Abstract
Sensing of nucleic acids by TLRs is crucial in the host defense against viruses and bacteria. Unc-93 homolog B1 (UNC93B1) regulates the trafficking of nucleic acid sensing TLRs from the ER to endolysosomes, where the TLRs encounter their respective ligands and become activated. Here we show that a carboxy-terminal tyrosine-based sorting motif (Yxx Φ) in UNC93B1 differentially regulates human nucleic acid sensing TLRs in a receptor- and ligand-specific manner. Destruction of the YxxΦ motif abolished TLR7, 8 and 9 activity towards nucleic acids in human B cells and monocytes, whereas TLR8 responses towards small molecules remained intact. The YxxΦ motif in UNC93B1 influenced the subcellular localization of human UNC93B1 via both adapter protein complex 1 (AP1)- and AP2-dependent trafficking pathways. However, loss of AP function was not causal for altered TLR responses, suggesting AP-independent functions of the YxxΦ motif in UNC93B1.
Introduction
One strategy to detect pathogens or tissue damage is nucleic acid recognition by TLR3, 7, 8 or 9 (1). As nucleic acids are not unique to microbes their sensing evokes the risk of autoimmunity (1). UNC93B1 regulates endoplasmic reticulum (ER) to endolysosome trafficking of nucleic acid sensing TLRs (2-4). A single point mutation in UNC93B1 (H412R) that prevents its ability to exit from the ER ablates endosomal TLR signaling (5), and patients lacking functional UNC93B1 are at risk to develop lethal HSV infections (6), a clinical phenotype resembled by TLR3 deficiency (7).
Proteins can either be directly delivered from the trans-Golgi network (TGN) to endosomes or indirectly via the plasma membrane (8). Recently, a YxxΦ motif in murine UNC93B1 was identified to interact with the major endocytic protein AP2, which was suggested to be required for murine TLR9 function and delivery to endosomes (9). In contrast, the function of other murine endosomal TLRs was reported to be unimpaired by mutation of the YxxΦ motif in UNC93B1 (9).
The cellular distribution and function of endosomal TLRs differs between species. Human TLR9 expression is restricted to pDCs and B cells (10) and TLRs 11-13 are present in mice, but not humans (11). Furthermore, human TLR8 signaling serves important roles in monocytes, dendritic cells and neutrophils, whereas murine TLR8 does not have the same functions (12). Here, we investigated the role of the YxxΦ motif in human UNC93B1 on the activation of human TLR7, 8 and 9 by nucleic acids and small molecule agonists in different human cell types. We found that the YxxΦ motif in human UNC93B1 bound to AP1 and AP2, both of which were involved in the correct localization of UNC93B1. Destruction of the Yxx Φ motif caused receptor- and ligand-specific defects of TLR responses. However, knockdown of AP1 or AP2 did not mimic the observed TLR defects, suggesting that the tyrosine-based motif in UNC93B1 likely serves additional roles in regulating TLR signaling.
Material and Methods
Cell lines and plasmids
TLR expressing HEK cells were purchased from InvivoGen. EBV-immortalized B cells derived from an UNC93B1-deficient patient were described previously (6), human UNC93B1 KO THP-1 monocytes were generated using CRISPR/Cas9-based gene editing (13). Plasmids for mCitrine, human UNC93B1-mCitrine wild-type (WT), H412R or Y539A L542A (AxxA), and mCherry-KDEL were engineered by standard cloning techniques. Stable cells generated by transduction were cell sorted for similar expression levels of UNC93B1 versions.
Cell stimulation and analysis
RNA interference was performed by lipofection (RNAiMax, Life Technologies) of 5 nM Silencer Select siRNAs (Life Technologies) for 72 h. Cells were stimulated for 14 h with CpG2006 (Metabion), R848 (InvivoGen), CL075 (InvivoGen), Pam2CSK4 (EMC microcollections), human TNF or IL-1β (R&D systems), TLR7-specific RNA (5′-ACUG1CG1AG1CUU-X-UUCG1AG1CG1UCA-5, G1 is 7-deazaguanosine, X is 1,2,3-propanetriol) (14) or TLR8-specific RNA (5′-YUGCUGCCUUUG-X-GUUUCCGUCGUY-5′, Y is 1,3-propanediol, X is 1,2,3-propanetriol) (15) (Idera Pharmaceuticals). Supernatants were analyzed by ELISA for hIL-8 (BD Biosciences), hIL-6 or hTNF (R&D Systems). Efficiency of RNA interference was analyzed by SYBR Green quantitative PCR (qPCR) for MyD88, AP1M1 and AP2M1 expression normalized to HPRT.
Surface plasmon resonance (SPR) spectroscopy
Soluble μ subunits of AP1 (mouse μ1A aa 158-423 in pET28b), AP2 (rat μ2 aa 158-435, pET28a), and AP3 (rat μ3a aa 166-418, pET28b) were expressed in E. coli BL21-DE3 and purified by Ni-NTA affinity followed by size exclusion chromatography (GE Healthcare Life Sciences). SPR was performed with a Biacore 3000 instrument (GE Healthcare Life Sciences). Biotinylated peptides containing the WT (YxxL) or mutant (AxxA) tyrosine-based motif of UNC93B1 or the tyrosine-based motif of TGN38 (21st Century Biochemicals) were immobilized on streptavidin sensor chips (GE Healthcare Life Sciences). Proteins were injected for 1 min at 40 μl/min flow rate at 25°C in 10 mM HEPES pH 7.4, 500 mM NaCl, 10 mM β-mercaptoethanol, 5 mM DTT. Data were analyzed by BIAevaluation 4.1.1 software.
Microscopy
Subcellular compartments were labeled using CellLight® Reagents, LysoTracker Red (L7528) or Transferrin-Alexa647 (T23366) from Life Technologies. Live cells were imaged using a Leica SP5 SMD confocal microscope with a 63×/1.2 water immersion objective. Data quantification was performed with a custom-designed Matlab-based analysis software.
Results and Discussion
The YxxΦ motif in UNC93B1 regulates human TLR signaling in a cell type-, receptor- and ligand-specific manner
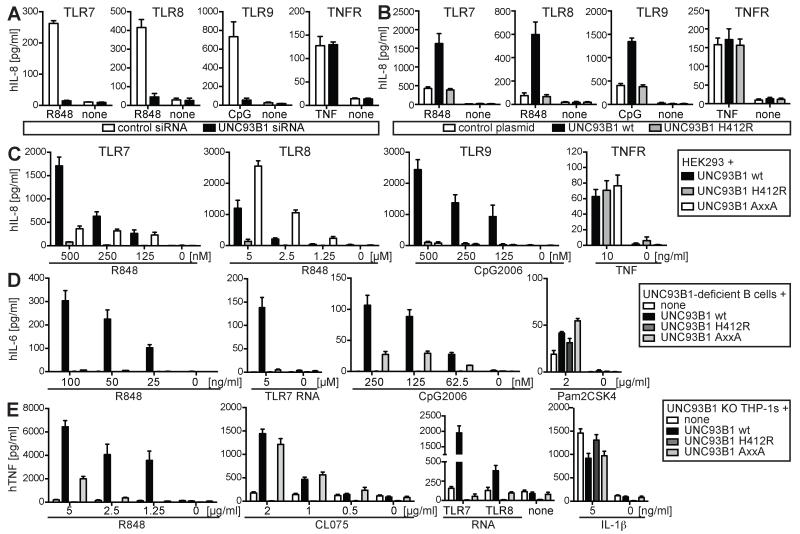
The Yxx Φ motif in UNC93B1 is conserved between species and was suggested to regulate TLR9 function in murine cells (9). We assessed the effects of the YxxΦ motif on TLR function in three different human cell types. To study UNC93B1-mediated regulation of human TLRs we first performed RNAi-mediated silencing of endogenous UNC93B1 or overexpression of WT or mutated UNC93B1 in HEK cells. As expected, knockdown of UNC93B1 abrogated TLR7, 8 and 9 signaling (Fig. 1A), while overexpression of WT but not the non-functional H412R mutant of UNC93B1 resulted in increased TLR7, 8 and 9 responses (Fig. 1B). Cellular activation by TNF remained unchanged (Fig. 1A, B). We next tested UNC93B1 WT, H412R or YxxΦ-mutant UNC93B1 (UNC93B1 AxxA) in stable HEK cells also expressing either TLR7, 8 or 9. Similar to what was found with murine macrophages (9), the destruction of the YxxΦ motif in human UNC93B1 abolished human TLR9 signaling (Fig. 1C). In contrast to murine TLR7, which was reported to be unaffected by changes in the YxxΦ motif of UNC93B1 (9), human TLR7 activity was also reduced (Fig. 1C). Conversely, signaling of TLR8 was increased in cells expressing UNC93B1 AxxA (Fig. 1C), suggesting that the Yxx Φ motif in UNC93B1 exerts differential effects on TLR7, 8 and 9 activation. Expression levels of all forms of UNC93B1 were comparable (Supplemental Fig. 1A) and TNFR signaling remained unaffected by the expression of either form of UNC93B1 (Fig. 1C). Previous studies on murine TLR9 showed cell-specific differences regarding the subcellular distribution (16) and signaling requirements of nucleic acid sensing TLRs (17). To study endogenous TLRs in human immune cells, we reconstituted EBV-transformed B cells from an UNC93B1-deficient patient (6) and UNC93B1-deficient human THP-1 monocytes (13) with fluorescent UNC93B1 WT, H412R or AxxA and sorted cells for UNC93B1 expression (Supplemental Fig. 1B and C). Similar to what we observed in HEK cells, TLR7 and 9 signaling was largely reduced in B cells expressing UNC93B1 AxxA, while TLR2 signaling in the same cells remained intact (Fig. 1D). Human THP-1 monocytes naturally express TLR7 and TLR8 (12), both of which may contribute to responses towards dual-specific ligands. As no strictly TLR8-specific small molecule activator is currently available, we chose to use R848 and CL075, which preferentially stimulate TLR7 and 8, respectively. THP-1 monocytes expressing UNC93B1 AxxA showed defective responses towards R848, while their response to CL075 was similar to cells expressing WT UNC93B1 (Fig. 1E), essentially reflecting the difference between TLR7 and 8 that we observed in HEK cells. However, unlike in HEK cells no hyper-responsiveness of TLR8 towards the small molecule agonists was found. This difference could be explained by the mixed TLR7/8 response in monocytes or by cell type-specific differences in small molecule diffusion or accumulation in subcellular compartments. To further elucidate TLR7 and 8 signaling in monocytes, we stimulated UNC93B1 knock-out THP-1 cells expressing different forms of UNC93B1 with TLR7- or 8-specific single-stranded RNA (14, 15). Notably, both TLR7 and 8 responses towards RNA agonists were completely abolished in cells expressing UNC93B1 AxxA (Fig. 1E) revealing differential regulation of small molecule-versus RNA-activated TLR8. Cellular responses towards IL-1β remained intact (Fig. 1E). Unlike in THP-1 monocytes, TLR8 responses towards RNA were found to be intact in HEK cells expressing UNC93B1 AxxA (Supplemental Fig. 1D). These results show that TLR7 and 8 responses are regulated in a cell type- and ligand-specific manner and that small molecule activators of TLRs should not be seen as simple surrogate ligands for nucleic acids.
FIGURE 1.
The YxxΦ motif of UNC93B1 regulates TLR7, 8 and 9 signaling in a cell type-, receptor- and ligand-specific manner. (A) HEK TLR7, 8 or 9 cells transfected with siRNA targeting UNC93B1 or control siRNA were stimulated with R848 (0.5 μM for TLR7, 5 μM for TLR8), CpG2006 (1 μM), TNF (10 ng/ml) or were left untreated and analyzed for IL-8 secretion. (B) HEK TLR7, 8HA or 9 cells transfected with mCitrine (control), UNC93B1-mCitrine WT or H412R were stimulated with R848 (0.5 μM for TLR7, 5 μM for TLR8), CpG2006 (1 μM), TNF (10 ng/ml) or were left untreated and analyzed for IL-8 secretion. (C) HEK cells stably expressing TLR7, 8HA or 9 and UNC93B1-mCitrine WT, H412R or AxxA (Y539A L542A) were stimulated as indicated and analyzed for IL-8 secretion. (D) EBV-immortalized human B cells from UNC93B1-deficient patients and (E) human UNC93B1 KO THP-1 monocytes generated by CRISPR/Cas9-based gene editing were retrovirally transduced with UNC93B1-mCitrine WT, H412R or AxxA and stimulated as indicated. B cells were analyzed for IL-6, monocytes for TNF secretion. All data are combined from three independent experiments (mean + s.e.m.).
The YxxΦ sorting motif in human UNC93B1 mediates both AP1- and AP2-dependent trafficking of UNC93B1
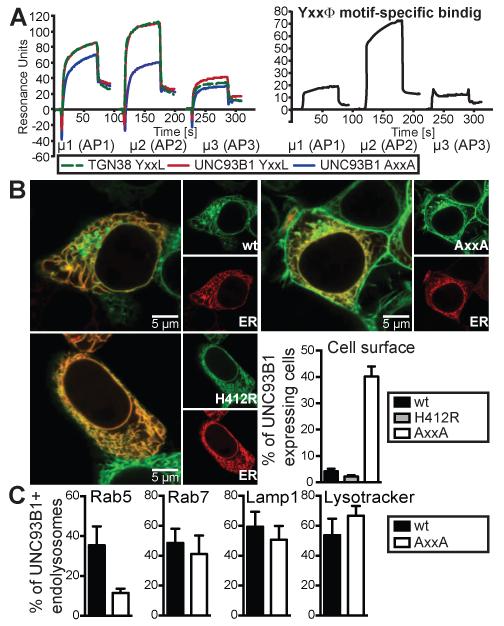
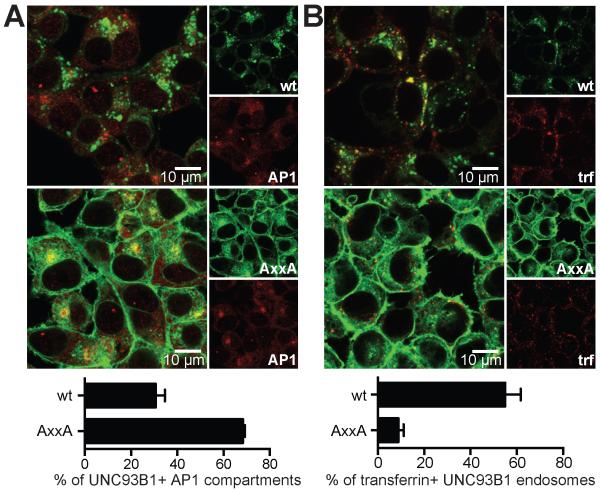
Tyrosine-based sorting motifs bind to μ subunits of AP complexes, which are heterotetrameric protein complexes that connect cargo to the clathrin coat of transport vesicles (18). To determine whether the Yxx Φ motif in human UNC93B1 interacts with specific μ subunits of AP1, AP2 or AP3, we assessed the binding of purified proteins and peptides containing sorting motifs by SPR. This analysis showed that the peptide derived from UNC93B1 containing the WT YxxΦ motif strongly interacted with the μ subunit of AP2 and to a lesser extent also with the μ subunits of AP1 and AP3. These interactions were reduced when the Yxx Φ motif was destroyed (Fig. 2A). Further studies showed that the interaction with the μ subunit of AP2 was comparable to that of a YxxΦ motif containing peptide derived from TGN38, one of the strongest AP2 interactors known (19). The μ subunit of AP2 is known to have the highest affinity and broadest specificity when compared to μ subunits of other AP complexes, whereas μ1 and μ3 subunits are weaker interactors of YxxΦ motifs, especially in in vitro studies (18). Given that in a cellular context the interaction of the Yxx Φ motif with AP proteins is multivalent, with other membrane proteins and lipids also contributing to the binding, the weaker interactions observed between μ1 and 3 with the YxxΦ motif of UNC93B1 could likely be relevant for the in vivo function. To assess whether the YxxΦ motif of human UNC93B1 indeed functions as a sorting motif, we analyzed the subcellular localization of WT and mutant UNC93B1. Confocal microscopy showed that the non-functional H412R UNC93B1 mutant was exclusively expressed in the ER, while fluorescent WT UNC93B1 was expressed both in the ER and in endosomes. No surface expression of either of these proteins could be discerned (Fig. 2B). In contrast, UNC93B1 AxxA was partially expressed on the plasma membrane (Fig. 2B). Further analysis showed diminished presence of UNC93B1 AxxA in early endosomal compartments when compared to WT UNC93B1, while markers for late endosomes and lysosomes still colocalized with both versions of UNC93B1 (Fig. 2C). AP3 is known to mediate trafficking of cargo to lysosomes and lysosome-related organelles (18), whereas AP1 is involved in the direct and AP2 in the indirect trafficking pathway to endosomal compartments (8). As the YxxΦ motif of human UNC93B1 was crucial for localization in early endosomal compartments, we further dissected the role of AP1 and AP2 for UNC93B1 trafficking. In steady state, AP1 is mostly localized to the TGN, where AP1-dependent sorting of proteins to their respective subcellular locations takes place (8). Consistent with functional AP1-dependent sorting of WT, but not mutant UNC93B1, UNC93B1 AxxA, but not WT accumulated at this subcellular sorting station (Fig. 3A). To elucidate whether human UNC93B1 entered the AP2-dependent trafficking pathway, we utilized fluorescent transferrin. Transferrin is endocytosed via the transferrin receptor in an AP2-dependent manner, thereby serving as a tracer molecule for AP2-dependent endosomes. Quantification of UNC93B1 and transferrin double-positive endosomes showed that UNC93B1 AxxA was largely absent from transferrin-positive endosomes, while approximately 60% of WT UNC93B1-positive endosomes contained transferrin (Fig. 3B). Our data support the notion that both AP1 and AP2 are both involved in the YxxΦ-mediated trafficking of UNC93B1.
FIGURE 2.
YxxL of UNC93B1 binds APs and functions as a trafficking motif. (A) SPR analysis of μ1, μ2 and μ3 (2 μM) binding to immobilized TGN38 YxxL (WT), UNC93B1 YxxL (WT), and UNC93B1 AxxA peptides. Specific binding to the Yxx Φ motif was determined by subtracting UNC93B1 AxxA from UNC93B1 WT SPR signals. Data are representative of three independent experiments. (B) HEK cells stably expressing UNC93B1-mCitrine WT, H412R or AxxA were transiently transfected with the ER marker mCherry-KDEL and analyzed by confocal microscopy. The graph represents combined data of three independent experiments (mean + s.e.m.). (C) HEK cells stably expressing UNC93B1-mCitrine WT or AxxA were transduced for 24 h with markers for early endosomes (Rab5-RFP), late endosomes (Rab7-RFP) or lysosomes (Lamp1-RFP) or incubated for 5 min with LysoTracker Red and analyzed by confocal microscopy. The graph shows combined data of four independent experiments (mean + s.e.m.).
FIGURE 3.
AP1 and AP2 are crucial for UNC93B1 trafficking. (A) HEK cells stably expressing UNC93B1-mCitrine WT or AxxA were fixed with 4% PFA, permeabilized with 0.5% Saponin, stained for AP1 (BD Transduction Laboratories, 611329) and analyzed by confocal microscopy. The graph represents combined data of two independent experiments (mean + s.e.m.) analyzing at least ten different image areas per experiment. (B) HEK cells stably expressing UNC93B1-mCitrine WT or AxxA were incubated for 10 min with transferrin-Alexa647 (1 μg/ml), fixed with 4% PFA and analyzed by confocal microscopy. The graph shows combined data of four independent experiments (mean + s.e.m.).
AP1- and AP2-dependent trafficking pathways are dispensable for human endosomal TLR responses
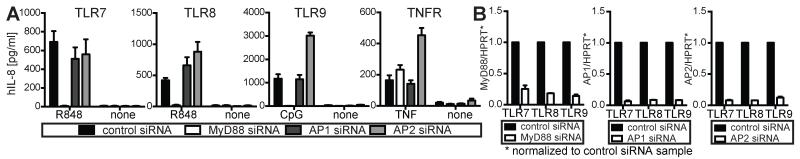
To assess whether the disability of UNC93B1 AxxA to use AP1- and AP2-dependent sorting pathways was causal for the observed TLR defects, we performed knockdown studies of AP1 and AP2 in HEK cells and analyzed TLR7, 8 or 9 responses. Knockdown of AP2 and to lesser extent of AP1 caused increased TLR8 activity (Fig. 4A), similar to the TLR8 phenotype observed in HEK cells expressing UNC93B1 AxxA. However, unlike mutating the YxxΦ motif of UNC93B1, neither AP1 nor AP2 knockdown abolished TLR7 and 9 responses (Fig. 4A). In stark contrast, TLR9 responses were increased upon silencing of AP2 (Fig. 4A). These data clearly demonstrate that loss of AP1 or 2 binding to UNC93B1 AxxA does not explain altered TLR responses upon destruction of the YxxΦ motif in UNC93B1, and suggest that the Yxx Φ motif of UNC93B1 regulates UNC93B1 trafficking versus TLR responses via two separate mechanisms. TNFR activity was increased upon AP2 knockdown (Fig. 4A), which is in accordance with a shutdown of pro-inflammatory TNFR signaling via AP2-dependent TNFR endocytosis (20). As expected, silencing of MyD88 or AP1 did not change TNFR responses compared to control siRNA-treated cells (Fig. 4A). qPCRs for MyD88, AP1 and AP2 established that the knockdown was very efficient with more than 90% knockdown at the mRNA level (Fig. 4B). Furthermore, immunofluorescence staining of AP1 in control versus AP1 siRNA-treated cells showed efficient silencing of AP1 at the protein level (Supplemental Fig. 2A). Loss of AP2 function was validated as transferrin uptake was completely abolished upon AP2 silencing, leading to transferrin accumulation on the cell surface in AP2 but not control siRNA-treated cells (Supplemental Fig. 2B). These results suggest that the Yxx Φ motif of UNC93B1 regulates TLR signaling independent of AP1 or AP2 and therefore must serve additional functions.
FIGURE 4.
AP1 and AP2 are dispensable for TLR responses. (A) HEK TLR7, 8 or 9 cells were transfected with siRNA targeting MyD88, AP1 (AP1M1 and AP1G1), AP2 (AP2M1) or control siRNA, stimulated with R848 (0.5 μM for TLR7, 10 μM for TLR8), 0.5 μM CpG2006, 10 ng/ml TNF or left unstimulated and analyzed for IL-8 secretion. All data are combined from three independent experiments (mean + s.e.m.). (B) In order to validate knockdown efficiency, cells from (A) were analyzed for expression levels of MyD88, AP1M1 and AP2M1 by qPCR. Expression levels are shown relative to control siRNA treated samples.
One possibility could be that the YxxΦ motif of UNC93B1 recruits other molecules that regulate TLR signaling. For example, YxxL of UNC93B1 could serve as a so-called hemITAM motif. Upon tyrosine phosphorylation ITAM or hemITAM motifs recruit Src Homology 2 domain containing proteins, such as spleen tyrosine kinase, which was previously suggested to regulate endosomal TLR signaling (21, 22).
Our study identifies a complex and versatile regulatory role of the YxxΦ motif in human UNC93B1, which is not only TLR-, but also ligand- and cell type-specific. The notion that UNC93B1 could serve multiple functions in trafficking and potentially signaling should spark future investigations.
Supplementary Material
Acknowledgements
We thank P. Wurst, A. Dolf, G. Horvarth and M. Beyer for help with the fluorescence-activated cell sorting and G. Engels for general technical support.
E.L. and A.M-R. were supported by a grant of the NIH (P01-AR050256). J.Z. and S.H. were supported by a grant of the Deutsche Forschungsgemeinschaft (HO2209/1-1).
Abbreviations used in this article
- UNC93B1
Unc-93 homolog B1
- YxxΦ
tyrosine-based sorting motif
- AP
adapter protein complex
- ER
endoplasmic reticulum
- TGN
trans-Golgi network
- WT
wild-type
- UNC93B1 AxxA
UNC93B1 YxxΦ-mutant Y539A L542A
Footnotes
The online version of this article contains supplemental material.
References
- 1.Kawasaki T, Kawai T, Akira S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol Rev. 2011;243:61–73. doi: 10.1111/j.1600-065X.2011.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim Y-M. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y-M, Brinkmann MM, Paquet M-E, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 4.Itoh H, Tatematsu M, Watanabe A, Iwano K, Funami K, Seya T, Matsumoto M. UNC93B1 Physically Associates with Human TLR8 and Regulates TLR8-Mediated Signaling. PLoS ONE. 2011;6:e28500. doi: 10.1371/journal.pone.0028500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, Shamel L, Herskovits AA, Portnoy DA, Cooke M, Tarantino LM, Wiltshire T, Steinberg BE, Grinstein S, Beutler B. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 6.Casrouge A, Zhang S-Y, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, Lorenzo L, Plancoulaine S, Sénéchal B, Geissmann F, Tabeta K, Hoebe K, Du X, Miller RL, Héron B, Mignot C, de Villemeur TB, Lebon P, Dulac O, Rozenberg F, Beutler B, Tardieu M, Abel L, Casanova J-L. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 7.Casanova J-L, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in Host Defense: Natural Insights from Evolutionary, Epidemiological, and Clinical Genetics. Annu Rev Immunol. 2011;29:447–491. doi: 10.1146/annurev-immunol-030409-101335. [DOI] [PubMed] [Google Scholar]
- 8.Braulke T, Bonifacino JS. Sorting of lysosomal proteins. BBA - Molecular Cell Research. 2009;1793:605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Lee BL, Moon JE, Shu JH, Yuan L, Newman ZR, Schekman R, Barton GM. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife. 2013;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barchet W, Wimmenauer V, Schlee M, Hartmann G. Accessing the therapeutic potential of immunostimulatory nucleic acids. Curr Opin Immunol. 2008;20:389–395. doi: 10.1016/j.coi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Qian C, Cao X. Regulation of Toll-like receptor signaling pathways in innate immune responses. Ann. N. Y. Acad. Sci. 2013;1283:67–74. doi: 10.1111/j.1749-6632.2012.06786.x. [DOI] [PubMed] [Google Scholar]
- 12.Sarvestani ST, Williams BRG, Gantier MP. Human Toll-Like Receptor 8 Can Be Cool Too: Implications for Foreign RNA Sensing. Journal of Interferon & Cytokine Research. 2012;32:350–361. doi: 10.1089/jir.2012.0014. [DOI] [PubMed] [Google Scholar]
- 13.Schmid-Burgk JL, Schmidt T, Gaidt MM, Pelka K, Latz E, Ebert TS, Hornung V. OutKnocker: a web tool for rapid and simple genotyping of designer nuclease edited cell lines. Genome Res. 2014 doi: 10.1101/gr.176701.114. In press, doi: 10.1101/gr.176701.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan T, Dai M, Wang D, Zhu F-G, Kandimalla ER, Agrawal S. Toll-like receptor 7 selective synthetic oligoribonucleotide agonists: synthesis and structure-activity relationship studies. J. Med. Chem. 2009;52:6871–6879. doi: 10.1021/jm901145s. [DOI] [PubMed] [Google Scholar]
- 15.Lan T, Kandimalla ER, Yu D, Bhagat L, Li Y, Wang D, Zhu F, Tang JX, Putta MR, Cong Y, Trombino AF, Sullivan T, Agrawal S. Stabilized immune modulatory RNA compounds as agonists of Toll-like receptors 7 and 8. Proc Natl Acad Sci USA. 2007;104:13750–13755. doi: 10.1073/pnas.0706059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onji M, Kanno A, Saitoh S-I, Fukui R, Motoi Y, Shibata T, Matsumoto F, Lamichhane A, Sato S, Kiyono H, Yamamoto K, Miyake K. An essential role for the N-terminal fragment of Toll-like receptor 9 in DNA sensing. Nat Commun. 2013;4:1949. doi: 10.1038/ncomms2949. [DOI] [PubMed] [Google Scholar]
- 17.Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, Ross C, Li X, Smart NG, Beutler B. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proceedings of the National Academy of Sciences. 2010;107:19973–19978. doi: 10.1073/pnas.1014051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 19.Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 20.Schütze S, Tchikov V, Schneider-Brachert W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nature Publishing Group. 2008;9:655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- 21.Liao C, Hsu J, Kim Y, Hu D-Q, Xu D, Zhang J, Pashine A, Menke J, Whittard T, Romero N, Truitt T, Slade M, Lukacs C, Hermann J, Zhou M, Lucas M, Narula S, DeMartino J, Tan S-L. Selective inhibition of spleen tyrosine kinase (SYK) with a novel orally bioavailable small molecule inhibitor, RO9021, impinges on various innate and adaptive immune responses: implications for SYK inhibitors in autoimmune disease therapy. Arthritis Res. Ther. 2013;15:R146. doi: 10.1186/ar4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.