Abstract
Insulin-like growth factor binding protein-3 (IGFBP-3), a secretory protein, is the most abundant IGF binding protein present in human serum among all IGF binding proteins. IGFBP-3 shows decreased level of expression in cancerous cells but has been known to be present in significant amounts in normal or non-cancerous cells. IGFBP-3 can induce apoptosis in prostate cancer cells either in an IGF-dependent manner or independently of IGF binding. Although putative cell death specific Insulin-like growth factor binding protein-3 (IGFBP-3R) receptor(s) has recently been identified by which IGFBP-3 may induce its anti-tumor effects, IGFBP-3 has also been known to activate various downstream intracellular signaling molecules via a different mechanistic pathway. Stat-1 has been known to be one of the candidate molecules activated by IGFBP-3. IGFBP-3 can also inhibit Akt/IGF-1 survival pathway in MCF- 7 breast cancer cells which ultimately leads to the induction of apoptosis in these cells. All these studies clearly demonstrate that IGFBP-3 regulates cell proliferation and promotes its pro-apoptotic effects in cancer cells in two different pathways,1) sequester IGF-I to bind to IGF-I receptor to inhibit cell proliferation and induce apoptosis, 2) independent of IGF-I pathway, IGFBP-3 binds to some putative receptor and activate various downstream pro-apoptotic molecules involved in cell death.
Keywords: Apoptosis, IGFBP-3, Stat-1, IGF-I, TGF-β
1. IGFBP-3 expression and function
Insulin like growth factor binding protein-3 (IGFBP-3) is one of the six known IGF binding proteins present in human serum and is comprised of 264-amino acid mature protein, with a 27-amino acid signal peptide [1]. IGFBP-3 is expressed in a wide variety of tissues. Epidemiological studies have clearly suggested that there is an inverse relationship between IGFBP-3 levels and occurrence of cancers [2] Increased expression levels of serum IGFBP-3 results in decreased prevalence of prostate [3, 4] and colorectal [5, 6]cancers. It has also been shown that IGFBP-3 overexpression results in decreased tumor formation in xenografts of non-small lung cancer cells [7] and M12 human prostate cancer cells [8]. Patient samples with hepatocellular and non-small cell lung carcinoma has been shown to have decreased expression of IGFBP-3[9, 10]. Besides, it has also been reported that there is an increased level of IGFBP-3 in senescence [11]. In addition, IGFBP-3 expression level was decreased in cells immortalized with the human papillomavirus type 16 oncoprotein E7 due to proteasomal degradation [12]. Although IGFBP-3 is a secretory protein, but active protein could also be found in nucleus and cytoplasm [13]. Besides its full length form, IGFBP-3 could also be found in a N-terminal truncated form [14, 15].
IGFBP-3 has been known to play an important role in cell proliferation by inducing its anti-proliferative and pro-apoptotic effects in breast and prostate cancer cells [16, 17, 18]. It has also been shown that IGFBP-3 not only triggers growth inhibitory effects by inducing apoptosis but it can also function in G1 cell cycle arrest in human breast, kidney and lung cancer cells [19, 20]. IGFBP-3 may also play an important role in mediating inhibitory effects of transforming growth factor (TGF)-β (Figure 1), retinoic acid, tumor necrosis factor (TNF)-α and p53 [16, 21–26], but modulation in IGFBP-3 synthesis or action could regulate these anti-proliferative effects.
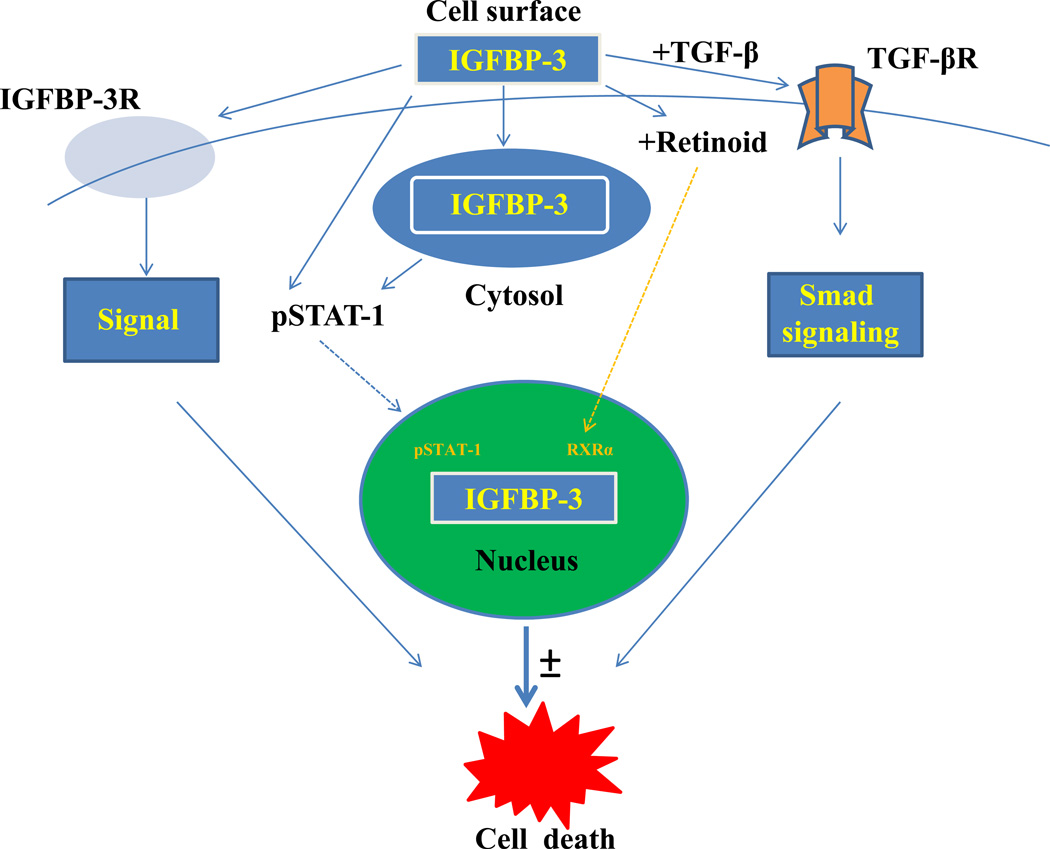
Figure 1. Key candidate molecules activated by IGFBP-3.
IGFBP-3 can activate various downstream signaling molecules either by binding to its putative IGFBP-3R (receptor) to monitor its signal and induce apoptosis or bind to TGF-β receptor resulting in smad activation which leads to apoptosis. IGFBP-3 can also activate Stat-1 or bind to RXR-α to induce its anti-proliferative and pro-apoptotic effects.
2. IGF-dependent and independent effect of IGFBP-3
IGF dependent studies revealed that Insulin-like growth factor binding protein (IGFBP)-3 could induce apoptosis by binding to IGF-I and form a binary complex with IGF-I and prevent it to activate (IGF-IR) IGF-1 receptor (Figure 2) to stimulate cell proliferation and survival [27, 28]. In addition, Insulin-like growth factor binding protein (IGFBP)-3 has been shown to potently inhibit cell proliferation and induce apoptosis in an insulin-like growth factor (IGF)-independent manner [13, 18, 29]. IGFBP-3 in the media may exert its pro-apoptotic effects by binding to some cell surface receptors resulting in the activation of various signal transduction pathways. Alternatively, IGFBP-3 may possibly enter into the cell by endocytosis (Figure 3). It has also been reported in some other studies that IGFBP-3 binds to RXRα (Figure 1) and induce its pro-apoptotic effects in PC-3 human prostate cancer cells [30]. Surprisingly, recent studies have shown that IGFBP-3 fail to gain entry or be internalized upon binding to IGF-1 in the extracellular media to induce its pro-apoptotic effects in non-transformed mammary epithelial cells [31].
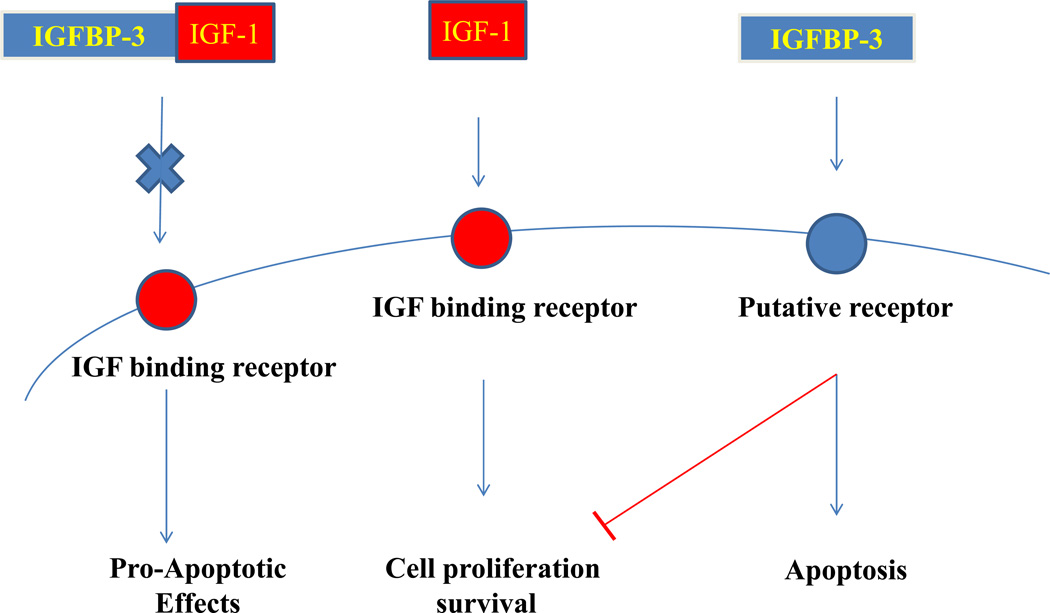
Figure 2. IGF dependent and Independent effects of IGFBP-3.
IGF-1 can bind to IGF-I receptor and stimulate cell proliferation. IGFBP-3 blocks IGF-I to bind to its receptor resulting in the induction of apoptosis. IGFBP-3 can also induce apoptosis on its own by IGF-I independent mechanism.
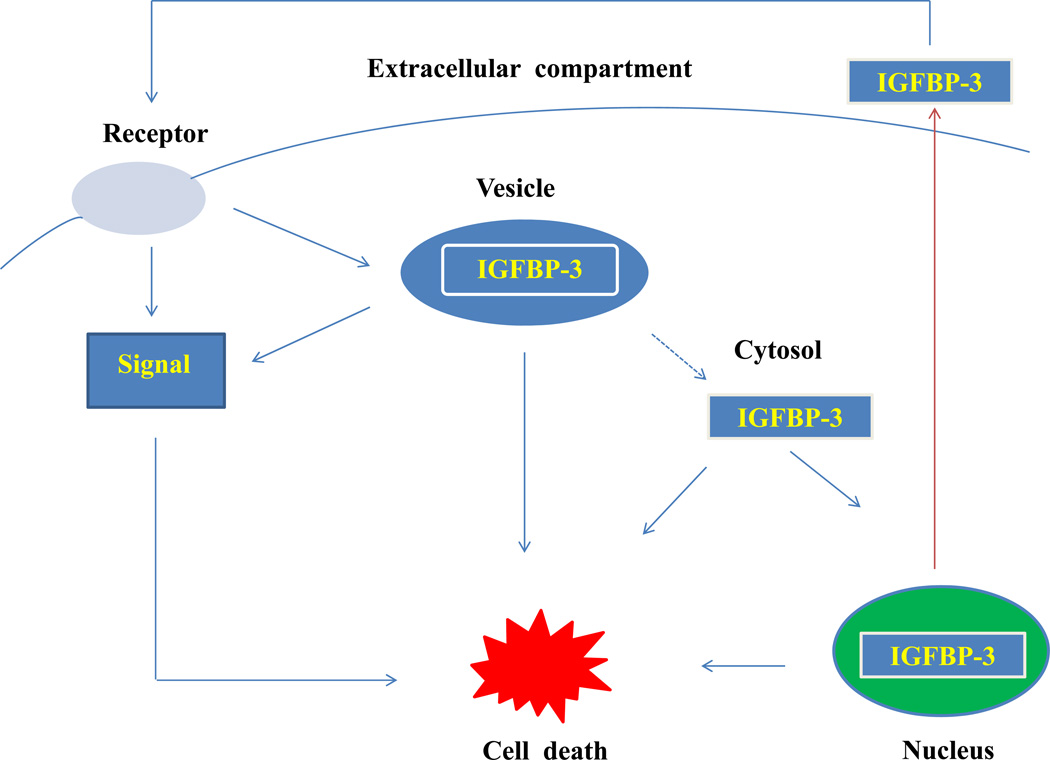
Figure 3. IGFBP-3 entry into the cell by different pathways.
IGFBP-3 in the media seemingly binds with plasma membrane IGFBP-3 receptor to activate signal transduction pathway or enters into the cell by endocytosis to induce apoptosis. IGFBP-3 may either directly activate signal transduction pathway by binding to some receptor to induce apoptosis or in the form of vesicular IGFBP-3 which enters through Endoplasmic reticulum membrane into the cytosol and possibly gets translocated into the nucleus
3. Pathways involved in IGFBP-3 signaling
IGFBP-3 action results in the activation of various signal transduction pathways [32] and Stat-1 (signal transducer and activator of transcription 1) has been known to have a functional role in IGFBP-3-induced apoptosis (Figure 1) in rat chondroprogenitor cells [33], although our previous studies [34] showed a protective role of Stat-1 on IGFBP-3 induced apoptosis in PC-3 human prostate cancer cells, implying the fact that role of Stat-1 may be cell type dependent.
Some other downstream signaling molecules of IGFBP-3 have also been reported in various other studies [16, 35, 36]. It has been shown from these studies that IGFBP-3 can bind to transforming growth factor-β (TGF-β) cell surface receptors and one of the studies have shown direct interaction of IGFBP-3 with TGFβ-receptor typeV (TGFβ-RV) in mink lung epithelial cells [37].
IGFBP-3 has also been known to bind and activate intracellular signaling by forming a hetero-meric complex with other TGF-β receptors (TGF-β RII and TGF-β RI) in T47D breast cancer cells [35, 36]. Besides, addition of IGFBP-3 resulted in the activation of Smad2 phosphorylation and cell growth inhibition in these cells. It has also been shown that IGFBP-3 mediates its pro-apoptotic effects via TGF-β in PC-3 human prostate cancer cells [16]. Results from our previous studies indicated the inhibition of TGF-β signaling in presence of IGFBP-3 in PC-3 human prostate cancer cells [34], although we did not study the Smad activation in these cells.
It has also been reported that IGFBP-3 down-regulates Akt activity in human epidermal growth factor receptor-2 (HER-2) overexpressed MCF-7 breast cancer cells [38], in this regard recent studies have shown that IGFBP-3 induces apoptosis in MCF-7 breast cancer cells by inhibiting IGF-I/Akt survival pathway [39]. Previous studies also indicated that IGFBP-3 can bind to a new cell death receptor (IGFBP-3R), a single-span membrane protein which specifically binds to IGFBP-3 but doesn’t bind to other IGFBP’s. Invivo studies using prostate and breast cancer xenografts in athymic nude mice, showed anti-tumor effects of IGFBP-3R [40]. It was shown from the invitro studies that IGFBP-3R triggers IGFBP-3 induced apoptosis in various cancer cells via a caspase-8 dependent pathway. IGFBP-3R directly interacts and activates caspase-8 in inducing apoptosis and knockdown of caspase-8 expression or activity can lead to inhibition of IGFBP-3/IGFBP-3R induced apoptosis. All these studies clearly indicate that IGFBP-3 induces its anti-proliferative and pro-apoptotic effects either by sequestering IGF-1 to prevent it to bind to Insulin-like growth factor-I receptor (IGF-IR) in an IGF dependent manner or by activating several candidate molecules via signal transduction pathway independent of IGF binding. Although Insulin-like growth factor binding protein-3 receptor (IGFBP-3R) has been known to be directly involved in IGFBP-3 action and may prove to be an important target molecule for the treatment of cancer, further studies are still needed to clarify the role of this receptor or other IGFBP-3 cell surface binding protein(s) in IGFBP-3 mediated cell signaling events.
4. Conclusions
IGFBP-3 is known to activate various downstream signaling molecules. Some of these signaling events could be initiated after binding to its receptor independent of IGF-I binding, to induce anti-proliferative and pro-apoptotic effects in certain type of cancer cells. On the other hand, IGFBP-3 can also bind to IGF-1 to modulate its ability to bind to IGF-1 receptor to inhibit cell proliferation and cell survival, resulting in cell death.
Acknowledgments
This work was supported by an intramural grant from NIDDK, NIH.
References
- 1.Wood WI, Cachianes G, Henzel WJ, Winslow GA, Spencer SA, Hellmiss R, Martin JL, Baxter RC. Cloning and expression of the growth hormone-dependent insulin-like growth factor-binding protein. Molecular Endocrinology. 1988;2:1176–1185. doi: 10.1210/mend-2-12-1176. [DOI] [PubMed] [Google Scholar]
- 2.Mehta HH, Gao Q, Galet C, Paharkova V, Wan J, Said J, Sohn JJ, Lawson G, Cohen P, Cobb LJ, Lee KW. IGFBP-3 is a metastasis suppression gene in prostate cancer. Cancer Research. 2011;71(15):5154–5163. doi: 10.1158/0008-5472.CAN-10-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 4.Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, Giovannucci E. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. Journal of the National Cancer Institute. 2002;94:1099–1106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 5.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. Journal of the National Cancer Institute. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Pollak MN, Platz EA, Willett WC, Stampfer MJ, Majeed N, Colditz GA, Speizer FE, Hankinson SE. A Prospective Study of Plasma Insulin-like Growth Factor-1 and Binding Protein-3 and Risk of Colorectal Neoplasia in Women. Cancer Epidemiological Biomarkers Preview. 2000;9:345–349. [PubMed] [Google Scholar]
- 7.Hochscheid R, Jaques G, Wegmann B. Transfection of human insulin-like growth factor-binding protein 3 gene inhibits cell growth and tumorigenicity: a cell culture model for lung cancer. Journal of Endocrinology. 2000;166:553–563. doi: 10.1677/joe.0.1660553. [DOI] [PubMed] [Google Scholar]
- 8.Devi GR, Sprenger CC, Plymate SR, Rosenfeld RG. Insulin-like growth factor binding protein-3 induces early apoptosis in malignant prostate cancer cells and inhibits tumor formation in vivo. Prostate. 2002;51:141–152. doi: 10.1002/pros.10068. [DOI] [PubMed] [Google Scholar]
- 9.Hanafusa T, Yumoto Y, Nouso K, Nakatsukasa H, Onishi T, Fujikawa T, Taniyama M, Nakamura S, Uemura M, Takuma Y, Yumoto E, Higashi T, Tsuji T. Reduced expression of insulin-like growth factor binding protein-3 and its promoter hypermethylation in human hepatocellular carcinoma. Cancer Letters. 2002;176(2):149–158. doi: 10.1016/s0304-3835(01)00736-4. [DOI] [PubMed] [Google Scholar]
- 10.Chang YS, Kong G, Sun S, Liu D, El-Naggar AK, Khuri FR, Hong WK, Lee HY. Clinical significance of Insulin-like growth factor-binding protein-3 expression in stage I non-small cell lung cancer. Clinical Cancer Research. 2002;8:3796–3802. [PubMed] [Google Scholar]
- 11.Lu XF, Jiang XG, Lu YB, Bai JH, Mao ZB. Characterization of a novel positive transcription regulatory element that differentially regulates the Insulin-like growth factor binding protein-3(IGFBP-3) gene in senescent cells. Journal of Biological Chemistry. 2005;280:22606–22615. doi: 10.1074/jbc.M412073200. [DOI] [PubMed] [Google Scholar]
- 12.Mannhardt B, Weinzimer SA, Wagner M, Fiedler M, Cohen P, Jansen-Durr P, Zwerschke W. Human Papillomavirus Type 16 E7 Oncoprotein Binds and Inactivates Growth-Inhibitory Insulin-Like Growth Factor Binding Protein 3. Molecular and Cellular Biology. 2000;20:6483–6495. doi: 10.1128/mcb.20.17.6483-6495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharyya N, Pechhold K, Shahjee H, Zappala G, Elbi C, Raaka B, Wiench M, Hong J, Rechler MM. Nonsecreted Insulin-like growth factor binding protein-3(IGFBP-3) can induce apoptosis in human prostate cancer cells by IGF-independent mechanisms without being concentrated in the nucleus. Journal of Biological Chemistry. 2006;281(34):24588–24601. doi: 10.1074/jbc.M509463200. [DOI] [PubMed] [Google Scholar]
- 14.Lalou C, Lassarre C, Binoux M. A proteolytic fragment of insulin-like growth factor (IGF) binding protein-3 that fails to bind IGFs inhibits the mitogenic effects of IGF-I and insulin. Endocrinology. 1996;137(8):3206–3212. doi: 10.1210/endo.137.8.8754741. [DOI] [PubMed] [Google Scholar]
- 15.Shahjee H, Bhattacharyya N, Zappala G, Wiench M, Prakash S, Rechler MM. An N-terminal fragment of Insulin-like growth factor binding protein-3(IGFBP-3) induces apoptosis in human prostate cancer cells in an IGF-independent manner. Growth Hormone & IGF Research. 2008;18(3):188–197. doi: 10.1016/j.ghir.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Rajah R, Valentinis B, Cohen P. Insulin-like growth factor IGF binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. Journal of Biological Chemistry. 1997;272(18):12181–12188. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 17.Butt AJ, Firth SM, King MA, Baxter RC. Insulin-like growth factor-binding protein-3 modulates expression of Bax and Bcl-2 and potentiates p53-independent radiation-induced apoptosis in human breast cancer cells. Journal of Biological Chemistry. 2000;275(50):39174–39181. doi: 10.1074/jbc.M908888199. [DOI] [PubMed] [Google Scholar]
- 18.Hong J, Zhang G, Dong F, Rechler MM. Insulin-like growth factor (IGF)-binding protein-3 mutants that do not bind IGF-I or IGF-II stimulate apoptosis in human prostate cancer cells. Journal of Biological Chemistry. 2002;277(12):10489–10497. doi: 10.1074/jbc.M109604200. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Lee WJ, Lee SW, Chae HW, Kim DH, Oh Y. Insulin-like growth factor binding protein-3 induces G1 cell cycle arrest with inhibition of cyclin-dependent kinase 2 and 4 in MCF-7 human breast cancer cells. Hormone and Metabolic Research. 2010;42(3):165–172. doi: 10.1055/s-0029-1243190. [DOI] [PubMed] [Google Scholar]
- 20.Wu C, Liu X, Wang Y, Tian H, Xie Y, Li Q, Zhang X, Liu F. Insulin-like factor binding protein-3 promotes the G1 cell cycle arrest in several cancer cell lines. Gene. 2013;512(1):127–133. doi: 10.1016/j.gene.2012.09.080. [DOI] [PubMed] [Google Scholar]
- 21.Gucev ZS, Oh Y, Kelley KM, Rosenfeld RG. Insulin-like growth factor binding protein-3 mediates retinoic acid-and transforming growth factor beta2-induced growth inhibition in human breast cancer cells. Cancer Research. 1996;56(7):1545–1550. [PubMed] [Google Scholar]
- 22.Rajah R, Lee KW, Cohen P. Insulin-like growth factor binding protein-3 mediates tumor necrosis factor-alpha-induced apoptosis: role of Bcl-2 phosphorylation. Cell Growth & Differentiation. 2002;13(4):163–171. [PubMed] [Google Scholar]
- 23.Vasylyeva TL, Chen X, Ferry RJ., Jr Insulin-like growth factor binding protein-3 mediates cytokine-induced mesangial cell apoptosis. Growth Hormone & IGF Research. 2005;15(3):207–214. doi: 10.1016/j.ghir.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan KM, Vousden KH. Characterization of structural p53 mutants which show selective defects in apoptosis but not cell cycle arrest. Molecular and Cellular Biology. 1998;18(7):3692–3698. doi: 10.1128/mcb.18.7.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimberg A, Liu B, Bannerman P, El-Deiry WS, Cohen P. IGFBP-3 mediates p53-induced apoptosis during serum starvation. International Journal of Oncology. 2002;21(2):327–335. [PMC free article] [PubMed] [Google Scholar]
- 26.Harms KL, Chen X. The C terminus of p53 family proteins is a cell fate determinant. Molecular and Cellular Biology. 2005;25(5):2014–2030. doi: 10.1128/MCB.25.5.2014-2030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rechler MM, Clemmons DR. Regulatory actions of Insulin-like growth factor-binding proteins. Trends in Endocrinology and Metabolism. 1998;9(5):176–183. doi: 10.1016/s1043-2760(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 28.Firth SM, Baxter RC. Cellular actions of the Insulin-like growth factor binding proteins. Endocrine Reviews. 2002;23(6):824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 29.Han J, Jogie-Brahmin S, Harada A, Oh Y. Insulin-like growth factor-binding protein-3 suppresses tumor growth via activation of caspase-dependent apoptosis and cross-talk with NF-kB signaling. Cancer Letters. 2011;307(2):200–210. doi: 10.1016/j.canlet.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Zappala G, Elbi C, Edwards J, Gorenstein J, Rechler MM, Bhattacharyya N. Induction of apoptosis in human prostate cancer cells by Insulin-like growth factor binding protein-3 does not require binding to retinoid X receptor-alpha. Endocrinology. 2008;149(4):1802–1812. doi: 10.1210/en.2007-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leibowitz BJ, Agostini-Dreyer A, Jetzt AE, Krumm CS, Cohick WS. IGF binding protein-3 mediates stress-induced apoptosis in non-transformed mammary epithelial cells. Journal of Cellular Physiology. 2013;228(4):734–742. doi: 10.1002/jcp.24220. [DOI] [PubMed] [Google Scholar]
- 32.Ricort JM. Insulin-like growth factor binding protein (IGFBP-3) signalling. Growth Hormone & IGF Research. 2004;14(4):277–286. doi: 10.1016/j.ghir.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Spagnoli A, Torello M, Nagalla SR, Horton WA, Pattee P, Chiarelli F, Roberts CT, Jr, Rosenfeld RG. Identification of STAT-1 as a molecular target of IGFBP-3 in the process of chondrogenesis. Journal of Biological Chemistry. 2002;277(21):18860–18867. doi: 10.1074/jbc.M200218200. [DOI] [PubMed] [Google Scholar]
- 34.Shahjee HM, Kefas B, Bhattacharyya N, Radwan MK. Signal transduction pathways mediated by secreted and non-secreted forms of intact Insulin-like growth factor binding protein-3 and its 1-97 N-terminal fragment in PC-3 human prostate cancer cells. Journal of Cancer Therapy. 2013;4(8):1–10. doi: 10.4236/jct.2013.48152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fanayan S, Firth SM, Butt AJ, Baxter RC. Growth inhibition by Insulin-like growth factor-binding protein-3 in T47D breast cancer cells requires transforming growth factor-beta (TGF-beta) and the type II TGF-beta receptor. Journal of Biological Chemistry. 2000;275(50):146–151. doi: 10.1074/jbc.M006964200. [DOI] [PubMed] [Google Scholar]
- 36.Fanayan S, Firth SM, Baxter RC. Signalling through the Smad pathway by Insulin-like growth factor-binding protein-3 in breast cancer cells. Relationship to transforming growth factor-beta 1 signalling. Journal of Biological Chemistry. 2002;277(9):7255–7261. doi: 10.1074/jbc.M108038200. [DOI] [PubMed] [Google Scholar]
- 37.Leal SM, Huang SS, Huang JS. Interactions of high affinity Insulin-like growth factor-binding proteins with the type V transforming growth factor-beta receptor in mink lung epithelial cells. Journal of Biological Chemistry. 1999;274(10):6711–6717. doi: 10.1074/jbc.274.10.6711. [DOI] [PubMed] [Google Scholar]
- 38.Jerome L, Alami N, Belanger S, Page V, Yu Q, Paterson J, Shiry L, Pegram M, Leyland-Jones B. Recombinant human Insulin-like growth factor binding protein-3 inhibits growth of human epidermal growth factor receptor-2 overexpressing breast tumors and potentiates herceptin activity in vivo. Cancer Research. 2006;66(14):7245–7252. doi: 10.1158/0008-5472.CAN-05-3555. [DOI] [PubMed] [Google Scholar]
- 39.Brosseau C, Pirianov G, Colston KW. Role of Insulin-like growth factor binding protein-3 in 1,25-dihydroxyvitamin-d 3-induced breast cancer cell apoptosis. International Journal of Cell Biology. 2013;2013:1–9. doi: 10.1155/2013/960378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingermann AR, Yang YF, Han J, Mikami A, Garza AE, Mohanraj L, Fan L, Idowu M, Ware JL, Kim HS, Lee DY, Oh Y. Identification of a novel cell death receptor mediating IGFBP-3-induced anti-tumor effects in breast and prostate cancer. Journal of Biological Chemistry. 2010;285(39):30233–30246. doi: 10.1074/jbc.M110.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]