors themselves may reflect their hyperactivity, an impaired sense of smell, a general cognitive deficit, or a combination of these and other things unrelated to social behavior. Nevertheless, the relationship between early subicular Aβ deposition and excess social activity is consistent with what is known about Aβ production, specifically that Aβ is generated in response to neural activity. Converging evidence from a number of studies suggests that early Aβ accumulation in Alzheimer's patients occurs in the default-mode network, i.e., those brain regions that remain active while we are passive (Greicius et al., 2004; Koch et al., 2012; Sorg et al., 2009; Wang et al., 2006; Zhang et al., 2010). Two studies have demonstrated a specific relationship between Aβ and vibrissal activity—specifically that Aβ production is increased by vibrissal stimulation and decreased by vibrissal deprivation (Bero et al., 2011; Tampellini et al., 2010). Given the excess social behavior exhibited by the 5xFAD mice, the importance of vibrissae to social communication in mice, and the role of the subiculum in mediating social behavior, it is not surprising to see such intense Aβ deposition in these two discrete areas.
Each layer IV barrel takes input from a single vibrissa, and has a complex network of interconnecting inputs and outputs to confer specificity and sensitivity for a given whisker. Layer V neurons are also active during vibrissal stimulation, but have larger, less-restricted receptive fields (Manns et al., 2004; Simons, 1978, 1995; Staiger et al., 2000; Wright & Fox, 2010). Layer V neurons are the major output neurons of the cortex, and receive inputs from and send afferents to all cortical regions and layers, as well as subcortical and brainstem areas such as the thalamus. Several characteristics of layer V pyramidal neurons are relevant to our behavioral results. First, these neurons send collaterals horizontally to other layer V pyramidal neurons. This and the amplified input from layer IV via thalamic nuclei serve to integrate and synchronize signals across layer V neurons (Simons, 1995; Staiger et al., 2000). Second, layer V pyramidal neurons arborize inhibitory interneurons in layer IV as well as throughout layer V (Tanaka et al., 2011; Thomson et al., 1996). These fast-spiking GABAergic interneurons in layers IV and V assist in the surround-inhibition that confers specificity in many sensory systsems, including vibrissal input (Connors et al., 1988; Kyriazi et al., 1998; Kyriazi et al., 1996). The loss of these inhibitory and integrative functions after degeneration of layer V pyramidal neurons and layer IV inhibitory interneurons may have made made presence in the closed arms of the plus maze more aversive with age for the 5xFAD mice. Instead of precise, meaningful input afforded by the finely-tuned neurons of the barrel cortex, they are overwhelmed with disorganized information that may not have meaning.
Although the disproportionate Aβ deposition in the subiculum and layer V in 5xFAD mice may be associated with whisker-mediated social activity, we don’t know why the transgenics exhibit excessive social behavior in the first place. We also do not know why the 5xFAD mice are hyperactive compared to controls. Indeed, these may be manifestations of the same behavior but measured in different ways. Mice are complex organisms with complex behaviors, and our understanding is always restricted by what and how we measure. In the present study we show that a relatively simple plus-maze task can be affected by non-anxiety-related processes such as hyperactivity and putatively aversive vibrissal hypersensitivity. The potential for obfuscating or confounding factors is even greater with the assessment of cognition. A valid assessment of memory is a critical component of an animal model of Alzheimer’s disease, and any possible involvement of non-cognitive processes such as anxiety or sensorimotor function must be ruled out (Mcdonald et al., 1996). Given the prevalence of impaired social behaviors and social cognition in Alzheimer’s disease and the importance of animal models in the development of novel treatments, issues such as these warrant further investigation.
Acknowledgements
Image production and stereological analyses were conducted at the Neuroscience Institute's Imaging Center (cns.uthsc.edu/imaging-center), and some behavioral studies were conducted in the Neuroscience Institute’s Behavioral Core (cns.uthsc.edu/behavioral-core). Funding was provided by the National Institutes of Aging grants R01AG031253, R01AG040230, and R21AG041935 to MM.
Footnotes
Disclosure statement. The authors have no actual or potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations within 3 years of beginning the work submitted that could inappropriately influence or bias their work. All in vivo procedures were approved by the Institutional Animal Care and Use Committee at UTHSC, and all animal housing and procedural facilities were approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
References
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKCγ mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- Bazalakova MH, Wright J, Schneble EJ, McDonald MP, Heilman CJ, Levey AI, Blakely RD. Deficits in acetylcholine homeostasis, receptors and behaviors in choline transporter heterozygous mice. Genes, brain, and behavior. 2007;6:411–424. doi: 10.1111/j.1601-183X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Harrison FE, McCord M, Zhao J, Bruchey A, Davies SS, Jackson Roberts L, 2nd, Mathews PM, Matsuoka Y, Ariga T, Yu RK, Thompson R, McDonald MP. Elimination of GD3 synthase improves memory and reduces amyloid-beta plaque load in transgenic mice. Neurobiol. Aging. 2009;30:1777–1791. doi: 10.1016/j.neurobiolaging.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Bernardo A, McCord M, Troen AM, Allison JD, McDonald MP. Impaired spatial memory in APP-overexpressing mice on a homocysteinemia-inducing diet. Neurobiol. Aging. 2007;28:1195–1205. doi: 10.1016/j.neurobiolaging.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat. Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, Collins AC, Tritto T, Wehner JM. Mice lacking PKCγ exhibit decreased anxiety. Behav. Genet. 2000;30:111–121. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Lu L, Williams RW, Holmes A. Genetic relationship between anxiety-related and fear-related behaviors in BXD recombinant inbred mice. Behav. Pharmacol. 2009;20:204–209. doi: 10.1097/FBP.0b013e32830c368c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. The hippocampus and spatial memory: findings with a novel modification of the water maze. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:6647–6654. doi: 10.1523/JNEUROSCI.0913-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Malenka RC, Silva LR. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer D. Basic Statistics for Social Research. New York: Routledge; 1997. [Google Scholar]
- Crowe SE, Ellis-Davies GC. In vivo characterization of a bigenic fluorescent mouse model of Alzheimer's disease with neurodegeneration. The Journal of comparative neurology. 2013;521:2181–2194. doi: 10.1002/cne.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Ohno M. Phospho-eIF2alpha level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice. PLoS One. 2010;5:e12974. doi: 10.1371/journal.pone.0012974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanushkodi A, Akano EO, Roguski EE, Xue Y, Rao SK, Matta SG, Rex TS, McDonald MP. A single intramuscular injection of rAAV-mediated mutant erythropoietin protects against MPTP-induced parkinsonism. Genes, brain, and behavior. 2013;12:224–233. doi: 10.1111/gbb.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanushkodi A, McDonald MP. Intracranial V. cholerae sialidase protects against excitotoxic neurodegeneration. PLoS One. 2011;6:e29285. doi: 10.1371/journal.pone.0029285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Qiao A, Wang Z, Goodwin JS, Lee ES, Block ML, Allsbrook A, McDonald MP, Fan GH. Retinoic acid attenuates β-amyloid deposition and rescues memory deficits in an Alzheimer disease rransgenic mouse model. J. Neurosci. 2008 doi: 10.1523/JNEUROSCI.3153-08.2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbinghaus H. Über das Gedächtnis. Untersuchungen zur Experimentellen Psychologie [Memory: A Contribution to Experimental Psychology] Leipzig: Duncker & Humblot; 1885. [Google Scholar]
- Eimer WA, Vassar R. Neuron loss in the 5XFAD mouse model of Alzheimer's disease correlates with intraneuronal Abeta42 accumulation and Caspase-3 activation. Mol Neurodegener. 2013;8:2. doi: 10.1186/1750-1326-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacology, biochemistry, and behavior. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav. Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- Flanigan TJ, Cook MN. Effects of an early handling-like procedure and individual housing on anxiety-like behavior in adult C57BL/6J and DBA/2J mice. PLoS One. 2011;6:e19058. doi: 10.1371/journal.pone.0019058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Ferchmin PA, Eterovic VA, Gonzalez-Lima EM. Metabolic activation of the brain of young rats after exposure to environmental complexity. Dev. Psychobiol. 1994;27:343–351. doi: 10.1002/dev.420270603. [DOI] [PubMed] [Google Scholar]
- Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci U. S. A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Harrison FE, Allard J, Bixler R, Usoh C, Li L, May JM, McDonald MP. Antioxidants and cognitive training interact to affect oxidative stress and memory in APP/PSEN1 mice. Nutritional neuroscience. 2009a;12:203–218. doi: 10.1179/147683009X423364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav. Brain Res. 2009b;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP, May JM. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol. Biochem. Behav. 2009c;93:443–450. doi: 10.1016/j.pbb.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, May JM, McDonald MP. Vitamin C deficiency increases basal exploratory activity but decreases scopolamine-induced activity in APP/PSEN1 transgenic mice. Pharmacol. Biochem. Behav. 2010;94:543–552. doi: 10.1016/j.pbb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Yu SS, Van Den Bossche KL, Li L, May JM, McDonald MP. Elevated oxidative stress and sensorimotor deficits but normal cognition in mice that cannot synthesize ascorbic acid. J. Neurochem. 2008;106:1198–1208. doi: 10.1111/j.1471-4159.2008.05469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inostroza M, Cid E, Brotons-Mas J, Gal B, Aivar P, Uzcategui YG, Sandi C, Menendez de la Prida L. Hippocampal-dependent spatial memory in the water maze is preserved in an experimental model of temporal lobe epilepsy in rats. PLoS One. 2011;6:e22372. doi: 10.1371/journal.pone.0022372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawhar S, Trawicka A, Jenneckens C, Bayer TA, Wirths O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer's disease. Neurobiol. Aging. 2012;33:196 e129–196 e140. doi: 10.1016/j.neurobiolaging.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Joyashiki E, Matsuya Y, Tohda C. Sominone improves memory impairments and increases axonal density in Alzheimer's disease model mice, 5XFAD. The International journal of neuroscience. 2011;121:181–190. doi: 10.3109/00207454.2010.541571. [DOI] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Benninghoff J, Wagner M, Bokde AL, Hampel H, Coates U, Reiser M, Meindl T. Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer's disease. Neurobiol. Aging. 2012;33:466–478. doi: 10.1016/j.neurobiolaging.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Kyriazi H, Carvell GE, Brumberg JC, Simons DJ. Laminar differences in bicuculline methiodide's effects on cortical neurons in the rat whisker/barrel system. Somatosens. Mot. Res. 1998;15:146–156. doi: 10.1080/08990229870871. [DOI] [PubMed] [Google Scholar]
- Kyriazi HT, Carvell GE, Brumberg JC, Simons DJ. Quantitative effects of GABA and bicuculline methiodide on receptive field properties of neurons in real and simulated whisker barrels. J. Neurophysiol. 1996;75:547–560. doi: 10.1152/jn.1996.75.2.547. [DOI] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Manns ID, Sakmann B, Brecht M. Sub- and suprathreshold receptive field properties of pyramidal neurones in layers 5A and 5B of rat somatosensory barrel cortex. J Physiol. 2004;556:601–622. doi: 10.1113/jphysiol.2003.053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MP, Overmier JB. Present imperfect: a critical review of animal models of the mnemonic impairments in Alzheimer's disease. Neurosci. Biobehav. Rev. 1998;22:99–120. doi: 10.1016/s0149-7634(97)00024-9. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Overmier JB, Bandyopadhyay S, Babcock D, Cleary J. Reversal of β-amyloid-induced retention deficit after exposure to training and state cues. Neurobiol. Learn. Mem. 1996;65:35–47. doi: 10.1006/nlme.1996.0004. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Watanabe S, Niki H. Increased fearfulness of Fyn tyrosine kinase deficient mice. Brain Res. Mol. Brain Res. 1994;27:179–182. doi: 10.1016/0169-328x(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- Moon M, Hong HS, Nam DW, Baik SH, Song H, Kook SY, Kim YS, Lee J, Mook-Jung I. Intracellular amyloid-beta accumulation in calcium-binding protein-deficient neurons leads to amyloid-beta plaque formation in animal model of Alzheimer's disease. J Alzheimers Dis. 2012;29:615–628. doi: 10.3233/JAD-2011-111778. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Cole SL, Yasvoina M, Zhao J, Citron M, Berry R, Disterhoft JF, Vassar R. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol. Dis. 2007;26:134–145. doi: 10.1016/j.nbd.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe+PSEN1ΔE9 bigenic mouse model of Alzheimer's disease. Genes, brain, and behavior. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Segev A, Akirav I. Differential effects of cannabinoid receptor agonist on social discrimination and contextual fear in amygdala and hippocampus. Learn. Mem. 2011;18:254–259. doi: 10.1101/lm.2110511. [DOI] [PubMed] [Google Scholar]
- Siesser WB, Cheng SY, McDonald MP. Hyperactivity, impaired learning on a vigilance task, and a differential response to methylphenidate in the TRβPV knock-in mouse. Psychopharmacology (Berl) 2005;181:653–663. doi: 10.1007/s00213-005-0024-5. [DOI] [PubMed] [Google Scholar]
- Siesser WB, Zhao J, Miller LR, Cheng SY, McDonald MP. Transgenic mice expressing a human mutant β1 thyroid receptor are hyperactive, impulsive, and inattentive. Genes, brain, and behavior. 2006;5:282–297. doi: 10.1111/j.1601-183X.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J. Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Neuronal integration in the somatosensory whisker/barrel cortex. In: Jones EGDIT, editor. The Barrel Cortex of Rodents. Cerebral Cortex. New York: Plenum Press; 1995. pp. 263–289. [Google Scholar]
- Sorg C, Riedl V, Perneczky R, Kurz A, Wohlschlager AM. Impact of Alzheimer's disease on the functional connectivity of spontaneous brain activity. Curr Alzheimer Res. 2009;6:541–553. doi: 10.2174/156720509790147106. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Kotter R, Zilles K, Luhmann HJ. Laminar characteristics of functional connectivity in rat barrel cortex revealed by stimulation with caged-glutamate. Neurosci. Res. 2000;37:49–58. doi: 10.1016/s0168-0102(00)00094-8. [DOI] [PubMed] [Google Scholar]
- Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, Gouras GK. Effects of synaptic modulation on beta-amyloid, synaptophysin, and memory performance in Alzheimer's disease transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:14299–14304. doi: 10.1523/JNEUROSCI.3383-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka YR, Tanaka YH, Konno M, Fujiyama F, Sonomura T, Okamoto-Furuta K, Kameda H, Hioki H, Furuta T, Nakamura KC, Kaneko T. Local connections of excitatory neurons to corticothalamic neurons in the rat barrel cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:18223–18236. doi: 10.1523/JNEUROSCI.3139-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC, Hahn J, Deuchars J. Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J Physiol. 1996;496(Pt 1):81–102. doi: 10.1113/jphysiol.1996.sp021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J. Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp HP. Spatial Memory and Learning in Transgenic Mice: Fact or Artifact? News Physiol Sci. 1998;13:118–123. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]
- Wright N, Fox K. Origins of cortical layer V surround receptive fields in the rat barrel cortex. J. Neurophysiol. 2010;103:709–724. doi: 10.1152/jn.00560.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Liu B, Ma ZL, Yang M, Zhang ZJ, Teng GJ. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology. 2010;256:598–606. doi: 10.1148/radiol.10091701. [DOI] [PubMed] [Google Scholar]