Abstract
Beta-glucans (β-glucans) are naturally occurring polysaccharides in cereal grains, mushrooms, algae, or microbes including bacteria, fungi, and yeast. Immune cells recognize these β-glucans through a cell surface pathogen recognition receptor (PRR) called Dectin-1. Studies using β-glucans and other Dectin-1 binding components have demonstrated the potential of these agents in activating the immune cells for cancer treatment and controlling infections. Here, we show that the β-glucan from Saccharomyces cerevisiae induces the expression of immune regulatory cytokines (IL-10, TGF-β1 and IL-2) and a tolerogenic enzyme (Indoleamine 2, 3-dioxygenase; IDO) in bone marrow derived DCs (BM DCs) as well as spleen cells. These properties can be exploited to modulate autoimmunity in non-obese diabetic (NOD) mouse model of type 1 diabetes (T1D). Treatment of pre-diabetic NOD mice with low dose β-glucan resulted in a profound delay in hyperglycemia and this protection was associated with increase in the frequencies of Foxp3-, LAP-, and GARP-positive T cells. Upon antigen presentation, β-glucan-exposed DCs induced a significant increase in Foxp3− and LAP− positive T cells in in vitro cultures. Further, systemic co-administration of β-glucan plus pancreatic β-cell-Ag resulted in an enhanced protection of NOD mice from T1D as compared to treatment with β-glucan alone. These observations demonstrate that the innate immune response induced by low dose β-glucan is regulatory in nature and can be exploited to modulate T cell response to β-cell-Ag for inducing an effective protection from T1D.
Keywords: Dectin-1, beta-glucan, Innate immunity, Type 1 diabetes, Insulitis, antigen presenting cells, Dendritic cells, T cells, regulatory T cells
Introduction
β-Glucans are primary components of the cell wall in various yeast, fungi, and certain bacteria and are recognized by the pathogen recognition receptors (PRRs) on innate immune cells (1, 2). β-glucans are polysaccharides with a backbone of β-1,3-linked D-glucose molecules (β-1,3-D-glucan) and β-1,6-linked side chains of varying sizes (1, 2). These polysaccharides have been shown to possess immune stimulatory properties that can enhance the innate immune function against tumors and infections (3, 4). Although β-glucan preparations from different sources can bind to many PRRs, purified 1-3,1-6-β-glucans signal primarily through the Dectin-1 receptor, which is expressed on dendritic cells (DCs), macrophages, and neutrophils (5, 6). The role of Dectin-1 in innate immunity against fungal pathogens and bacteria, mycobacteria in particular, is widely recognized (5-8). Studies have shown that β-glucan can activate both the innate and adaptive immunity and hence, can be used as an effective adjuvant in immunotherapies for treatment of cancer and infections (3, 4). However, recent studies have shown that the role of Dectin-1 in immune potentiation activities of β-glucan rich ligands against some bacterial and fungal infections is redundant (9-11).
While previous studies were mostly focused on the pro-inflammatory properties of β-glucan containing microbial and plant products and host defense aspects of Dectin-1-dependent signaling, we and others have shown that β-glucan containing fungal cell-wall preparations such as zymosan can promote regulatory innate immune responses and modulate autoimmunity (12-16). Further, immune potentiation using β-glucan, starting at the pre-insulitis stage (4-week old), caused a delay in Th1 dominated type 1 diabetes (T1D) in a rat model (17). On the other hand, β-glucan-containing compounds have shown the ability to promote Th17 dominated autoimmunity, autoimmune arthritis in particular, in genetically susceptible mice under specific pathogen-free as well as germ-free conditions (18-21). These reports suggest that signaling through PRRs like Dectin-1 by environmental factors such as microbial products and food components can influence the incidence of autoimmune diseases in genetically susceptible subjects.
Like many other autoimmune conditions, T1D can be triggered by environmental factors such as microbes, affecting the genetic susceptibility of individuals, leading to immune cell-mediated destruction of insulin-secreting β cells of the pancreatic islets (22, 23). Innate immunity induced by these microbial factors could play a key role in both initiating an effector T cell response and maintaining tolerance to the pancreatic β-cell antigens (β-cell-Ag). In fact, increase in the incidence of T1D in developed countries, possibly due to, better sanitary and healthcare practices indicate a role for microbial exposure and innate immunity in preventing autoimmunity and T1D (2, 3).
Innate immune response is mediated through a number of PRRs, predominantly the Toll-like receptors (TLRs) and C-type lectin receptors (CLRs) (24). Dectin-1, a CLR, plays a crucial role in the recognition of fungi such as Candida and Aspergillus by binding to the β-glucan-like polysaccharides in their cell walls. This receptor has been shown to collaborate with TLR2 in inducing balanced pro- and anti-inflammatory innate immune responses by the antigen presenting cells (APCs) (5, 6, 25). Importantly, TLR2 deficiency in non-obese diabetic (NOD mice) leads to reduced susceptibility to T1D (26). Paradoxically administration of TLR2 ligands results in enhanced Treg function and protection of NOD mice from diabetes (27, 28). However, co-engagement of TLR2 and Dectin-1 by zymosan, a fungal cell wall component, results in expression of the immune regulatory cytokines IL-10, IL-2 and TGF-β1 and suppression of the pro-inflammatory cytokines like IL-12 (12-16),(25, 29, 30). However, independent engagement of TLR2 in immune cells results in the induction of IL-10, but not IL-2 or TGF-β1. This indicates that signaling through Dectin-1 may be critical for the expression of these immune regulatory cytokines in innate immune cells. In fact, previous reports have shown that IL-2 expression induced by zymosan in APCs requires Dectin-1 engagement (1, 31). Therefore, in this study, we examined the immunoregulatory properties of the Dectin-1 ligand, β-glucan derived from S. cerevisiae and its ability to modulate T1D in NOD mice.
Our observations show that β-glucan induces mixed pro- and anti-inflammatory responses and this mixed innate immune response promotes regulatory T cell (Treg) and Th17 responses both in vitro and in vivo. Treatment of 12 week-old NOD mice at the pre-diabetic stage) with low dose β-glucan resulted in the protection of pancreatic β-cells from immune destruction and the mice from T1D. Importantly, co-administration of β-cell-Ag along with β-glucan resulted in a better protection of NOD mice from diabetes as compared to treatment with β-glucan alone. These observations show that the Dectin-1-dependent innate immune response induced by β-glucan is regulatory in nature and it could be exploited to modulate the immune response to self-Ag for preventing Th1 dominated autoimmune conditions like T1D.
Materials and Methods
Mice
Wild-type NOD/LtJ, NOD-BDC2.5 TCR-transgenic, NOD-Scid, and OT-II-TCR-transgenic (OT-II-TCR-Tg) mice were purchased from the Jackson laboratory (Maine, USA). Foxp3-GFP-knockin (ki) (32) mice in the B6 background were kindly provided by Dr. Kuchroo (Harvard Medical School). Breeding colonies of these strains were established and maintained in the pathogen free facility of University of Illinois at Chicago (UIC) or Medical University of South Carolina (MUSC). NOD (NOD/LtJ) mice from our breeding colony or freshly purchased from Jackson Lab were used in this study. Dectin 1 deficient mice in C57BL6 background (29) were kindly provided by Dr. Iwakura, University of Tokyo, Japan. To generate OT-II-TCR-Tg-Foxp3-GFP- ki F1 (OT-II-Foxp3-GFP-F1) generation mice, OT-II mice were crossed with Foxp3-GFP-ki mice. NOD-Foxp3-GFP-ki mice were generated by backcrossing B6-Foxp3-GFP-ki mice to NOD background for 12 generations. To detect hyperglycemia in NOD mice, glucose levels in blood collected from the tail vein of wild-type NOD-Ltj or NOD-Scid mice were monitored using the Ascensia Micro-fill blood glucose test strips and an Ascensia Contour blood glucose meter (Bayer, USA). All animal studies were approved by the animal care and use committee of UIC and MUSC.
Peptide antigens, cell lines, and antibodies
Immunodominant β-cell antigen peptides [viz., 1. Insulin B (9-23), 2. GAD65 (206-220), 3. GAD65 (524-543), 4. IA-2beta (755-777), 5. IGRP (123-145), 6. BDC2.5 TCR reactive peptide (YVRPLWVRME; referred to as BDC-peptide), and 7. OVA (323-339) peptides] were custom synthesized (Genescript Inc) and used in this study. Peptides 1-5 were pooled at an equal molar ratio and used as β-cell-Ag as described in our earlier studies (33-35). MFB-F11 TGF-β1 activity reporter cell line was provided by Dr. Wyss-Coray, Stanford University.
Zymosan of Saccharomyces cerevisiae origin was purchased from Sigma-Aldrich, boiled for 30 mins, washed extensively, and suspended in PBS as described earlier (12, 13). β-glucan (glucan from baker's yeast, S. cerevisiae), ≥98% pure, was purchased from Sigma-Aldrich for this study and tested for specific activity using thioglycolate-activated macrophages as described before (25, 36). Unlike zymosan, β-glucan from Sigma-Aldrich failed to induce considerable amounts of IL-12 and IL-10 in thioglycolate-activated mouse peritoneal macrophages (Supplemental Fig. 1) indicating that this β-glucan preparation is of acceptable purity. Bacterial lipopolysaccharide (LPS), CpG-ODN, poly I:C, L-tryptophan, recombinant TGF-β1, PMA, ionomycin, Brefeldin A, and monensin were purchased from Sigma-Aldrich, BD Biosciences, eBioscience, Invivogen, and Invitrogen. β-glucan stock was suspended in PBS and incubated at 56°C for 30 min, washed with sterile PBS, before making aliquots and storing at −20°C. Normal rat serum, purified anti-mouse TGF-β1 (clone A75-2), anti-CD16/CD32 (Fc block), various fluorochrome conjugated reagents and anti-mouse CD11c, CD4, CD25, CD80, CD86, CD40, MHC II, Foxp3, IFN-γ, IL-17, IL-4, IL-10, TGF-β1, GARP, Helios, LAP (latent TGF-β1) antibodies (Abs) and isotype control Abs (Invitrogen, BD Biosciences, eBioscience, R&D Systems, and Biolegend Laboratories) were used for FACS. Magnetic bead-based total and CD4+ T cells and CD11c+ dendritic cell isolation kits (Miltenyi Biotec and Invitrogen) were used for enriching or depleting T cells and DCs. Paired Abs and standards for ELISA to detect TGF-β1, TNF-α, IL-10, IL-2, IL-4, IL-17, IFN-γ, IL-12, IL-1β, and IL-6 were purchased from R&D Systems, BD Biosciences, Invitrogen, and eBioscience.
Treating mice with TLR ligands, β-glucan and β-cell-Ag
In a trend-finding experiment, 10-week old female NOD mice were injected (i.p.) with zymosan (5 μg/mouse/day), Pam3Cys (0.5 μg/mouse/day), β-glucan (5 μg/mouse/day) or bacterial LPS (1 μg/mouse/day) for 3 alternate days and monitored for blood glucose levels. In a dose determining pilot experiment, NOD mice were treated with varying amounts of β-glucan for 3 alternate days (Supplemental Fig. 2). In some experiments, mice that were treated on 3 alternate days were euthanized 24 h post-injection and spleen cells were examined for innate immune response. Since in our previous study (12), zymosan was used at >25 μg/mouse/day, 5 μg zymosan or β-glucan per mouse per injection is considered a significantly low dose. In one set of experiments, 12-week-old prediabetic stage mice were treated with β-glucan (i.p.; 5 μg/mouse/day) every other day, for 30 days (total of 15 injections). Some of these treated mice were monitored for up to 30 weeks post-treatment for hyperglycemia. The remaining mice were euthanized 30 days post-treatment and examined for insulitis and immune cell characteristics. In another set of experiments, 12-week-old prediabetic mice were treated with β-glucan (5 μg/mouse/day; i.v., on days 1, 3, 5, 13, 15 and 17) and/or β-cell-Ag (0.5 μg/mouse/day; i.v., on days 5 and 17). These mice were monitored weekly for hyperglycemia. Additional mice from parallel experiments were euthanized 30 days post-treatment and examined for insulitis and immune cell characteristics.
Dendritic cells and T cells
Peripheral CD11c+ DCs were enriched from spleen cells using magnetic bead linked-anti-CD11c Ab reagent and magnetic columns. CD11c+ population was enriched to >90% using magnetic separation reagents. To generate bone marrow (BM)-derived DCs, RBC-depleted BM cells were cultured in complete RPMI 1640 medium containing 10% heat-inactivated FBS in the presence of 20 ng/ml GM-CSF for 2 days and for an additional 3 days in fresh medium containing 20 ng/ml GM-CSF and 5 ng/ml IL-4. Cells from 5-day cultures were used for this study. T cells were enriched to >95% using negative selection kits from Miltenyi Biotec or Invitrogen.
DC assays
Splenic CD11c+ DCs and bone marrow (BM) DCs were incubated with β-glucan (25 μg/ml), zymosan (25 μg/ml), Pam3Cys (1.0 μg/ml), and LPS (2 μg/ml) for different time points. Initial experiments have used varying amounts of these agents to optimize the long term culture conditions for cytokine measurement and for antigen presentation assays. Optimum dose was determined by assessing the concentration of a specific agent required in the culture for inducing the highest amount of TNF-α without affecting the cell viability during the 48 h culture (Supplemental Fig. 3). For short-term cultures to induce IDO in vitro, β-glucan was used at 100 μg/ml concentration. Cells collected at 6h or 12h time-points were used for examining transcript levels of indoleamine-2,3-dioxygenase (IDO) and a housekeeping gene β-actin by qualitative or quantitative RT-PCR assays. In some assays, spleen cells were obtained from NOD mice that were injected with β-glucan or Pam3Cys for 24 h and processed for RT-PCR or cultured overnight for obtaining supernatants to determine in vivo induced cytokines. RNA was prepared using Trizol reagent (Invitrogen), first strand cDNA was synthesized using the cDNA synthesis kit (Fermentas), and real-time PCR assays were performed using transcript-specific primer sets and SYBR-green PCR mastermix (ABI prism or Bio-Rad). In some assays, cDNA samples were also used in a qualitative PCR analysis. DCs, obtained from the above described overnight cultures, were pulsed with specific Ags and used in Ag-presentation assays as described below. DCs from 36h cultures were examined for the levels of activation markers on the surface, after staining with fluorochrome-labeled specific Abs. Spent medium from these cultures were also tested for secreted cytokine levels by ELISA.
T cell assays
Purified total T cells from diabetic NOD mice (1×105 cells/well) were incubated with β-glucan (25 μg/ml) or LPS (2 μg/ml) exposed or unexposed DCs (2×104 DCs/well) in the presence of anti-CD3 Ab (2 μg/ml). In some assays, DCs were incubated with β-cell-Ag (5 μg/ml) and β-glucan (25 μg/ml) or LPS (2 μg/ml) overnight and then washed and cultured with CD4+ T cells from diabetic NOD mice. β-glucan exposed DCs were also cultured with FACS sorted GFP+ and GFP− CD4+ T cells from NOD-Foxp3-GFP mice in anti-CD3 Ab coated 96 well plates. Specific Ag peptide-pulsed and ligand-exposed B6 or NOD DCs (2×104 cells/well) were also cultured in 96-well round-bottom plates along with CFSE labeled- or unlabeled- purified CD4+ T cells (1 × 105/well) from OT-II or BDC2.5 TCR-Tg mice. After 4 days of culture, cells were stained using PE-linked CD4 specific Ab and examined for CFSE dilution by FACS. For some assays, in vitro stimulated or freshly isolated T cells were re-stimulated using PMA (50 ng/ml) and ionomycin (500 ng/ml) in the presence of brefeldin A (1 μg/ml) for 4h before staining for intracellular IFN-γ, IL-10, TGF-β1, IL-17 and IL-4. Recombinant IL-2 (2 units/ml), GM-CSF (5ng/ml), TGF-β1 (1 ng/ml) were added to the culture of selected assays. In some assays, spleen and pancreatic lymph node (PnLN) cells (2 × 105 cells/well) from treated and control mice were stimulated with anti-CD3 Ab (2 μg/ml) or β-cell-Ag (5 μg/ml) for 48h. Spent media from these cultures were tested for cytokines.
FACS analysis
Freshly isolated and ex vivo cultured cells were washed using PBS supplemented with 2% FBS and 10 mM EDTA (pH 7.4) and blocked with anti-CD16/CD32 Fc block Ab or 5% rat serum on ice for 15 min. For surface staining, cells were incubated with FITC-, PE-, and PECy5 or PE-TR-labeled appropriate Abs in different combinations and washed three times before analysis. For intracellular staining, surface-stained cells were fixed and permeabilized using in-house reagents (2% paraformaldehyde and 0.1% saponin) or reagents from eBioscience, incubated with fluorochrome-labeled appropriate Abs, and washed three times before analysis. Stained cells were acquired using a FACSCalibur or LSR (BD Biosciences) or Cyan (Dako-cytomation) flow cytometer, and the data were analyzed using WinMDI or Summit applications. Cells were also stained using isotype-matched control Abs for determining the background. Specific regions were marked and the gates and quadrants were set while analyzing the data based on the isotype control background staining. At least 10,000 cells were analyzed for each sample.
Cytokine detection
Culture supernatants were tested for pro- and anti-inflammatory cytokines by ELISA using paired antibody sets and kits from eBioscience, BD Biosciences, Invitrogen and R&D systems. Bioassay for TGF-β1 activity was performed using the MFB-F11 cell line which secretes alkaline phosphatase upon stimulation with TGF-β1. Cells were cultured at 2×106/well in a 24 well plate overnight, spent medium was replaced with fresh medium containing recombinant TGF-β1, or non-treated or HCl/NaOH treated (to release active TGF-β1) culture supernatants, and cultured for an additional 24 h. 25 μl of clarified supernatants from these cultures were incubated with 225 μl p-nitrophenyl phosphate substrate for 2 h in a 96 well plate, and the optical density values were measured at 405nm using an ELISA reader. Blank values were subtracted from test values and fold induction was calculated against values from untreated group.
T cell transfer experiment
Total T cells were enriched from spleen of control and treated mice using a magnetic bead-based pan T cell isolation (negative selection) kit. Enriched cells, which contained both CD4+ and CD8+ populations, were transferred into 8-week old NOD-scid mice or pre-diabetic female NOD mice. Recipient mice were tested for blood glucose every week. In some experiments, freshly isolated T cells from hyperglycemic mice (2×105 cells/well) were cultured ex vivo along with CD11c+ DCs (5×104 cells/well) in the presence of anti-CD3 Ab (2 μg/ml) and β-glucan (25 μg/ml). T cells were purified from these cultures and injected into 8-week-old NOD-Ltj mice (i.v) and monitored as described above.
Histochemical and Immunofluorescence analysis of pancreatic tissues
Pancreata were fixed in 10% formaldehyde, 5-μm paraffin sections were made, and stained with hematoxylin and eosin (H&E). Stained sections were analyzed using a grading system in which 0 = no evidence of infiltration, 1 = peri-islet infiltration (<5%), 2= 5-25% islet infiltration, 3 = 25–50% islet infiltration, and 4 = >50% islet infiltration as described in our earlier studies (33-35, 37). Areas that appeared to have completely lost islets were not included in this grading approach.
Statistical analysis
Mean, SD, and statistical significance (p-value) were calculated using Microsoft Excel, GraphPadonline, and/or other online statistical applications. Two-tailed t-test was employed, unless specified for values from in vitro and ex vivo assays. Log-rank analysis was performed to compare T1D incidence (hyperglycemia) of the test group with that of control group. Fisher’s exact test was used for comparing the total number of infiltrated islets in test vs. control groups. A p value of ≤0.05 was considered significant.
Results
β-glucan exposed DCs express both immune regulatory and pro-inflammatory factors
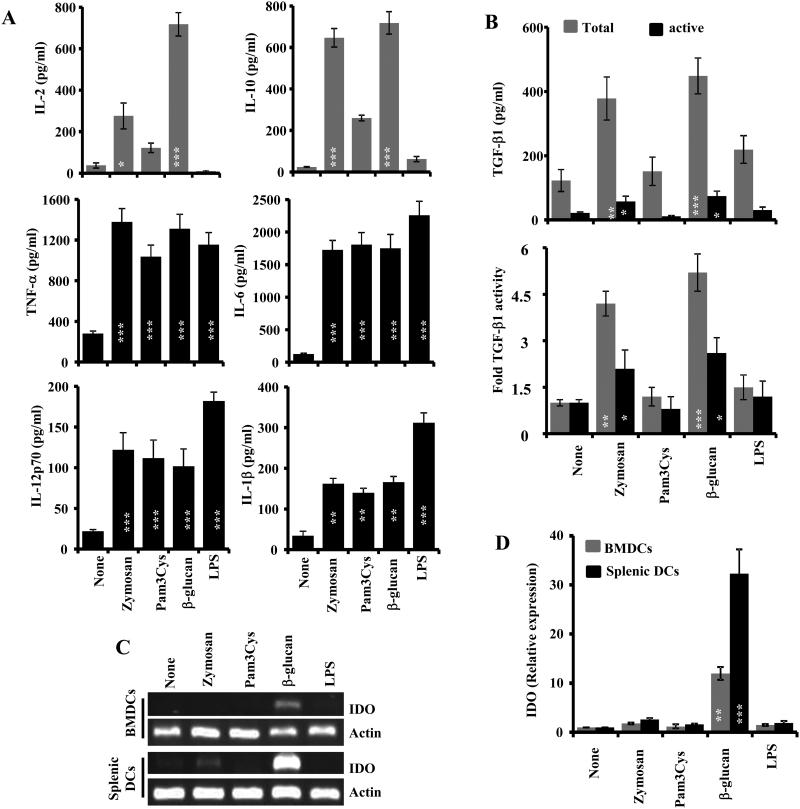
We and others have reported that simultaneous engagement of TLR2 and Dectin-1 by zymosan from the fungal cell wall can induce both anti- and pro-inflammatory cytokines in APCs (12-16). Therefore, we examined whether engagement of these two receptors, independently, can produce tolerogenic factors in APCs. The BM derived and splenic DCs were stimulated with optimized dose of β-glucan and Pam3Cys, as the primary Dectin-1- and TLR2- interacting ligands, along with other control agents (zymosan and bacterial LPS) for different durations and examined for the expression of cytokines. As observed in Fig. 1A and 1B, BM-derived DCs that were exposed to β-glucan produced large amounts of IL-2, IL-10, and TGF-β, similar to DCs that were exposed to zymosan, as compared to the untreated control. However, Pam3Cys induced the expression of high levels of IL-10 and TNF-α, but not IL-2 or TGF-β1. Importantly, β-glucan induced higher levels of IL-2 and TGF-β1 in BM DCs, compared to zymosan. Importantly, although the ELISA and bioassay results suggest that upon treatment of BM DCs with either β-glucan or zymosan, only a portion of the secreted TGF-β1 is in its active form, but the total TGF-β1 produced by β-glucan exposed BMDCs is relatively higher than that induced by zymosan (Fig. 1B). Bacterial LPS, a TLR4 ligand, failed to induce significant amounts of regulatory cytokines suggesting a dominant and specific effect of Dectin-1 engagement on the expression of these cytokines. Splenic DCs that were exposed to β-glucan and zymosan also produced considerable amounts of IL-10 and TGF-β1, albeit much lower than that produced by BM DCs (not shown). Zymosan, Pam3Cys, β-glucan, and LPS-exposed DCs induced comparable levels of the pro-inflammatory cytokine TNF-α (Fig. 1A). To realize the other properties of Dectin-1- and TLR2-engaged DCs, expression level of the tolerogenic enzyme IDO was also examined in similarly treated DCs. Fig. 1C and ID show that only the β-glucan-exposed, but not Pam3Cys-, zymosan- or LPS-exposed, BM-derived and splenic DCs expressed considerable levels of IDO. Overall, these observations show that Dectin-1, but not TLR2 or TLR4 engagement, induces a combination of immune regulatory and pro-inflammatory factors in APCs.
FIGURE 1. Fungal β-glucan induces regulatory innate immune response in DCs.
A) DCs were generated in vitro from BM cells (BMDCs) using GM-CSF and IL-4 and left untreated or exposed to indicated agents for 48h, and cytokine levels were measured in the supernatants by ELISA. Mean±SD of values from 2 individual representative experiments carried out in triplicate are shown. B) Non-activated (to detect active form) and activated (to detect total amount) culture supernatants were examined for TGF-β1 levels ELISA (upper panel) and TGF-β1 activity by bioassay using F-11 reporter cells (lower panel). TGF-β1 activity of control (none) sample was considered as 1 for calculating the fold activity. C) BMDC and splenic CD11c+ cells were exposed to indicated agents as described above, cDNA was prepared using cells collected from 18h cultures and were subjected to qualitative PCR using IDO and housekeeping gene, actin specific primer sets. Gel images from representative experiments are shown. D) cDNA prepared from splenic and BM DCs were also used in real-time qPCR for detecting IDO and actin. Expression levels were calculated relative to actin expression and the values of ligand-treated samples were compared against the value of untreated (none) sample which was considered as 1. Mean±SD values of 2 independent assays are shown. Statistical significance of treated group was calculated against untreated (none) group. *, p<0.05; **, p <0.01; ***, p <0.001.
Regulatory innate immune response induced by β-glucan in DCs is Dectin-1 dependent
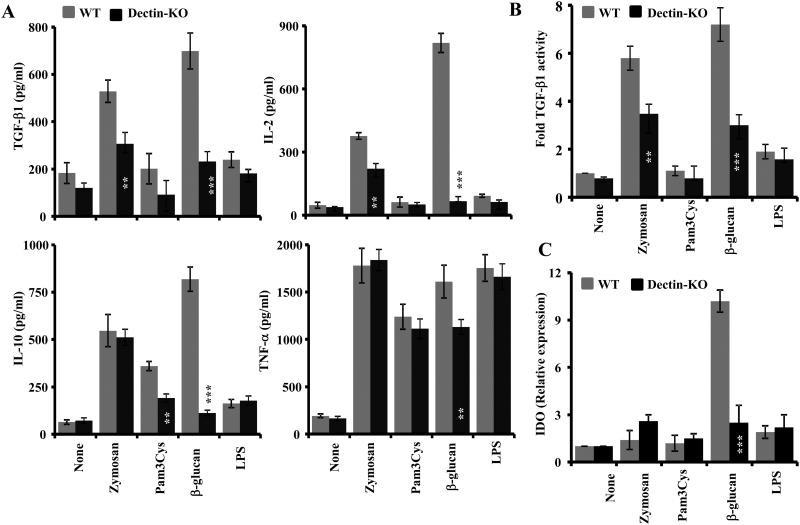
To assess whether the β-glucan induced regulatory innate immune response is primarily Dectin-1 dependent, DCs were generated from BM of WT and Dectin-1 KO mice and activated using the Dectin-1 ligand, β-glucan or TLR2 ligand, Pam3Cys and co-ligands of both receptors, zymosan. Cytokine levels in the spent medium from these cultures were examined by ELISA and their IDO expression was determined by qPCR. Fig. 2 shows that β-glucan treated Dectin 1 KO DCs secrete/express profoundly low levels of TGF-β1, IL-2, IL-10, and IDO, as well as the pro-inflammatory cytokine TNF-α compared to their WT counterparts and the levels of these factors were comparable to that of untreated cells. On the other hand, WT and Dectin 1 KO DCs that were exposed to TLR2 ligand, Pam3Cys showed a comparable up-regulation of IL-10 and pro-inflammatory cytokine, TNF-α. Further, Pam3Cys failed to induce IDO in WT and Dectin-1 KO DCs. Importantly, zymosan induced the expression of considerable levels of IL-2 and TGF-β1 in WT DCs but at a significantly lower level in Dectin-1 KO DCs. These observations show that immune-regulatory and pro-inflammatory responses induced by β-glucan and zymosan in DCs are primarily Dectin 1 dependent.
FIGURE 2. Fungal β-glucan induced regulatory innate immune response in DCs is Dectin-1 dependent.
A) DCs were generated in vitro from BM cells (BMDCs) of WT and Dectin-KO B6 mice using GM-CSF and IL-4 and left untreated or exposed to indicated agents for 48h, and cytokine levels were measured in the supernatants by ELISA. Mean±SD of values from 2 individual representative experiments carried out in triplicate are shown. Statistical significance of treated group was calculated against untreated (none) group. *, p<0.05; **, p <0.01; ***, p <0.001. B) Activated culture supernatants were examined for total TGF-β1 activity by bioassay using F-11 reporter cells. TGF-β1 activity of control (none) sample was considered as 1 for calculating the fold activity. C) cDNA was prepared using cells collected from 18h cultures and were subjected to real-time qPCR for detecting IDO and actin. Expression levels were calculated relative to actin expression and the values of ligand-treated samples were compared against the value of untreated (none) sample which was considered as 1. Mean±SD values of 2 independent assays are shown. Statistical significance of treated group was calculated against untreated (none) group. *, p<0.05; **, p <0.01; ***, p <0.001.
Treatment with low dose β-glucan promotes relatively better protection of NOD mice from T1D than by zymosan and Pam3Cys
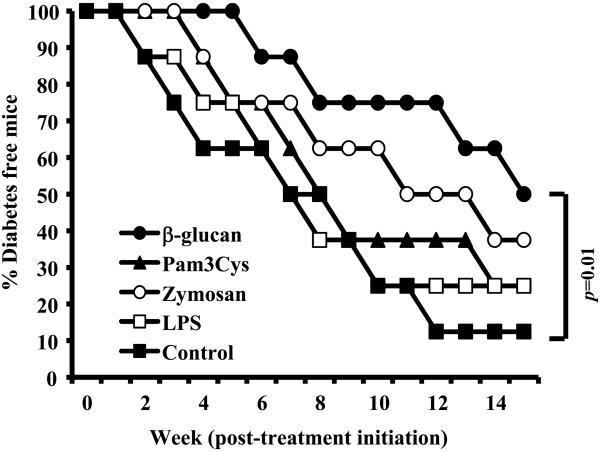
Previously, we showed that treatment with zymosan, which is known to interact with TLR2 and Dectin-1, induces a regulatory innate immune response and prevents T1D in NOD mice (12-16). In addition, other studies have shown that the innate immune response induced by Pam3Cys, which targets TLR2, can suppress autoimmunity even in the mouse model of T1D (27, 28). Since Dectin-1 signaling is necessary for zymosan induced TGF-β1 and IL-2 responses in APCs, the effect of β-glucan-induced innate immune response on T1D incidence in pre-diabetic NOD mice was compared to that of LPS, Pam3Cys and zymosan. Ten-week old NOD mice were treated with above agents for three alternate days and monitored for hyperglycemia. As observed in Fig. 3, mice that received only β-glucan, but not LPS, Pam3Cys or zymosan showed a statistically significant delay in diabetes compared to control group mice during the 15 week monitoring period. While treatment with low dose zymosan also caused considerable delay in hyperglycemia, albeit not significant statistically, Pam3Cys and LPS treatment showed little or no protective effect in 10 week old pre-diabetic NOD mice. These observations confirm that the Dectin-1 ligand, β-glucan has a better immunoregulatory property in T1D model as compared to TLR2 and TLR4 ligands.
FIGURE 3. Innate immune response induced using β-glucan, but not Pam3Cys or LPS, produced significant delay in hyperglycemia.
A) 10-week-old pre-diabetic female NOD mice were injected with β-glucan (5 μg/mouse/day), zymosan (5 μg/mouse/day), Pam3Cys (1.0 μg/mouse/day) and bacterial LPS (0.5 μg/mouse/day); for three alternate days or left untreated (control). Mice were checked every week for hyperglycemia for up to 15 weeks and mice with glucose level of 250 mg/dl for two consecutive weeks were considered diabetic. Log-rank test was performed to compare the hyperglycemia incidence in treated and control groups of mice and the statistically significant p-value is shown.
β-glucan induces regulatory immune response in vivo
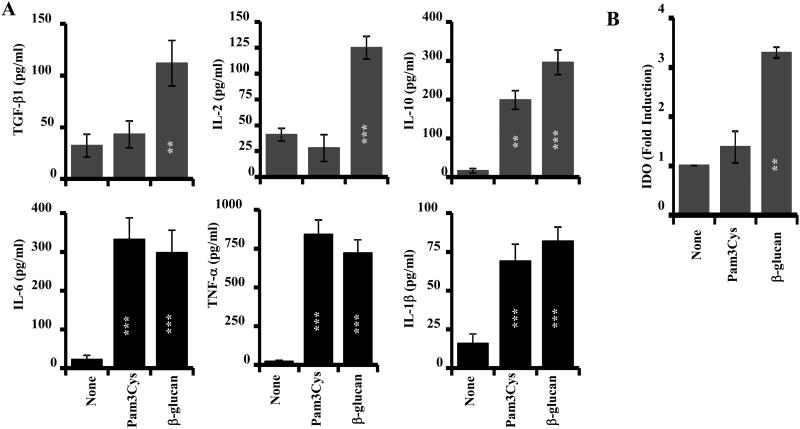
Results presented in Fig.3, in association with earlier reports, including ours, on the therapeutic effects of zymosan in NOD mice (12, 14), indicate that Dectin-1, but not TLR2, dependent innate immune response is the primary player in zymosan induced prevention of T1D. In addition, Figs 1 and 3 shows that β-glucan, but not Pam3Cys, induces immune regulatory factors such as IL-2 and TGF-β1 in DCs, in a similar manner to zymosan. Interestingly, only β-glucan, but not zymosan or Pam3Cys, induced IDO expression in DCs. Therefore, we compared β-glucan and Pam3Cys for their abilities to induce immune regulatory cytokines and IDO in vivo. Pre-diabetic NOD mice were left untreated or treated with β-glucan and Pam3Cys for 3 alternate days as described for Fig. 3. Spleen cells were harvested 24 h post-treatment and subjected to qPCR or cultured to examine the levels of spontaneously released cytokines. As observed in Fig. 4, cells from β-glucan, but not Pam3Cys, treated mice showed significant induction of IL-2, TGF-β1 and IDO. On the other hand, spleen cells from both β-glucan and Pam3Cys treated mice produced significant similar amounts of IL-10 and TNF-α as compared to cells from untreated control mice. These observations, in association with the results of Figs. 1-3, show that the Dectin-1 ligand, β-glucan has better immune regulatory properties than the TLR2 ligand, Pam3Cys.
FIGURE 4. β-glucan, but not Pam3Cys, induced IL-10, IL-2 and IDO in vivo.
A) 10-week-old pre-diabetic female NOD mice (3/group) were injected with β-glucan and Pam3Cys for three alternate days or left untreated (control) as described in Fig. 3 and euthanized after 24 h. A) Spleen cells were cultured for 48h and supernatants were tested for spontaneously secreted cytokines by ELISA. B) cDNA prepared using spleen cells was subjected to real-time qPCR for detecting IDO. Expression levels were calculated relative to actin expression and the values of ligand-treated mice were compared against the value of untreated (none) group which was considered as 1. This experiment was repeated twice. Statistical significance of treated group was calculated against untreated (none) group. *, p<0.05; **, p <0.01; ***, p <0.001.
Treatment with β-glucan protects pre-diabetic NOD mice from T1D
Our trend finding study using 10-week old NOD mice (Fig. 3) showed that treatment with low dose of β-glucan, but not the TLR2 and TLR4 ligands (Pam3Cys and LPS respectively) for 3 days, delayed hyperglycemia in NOD mice. Further, as compared to zymosan, low dose β-glucan showed a superior ability to delay hyperglycemia when treated at pre-hyperglycemic stage. Therefore, the effect of prolonged treatment with this Dectin-1 ligand was examined in pre-diabetic stage NOD mice. As observed in Fig. 5A, prolonged treatment of 12-week old pre-diabetic NOD mice with low-dose of β-glucan could delay hyperglycemia in NOD mice for a significant duration and prevented the disease in 50% of the mice for at least 30 weeks post-treatment as compared to non-injected controls whereas, 100% of the untreated control mice developed diabetes by 26 weeks of age (within 14 weeks post-treatment initiation).
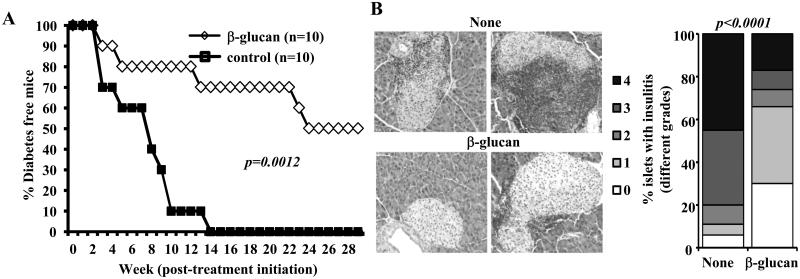
FIGURE 5. Activation of innate immune response using β-glucan prevents hyperglycemia in NOD mice.
A) Twelve-week old pre-diabetic female NOD mice were treated i.p. with β-glucan (5 μg/mouse/day; every other day for 30 days; total of 15 injections) or left untreated (control). Mice were checked every week for hyperglycemia and glucose level of 250 mg/dl for two consecutive weeks was considered diabetic. Log-rank test was performed to compare the hyperglycemia incidence in treated and control groups of mice and the p-value is shown. B) Euglycemic β-glucan-treated and control mice from parallel experiments were euthanized 30 days after the last injection. Pancreatic tissues were processed for H&E staining to evaluate insulitis. Representative islets (left panel) and percentages of islets with different grades of insulitis plotted as bar diagram (right panel) are shown. Sections of pancreatic tissues from at least 5 mice/group were examined for insulitis and at least 180 islets/group were examined.
The pancreatic islets of pre-diabetic mice that received β-glucan showed significantly less severe immune cell infiltration and insulitis, compared to untreated control mice, 30 days post-treatment (Fig. 5B). While about 70% of the islets in mice that received β-glucan showed insulitis grade ≤1, at least 80% of the islets in untreated mice showed insulitis severity grade >2. Importantly, while approximately 30% of the islets in β-glucan recipient mice were found to be free from immune cell infiltration, <5% of islets of untreated mice were insulitis-free. These results suggest that β-glucan induced-innate immune response in vivo is regulatory in nature and protects NOD mice from T1D.
T cells from β-glucan treated mice produce IL-10, TGF-β1 and IL-17
Since mice that were treated with β-glucan showed significant protection from hyperglycemia, T cells from these mice were examined for their functional properties in comparison with their counterparts from control mice. Spleen and PnLN cells obtained from mice that were euthanized 30 days post-treatment were examined for their cytokine secretion profiles upon activation using anti-CD3 Ab. The cytokine profiles of cells from β-glucan recipient groups were significantly different as compared to control mice (Fig. 6A). While cells from the β-glucan recipient and control mice produced comparable levels of IFN-γ in both the spleen and PnLN, cells from β-glucan-treated mice, PnLN cells in particular, produced significantly higher amounts of IL-10, IL-17 and TGF-β1, as compared to T cells from control mice. These observations show that Dectin-1 dependent innate immune response induced by β-glucan modulates T cell function in vivo leading to the protection of NOD mice from T1D.
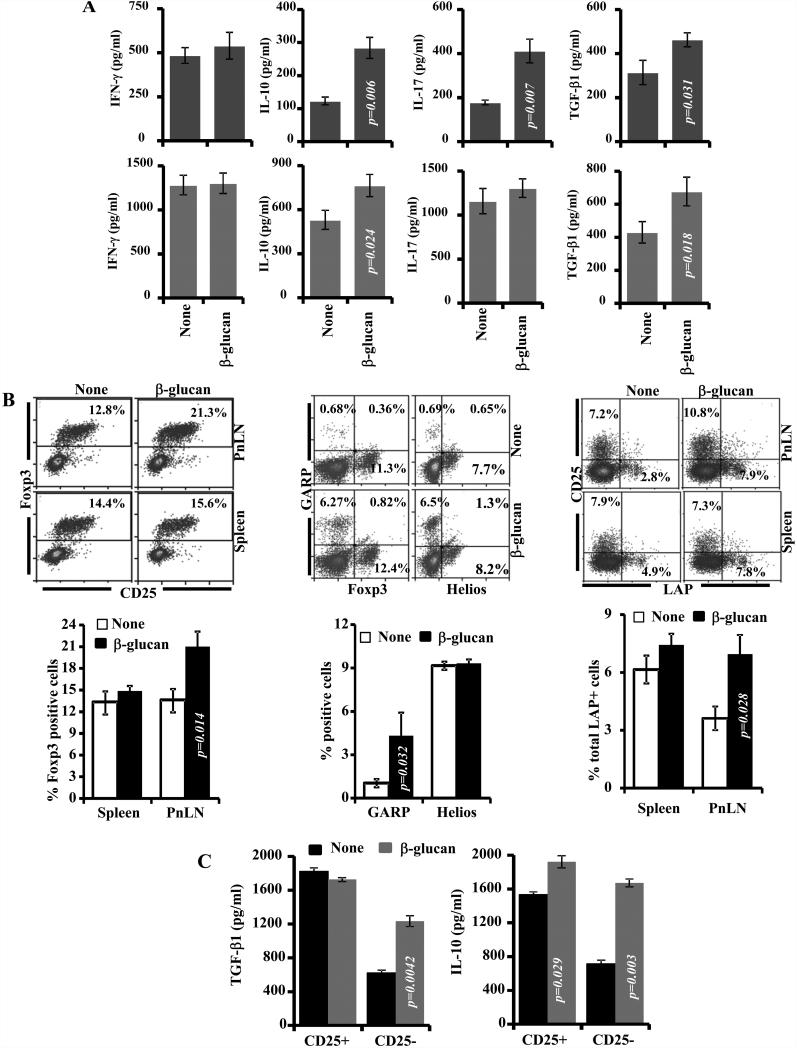
FIGURE 6. T cells from β-glucan-treated mice produce TGF-β1 and IL-10 and show regulatory phenotype.
A) Spleen and pancreatic LN (PnLN) cells obtained from control and β-glucan treated 12-week old NOD mice (treated as described above for Fig. 5; cells were obtained 15 days after the final dose of β-glucan) were stimulated using anti-CD3 Ab (1μg/ml) for 48h in a 96-well round bottom plate (1×105cells/well), supernatants were tested for IL-10, TGF-β1, IL-17, and IFN-γ by ELISA. Background cytokine values of supernatants from non-stimulated cultures were subtracted from respective anti-CD3 Ab-stimulated cultures to exclude cytokines released by APCs spontaneously and plotted as bar-diagrams. Mean±SD of values from a total of 8-9 individual mice tested in triplicate are shown. B) Fresh cells were also examined for the expression of Foxp3, GARP and Helios, and surface LAP by FACS. Representative FACS graphs (upper panels) and mean ± SD of cells from at least 4 mice of a representative experiment tested in duplicate (lower panels) are shown. C) Splenic CD4+CD25+ and CD4+CD25− T cells from control and β-glucan-treated mice were examined for cytokine profiles. Enriched T cell populations were cultured with anti-CD3 and anti-CD28 Abs (2 μg/ml) in a 96 well round bottom plate (1×105 cells/well) for 72h and the spent medium was tested for TGF-β1 and IL-10. Each bar represents mean ± SD of cells from 3-4 mice of a representative experiment tested in triplicate. Treated and control groups of mice were compared and the p-value is shown.
β-glucan treated mice show increased frequency of T cells with regulatory phenotype
To realize whether β-glucan treatment induced Tregs, spleen and PnLN cells from mice that were euthanized 15 days post-treatment were examined for Foxp3+, LAP+, and GARP+ T cells by FACS. As observed in Fig. 6B, significantly higher numbers of Foxp3+CD4+ T cells were found in the PnLN of mice treated with β-glucan as compared to the control mice. However, the splenic Foxp3+CD4+ T cell frequencies in β-glucan treated and control mice were comparable. Interestingly, significantly higher numbers of splenic CD4+Foxp3− T cells from mice that were treated with β-glucan were positive for another Treg marker, GARP. However, PnLN cells from β-glucan treated and control mice showed no significant difference in the GARP expression (not shown). Further, similar to Foxp3, the expression of another natural Treg marker, Helios was not significantly different in spleen cells from β-glucan treated and control mice. Splenic and PnLN cells from β-glucan treated and control mice were also examined for surface bound LAP by FACS. Significantly higher frequencies of CD4+ CD25− T cells from the PnLNs and spleen of β-glucan treated mice were positive for surface LAP
Since spleen cells of β-glucan treated mice showed Foxp3− and CD25− T cells with Treg frequencies, CD4+CD25+ and CD4+CD25− T cells were enriched from spleens of β-glucan treated and control mice, activated using anti-CD3 Ab, and examined for secreted cytokines. Fig. 6C shows that splenic CD4+CD25− T cells from β-glucan treated mice produced profoundly higher amounts of TGF-β1 and IL-10, as compared to their counterparts from control mice. Interestingly, only IL-10, but not TGF-β1, secretion by CD4+CD25+ T cells from β-glucan treated mice was significantly higher compared to these cells from control mice. As indicated by the p-values, the difference in IL-10 production between T cells from β-glucan treated and control mice was more pronounced with CD4+CD25− than CD4+CD25+ T cells. These observations along with the cytokine profile described in Fig. 6A indicate that treatment with β-glucan induces both Foxp3+ and Foxp3− Treg populations and the Foxp3+ and LAP+ populations appear to be trafficking selectively to the pancreatic microenvironment and suppression of insulitis.
T cells from β-glucan treated mice are less diabetogenic and suppress hyperglycemia in NOD mice
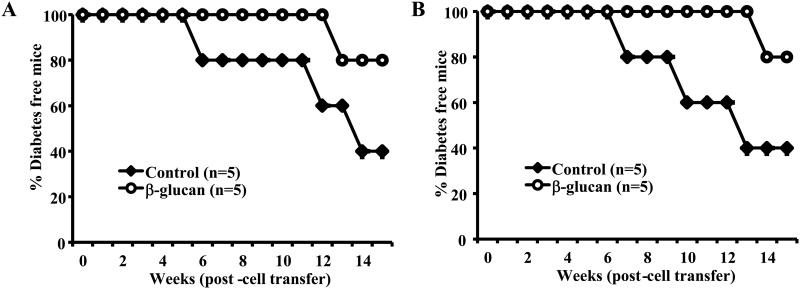
T cells isolated from mice that were euthanized 30 days post-treatment with β-glucan were adoptively transferred into 10-week-old female NOD mice to examine their ability to modulate ongoing autoimmunity and prevent hyperglycemia. Our initial experiments showed that the CD4+CD25+ T cell population isolated from the spleen of treated and untreated mice did not have a noticeable difference in their ability to suppress autoimmunity, when transferred alone to WT female mice or along with diabetogenic T cells into NOD-Scid mice (not shown), perhaps due to the lack of difference in Foxp3+ cell frequencies in the spleen. As observed in Fig. 6B, CD25+Foxp3+ Treg frequency was significantly higher in the PnLN, but not in the spleen, of mice that were treated with β-glucan as compared to controls. Since it is extremely difficult to obtain sufficient number of CD25+ (Foxp3+) T cells from PnLN for adoptive transfer studies and considering the ability of splenic T cells from β-glucan treated mice to produce large amounts of IL-10 and TGF-β1 (Fig. 6), total T cells from the spleens of β-glucan treated and control mice were used in adoptive transfer experiments. As observed in Fig. 7A, albeit not statistically significant, NOD-Scid mice that received T cells from β-glucan treated mice developed hyperglycemia, relatively slowly, compared to mice that received T cells from control mice. Similarly, WT NOD mice that received T cells from β-glucan treated mice also developed hyperglycemia relatively slower than those that received control T cells (Fig. 7B). These observations, in conjunction with Fig. 6, suggest that considerable numbers of T cells with regulatory properties are induced upon treatment with β-glucan and these cells contribute to the prevention of T1D in β-glucan treated mice.
FIGURE 7. Treatment with β-glucan suppresses the diabetogenicity of T cells.
Purified T cells from euglycemic control or β-glucan treated mice (as described above for Fig. 5 and splenic T cells isolated 15 days after the final dose of β-glucan injection) were adoptively transferred to A) 8-week old NOD-Scid mice (i.v.; 2×106 T cells/mouse) and B) 8-week old female NOD/Ltj mice (i.v.; 2×106 T cells/mouse) and monitored for hyperglycemia every week. Mice with glucose levels 250 mg/dl for two consecutive weeks were considered diabetic. Five mice were included in each group and the experiment was repeated with similar results.
β-glucan- and LPS-exposed DCs induce similar levels of T cell proliferation, despite phenotypic differences
To understand the antigen presenting properties of β-glucan-exposed splenic DCs, surface levels of activation markers on them, in comparison with untreated and LPS treated DCs, were examined. A considerable increase in the expression of CD80 and CD40 was observed on β-glucan activated DCs as compared to untreated DCs (Supplemental fig. 4A). However, LPS-exposed DCs showed an upregulation of CD40, CD86, MHC II and PDL2, but not CD80. Expression of high CD80 on β-glucan, but not LPS, exposed DCs suggests the preferential engagement of CTLA-4 on T cells by these DCs upon antigen presentation, leading to a better immune regulation. Our previous studies have shown that B7.1 is a preferential ligand for CTLA-4 and dominant engagement of CTLA-4 by B7.1 or CTLA-4 agonists upon antigen presentation leads to the induction and expansion of Tregs (33, 34).
As described in Fig. 1, β-glucan-exposed DCs produced a relatively superior regulatory innate immune response as compared to other ligand-exposed DCs. Therefore, to further understand β-glucan induced pro- and anti-inflammatory cytokine profiles of DCs, splenic CD11c+ cells were treated with β-glucan for 48h and the levels of secreted cytokines were compared to that of LPS-treated DCs. While β-glucan exposure induced both regulatory (IL-10 and TGF-β1) and pro-inflammatory (IL-1β and TNF-α) cytokines in these DCs, LPS-treatment led to an innate immune response involving primarily pro-inflammatory cytokines (IL-6, IL-1β and TNF-α) (Supplemental Fig. 4B). These observations suggest that the Ag presenting potential of peripheral DCs is enhanced upon exposure to β-glucan through higher expression of costimulatory molecules as well as cytokines. To validate this notion, T cell proliferation assay was carried out using Ag pulsed, β-glucan or LPS treated, DCs and OT-II TCR-Tg T cells. As observed in Supplemental Fig. 4C, no significant difference in proliferation was observed in the OT-II T cells, upon activation using either β-glucan or LPS treated DCs. Overall, these observations indicate that although untreated and β-glucan treated DCs show comparable abilities to induce T cell proliferation, they may influence T differentiation differently.
Ag presentation by β-glucan exposed DCs induces Foxp3+ and IL-10-secreting T cells
Since β-glucan- and LPS-exposed splenic DCs, in spite of their phenotypic differences, induced similar levels of T cell proliferation, these DCs were further tested for their ability to promote T cell differentiation using OT-II and/or OT-II-Foxp3-GFP-ki T cells. The DCs that were exposed to β-glucan could induce significantly higher frequencies of Foxp3+ and LAP+ T cells in cultures, compared to unexposed- or LPS-exposed DCs. Although β-glucan-exposed DCs could promote higher GARP expression in T cells as compared to untreated DCs, LPS-exposed DCs also promoted a significant increase in the expression of GARP on CD4+ T cells (Fig. 8A). Importantly, culture supernatants from similar primary cultures, where β-glucan-exposed DCs were used, showed significantly high levels of IL-10, IL-4, TGF-β1 and IL-17 as compared to supernatants from cultures where LPS-treated or -untreated DCs were used (Fig. 8B). OT-II T cells from similar primary cultures were also examined for intracellular cytokines after restimulation using PMA/ionomycin. Fig. 8C shows that β-glucan treated and untreated DCs induced IFN-γ in a comparable percentage of T cells, but at relatively lower frequencies than by LPS-treated DCs. However, β-glucan-exposed DCs induced significantly higher number of IL-10- and IL-4- producing T cells in these in vitro cultures as compared to untreated or LPS treated DCs.
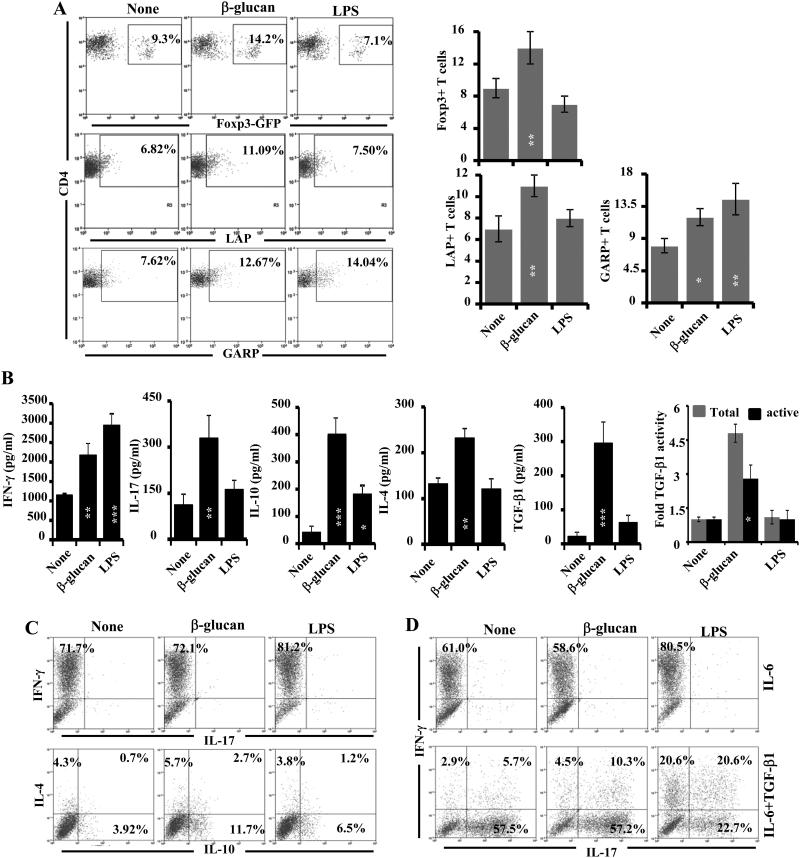
FIGURE 8. Ag presentation by β-glucan-exposed DCs modulates T cell response.
CD11c+ splenic DCs from B6 mice were incubated with β-glucan or LPS and OVA (323-339) peptide for 24h, washed and incubated with purified T cells from OT-II TCR-Tg or OT-II-Foxp3-GFP-ki mice for 96 h. A) Cells were stained for surface CD4 and intracellular Foxp3 and examined by FACS (upper panel). Fresh cells from the cultures were also stained for surface LAP and GARP and analyzed by FACS (middle and lower panels). Representative FACS graphs (left panels) and mean±SD of values from 2-3 experiments carried out in triplicate are shown as bar diagrams (right panels). B) Spent medium from similar primary cultures was tested for cytokines by ELISA. Mean±SD of values from 3 individual experiments carried out in triplicate are shown as bar diagrams. Non-activated and activated culture supernatants were also examined for TGF-β1 activity by bioassay as described in Fig. 1 (extreme right panel). Statistical significance of treated group was calculated against untreated (none) group. *, p<0.05; **, p <0.01; ***, p <0.001. C) Cells from primary cultures were activated using PMA and ionomycin in the presence of brefeldin A and stained for surface CD4 and intracellular cytokines and subjected to FACS analysis. D) Primary cultures were also carried out in the presence of IL-6 alone (20 ng/ml) or along with TGF-β1 (2 ng/ml) for 96h and T cells were analyzed for intracellular IFN-γ and IL-17 as described for panel B. For C and D panels, representative FACS graphs from 2-3 experiments carried out in triplicate are shown. The β-glucan and LPS groups were compared for calculating p-values for panels A and B.
Since β-glucan-exposed DCs produced significant amounts of TGF-β1 along with pro-inflammatory cytokines and induced Foxp3, TGF-β1 and LAP expression in T cells upon Ag presentation, whether addition of IL-6 can result in Th17 cell differentiation in these cultures was examined. Fig. 8D shows that addition of IL-6 alone did not help β-glucan-exposed DCs or untreated as well as LPS exposed DCs to skew the OT-II T cell response to Th17 type. However, addition of exogenous TGF-β1 along with IL-6 skewed almost all IFN-γ-producing cells to IL-17-producing cells in cultures where untreated DCs were used. Similarly, β-glucan-exposed DCs also skewed the T cell response to Th17 type in the presence of exogenous IL-6 and TGF-β1. A considerable number of IL-17+ T cells also expressed IFN-γ in these cultures. Importantly, LPS-exposed DCs appeared to be more resistant in their ability to induce Th17 cells, even in the presence of exogenous IL-6 and TGF-β1. About 70% of cytokine-producing cells in these cultures were either IFN-γ or IFN-γ and IL-17 positive. On the other hand, more than 70% of the cytokine-producing T cells that were activated using β-glucan-exposed DCs, in the presence of IL-6 and TGF-β1, expressed only IL-17. Overall, these observations suggest that β-glucan- exposed APCs can promote a combination of Tregs and IL-17-producing T cells upon Ag presentation under appropriate conditions. On the other hand, LPS-exposed APCs mainly promote differentiation of the IFN-γ-producing T cells. Importantly, while Tregs can promote protection from autoimmunity, IL-17- and IL-4- producing cells are considered less pathogenic than IFN-γ- producing cells in T1D.
Co-administration of β-glucan and β-cell-Ag produces enhanced protection of NOD mice from T1D as compared to β-glucan treatment
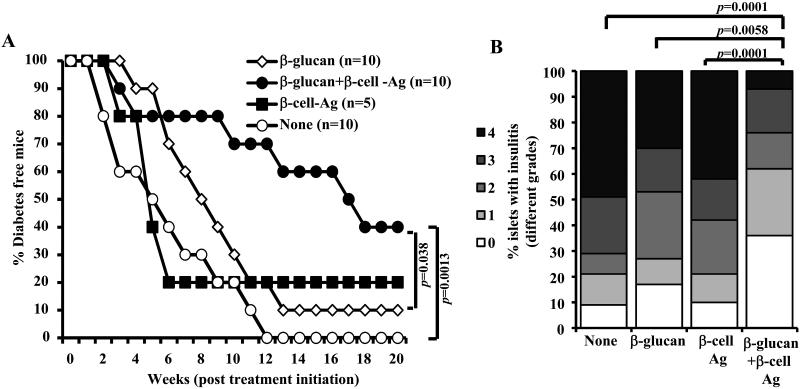
Since β-glucan-exposed DCs could induce T cells that have a regulatory phenotype as well as express IL-17 in vitro and β-glucan treatment resulted in an increase in Foxp3, LAP, and GARP positive populations, we examined whether co-administration of β-glucan and β-cell-Ag produces better protection of NOD mice from T1D. It is assumed that if T cell response against β-cell-Ag is modulated, then long-lasting protection from T1D will be achieved without the need for long-term treatment with β-glucan alone. Pre-diabetic mice were treated with β-glucan plus β-cell-Ag alone or in combination, for a shorter duration than that described for Fig. 5. As observed in Fig. 9A, mice that received β-glucan plus β-cell-Ag showed significantly delayed hyperglycemia as compared to β-glucan recipients. On the other hand, majority of the mice that received β-cell-Ag alone developed hyperglycemia as rapidly as non-injected controls.
FIGURE 9. Treatment using β-glucan and β-cell-Ag resulted in better protection of NOD mice from hyperglycemia as compared to treatment with β-glucan or β-cell-Ag alone.
Twelve-week old euglycemic female NOD mice were left untreated (control) or treated with β-glucan (i.v.; on days 1, 3, 5, 13, 15, and 17 with 5 μg/mouse/day). Some groups of animals received β-cell-Ag on days 5 and 17. A) Mice were checked every week for hyperglycemia and blood glucose level of 250 mg/dl for two consecutive weeks was considered diabetic. Log-rank test was performed to compare hyperglycemia incidence in β-glucan and β-glucan plus β-cell-Ag treated groups with control and the p-value is shown on each graph. The group that received β-cell-Ag was also compared to control mice. B) One set of treated and control mice from parallel experiments were euthanized 4 weeks after the last injection. Pancreatic tissues were processed for H&E staining to evaluate insulitis as described above for Fig. 2. The islets with representative insulitis grades and percentages of islets with different grades of insulitis plotted as bar diagram are shown. Sections of pancreatic tissues from at least 5 mice/group were examined for insulitis and the insulitis score of at least 150 islets/group were plotted as bar diagram.
One set of euglycemic mice from a similar experiment was euthanized 30 days post-treatment and pancreatic tissues were examined for insulitis. The pancreatic islets of mice that received β-glucan plus β-cell-Ag showed significantly less severe immune cell infiltration and insulitis as compared to β-cell-Ag treated and untreated control mice (Fig. 9B). While about 70% of the islets in mice that received β-glucan plus β-cell-Ag showed insulitis grade ≤2, at least 80% of the islets in untreated mice and β-cell-Ag recipient mice showed insulitis severity grade ≥2. Importantly, mice that received short-term treatment (compared to that of Fig. 5) with β-glucan alone also showed relatively less severe insulitis, albeit not statistically significant. However, mice that were treated with β-glucan plus β-cell-Ag showed profoundly less severe insulitis as compared with mice that received β-glucan or β-cell-Ag alone. These results suggest that β-glucan-induced innate immune response promotes β-cell-Ag specific immunomodulation leading to better protection of NOD mice from T1D.
β-glucan plus β-cell-Ag treatment causes Th cell skewing and increase in the frequency of T cells with regulatory phenotype
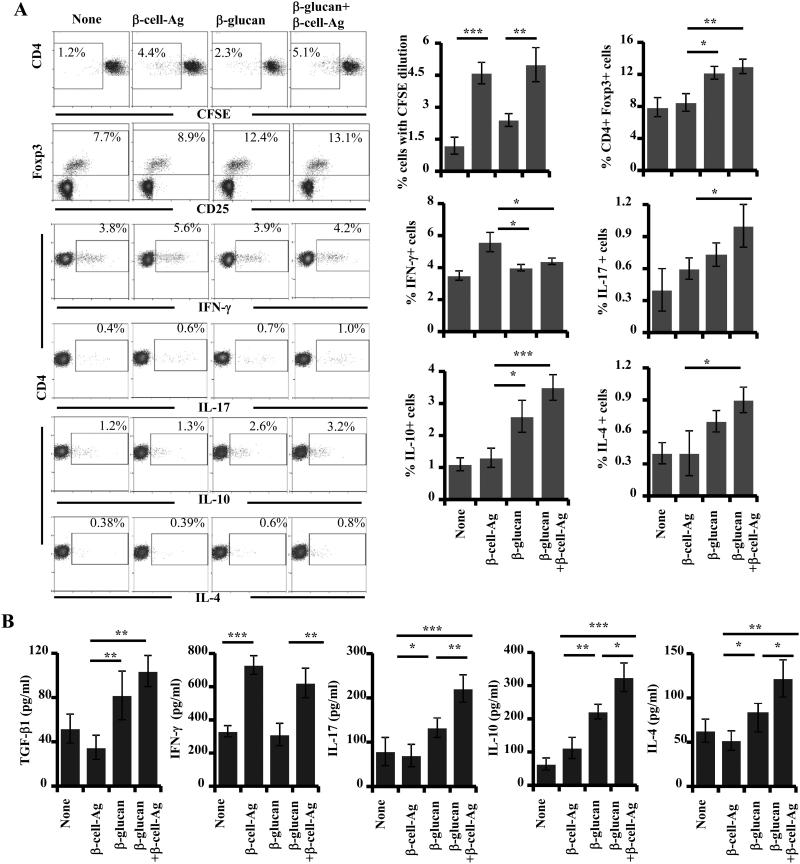
Since mice that were treated with β-glucan plus β-cell-Ag showed significant protection from hyperglycemia, T cells from these mice were examined for their phenotypic and functional properties, in comparison with their counterparts from control groups. PnLN cells obtained from mice that were euthanized 30 days post-treatment were examined for their ability to respond to ex vivo challenge with β-cell-Ag. CD4+ T cells from mice that received β-cell-Ag or β-glucan plus β-cell-Ag showed comparable extent of CFSE dilution upon ex vivo challenge, which was significantly higher than their counterparts from untreated control mice (Fig. 10A). PnLN cells from β-glucan and/or β-cell-Ag treated mice were examined for Foxp3+ T cells by FACS. Significantly higher numbers of Foxp3+CD4+ T cells were found in the PnLN of mice treated with β-glucan alone or along with β-cell-Ag as compared to β-cell-Ag treated mice. To examine the functionality of T cells, freshly prepared PnLN cells from treated and untreated mice were stimulated with PMA and ionomycin and stained for intracellular cytokines. Fig. 10A shows that while the CD4+IFN-γ+ T cell frequencies were significantly low in β-glucan and β-glucan plus β-cell-Ag recipient mice, higher percentage of PnLN cells from the latter group showed IL-10, IL-17 and IL-4 expression compared to their counterparts from β-glucan or β-cell-Ag treated mice. Importantly, levels of cytokines secreted by PnLN cells from β-glucan plus β-cell-Ag recipient groups, when challenged with β-cell-Ag ex vivo, were also significantly different from that of β-glucan or β-cell-Ag recipients (Fig. 10B). While all β-cell-Ag recipient groups produced comparable levels of IFN-γ, which is significantly higher than that of control and β-glucan treated mice, β-glucan plus β-cell-Ag recipients produced significantly higher amounts of IL-10 and IL-17 as compared to mice that received β-cell-Ag alone. These results suggest that exposure of APCs to β-glucan in vivo leads to the skewing of T cell response from pathogenic to protective/less pathogenic type and these T cells with altered function may eliminate the need for continuous long term-treatment with a non-specific immune-modulator.
FIGURE 10. T cells from β-glucan and β-cell-Ag treated mice produce higher IL-17, IL-10 and IL-4 as compared to control groups.
Pancreatic LN (PnLN) cells were obtained from control and treated 12-week old NOD mice (treated as described above for Fig. 9; cells were obtained 30 days after the final injection with agents ). A) Cells were examined for peptide specific proliferation by CFSE dilution assay. Percentage values of CFSE low cells at 96 h time-point are shown. Cells were also examined for intra-cellular Foxp3 expression by FACS. Fresh PnLN cells were stimulated using PMA and ionomycin for 4 h in the presence of brefeldin A and stained for intracellular cytokines for FACS analysis. Percentage values of CD4+ T cells with cytokine expression are shown. Representative FACS graphs (left panels) and mean ± SD of cells from 3-4 mice of a representative experiment tested in duplicate (right panels) are shown. B) Fresh cells were also stimulated using β-cell-Ag for 48h in 96-well round bottom plates (2×105cells/well) and the supernatants were tested for cytokines by ELISA. Mean±SD of values from a total of 3-4 individual mice tested at least in duplicate are shown. *, p<0.05; **, p <0.01; ***, p <0.001.
Discussion
The immunostimulatory properties of β-glucans of fungal, bacterial and plant origin have been recognized for many decades (3). Primary sources of β-glucans include baker’s yeast (S. cerevisiae), mushrooms, bacteria (Alcaligenes faecalis), barley, and seaweed (Laminaria digitata) (3, 4). β-glucans from these and other sources have the ability to stimulate immune cells. Although several PRRs have been described for their ability to interact with β-glucans and β-glucan-containing microbial products, Dectin-1 is thought to be the primary receptor for fungal β-glucans (1, 4). With the exception of a few reports that show Dectin-1-dependent IL-10 and IL-2 responses by innate immune cells (31, 38), previous studies were primarily focused on the pro-inflammatory and host defense aspects of β-glucan and Dectin-1-associated innate immune response. Further, β-glucan and β-glucan-containing fungal cell wall preparations such as zymosan have been used for inducing autoimmune arthritis in a genetically susceptible mouse model (18-21), suggesting that Dectin-1-dependent innate immune response involves pro-inflammatory factors. Here, we show that the β-glucan-induced innate immune response comprises of both pro-inflammatory cytokines and immune-regulatory factors that can prevent T1D in NOD mice. We also show that the innate immune response induced by β-glucan promotes the generation and/or expansion of T cells with regulatory phenotype and could be exploited for achieving pancreatic β-cell-Ag specific modulation of autoimmunity and lasting protection from T1D.
Previously, we and others have reported that inducing regulatory innate immune response through TLR2 and Dectin-1 using a fungal cell wall agent, zymosan could suppress autoimmunity and prevent T1D in NOD mice (12, 14). However, zymosan is also known to bind to many other receptors including TLR2, and the IL-10, TGF-β1 and IL-2 responses induced by this agent is thought to be the result of co-operative signaling through TLR2 and Dectin-1 receptors (13, 16, 38). In addition, a tolerogenic enzyme, Raldh1A2, induced in DCs by zymosan was reported to be TLR2-dependent (16). Yeast β-glucan does not induce signaling through TLR2 and can trigger the expression of IL-10, TGF-β1, and IL-2 along with the tryptophan-catabolizing tolerogenic enzyme, IDO as well as pro-inflammatory cytokines including TNF-α in DCs. Our observations highlight the regulatory nature of Dectin-1-dependent innate immune responses. In fact, unlike zymosan (15, 16), β-glucan failed to induce Raldh1A2 expression in peripheral DCs (not shown) confirming that TLR2 engagement may be necessary for Raldh1A2 induction. On the other hand, previous studies have shown that engagement of TLR2 by its ligands induces, along with pro-inflammatory cytokines, IL-10, but not IL-2 or TGF-β1 in APCs (16, 27, 39), further substantiating the notion that Dectin-1 engagement is responsible for zymosan-induced IL-2 and TGF-β1 production. Importantly, the observation that β-glucan, but not zymosan, induces IDO in DCs suggests that interaction of zymosan with other receptors may have counteractive effects on Dectin-1-dependent IDO expression.
Previous studies have shown that interaction with fungi, Candida in particular, can induce IDO expression in innate immune cells (40, 41). However, the specific ligand-receptor interaction responsible for IDO expression was not known. Our observation that purified β-glucan can induce IDO expression in peripheral DCs shows that engagement of Dectin-1 may be responsible for fungal recognition-associated IDO expression. IDO-dependent immune tolerance involves at least two major mechanisms: 1) Depletion of tryptophan by IDO in the immune microenvironment can dampen T cell proliferation and their effector function (42) and 2) IDO induced kynurenines, which are L-tryptophan-derived metabolites, can drive the conversion of naïve T cells to Tregs in the presence of TGF-β1 (42, 43). Our in vitro and in vivo studies show that β-glucan-induced innate immune response promotes the induction and/or expansion of Foxp3+ and LAP+ T cells suggesting the involvement of TGF-β1, IL-2 and/or IDO in protection of β-glucan treated NOD mice from T1D.
Treatment of NOD mice at young ages with TLR2, TLR3, TLR4 and TLR9 ligands resulted in a considerable delay in T1D by activating both innate and adaptive immune responses (27, 28, 44-46). However, these ligands were not known to be effective in inducing protection from the disease at later stages. Although it has been shown that treatment with β-glucan, starting at pre-insulitis stage, caused a significant delay in diabetes in BB rats (17), the effect of innate immune response induced by this Dectin-1 ligand at later stages of disease progression in this rat model or NOD mice was not studied before. Our current study shows that treatment of 12 week- old pre-diabetic NOD mice with a low dose of β-glucan results in protection from T1D and this effect appears to be associated with anti-inflammatory cytokines (IL-10, TGF-β1 and IL-2) and IDO- mediated enhanced immune regulation. The immune regulatory cytokine- (IL-10, TGF-β1 and IL-2) and IDO-induced metabolic products such as kynurenines are known to play a role in the induction, expansion, survival, and/or functioning of both natural and adaptive Tregs (47, 48). Therefore, we anticipated that Ag presentation by β-glucan-exposed DCs can modulate the T cell response from pro-inflammatory to regulatory type. Our observations that β-glucan-exposed DCs can promote Foxp3 and LAP expression in OT-II TCR-Tg T cells upon Ova peptide presentation support this notion.
Interestingly, T cells from β-glucan treated mice also produced higher amounts of IL-17, a cytokine which is known to play a protective, or less pathogenic, role in T1D. However, this cytokine and Th17 cells have a pathogenic role in many autoimmune diseases including multiple sclerosis and arthritis (49-52). It appears that TGF-β1, along with some of the pro-inflammatory cytokines such as IL-6, IL-1β, TNF-α, and/or IL-23 induced upon Dectin-1 engagement on innate immune cells, could be responsible for the IL-17 response in β-glucan treated NOD mice. While purified splenic CD11c+ DCs do not produce considerable amounts of IL-6, BM DCs produce IL-6 when exposed to β-glucan (not shown). Therefore, it is possible that IL-6 is produced by other innate immune cells in vivo upon treatment with β-glucan. Further, Dectin-1-dependent production of IL-23, a cytokine responsible for the maintenance of Th17 cells, has been reported in the past (25). Importantly, our in vitro Ag presentation assays show that although secreted IL-17 levels were high in the primary T cell cultures, β-glucan exposed DCs did not induce large number of IL-17+ T cells, without exogenous IL-6 and TGF-β1 indicating complex interactions between various pro-inflammatory and anti-inflammatory factors produced upon Dectin-1 engagement. On the other hand, as compared to LPS-treated DCs, T cells activated using β-glucan exposed DCs were found to be more susceptible to Th17 skewing in the presence of exogenous IL-6 and TGF-β1. These effects of β-glucan induced innate immune response may explain why β-glucan treatment produces contrasting disease outcomes in arthritis and T1D models.
In T1D, autoreactive T cells, primarily IFN-γ-producing CD4 (Th1) and CD8 (Tc1) T cells, progressively expand and cause destruction of the insulin producing β-cells. Further, in the inflammatory microenvironment, the remaining β-cells fail to meet the metabolic need of insulin production. While systemic delivery of β-cell-Ag, at later stages, can result in aggravated disease progression due to a pro-inflammatory environment and activated APCs, treatment at young ages or early stages of insulitis can produce a disease protective effect in NOD mice (53, 54). Our observation that treatment with β-glucan plus β-cell-Ag induces a relatively better protection of the pre-diabetic stage NOD mice from T1D as compared to treatment with β-glucan or β-cell-Ag alone suggests that β-glucan-exposed APCs skew the T cell response to β-cell-Ag from pathogenic Th1 to protective/less pathogenic Treg, Th17 and Th2 types.
As mentioned above, β-glucan and the β-glucan-containing fungal cell wall preparation, zymosan, are known to promote arthritis in genetically susceptible SKG mouse model (18-21). Importantly, Th17 cells play an important role in arthritis of SKG mice (51, 55). In this context, our observations that β-glucan treatment and β-glucan exposed DCs promote an increase in the Treg frequency and IL-17, IL-10 and IL-4 production and protect pre-diabetic NOD mice from T1D show that microbial factors like β-glucan can trigger specific types of innate and adaptive immune responses in individuals who are genetically susceptible to different autoimmune diseases and produce different outcomes.
In this context, β-glucans are known, for a long-time, for their ability to trigger both protective and destructive immune responses in various clinical conditions (3, 4). However, recent reports have shown that the β-glucan receptor, Dectin-1 has a redundant role in protective responses against certain fungal infections, and β-glucan rich ligand induced immunomodulatory response against systemic Staphylococcus infection as well as in zymosan induced multiple organ dysfunction syndrome (9-11). Our observations that β-glucan induced innate immunity can promote Treg and Th17 and Th2 responses demonstrate the immune regulatory nature of Dectin-1 dependent innate immune response and confirm the redundant role of Dectin-1, especially in conditions that involve strong inflammatory and Th1 responses.
In conclusion, our observations show that Dectin-1 dependent innate immune response induced by β-glucan is significantly different from that induced by other PRRs. Further, Dectin-1 signaling not only appears to be the primary contributor of previously reported zymosan-induced regulatory innate immune response (12-16), but also induces IDO expression in DCs. The complex pro- and anti-inflammatory responses triggered by β-glucan can promote both Treg and Th17 responses, leading to suppressed Th1 dominated β-cell Ag specific autoimmunity and protection of NOD mice from TID. Most importantly, Dectin-1 induced innate immune response can be exploited to modulate the T cell response against pancreatic β-cell-Ag for achieving long-term protection from T1D.
Supplementary Material
Acknowledgments
This work was supported by internal funds from MUSC and UIC, National Institutes of Health (NIH) grants R01AI073858, American association of diabetes grant ADA-1-13-IN-57, and Juvenile Diabetes Research Foundation regular grants JDRF-32-2008-343 to CV. Dr. Vasu is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Abbreviations
- TID
Type 1 Diabetes
- NOD
non-obese diabetic
- TLR
Toll-like receptor
- PRR
pathogen recognition receptor
- IDO
Indoleamine 2, 3-dioxygenase
- Raldh1A2
Retinaldehyde dehydrogenase 1A2.
Footnotes
Conflict of Interest statement: Authors do not have any conflict(s) of interest to disclose.
References
- 1.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 2.Reid DM, Gow NA, Brown GD. Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol. 2009;21:30–37. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Seviour R. Medicinal importance of fungal beta-(1-->3), (1-->6)-glucans. Mycological research. 2007;111:635–652. doi: 10.1016/j.mycres.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Vannucci L, Krizan J, Sima P, Stakheev D, Caja F, Rajsiglova L, Horak V, Saieh M. Immunostimulatory properties and antitumor activities of glucans (Review) International journal of oncology. 2013;43:357–364. doi: 10.3892/ijo.2013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plato A, Willment JA, Brown GD. C-type lectin-like receptors of the dectin-1 cluster: ligands and signaling pathways. Int Rev Immunol. 2013;32:134–156. doi: 10.3109/08830185.2013.777065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herre J, Willment JA, Gordon S, Brown GD. The role of Dectin-1 in antifungal immunity. Crit Rev Immunol. 2004;24:193–203. doi: 10.1615/critrevimmunol.v24.i3.30. [DOI] [PubMed] [Google Scholar]
- 7.Herre J, Gordon S, Brown GD. Dectin-1 and its role in the recognition of beta-glucans by macrophages. Mol Immunol. 2004;40:869–876. doi: 10.1016/j.molimm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 8.van den Berg LM, Gringhuis SI, Geijtenbeek TB. An evolutionary perspective on C-type lectins in infection and immunity. Ann N Y Acad Sci. 2012;1253:149–158. doi: 10.1111/j.1749-6632.2011.06392.x. [DOI] [PubMed] [Google Scholar]
- 9.Vautier S, Drummond RA, Redelinghuys P, Murray GI, MacCallum DM, Brown GD. Dectin-1 is not required for controlling Candida albicans colonization of the gastrointestinal tract. Infection and immunity. 2012;80:4216–4222. doi: 10.1128/IAI.00559-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marakalala MJ, Williams DL, Hoving JC, Engstad R, Netea MG, Brown GD. Dectin-1 plays a redundant role in the immunomodulatory activities of beta-glucan-rich ligands in vivo. Microbes and infection / Institut Pasteur. 2013;15:511–515. doi: 10.1016/j.micinf.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura K, Kinjo T, Saijo S, Miyazato A, Adachi Y, Ohno N, Fujita J, Kaku M, Iwakura Y, Kawakami K. Dectin-1 is not required for the host defense to Cryptococcus neoformans. Microbiology and immunology. 2007;51:1115–1119. doi: 10.1111/j.1348-0421.2007.tb04007.x. [DOI] [PubMed] [Google Scholar]
- 12.Karumuthil-Melethil S, Perez N, Li R, Vasu C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J Immunol. 2008;181:8323–8334. doi: 10.4049/jimmunol.181.12.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S, Unutmaz D, Pulendran B. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton OT, Zaccone P, Phillips JM, De La Pena H, Fehervari Z, Azuma M, Gibbs S, Stockinger B, Cooke A. Roles for TGF-beta and programmed cell death 1 ligand 1 in regulatory T cell expansion and diabetes suppression by zymosan in nonobese diabetic mice. Journal of immunology. 2010;185:2754–2762. doi: 10.4049/jimmunol.1001365. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Gonnella P, Safavi F, Vessal G, Nourbakhsh B, Zhou F, Zhang GX, Rostami A. Low dose zymosan ameliorates both chronic and relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2013;254:28–38. doi: 10.1016/j.jneuroim.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nature medicine. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kida K, Inoue T, Kaino Y, Goto Y, Ikeuchi M, Ito T, Matsuda H, Elliott RB. An immunopotentiator of beta-1,6;1,3 D-glucan prevents diabetes and insulitis in BB rats. Diabetes Res Clin Pract. 1992;17:75–79. doi: 10.1016/0168-8227(92)90152-h. [DOI] [PubMed] [Google Scholar]
- 18.Yoshitomi H, Sakaguchi N, Kobayashi K, Brown GD, Tagami T, Sakihama T, Hirota K, Tanaka S, Nomura T, Miki I, Gordon S, Akira S, Nakamura T, Sakaguchi S. A role for fungal {beta}-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J Exp Med. 2005;201:949–960. doi: 10.1084/jem.20041758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hida S, Miura NN, Adachi Y, Ohno N. Cell wall beta-glucan derived from Candida albicans acts as a trigger for autoimmune arthritis in SKG mice. Biol Pharm Bull. 2007;30:1589–1592. doi: 10.1248/bpb.30.1589. [DOI] [PubMed] [Google Scholar]
- 20.Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. Eur J Immunol. 2009;39:2040–2044. doi: 10.1002/eji.200939578. [DOI] [PubMed] [Google Scholar]
- 21.Ruutu M, Thomas G, Steck R, Degli-Esposti MA, Zinkernagel MS, Alexander K, Velasco J, Strutton G, Tran A, Benham H, Rehaume L, Wilson RJ, Kikly K, Davies J, Pettit AR, Brown MA, McGuckin MA, Thomas R. beta-glucan triggers spondylarthritis and Crohn's disease-like ileitis in SKG mice. Arthritis Rheum. 2012;64:2211–2222. doi: 10.1002/art.34423. [DOI] [PubMed] [Google Scholar]
- 22.Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwright R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ. 1992;304:1020–1022. doi: 10.1136/bmj.304.6833.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahromi MM, Eisenbarth GS. Cellular and molecular pathogenesis of type 1A diabetes. Cell Mol Life Sci. 2007;64:865–872. doi: 10.1007/s00018-007-6469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Berg TK, Yoder JA, Litman GW. On the origins of adaptive immunity: innate immune receptors join the tale. Trends Immunol. 2004;25:11–16. doi: 10.1016/j.it.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. European journal of immunology. 2009;39:1379–1386. doi: 10.1002/eji.200838543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Filippi CM, Ehrhardt K, Estes EA, Larsson P, Oldham JE, von Herrath MG. TLR2 signaling improves immunoregulation to prevent type 1 diabetes. Eur J Immunol. 2011;41:1399–1409. doi: 10.1002/eji.200939841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DH, Lee JC, Kim S, Oh SH, Lee MK, Kim KW, Lee MS. Inhibition of autoimmune diabetes by TLR2 tolerance. J Immunol. 2011;187:5211–5220. doi: 10.4049/jimmunol.1001388. [DOI] [PubMed] [Google Scholar]
- 29.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 31.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–3115. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 32.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karumuthil-Melethil S, Perez N, Li R, Prabhakar BS, Holterman MJ, Vasu C. Dendritic cell-directed CTLA-4 engagement during pancreatic beta cell antigen presentation delays type 1 diabetes. Journal of immunology. 2010;184:6695–6708. doi: 10.4049/jimmunol.0903130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez N, Karumuthil-Melethil S, Li R, Prabhakar BS, Holterman MJ, Vasu C. Preferential costimulation by CD80 results in IL-10-dependent TGF-beta1(+) - adaptive regulatory T cell generation. Journal of immunology. 2008;180:6566–6576. doi: 10.4049/jimmunol.180.10.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sofi MH, Gudi RR, Karumuthil-Melethil S, Perez N, Johnson BM, Vasu C. pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes. 2013 doi: 10.2337/db13-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, Adema GJ, Kullberg BJ, Schweighoffer E, Tybulewicz V, Mora-Montes HM, Gow NA, Williams DL, Netea MG, Brown GD. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. European journal of immunology. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R, Perez N, Karumuthil-Melethil S, Vasu C. Bone marrow is a preferential homing site for autoreactive T-cells in type 1 diabetes. Diabetes. 2007;56:2251–2259. doi: 10.2337/db07-0502. [DOI] [PubMed] [Google Scholar]
- 38.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozza S, Fallarino F, Pitzurra L, Zelante T, Montagnoli C, Bellocchio S, Mosci P, Vacca C, Puccetti P, Romani L. A crucial role for tryptophan catabolism at the host/Candida albicans interface. J Immunol. 2005;174:2910–2918. doi: 10.4049/jimmunol.174.5.2910. [DOI] [PubMed] [Google Scholar]
- 41.De Luca A, Montagnoli C, Zelante T, Bonifazi P, Bozza S, Moretti S, D'Angelo C, Vacca C, Boon L, Bistoni F, Puccetti P, Fallarino F, Romani L. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J Immunol. 2007;179:5999–6008. doi: 10.4049/jimmunol.179.9.5999. [DOI] [PubMed] [Google Scholar]
- 42.Munn DH. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol. 2006;18:220–225. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Belladonna ML, Orabona C, Grohmann U, Puccetti P. TGF-beta and kynurenines as the key to infectious tolerance. Trends in molecular medicine. 2009;15:41–49. doi: 10.1016/j.molmed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Montandon R, Korniotis S, Layseca-Espinosa E, Gras C, Megret J, Ezine S, Dy M, Zavala F. Innate pro-B-cell progenitors protect against type 1 diabetes by regulating autoimmune effector T cells. Proc Natl Acad Sci U S A. 2013;110:E2199–2208. doi: 10.1073/pnas.1222446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quintana FJ, Rotem A, Carmi P, Cohen IR. Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: modulation of spontaneous 60-kDa heat shock protein autoimmunity. J Immunol. 2000;165:6148–6155. doi: 10.4049/jimmunol.165.11.6148. [DOI] [PubMed] [Google Scholar]
- 46.Fukushima K, Abiru N, Nagayama Y, Kobayashi M, Satoh T, Nakahara M, Kawasaki E, Yamasaki H, Ueha S, Matsushima K, Liu E, Eguchi K. Combined insulin B:9-23 self-peptide and polyinosinic-polycytidylic acid accelerate insulitis but inhibit development of diabetes by increasing the proportion of CD4+Foxp3+ regulatory T cells in the islets in non-obese diabetic mice. Biochem Biophys Res Commun. 2008;367:719–724. doi: 10.1016/j.bbrc.2007.12.191. [DOI] [PubMed] [Google Scholar]
- 47.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng XX, Steele AW, Hancock WW, Stevens AC, Nickerson PW, Roy-Chaudhury P, Tian Y, Strom TB. A noncytolytic IL-10/Fc fusion protein prevents diabetes, blocks autoimmunity, and promotes suppressor phenomena in NOD mice. J Immunol. 1997;158:4507–4513. [PubMed] [Google Scholar]
- 49.Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2011;108:11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nikoopour E, Schwartz JA, Huszarik K, Sandrock C, Krougly O, Lee-Chan E, Singh B. Th17 polarized cells from nonobese diabetic mice following mycobacterial adjuvant immunotherapy delay type 1 diabetes. J Immunol. 2010;184:4779–4788. doi: 10.4049/jimmunol.0902822. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto Y, Uga H, Tanaka S, Kadowaki M, Ikeda M, Saegusa J, Morinobu A, Kumagai S, Kurata H. Podoplanin is an inflammatory protein upregulated in Th17 cells in SKG arthritic joints. Mol Immunol. 2013;54:199–207. doi: 10.1016/j.molimm.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han B, Serra P, Amrani A, Yamanouchi J, Maree AF, Edelstein-Keshet L, Santamaria P. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med. 2005;11:645–652. doi: 10.1038/nm1250. [DOI] [PubMed] [Google Scholar]
- 54.Hartemann-Heurtier A, Mars LT, Bercovici N, Desbois S, Cambouris C, Piaggio E, Zappulla J, Saoudi A, Liblau RS. An altered self-peptide with superagonist activity blocks a CD8-mediated mouse model of type 1 diabetes. J Immunol. 2004;172:915–922. doi: 10.4049/jimmunol.172.2.915. [DOI] [PubMed] [Google Scholar]
- 55.Duarte J, Agua-Doce A, Oliveira VG, Fonseca JE, Graca L. Modulation of IL-17 and Foxp3 expression in the prevention of autoimmune arthritis in mice. PLoS One. 2010;5:e10558. doi: 10.1371/journal.pone.0010558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.