Abstract
Introduction
Chronic infection with HBV and HCV as well as cigarette smoking are established risk factors of hepatocellular carcinoma (HCC), but it is unclear whether an interaction exists between these factors in causing hepatocellular carcinogenesis. We conducted a meta-analysis to evaluate the interaction of HBV and HCV infection and cigarette smoking on the risk of HCC.
Methods
We systematically searched the PUBMED and the China National Knowledge Infrastructure (CNKI) databases. A total of 16 eligible publications were identified. Cigarette smoking, and chronic HBV and HCV infections were dichotomized into present or absent. Additive (S) and multiplicative interaction indexes (V) between smoking and each of the two infections and their 95% confidence intervals (95% CI) were calculated for each study and then combined in a meta-analysis.
Results
We found a more than additive interaction between HBV infection and cigarette smoking (S=1.44, 95% CI=1.00–2.06; 9 studies) and a more than multiplicative interaction (V=1.60, 95% CI=1.16–2.20; 6 studies) between HCV infection and cigarette smoking. No publication bias was detected.
Conclusion
Smoking appears to interact with both HBV and HCV in determining HCC risk. A pooled analysis of individual subject data, with appropriate adjustment with other risk factors is warranted to confirm these results.
Impact
Chronic carriers of HBV and HCV are suggested to avoid smoking.
Keywords: Cigarette smoking, HBV, HCV, HCC, interaction
Introduction
Liver cancer is the sixth most common cancer and the third most common cause of death from cancer worldwide with about 600,000 estimated new cancer cases and about the same number of deaths in 2002 (1). China alone accounts for about 55% of the world burden of liver cancer (1). The 5-year survival rates for liver cancer are low, at 12% in the United States during 1996–2004(2), 9% in Europe during 1995–1999 (3), and 5% in developing countries in 2002 (1). Hepatocellular carcinoma (HCC) represents the main histologic type of liver cancer. The main risk factors for HCC are chronic infection with hepatitis B and C viruses (HBV and HCV), alcohol drinking, tobacco smoking, and aflatoxin exposure. Oral contraceptive usage, iron overload, overweight and diabetes are also known or suspected risk factors of the disease (4)
The risk of HCC in people infected with HBV or HCV is about 20 times higher than in those who are not (5). HCC cases from Asia (except Japan), Africa, Latin America, and Greece are mainly attributed to HBV infection while those cases from other European countries, northern America and Japan are mainly attributed to HCV (6). Overall, the attributable fraction of HBV on HCC is 54.4%, with 23.3% in high-income countries and 58.8% in low- and middle-income countries (7). Thirty-one percent of HCC cases worldwide are attributed to HCV, with 19.9% in high income countries and 33.4% in low- and middle-income countries.
The International Agency for Research on Cancer (IARC) had classified HCC as one of the tobacco-related cancers in 2004 (8). A recent meta-analysis reported a moderate risk of HCC with current cigarette smoking status (meta-RR=1.51, 95% CI=1.37–1.67) (9). Residual confounding from HBV and HCV infection has long been an issue to establish whether cigarette smoking is a risk factor of HCC. Adjustment for and stratification by HBV or HCV status were considered to evaluate the effect of smoking on the risk of HCC (8, 9).
Though the independent effects of HBV and HCV infection, and of cigarette smoking on the risk of HCC have been established, the possible interaction between these factors is not well characterised. The data from individual studies on the interaction between HBV infection and smoking are not fully consistent. Some studies observed an association between cigarette smoking and HCC only among HBV negative (HBV−) persons (10–13), some studies reported associations in HBV carriers (HBV+) (14, 15), but other studies reported no interaction (16–18). Nevertheless, most studies observed an interaction between cigarette smoking and HCV infection on the risk of HCC (18–21). In consistencies among studies can be due to random fluctuations, because of small number of cases, or to systematic differences in study design.
To better elucidate the independent and combined effect of cigarette smoking and HBV and HCV infection in the etiology of HCC, we conducted a meta-analysis to evaluate the interactions between these factors in determining HCC risk.
Material and Methods
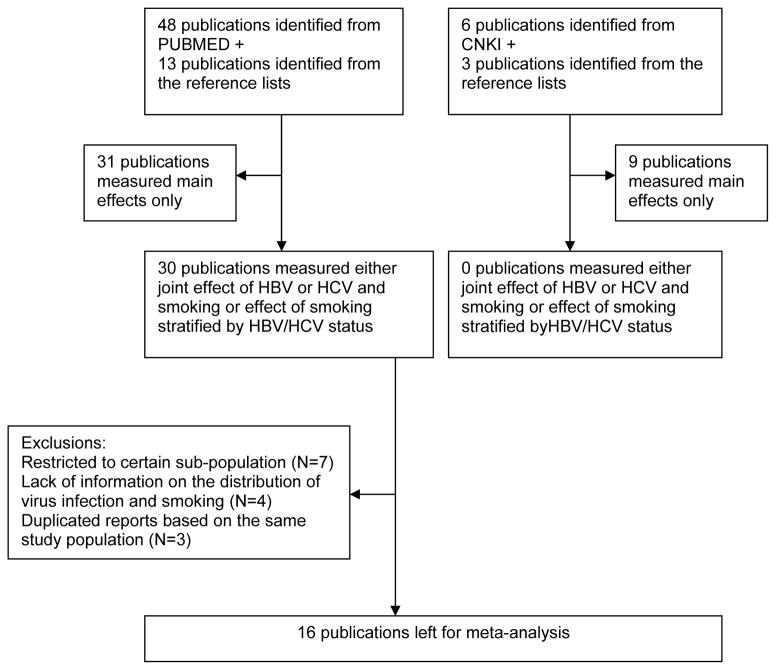
Search Strategy and Selection Criteria (Figure 1)
Figure 1.
Flow-chart of selection of publications included in the meta-analysis
We systematic searched the PUBMED database with the following keywords: (HBV OR HCV) AND (Smoking [Mesh] OR Tobacco [Mesh]) AND (Liver cancer [Mesh] OR HCC [Mesh]) from 1966 to May 2009. The search was not restricted as to language. In view of the large number of HCC cases arising in China, we aimed at including also studies conducted in this country and reported in national scientific journal not indexed in PUBMED. Therefore, we also searched the China National Knowledge Infrastructure (CNKI) database, with the same keywords. The CNKI database includes papers published in Chinese journals after 1994. In addition to the databases, we checked the references list of the articles retrieved from PUBMED and CNKI.
Overall, 48 publications were identified in PUBMED and an additional 13 articles were identified from their reference lists. In 30 of these publications, results on either joint effect of HBV or HCV and smoking or effect of smoking stratified by HBV/HCV status were reported. We excluded publications in which the study population was restricted to HBV carriers (four publications) or non-carriers only (three publications). In addition, we excluded seven publications due to the following reasons: only stratified results were reported, which made it impossible to estimate the variance of the interaction indexes; inclusion in later reports of the same studies; and information on HBV and smoking collected at the baseline without exposure distribution at end point.
Of the 9 additional publications which were identified from the CNKI (including one meta-analysis of risk factors of HCC), none presented detailed information on the combined effect of HBV, HCV, and cigarette smoking. Thus, all identified publications from CNKI were excluded from this analysis.
In total, 16 publications were included in this current meta-analysis. Their characteristics are listed in Table 1. Nine studies provided results on the interaction between cigarette smoking and HBV infection and six studies were considered to estimate the interaction with HCV infection on the risk of HCC. Since the fatality of HCC is high, results based on mortality or incidence were combined. No studies provided results on the interaction between cigarette smoking and combined HBV and HCV infections.
Table 1.
Summary characteristics of the studies selected
| Author, year | Study Period | Country | Age | Study Design | Case | Number of case | HBV/HCV markers | Smoking definition |
|---|---|---|---|---|---|---|---|---|
| For HBV analysis | ||||||||
| Hassan, 2008 (18) | 2000–2006 | US | All ages | Case-control | Incident | 319 | HBsAg, anti-HBc | ≥100 cigarettes lifetime |
| Jee, 2004 (17) | 1993–2002 | Korea | 30–95 | Cohort | Mortality | 3807 | HBsAg | Ever smoker |
| Wang, 2003 (10) | 1991–2000 | Taiwan | 30–65 | Cohort | Incident | 115 | HBsAg | 4 days per week for a year |
| Mori, 2000 (16) | 1992–1997 | Japan | 30+ | Cohort | Incident | 22 | HBsAg | ≥100 cigarettes lifetime |
| Goritsas, 1995 (44) | 1989–1992 | Greece | All ages | Case-control | Incident/Prevalent | 51 | HBsAg | Ever smoker |
| Tzonou, 1991 (45) | 1976–1984 | Greece | All ages | Case-control | Incident/Prevalent | 185 | HBsAg | Smoker or stopped smoking for less than 3 years |
| Chen, 1991 (42) | 1985–1987 | Taiwan | All ages | Case-control | Incident | 200 | HBsAg, HBeAg | Ever smoker |
| Tu, 1985 (14) | 1980–1982 | China | 40+ | Cohort | Mortality | 70 | HBsAg, anti-HBc | Ever smoker |
| Lam, 1982 (12) | 1977–1980 | Hong Kong | All ages | Case-control | Incident/Prevalent | 107 | HBsAg | Ever smoker |
| For HCV analysis | ||||||||
| Hassan, 2008 (18) | 2000–2006 | US | All ages | Case-control | Incident | 319 | Third generation anti-HCV | ≥100 cigarettes lifetime |
| Fujita, 2006 (20) | 1988–1999 | Japan | 40–79 | Nested case-control | Mortality | 94 | Anti-HCV | Ever smoker |
| Sun, 2003 (21) | 1991–2001 | Taiwan | 30–65 | Cohort | Incident | 112 | Second generation anti-HCV | 4 days per week for a year |
| Mori, 2000 (16) | 1992–1997 | Japan | 30+ | Cohort | Incident | 22 | Second generation anti-HCV | ≥100 cigarettes lifetime |
| Yu, 1991 (19) | 1986–1987 | Taiwan | All ages | Case-control | Incident | 127 | Anti-HCV | Ever smoker |
| Tzonou, 1991 (45) | 1976–1984 | Greece | All ages | Case-control | Incident/Prevalent | 185 | Anti-HCV | Ever smoker |
| HBV or HCV not specified | ||||||||
| Franceschi, 2006 (46) | 1999–2002 | Italy | 41–84 | Case-control | Incident | 229 | HBsAg, anti-HCV | 1 cigarette per day for a year |
| Yuan, 2004 (25) | 1984–2001 | US | 18–74 | Case-control | Incident | 295 | HBsAg, anti-HBc, anti-HCV | Smoker or stopped smoking for less than 10 years |
| HBV− and HCV− | ||||||||
| Wang, 2009 (27) | 1996–2004 | Taiwan | 35+ | Cohort | Incident | 111 | HBsAg, anti-HCV | Smoker or stopped smoking for less than 6 months |
| Chen, 2008 (26) | 1991–2004 | Taiwan | 30–65 | Cohort | Incident | 291 | HBsAg, HBeAg, anti-HCV | 4 days per week for a year |
| Yuan, 2004 (25) | 1984–2001 | US | 18–74 | Case-control | Incident | 295 | HBsAg, anti-HBc, anti-HCV | Smoker or stopped smoking for less than 10 years |
Statistical Analysis
We categorized study subjects into four groups with respect to infection and smoking: Non-HBV/HCV infected and never-smokers (reference category), non-HBV/HCV infected and ever-smokers (RR01), HBV/HCV infected and never-smokers (RR10), and HBV/HCV infected and ever-smokers (RR11). The number of subjects in each stratum and the adjusted risk estimates (if available) were recorded. If the latter were not available, crude relative risks were calculated from the numbers of subjects and person-years reported in the tables. Additive (S) and multiplicative interaction indexes (V) between each infection and cigarette smoking status and their 95% confidence intervals (95% CI) were calculated for each study (22).
Where F1=(Var(R11)+Var(R00))/(R11−R00)2
F2=(Var(R01)+Var(R10)+4Var(R00))/(R01+R10−2R00)2
F3=Var(R00)/((R11−R00)(R01+R10−2R00))
Var(Rij)=Rij/Mij, where Mij is the total number in the joint category
Rij is the risk of the specific category, where i=1 refers to the exposure of HBV or HCV infection, i=0 refers to no virus exposure; j=1 refers to the exposure of the cigarette smoking, j=0 refers to no cigarette smoking exposure.
Where F4=Var(RR11)/(RR11−1)2
F5=Var(RR01)+(Var(RR10)+2cov(RR01, RR10))/(RR01+RR10−2)2
F6=2cov(RR11, RR01+RR10)/((RR11−1)(RR01+RR10−2))
Var(RRij)=RRij2×(1/aij+1/cij+1/b+1/d)
Cov(RR01, RR10)=RR01×RR10×(1/b+1/d)
Cov(RR11, RR01+RR10)=RR11×(RR01+RR10)×(1/b+1/d)
Where “b” and “d” are the frequency of cases and controls, respectively, in the reference category and “aij” and “cij” are the frequency of cases and controls in the exposed category.
Where “a” to “d” are the numbers of cases and controls classified with respect to HBV or HCV infection and “e” to “h” are the corresponding numbers with respect to smoking, and the two exposures are assumed to be independent.
Overall additive and multiplicative interaction indexes were then calculated using random-effects models to combine the study-specific interaction estimates.
In order to explore sources of heterogeneity, subgroup analysis were performed by study design (case-control and cohort studies), region (Asia and non-Asia), and vital status of cases (incidence and mortality). Heterogeneity of the estimates across studies was tested, using a non-iterative weighted method (23). Egger’s test was utilized to assess the presence of publication bias (24). Sensitivity analyses were performed by removing one study at a time to assess whether the meta-estimates were strongly influenced by any particular study.
To explore the effect of cigarette smoking, independent of both HBV and HCV infection, on the risk of HCC, we also abstracted results from publications on the HBV and HCV negative or HBV and HCV positive groups.
Results
Interaction between HBV infection and Cigarette Smoking
Among the nine studies selected for the HBV analysis, five were case-control studies, and the other four were cohort studies. Six of the studies were from Asia (China, Hong Kong, Japan, Korea, and Taiwan), two were from Greece, and the remaining study was from USA. Two of the studies used mortality data (Table 1).
Overall, relative to HBV-negative nonsmokers, the risk of HCC was 1.87 (95% CI=1.30–2.69) for HBV negative smokers, 15.8 (95% CI=9.69–25.7) for HBV positive non-smokers and 21.6 (95% CI=15.2–30.5) HBV positive smokers. These results suggested a more than additive interaction between these two risk factors (S=1.44, 95% CI=1.00–2.06), and were compatible with a multiplicative interaction (V=0.87, 95% CI=0.58–1.29) (Table 2). The results were similar after exclusion of a large cohort study from Korea, thought the test for the departure from the additive model of interaction included the null value (S=1.51, 95% CI=0.85–2.66 and V=0.78, 95% CI=0.47–1.29). No heterogeneity in the results of the meta-analysis were suggested by study design, region, and source of cases (supplement Table 1)
Table 2.
Risk estimates and 95% confidence intervals for the joint effects and interaction indexes between HBV and smoking
| HBV−/Tob− | HBV−/Tob+ | HBV+/Tob−* | HBV+/Tob+ | Interaction Index | ||
|---|---|---|---|---|---|---|
| Additive | Multiplicative | |||||
| All studies (N=9) | ||||||
| No. of cases | 272 | 960 | 419 | 1680 | 3331 | 3309 |
| Random effect | 1.00 | 1.87 (1.30–2.69) | 15.8 (9.69–25.7) | 21.6 (15.2–30.5) | 1.44 (1.00–2.06) | 0.87 (0.58–1.29) |
| Adjusted random effects* | 1.00 | 1.59 (0.94–2.70) | 18.27 (14.5–23.0) | 21.7 (11.8–40.0) | 1.53 (1.34–1.75) | 0.77 (0.36–1.67) |
| p for heterogeneity | 0.001 | 0.011 | 0.050 | 0.049 | <0.001 | |
| Egger’s test for publication bias | 0.609 | 0.105 | ||||
One cohort study in Asia reported 0 cases in the HBV+/Tob− category. Multiplicative interaction was not able to be calculated for this study.
Abbreviation: HBV−/Tob−: Reference category, non-HBV infection and non-smoker; HBV−/Tob+: Non-HBV infection and smoker; HBV+/Tob−: HBV infection and non-smoker; HBV+/Tob+: HBV infection and smoker
Three publications provided adjusted estimates (10, 17, 18). The estimate was based on male population. All three publications adjusted for age. Other adjustments included race (18), education, residence, anti-HCV, (10, 18), marital status (18), alcohol, diabetes (17, 18), family history of HCC (10), family history of cancer (18), and liver function (10)
Interaction between HCV infection and Cigarette Smoking
Six studies provided data relevant to the evaluation of the joint effect of HCV infection and cigarette smoking on the risk of HCC (Table 3). Four of them were case-control studies and two were cohort studies. The relative risk of HCC was 1.50 (95% CI=1.25–1.80) for cigarette smokers among HCV negative subjects, 7.94 (95% CI=4.40–14.3) for HCV positive subjects among non-smokers, and 23.1 (95% CI=9.43–56.8) for the joint effect of cigarette smoking and HCV infection. The overall interaction terms were 3.32 (95% CI=2.23–4.94) based on additive model and 1.60 (95% CI=1.16–2.20) on the multiplicative model. The Egger’s test suggested no publication bias in these studies (p=0.511 for S and 0.696 for V). No heterogeneity in the results of the meta-analysis was suggested by study design or by region (supplement Table 2)
Table 3.
Risk estimates and 95% confidence intervals for the joint effects and interaction indexes between HCV and smoking
| HCV−/Tob− | HCV−/Tob+ | HCV+/Tob− | HCV+/Tob+ | Interaction Index | ||
|---|---|---|---|---|---|---|
| Additive | Multiplicative | |||||
| All studies (N=6) | ||||||
| No. of cases | 197 | 373 | 62 | 200 | 832 | 832 |
| Random effect | 1.00 | 1.50 (1.25–1.80) | 7.94 (4.40–14.3) | 23.1 (9.43–56.8) | 3.32 (2.23–4.94) | 1.60 (1.16–2.20) |
| Adjusted random effects* | 1.00 | 1.42 (1.05–1.96) | 6.90 (1.12–42.7) | 19.6 (1.55–247.0) | 3.36 (1.09–10.4) | 1.83 (1.00–3.34) |
| p for heterogeneity | 0.471 | 0.064 | <0.001 | 0.755 | 0.697 | |
| Egger’s test for publication bias | 0.511 | 0.696 | ||||
Abbreviation: HCV−/Tob−: Reference category, non-HCV infection and non-smoker; HCV−/Tob+: Non-HCV infection and smoker; HCV+/Tob−: HCV infection and non-smoker; HCV+/Tob+: HCV infection and smoker
Three publications provided adjusted estimates (18, 19, 21). One of the publication was based on male (21), one is stratified by gender (18), and the other one adjusted for sex (19). All three publications adjusted for age. Other adjustments included race, residence (18, 19), marital status (18), education, HBV (18, 21) alcohol, diabetes, family history of cancer (18), and family history of HCC (21).
Smoking effects among HBV and HCV negative subjects
The combined result based on the crude estimates from the two (25, 26) studies in which ever smoker was compared to never smoker among the HBV and HCV negative subjects was 2.47 (95% CI=1.41–4.32) and it was 0.80 (95% CI=0.15–4.30) based on the adjusted estimates from another set of publications (25, 27) (Table 4). The negative result of the meta-analysis of the adjusted RR is due to a single study (27).
Table 4.
Crude and adjusted risk estimates and 95% confidence intervals for smoking among HBV and HCV negative populations
Smoking effects among HBV and HCV positive subjects
There were few publications on the three-way interaction among HBV, HCV, and smoking. This was probably due to the difficulty of identifying an appropriate control group with co-infection by HBV and HCV. In a cohort study in southern Taiwan (27), no association was reported between smoking and HCC (RR=1.1, 95% CI=0.3–4.4,) in the coinfected population (n=134, 2% of the cohort, 12 HCC cases) after 8 years of follow-up.
Discussion
The results from our study suggest an interaction on the additive scale between cigarette smoking and HBV infection and an interaction on the multiplicative scale with HCV infection. In addition, our results support the notion that cigarette smoking has a measurable effect on HCC risk even in the absence of HBV or HCV infection.
Several theories have been proposed for the role of cigarette smoking in liver carcinogenesis and its potential interaction with viral infection. Cigarette smoke contains several chemicals which are metabolized and activated as carcinogens in the liver (28), and it can therefore act as an initiator in the liver carcinogenesis, while HBV and HCV mainly act as a promoter through chronic inflammation and cell 13 proliferation through chronic hepatitis and liver cirrhosis (5). In addition, cigarette smoking may contribute to progression from chronic HBV and HCV infection to HCC (15, 29, 30). An action on different stages of carcinogenesis would be compatible with a multiplicative interaction index close or equal to 1, as in the case of HBV infection and cigarette smoking. A multiplicative interaction index >1, as in the case of HCV infection and cigarette smoking, if real, would imply a biological interaction between the two factors.
A cohort study conducted in southern Taiwan showed that cigarette smokers had higher prevalence of HCV infection, but such an association was not observed with HBV prevalence (31). In addition, smoking tended to be associated with elevated alanine aminotransferase levels only among HCV infected individuals (32) Cigarette smoking may worsen the prognosis of chronic HCV infection, possibly through the accumulation of oxidative stress (33, 34, 34), impaired immune response (35), and generation of insulin resistance (36, 37), which are also associated with HCV-related HCC (38).
An alternative explanation could be uncontrolled confounding, particularly by alcohol drinking. In one study, the interaction of alcohol drinking with HCV on HCC risk was observed to be stronger than that with HBV (39). As smoking and drinking are correlated in many populations, it is difficult to rule out a potential confounding effect by alcohol drinking.
The difference between the crude and adjusted estimates of cigarette smoking on the risk of HCC among the HBV and HCV negative population is difficult to interpret because the two estimates were based on different publications and should be interpreted with caution. Only one study contributed to both estimates (25) and there was only a small change in the risk estimate after adjusting for age, sex, race, education, alcohol, and diabetes (1.88 vs. 1.70). The two studies, which showed conflicting results, were both from Taiwan. In the Seven-Township study (26), no effect was shown for former smokers (RR=1.00, 95% CI=0.22–4.59, compared to never smokers) but there was an increased association for current smokers after adjustment for age and sex (RR=2.44, 95% CI=1.17–5.00). In the A-Lein study (27), smoking habit was not associated with HCC risk (RR=0.3, 95% CI=0.1–1.4) after adjusting for age, sex, alcohol consumption, body mass index, and diabetes status. Whether the difference in results was due to the definition of smoking, change of smoking habit during follow-up, the characteristics of the study populations, or by chance needs further investigations.
One limitation of our meta-analysis was the fact that the authors of some studies did not provide adjusted risk estimates and only crude risk estimates was calculated based on the raw numbers reported in the original publications, leaving open to the possibility residual confounding in particular by age, sex, and alcohol drinking. A pooled analysis of individual data is warranted to overcome such a limitation.
Another limitation was the fact that methods used to measure HBV and HCV infection were different across studies. HBsAg positive for six months are generally considered as HBV carrier (40). Negativity for HBsAg combined with positivity for anti-HBs or anti-HBc indicates vaccination or ability to clear the infection (41). HBeAg is usually related to virus replication and infectivity (41). In all studies included in the present meta-analysis, test for HBsAg was one of the criteria for HBV infection; but in some studies, tests for anti-HBc (14, 18) and HBeAg (42) were also used. However, sensitivity analysis by excluding these studies did not reveal differences in the overall results.
Anti-HCV is the most common marker used to test for HCV infection. Two studies in the analysis used second-generation ELISA (16, 21), one study used the third-generation (18), and the others did not specify the assays used to measure anti-HCV. As the technique improves, the third-generation ELISA can identify 97% HCV infection but might be less specific than the second-generation ELISA (43). However, the results did not differ by excluding the study using third generation ELISA.
In conclusion, our meta-analysis found an interaction between cigarette smoking and both HBV and HCV infection, respectively. The pattern of the interaction seems different between the two infections, which might reflect their different roles in liver carcinogenesis. In addition, the carcinogenic effect of cigarette smoking on HCC risk appeared to be independent from infection with either HBV or HCV.
Supplementary Material
Acknowledgments
Shu-Chun Chuang worked on this study during the tenure of a postdoctoral fellowship from the International Agency for Research on Cancer Cancer (IARC). Yuan-Chin Amy Lee worked on this project during the fellowship in the Cancer Epidemiology Training Program (NIH-T32 CA09142) at the University of California at Los Angeles (UCLA). The authors thank Dr. Menghua Tao at University at Buffalo, New York, USA for her assistance in translating the references of the Chinese articles cited/referenced in this article.
Reference List
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Ries L, Melbert D, Krapcho M, et al. SEER Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 3.Berrino F, De Angelis R, Sant M, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773–83. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- 4.Chuang SC, Vecchia CL, Boffetta P. Liver cancer: Descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 5.IARC Working Group. Hepatitis Viruses. 1994 [Google Scholar]
- 6.Raza SA, Clifford GM, Franceschi S. Worldwide variation in the relative importance of hepatitis B and hepatitis C viruses in hepatocellular carcinoma: a systematic review. Br J Cancer. 2007;96:1127–34. doi: 10.1038/sj.bjc.6603649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 8.IARC Working Group. Tobacco smoke and involuntary smoking. 2004:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YC, Cohet C, Yang YC, et al. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009 doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- 10.Wang LY, You SL, Lu SN, et al. Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg-seropositive and 9421 HBsAg-seronegative male residents in Taiwan. Cancer Causes Control. 2003;14:241–50. doi: 10.1023/a:1023636619477. [DOI] [PubMed] [Google Scholar]
- 11.Trichopoulos D, Day NE, Kaklamani E, et al. Hepatitis B virus, tobacco smoking and ethanol consumption in the etiology of hepatocellular carcinoma. Int J Cancer. 1987;39:45–9. doi: 10.1002/ijc.2910390109. [DOI] [PubMed] [Google Scholar]
- 12.Lam KC, Yu MC, Leung JW, Henderson BE. Hepatitis B virus and cigarette smoking: risk factors for hepatocellular carcinoma in Hong Kong. Cancer Res. 1982;42:5246–8. [PubMed] [Google Scholar]
- 13.Tanaka K, Hirohata T, Takeshita S. Blood transfusion, alcohol consumption, and cigarette smoking in causation of hepatocellular carcinoma: a case-control study in Fukuoka, Japan. Jpn J Cancer Res. 1988;79:1075–82. doi: 10.1111/j.1349-7006.1988.tb01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu JT, Gao RN, Zhang DH, Gu BC. Hepatitis B virus and primary liver cancer on Chongming Island, People’s Republic of China. Natl Cancer Inst Monogr. 1985;69:213–5. [PubMed] [Google Scholar]
- 15.Oshima A, Tsukuma H, Hiyama T, et al. Follow-up study of HBs Ag-positive blood donors with special reference to effect of drinking and smoking on development of liver cancer. Int J Cancer. 1984;34:775–9. doi: 10.1002/ijc.2910340607. [DOI] [PubMed] [Google Scholar]
- 16.Mori M, Hara M, Wada I, et al. Prospective study of hepatitis B and C viral infections, cigarette smoking, alcohol consumption, and other factors associated with hepatocellular carcinoma risk in Japan. Am J Epidemiol. 2000;151:131–9. doi: 10.1093/oxfordjournals.aje.a010180. [DOI] [PubMed] [Google Scholar]
- 17.Jee SH, Ohrr H, Sull JW, Samet JM. Cigarette smoking, alcohol drinking, hepatitis B, and risk for hepatocellular carcinoma in Korea. J Natl Cancer Inst. 2004;96:1851–6. doi: 10.1093/jnci/djh334. [DOI] [PubMed] [Google Scholar]
- 18.Hassan MM, Spitz MR, Thomas MB, et al. Effect of different types of smoking and synergism with hepatitis C virus on risk of hepatocellular carcinoma in American men and women: case-control study. Int J Cancer. 2008;123:1883–91. doi: 10.1002/ijc.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu MW, You SL, Chang AS, et al. Association between hepatitis C virus antibodies and hepatocellular carcinoma in Taiwan. Cancer Res. 1991;51:5621–5. [PubMed] [Google Scholar]
- 20.Fujita Y, Shibata A, Ogimoto I, et al. The effect of interaction between hepatitis C virus and cigarette smoking on the risk of hepatocellular carcinoma. Br J Cancer. 2006;94:737–9. doi: 10.1038/sj.bjc.6602981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun CA, Wu DM, Lin CC, et al. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. Am J Epidemiol. 2003;157:674–82. doi: 10.1093/aje/kwg041. [DOI] [PubMed] [Google Scholar]
- 22.Wraith D, Mengersen K. A Bayesian approach to assess interaction between known risk factors: the risk of lung cancer from exposure to asbestos and smoking. Stat Methods Med Res. 2008;17:171–89. doi: 10.1177/0962280206075525. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U. S Cancer. 2004;101:1009–17. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 26.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–21. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 27.Wang CS, Yao WJ, Chang TT, Wang ST, Chou P. The impact of type 2 diabetes on the development of hepatocellular carcinoma in different viral hepatitis statuses. Cancer Epidemiol Biomarkers Prev. 2009;18:2054–60. doi: 10.1158/1055-9965.EPI-08-1131. [DOI] [PubMed] [Google Scholar]
- 28.Staretz ME, Murphy SE, Patten CJ, et al. Comparative metabolism of the tobacco-related carcinogens benzo[a]pyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and N′-nitrosonornicotine in human hepatic microsomes. Drug Metab Dispos. 1997;25:154–62. [PubMed] [Google Scholar]
- 29.Yu MW, Hsu FC, Sheen IS, et al. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol. 1997;145:1039–47. doi: 10.1093/oxfordjournals.aje.a009060. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZM, Liu BQ, Boreham J, et al. Smoking and liver cancer in China: case-control comparison of 36,000 liver cancer deaths vs. 17,000 cirrhosis deaths. Int J Cancer. 2003;107:106–12. doi: 10.1002/ijc.11342. [DOI] [PubMed] [Google Scholar]
- 31.Wang CS, Chang TT, Yao WJ, Chou P. Comparison of hepatitis B virus and hepatitis C virus prevalence and risk factors in a community-based study. Am J Trop Med Hyg. 2002;66:389–93. doi: 10.4269/ajtmh.2002.66.389. [DOI] [PubMed] [Google Scholar]
- 32.Wang CS, Wang ST, Chang TT, Yao WJ, Chou P. Smoking and alanine aminotransferase levels in hepatitis C virus infection: implications for prevention of hepatitis C virus progression. Arch Intern Med. 2002;162:811–5. doi: 10.1001/archinte.162.7.811. [DOI] [PubMed] [Google Scholar]
- 33.Jaimes EA, Tian RX, Raij L. Nicotine: the link between cigarette smoking and the progression of renal injury? Am J Physiol Heart Circ Physiol. 2007;292:H76–H82. doi: 10.1152/ajpheart.00693.2006. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal R. Smoking, oxidative stress and inflammation: impact on resting energy expenditure in diabetic nephropathy. BMC Nephrol. 2005;6:13. doi: 10.1186/1471-2369-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sopori ML, Kozak W, Savage SM, et al. Effect of nicotine on the immune system: possible regulation of immune responses by central and peripheral mechanisms. Psychoneuroendocrinology. 1998;23:189–204. doi: 10.1016/s0306-4530(97)00076-0. [DOI] [PubMed] [Google Scholar]
- 36.Houston TK, Person SD, Pletcher MJ, et al. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ. 2006;332:1064–9. doi: 10.1136/bmj.38779.584028.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anan F, Takahashi N, Shinohara T, et al. Smoking is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patients. Eur J Clin Invest. 2006;36:459–65. doi: 10.1111/j.1365-2362.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- 38.Mason AL, Lau JY, Hoang N, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–33. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 39.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–31. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 40.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 41.Bonino F, Chiaberge E, Maran E, Piantino P. Serological markers of HBV infectivity. Ann Ist Super Sanita. 1987;24:217–23. [PubMed] [Google Scholar]
- 42.Chen CJ, Liang KY, Chang AS, et al. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991;13:398–406. [PubMed] [Google Scholar]
- 43.Chou R, Clark EC, Helfand M. Screening for hepatitis C virus infection: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:465–79. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 44.Goritsas CP, Athanasiadou A, Arvaniti A, Lampropoulou-Karatza C. The leading role of hepatitis B and C viruses as risk factors for the development of hepatocellular carcinoma. A case control study. J Clin Gastroenterol. 1995;20:220–4. doi: 10.1097/00004836-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Tzonou A, Trichopoulos D, Kaklamani E, et al. Epidemiologic assessment of interactions of hepatitis-C virus with seromarkers of hepatitis-B and -D viruses, cirrhosis and tobacco smoking in hepatocellular carcinoma. Int J Cancer. 1991;49:377–80. doi: 10.1002/ijc.2910490311. [DOI] [PubMed] [Google Scholar]
- 46.Franceschi S, Montella M, Polesel J, et al. Hepatitis viruses, alcohol, and tobacco in the etiology of hepatocellular carcinoma in Italy. Cancer Epidemiol Biomarkers Prev. 2006;15:683–9. doi: 10.1158/1055-9965.EPI-05-0702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.