Abstract
This study investigates the effect of dietary fat on the testosterone (T) pharmacokinetics in hypogonadal men following administration of a self-emulsifying capsule formulation of oral T undecanoate (TU). In an open-label, 2-center, 5-way crossover study, a single oral dose of TU containing 300-mg equivalents of T (maximum anticipated human dose per administration) was administered to 16 hypogonadal men with a washout period of at least 5 days between doses. All participants were randomized to receive the TU capsules fasting or 30 minutes after an approximately 800-calorie meal containing 10%, 20%, 30%, or 50% fat. Serial blood samples were collected from 2 hours predose to 25 hours postdose to determine serum T and dihydrotestosterone (DHT) by liquid chromatography tandem mass spectrometry. Administering TU with a meal increased serum T concentrations, with the magnitude of the increase being directly dependent on the amount of fat in the meal. Average and peak serum T concentrations and area under the curve increased as the fat content of the meal was increased. Neither the high-fat meal (50% fat) nor the lower-fat meal (20% fat) showed a significant food effect relative to the normal-fat (Western diet) meal (30% fat). However, administering TU while fasting resulted in 50% or less of the cumulative exposure obtained when administered with 20%- to 50%-fat meals (albeit still substantial). A very-low-fat meal (10% fat) showed a significant food effect relative to the normal meal, but still exceeded the fasting condition by approximately 50%. Serum DHT concentrations showed corresponding increases to the serum T. As expected with the maximum anticipated clinical dose of TU (300 mg T), oral administration of this new formulation with food containing 20% to 50% dietary fat produced T levels at or above the upper range of adult men, and T levels trended higher as dietary fat content increased. Only with a very-low-fat diet (10%) or in a fasted state did a clinically significant food effect occur, but even then sufficient TU was absorbed with the self-emulsifying TU formulation to produce average serum T concentration predicted to be in the normal reference range (10 to 35 nmol/L).
Keywords: Male hypogonadism, food effect
Oral testosterone (T) is an attractive option for hypogonadal men because of convenience, ease of administration, and patient preference compared with T patches, gels, injections, and implants. A major disadvantage limiting the use of oral T replacement is extensive first-pass hepatic metabolism that limits the bioavailability of T and its esters. Recent effort has focused on developing T prodrug formulations to enhance delivery via intestinal lymphatics, thereby bypassing hepatic metabolism and optimizing bioavailability. A castor oil–based oral T undecanoate (TU) preparation with an excellent safety profile has been available in parts of the world since the 1970s (Skakkebaek et al, 1981; Gooren, 1994; Emmelot-Vonk et al, 2008; Legros et al, 2009), although there is at present no commercially available oral TU formulation marketed in the United States.
The bioavailability of TU is affected by food and dietary fat content. With the castor oil formulation, minimal TU is absorbed in the fasting state, and coadministration with a standardized meal dramatically increases TU bioavailability (Bagchus et al, 2003). Administration of 80 mg TU with a “normal” meal (containing 19 g of lipid per meal) in postmenopausal women raised serum T levels 10-fold over those during fasting. No additional increases in T or dihydrotestosterone (DHT) exposure were seen with coadministration with meals having lipid content greater than 19 g (Schnabel et al, 2007).
Previously we reported the pharmacokinetics (PK) of serum T, DHT, TU, and DHT undecanoate (DHTU) after single-, 7-, and 28-day oral dosing of a new TU formulation incorporating a self-emulsifying drug delivery system (SEDDS; Yin et al, 2011). We demonstrated that serum T levels within the physiologic range are achieved in most hypogonadal men (>75%) after dosing with TU containing 200-mg equivalents of T twice per day. This dosing regimen resulted in 2.1- to 2.4-fold higher average (Cavg) and maximum (Cmax) serum T levels in the fed state (ie, after a meal containing 30% fat) compared to when administered while fasting. Average serum TU and DHTU concentrations were 6.2- and 8.8-fold higher, respectively, in the fed state compared to fasting. However, the amount of dietary fat required to achieve adequate absorption was not determined. In this study we characterize the effect of varying fat content (from 0% to 50%) in a fixed-calorie meal on the PK of the new SEDDS TU formulation in hypogonadal men.
Materials and Methods
Experimental Medication
A prodrug formulation SEDDS including the active ingredient TU was developed by Clarus Therapeutics Inc (Northbrook, Illinois). Each TU capsule contains 158.3 mg TU, equivalent to 100 mg T. The TU dose henceforth refers to the T dose as mg equivalents. TU is highly lipophilic and the proprietary formulation incorporates a complex lipid matrix and an emulsifying agent. In each study period, participants received single doses of 3 capsules of the study medication 300 mg T total—the maximum anticipated morning dose (as TU) for T-replacement therapy and 50% above that tested in prior studies (Yin et al, 2011), 30 minutes after initiation of a protocol-defined breakfast.
Participants
Sixteen hypogonadal men (ie, serum total T ≤ 10.4 nmol/L on 2 separate measures) with mean baseline serum T of 7.0 ± 3.6 nmol/L and aged 25 to 64 years participated. approval was obtained by the institutional review board at each site before men were screened for participation. All participants provided written consent and were hypogonadal (ie, total serum T < 10.4 nmol/L on 2 consecutive occasions). Participants agreed to cease existing T treatment and remain off all forms of T replacement except the study medication throughout participation. If participants were on replacement therapy for hypopituitarism or multiple endocrine deficiencies, doses of thyroid and adrenal replacement were stable for at least 14 days prior to enrollment. Individuals were excluded if they had significant liver, kidney or heart disease; uncontrolled diabetes mellitus; evidence of prostate disease/cancer; abnormal bleeding; use of noninjectable T replacement in the previous week or use of T injections in the previous 4 weeks; known malabsorption; or history of substance abuse.
Study Design
This was an open-label, 5-way crossover study conducted at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (Torrance, California) and Anapharm Inc (a PharmaNet company; Montreal, Canada). A minimum washout period of 5 days separated treatments. All participants at each site were randomly assigned to one of the following sequences: ABCDE, BDCEA, CBEDA, or DBAEC, where A was fasting, and B, C, D, and E were 800-calorie meals containing 10%, 20%, 30%, or 50% of calories as fat, respectively. Treatment D (the 30% fat meal) served as the reference, normal Western diet. Participants received a single dose of 300 mg T as TU with 240 mL of water 30 minutes after initiating the meal, or with just 240 mL of water after at least a 12-hour fast if randomized to the fasted treatment. Serial blood samples were taken 1 and 2 hours before dosing and 0, 1.5, 3, 4, 5,6,8,11,14,18,24, and 25 hours postdose for determination of serum T, DHT, TU, and DHTU. Estradiol levels were not measured because the study was designed to study food effects on serum T PK.
Analytical Methods
Serum total T and DHT concentrations were measured simultaneously using a sensitive, specific, and validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method without modification as previously described (Shiraishi et al, 2008). Intra-assay and interassay coefficients of variation for T and DHT are less than 5%. The lower limits of quantification (LLOQ) for T and DHT are 0.096 nmol/L (2 ng/dL). The reference range for normal serum T is 10.4 to 34.7 nmol/L (300 to 1000 ng/dL, based on regulatory guidelines from the Food and Drug Administration and similar to the laboratory reference ranges in adult men); for DHT the normal reference range is 0.47 to 2.7 nmol/L (13.7 to 77 ng/dL, determined in our laboratory from 113 healthy adult men). Serum TU and DHTU were measured by LC-MS/MS without modification as previously described (Yin et al, 2011).
Pharmacokinetic Parameters and Statistical Analysis
PK parameters were calculated for each participant by treatment using the assayed serum T, TU, DHT, and DHTU concentrations. The maximum serum concentration (Cmax) and time to reach Cmax (Tmax) were identified from assayed serum concentrations, without interpolation. The area under the curve (AUC) was calculated using the linear trapezoidal method. The baseline corrected AUC was calculated by subtracting the mean of the predose concentrations from each postdose concentration (the baseline corrected concentration being set equal to 0.00 nmol/L if the result was negative). The baseline corrected AUC was extrapolated to infinite time AUC∞) by dividing the last quantifiable baseline corrected concentration by the terminal elimination rate constant, and adding that value to the baseline corrected AUCt, where t is the time of the last concentration greater than the assay LLOQ. The terminal elimination rate constant (λz) was calculated as the negative of the slope of the terminal elimination phase when the natural log of the concentrations were plotted vs time. At least 3 final quantifiable concentrations were required to define an approximately straight line decline (r ≤ −0.80) in order for the terminal elimination rate constant to be considered evaluable. The terminal half-life (T1/2) was calculated as ln(2)/λz.
The study was conducted using single doses of TU, and following the PK for 25 hours. The anticipated average concentration over a dosing interval (Cavg), if the dosing were continued according to a twice-a-day dosing schedule, was calculated as the baseline corrected AUC∞/12, plus the baseline T concentration. Analysis of variance (ANOVA) was performed on the natural logarithms of Cavg, AUC, and Cmax. The serum T concentrations in Figure 1 and Figure 2 are antilogarithms of the geometric mean ± SEM. Factors included in the ANOVA model were Group, Sequence, Sequence * Group, Subject(Sequence * Group), Period(Group), Treatment, and Treatment * Group. If the Treatment * Group effect was not found to be statistically significant at the 5% level it was removed from the statistical model. All PK parameters are reported as the arithmetic mean and the coefficient of variation. Comparisons between the PK parameter values for the different treatments were made using least squares geometric means. For evaluation of a food effect, the 30% fat diet (D), representative of the typical Western meal, served as the reference treatment for each comparison (A/D, B/D, C/D, and E/D). Notably, 2 a priori criteria must have been met in order to conclude the absence or existence of a significant food effect. First, the 90% confidence interval (CI) of the ratio of least-squares geometric means needed to be totally contained in the interval 0.80 to 1.25 to conclude in favor of no food effect. Second, the point estimate of the ratio had to be outside the range of 0.70 to 1.43, and the 90% CI could not include unity (ie, 1.00). By definition, in all cases where the value of 1.00 (100%) is not within the 90% CI, the difference between the 2 treatments would be statistically significant at P < .05.
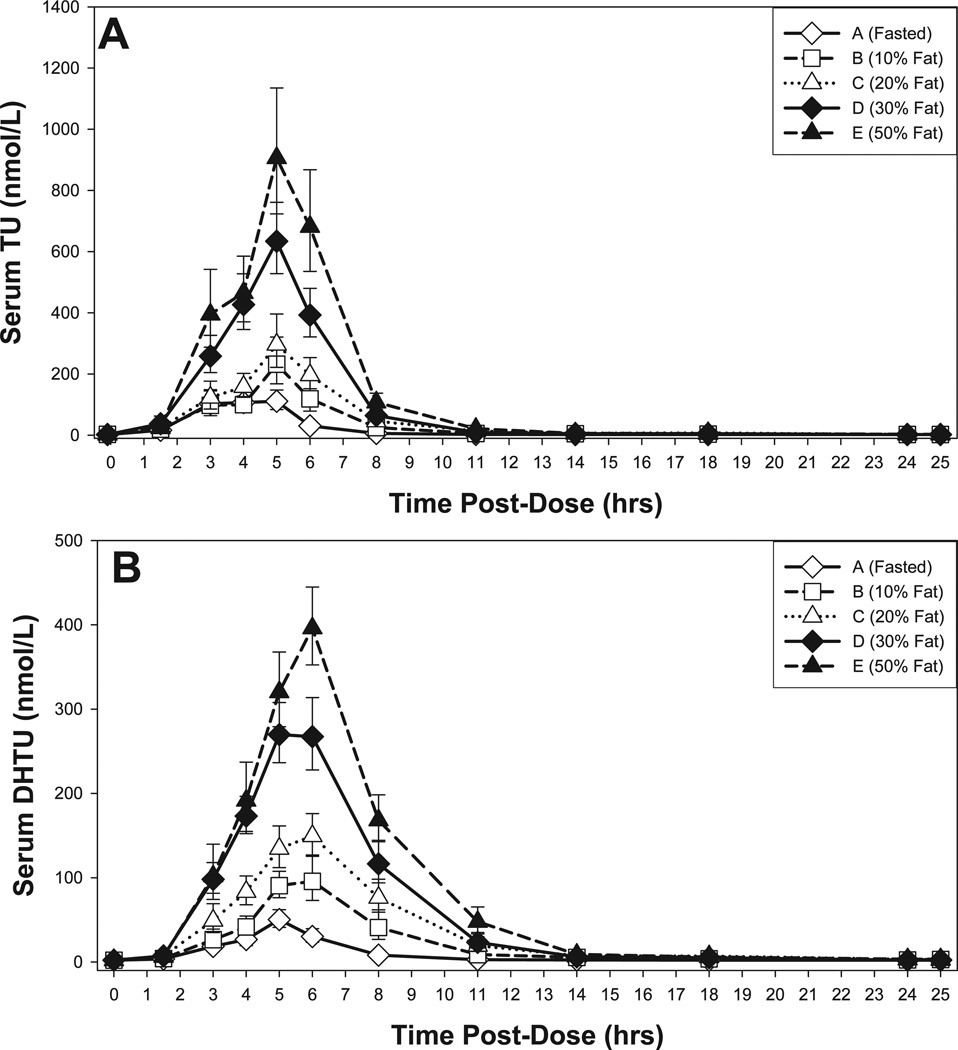
Figure 1.
Mean (A) serum testosterone (T) and (B) serum dihydrotestosterone (DHT) concentrations for 25 hours after administration of 300 mg T equivalents as TU while fasting or with a meal with a selected fat content. The data represent the antilogarithmic transformed geometric mean ± SEM of serum TU and DHT levels.
Figure 2.
Mean (A) serum testosterone undecanoate (TU) and (B) serum dihydrotestosterone undecanoate (DHTU) concentrations for 25 hours after administration of 300 mg testosterone equivalents as TU while fasting or with a meal with a selected fat content. The data represent antilogarithmic conversion of the geometric mean ± SEM of serum TU and DHTU levels.
Results
Participants
The average age of the 16 hypogonadal men enrolled into and completing the study was 44 (range 25 to 64) years, average height 174 (range 161 to 185) cm, and average weight 91.1 (55.2 to 112.1) kg. The hypogonadal men were obese, with a mean ± SD body mass index of 30.1 ± 4.8 (range 16 to 36) kg/m2. The mean pretreatment baseline serum T and DHT concentrations were 7.0 ± 3.6 nmol/L and 0.51 ± 0.34 nmol/L (mean ± SD), respectively.
Serum Concentrations and PK Parameters
Figure 1 illustrates the response in serum T and DHT concentration-time profiles to differences in dietary fat in the meal administered with oral TU capsules. Mean serum T concentrations increased above the lower threshold of the adult reference range (10.4 nmol/L) by 1.5 hours in all the meal settings following this single dose of TU, and remained above that threshold for 14 to 18 hours when the dose was taken with a meal, or for approximately 8 hours when taken while fasting. The greater the fat content of the meal, the higher the observed mean concentrations, with the general shape of the profile being similar for all of the meal conditions (Figure 1A). Median Tmax was 4 to 6 hours in all the treatment groups.
Dietary fat also modulated the AUC, Cavg (generally regarded as the most clinically meaningful PK parameter), and Cmax for serum T and DHT (Table 1). The predicted Cavg values for T appearing in Table 1 values assume twice-a-day dosing with 300 mg of T equivalents as TU. Baseline values are included in the predicted Cavg, although with chronic dosing some suppression of that portion of the T concentration can be anticipated.
Table 1.
Mean (CV%) pharmacokinetic parameters for serum T by meals with different percentage fat
| Cbaseline, nmol/L |
Cmax, nmol/L |
Tmax, h |
AUCt, nmol·h/L |
AUC∞, nmol·h/L |
Predicted Cavg, nmol/La |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | |
| A (fasting) | 6.10 | 51.8 | 32.9 | 84.2 | 4.13 | 23.2 | 123 | 99.3 | 147 | 94.6 | 18.2 | 61.6 |
| B (10% fat) | 7.00 | 54.3 | 47.5 | 53.4 | 4.88 | 36.1 | 207 | 77.1 | 244 | 71.5 | 27.1 | 49.3 |
| C (20% fat) | 7.00 | 48.0 | 52.7 | 46.7 | 6.27 | 61.5 | 264 | 70.5 | 291 | 76.2 | 30.6 | 57.2 |
| D (30% fat) | 6.31 | 52.5 | 61.0 | 34.0 | 5.08 | 28.5 | 315 | 48.7 | 346 | 46.6 | 35.0 | 35.4 |
| E (50% fat) | 6.28 | 53.2 | 74.2 | 42.2 | 6.43 | 76.6 | 414 | 51.4 | 450 | 47.1 | 43.7 | 37.9 |
Abbreviations: AUCt, area under the curve; AUC∞ baseline corrected area under the curve extrapolated to infinite time; Cavg, average serum T level; Cbaseline, baseline serum T level; Cmax, maximum serum T level; CV, coefficient of variation; Tmax, time to reach Cmax; T, testosterone.
Predicted Cavg at steady state = (baseline corrected AUC∞ following a single dose)/12 h + baseline T concentration. This assumes dosing to steady state with 300 mg twice a day of T equivalents as T undecanoate.
PK trends for serum DHT were generally similar to those for T (Figure 1B; Table 2). Cmax for serum DHT was above the reference range (0.47 to 2.7 nmol/L) for all but the fasted groups. The mean Tmax for DHT occurred approximately 1.0 to 1.5 hours after that of T, except when the TU dose was administered while fasting. The mean DHT/T ratio with higher levels of dietary fat ranged from 0.123 (fasting) to 0.160 (30% fat).
Table 2.
Mean (CV) pharmacokinetic parameters for serum DHT by treatment group
| Cmax, nmol/L |
Tmax, h |
AUCt, nmol·h/L |
Cavg, nmol/La |
DHT/T, ratiob |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | |
| A (fasting) | 3.51 | 76.7 | 4.09 | 40.7 | 34.0 | 68.5 | 2.35 | 82.2 | 0.123 | 36.4 |
| B (10% fat) | 5.18 | 66.8 | 5.84 | 45.0 | 57.1 | 68.0 | 4.20 | 75.9 | 0.146 | 32.1 |
| C (20% fat) | 5.98 | 53.7 | 7.13 | 52.2 | 69.6 | 62.9 | 5.20 | 69.0 | 0.156 | 27.8 |
| D (30% fat) | 6.94 | 50.7 | 5.81 | 26.8 | 79.3 | 61.0 | 6.10 | 65.3 | 0.160 | 30.9 |
| E (50% fat) | 7.93 | 51.2 | 8.19 | 57.3 | 94.3 | 67.9 | 7.37 | 71.3 | 0.157 | 34.8 |
Abbreviations: AUCt, area under the curve; Cavg, average serum T level; Cmax, maximum serum T level; CV, coefficient of variation; DHT, dihydrotestosterone; Tmax, time to reach Cmax; T, testosterone.
Predicted Cavg at steady state = (baseline corrected AUC∞ following a single dose)/12 hours + baseline T concentration. This assumes dosing to steady state with 300 mg twice a day of T equivalents as T undecanoate.
Ratio of AUCt values, without baseline correction, for DHT and T.
Serum concentrations of TU and DHTU are depicted in Figure 2, with associated PK parameters summarized in Table 3. As one would predict for the parent prodrug and its 5α-reduced metabolite, both serum TU and DHTU exhibited peak serum concentrations across all diets that preceded the corresponding peak concentrations for T and DHT. Furthermore, the time to peak concentration for TU preceded that of DHTU. In general, serum TU concentrations were about 2.5 times those observed for DHTU and increased in a proportional manner to the amount of dietary fat.
Table 3.
Mean (CV) pharmacokinetic parameters for serum TU and DHTU by treatment group
| TU Cmax, nmol/L |
TU Tmax, h |
TU AUC∞, nmol·h/L |
DHTU Cmax, nmol/L |
DHTU Tmax, h |
DHTU AUC∞, nmol·h/L |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | |
| A (fasting) | 402 | 126 | 4.03 | 30.9 | 1170 | 129 | 121 | 181 | 4.50 | 19.9 | 629 | 154 |
| B (10% fat) | 906 | 77.5 | 4.75 | 35.6 | 2050 | 72.2 | 251 | 91.9 | 5.19 | 30.0 | 2490 | 121 |
| C (20% fat) | 939 | 68.1 | 5.47 | 67.1 | 3050 | 58.6 | 296 | 47.8 | 6.09 | 60.2 | 3540 | 99.9 |
| D (30% fat) | 1250 | 57.5 | 4.53 | 33.6 | 3410 | 33.8 | 410 | 43.3 | 5.19 | 25.6 | 2940 | 58.7 |
| E (50% fat) | 1660 | 55.0 | 5.34 | 68.2 | 5520 | 37.4 | 473 | 36.5 | 6.38 | 50.5 | 5090 | 56.0 |
Abbreviations: AUC∞, baseline corrected area under the curve extrapolated to infinite time; Cmax, maximum serum testosterone level; CV, coefficient of variation; DHTU, dihydrotestosterone undecanoate; Tmax time to reach Cmax; TU, testosterone undecanoate.
Comparison of Dietary Effects on Serum T PK Parameters Using 30% Fat Meal as the Reference
The observed Cmax, Cavg (average serum T over the dosing interval and most clinically relevant parameter), and AUCt for serum T obtained following oral TU administered while fasting were significantly lower than the values observed following administration of the TU with a breakfast containing 30% fat. The point estimates of the ratio of the least-squares geometric means for Cmax and AUCt are presented in Table 4. Because the point estimates for the ratios of both parameters were outside the range (70%, 143%) and the 90% CI did not include the value 1.0 (ie, 100%), a significant food effect was found to exist for both parameters for TU taken while fasting or with a diet containing 10% fat compared to TU taken with a meal containing 30% fat.
Table 4.
Between-diet comparisons for serum T exposure metrics
| 90% CIb |
Criteria Met for Clinically Significant Food Effect?c |
||||
|---|---|---|---|---|---|
| Parameter | Comparison | Point Estimate, %a | Lower, % | Upper, % | |
| Cmax | Fasting (A) vs 30% fat meal (D) | 60.79 | 44.38 | 83.27 | Yes |
| 10% fat (B) vs 30% fat meal (D) | 68.15 | 50.60 | 91.79 | Yes | |
| 20% fat (C) vs 30% fat meal (D) | 114.17 | 83.35 | 156.38 | No | |
| 50% fat (E) vs 30% fat meal (D) | 139.75 | 105.78 | 184.63 | No | |
| AUC, | Fasting (A) vs 30% fat meal (D) | 62.46 | 53.24 | 73.29 | Yes |
| 10% fat (B) vs 30% fat meal (D) | 73.55 | 63.23 | 85.55 | Yes | |
| 20% fat (C) vs 30% fat meal (D) | 101.58 | 86.58 | 119.18 | No | |
| 50% fat (E) vs 30% fat meal (D) | 130.91 | 113.65 | 150.80 | No | |
| Cavg | Fasting (A) vs 30% fat meal (D) | 51.12 | 40.88 | 63.93 | Yes |
| 10% fat (B) vs 30% fat meal (D) | 66.59 | 53.49 | 82.90 | Yes | |
| 20% fat (C) vs 30% fat meal (D) | 97.43 | 78.60 | 120.77 | No | |
| 50% fat (E) vs 30% fat meal (D) | 138.89 | 115.28 | 167.32 | No | |
Abbreviations: AUCt, area under the curve; Cavg, average serum T level; CI, confidence interval; Cmax, maximum serum T level; T, testosterone.
Ratio of the least squares geometric means.
Antilogs of the 90% confidence interval of the log-transformed values. If the value 100 is not within the 90% CI, then the difference between the 2 treatments is statistically significant at P < .05.
For no food effect, the 90% CI of the ratio of least squares geometric means should be within 80% to 125%. For clinically significant food effect, the point estimate of the ratio had to be outside the range of 70% to 143% and the 90% CI could not include unity (ie, 100%).
None of the 3 PK parameters for TU given with a 20% fat meal (treatment C) or with a 50% fat meal (treatment E) were found to show a significant food effect relative to the reference meal comprised of 30% fat (treatment D) although there was a distinct trend for all 3 parameters to increase with increasing fat content. In fact, the similarity in PK parameters between the 20% fat meal and the 30% fat meal was so close that the AUCt and Cavg parameters met the strict definition of bioequivalence (ie, 90% CI of the ratios being totally within the range 80% to 125%).
The ANOVA detected a statistically significant difference between treatments for baseline uncorrected PK parameters of Cmax, AUCt, and Cavg (P values < .05), and Dunnett’s 2-tailed t test indicated that AUCs and Cavg were statistically lower when TU was taken when fasting (treatment A) or with a 10% fat meal (treatment B) when compared to the 30% fat meal (treatment D). When compared to the 30% fat diet (treatment D), substantially reducing the amount of fat in the meal (treatments A and B) led to a 33% decrease in the T exposure metrics (AUCs, Cavg, and Cmax). Conversely, increasing the amount of fat in the meal (treatment E) led to an increase in T exposure metrics of about 33%. Tmax was similar for all treatment groups and no trend was apparent related to the fat content of the meal (Table 1).
Safety Measurement/Adverse Events
Seventeen mild or moderate adverse events (AE) were reported; none were severe or serious. Venous catheter site pain (n = 2 or 12.5% in treatment E), oropharyngeal pain (n = 2 in treatment D), contact dermatitis (n = 2 in treatment C), and somnolence (1 participant each in treatments A and C) were the most common. All other AEs (otorrhea, conjunctivitis, eye swelling, ocular hyperemia, catheter site swelling, headache, rhinorrhea, and pain in an extremity) were each reported by 1 participant. No trend was observed among treatment groups or with respect to fasting or fat content, and the majority of AEs were considered unrelated to the study medication. All AEs of moderate intensity were reported during treatment D by a single participant and were considered unrelated to the study medication.
No safety issues were noted with respect to physiological and clinical chemistry measurements. There were no clinically relevant differences with other clinical laboratory results and vital signs from baseline to postdose periods. No significant changes were observed in other clinical chemistry tests, including liver function tests.
Discussion
In our prior 4-week steady-state PK investigation with this SEDDS formulation of TU, 86% of hypogonadal men with a mean serum pretreatment baseline T of 8.09 ± 1.17 nmol/L achieved mean serum T levels within the adult male range with 200 mg twice a day of T equivalents as TU (Yin et al, 2011). Consistent with recommendations by regulatory agencies, this food effect study was conducted using a randomized sequence of single TU doses, each of the 5 treatments consisting of the maximum anticipated clinical TU dose administered in conjunction with a defined, standardized meal, or while fasting. The washout interval between doses was typically 7 days but ranged from 5 to 10 days. Previous studies have demonstrated that T PK are linear over at least the 100- to 300-mg dosage range, so findings obtained in the current study are expected to hold proportionally for lower doses also (Yin et al, 2011).
In this study, we showed that circulating serum T, DHT, TU, and DHTU concentrations achieved following a dose increased as the amount of dietary fat in the meal consumed at the time of the dose increased. Although the effect increase was progressive with increasing fat content, using predefined criteria of food effect, the PK parameters (Cavg, Cmax, and AUCt) of serum T were significantly lower when compared to TU administered with the reference meal (30% fat) only when TU was administered while fasting or with a very-low-fat meal (10% fat). The serum T concentrations achieved when the dose was taken with a 20% fat meal were bioequivalent to those of a dose taken with a 30% fat meal, a fat content typical of the “normal” meal in the Western diet. This later finding is important given public health efforts to reduce dietary fat intake. Consistent with the data of Schnabel et al (2007), although significant circulating concentrations of TU and DHTU were observed in the present study, presence of the undecanoyl moiety at the C-17 position of the molecules precludes binding to the androgen receptor (Attardi et al, 2006; Clarus Therapeutics, unpublished data).
Although the serum T AUC observed in response to a 50% fat diet was higher than that observed with a 30% diet, the difference did not meet the pre-established criteria for demonstration of a food effect. Thus, across diets containing low, normal, and high fat, clinically meaningful fluctuations in average serum T levels are not likely in men taking oral TU in a self-emulsifying formulation. Should an individual take the oral TU formulation evaluated in this study exclusively with a low-fat diet, it is possible an upward dose adjustment may be needed. Conversely, a man taking oral TU persistently with a high-fat diet may need a reduction in dose. Nonetheless, it is probable that the meals consumed by hypogonadal men, even if variable day to day in fat content, will contain sufficient fat to work in concert with the components of the self-emulsifying TU formulation to yield consistent average serum T concentrations in the eugonadal range if the meal compositions reflect those of the adult population at large. This is particularly true during chronic therapy.
As with other, lower-dose formulations of oral TU, the lipid content of a meal has a significant effect on a lipophilic medication’s absorption and PK. Higher levels of T and its metabolite, DHT, were observed when the TU dose was taken with a higher lipid content meal in our study, a result consistent with similar investigations with a marketed oral TU formulation in castor oil but containing substantially less TU (Andriol Testocaps, MSD, Haarlem, The Netherlands, hereafter Andriol; Schnabel et al, 2007). The median Tmax values observed when the TU SEDDS formulation was administered (4 to 6 hours) were also similar to those reported for Andriol (3 to 6 hours; Schnabel et al, 2007).
Despite these similarities, there were some notable differences when compared to Andriol. First, we did not observe as distinct a plateau effect at high fat levels as reported for Andriol (Schnabel et al, 2007). Reasons for this are unclear, but it is possible that dietary fat acted synergistically with fat in the self-emulsifying formulation to foster TU absorption. Second, unlike Andriol, the serum T observed when the SEDDS TU was taken with either a 20% or a 30% fat meal showed the 2 dietary fat settings resulted in bioequivalent serum T levels. Finally, the SEDDS TU formulation results in significant TU absorption and consequently serum T concentrations, even at low levels of dietary fat or when no food is present. This difference is not surprising in light of the fact that Andriol lacks components present in a self-emulsifying TU formulation that foster solubility of TU in the gastrointestinal tract even in the absence of dietary fat. However, the TU dose administered to men in this study was substantially higher than the standard Andriol dose (ie, 80 to 120 mg, twice or three times a day), accounting for some of the difference.
Similar to other methods of administering exogenous T, oral TU resulted in an increase in the circulating levels of DHT. Based on the PK patterns for serum TU and DHTU, it is apparent that the increase in serum DHT is driven, in part, by the hydrolysis of DHTU by nonspecific esterases. After oral administration, TU is metabolized to DHTU in the intestine, whereupon it (like TU) is absorbed into the circulation exclusively via the intestinal lymphatic system (Hirschhauser et al, 1975; Horst et al, 1976). A recent study showed that coadministration of a the 5α-reductase inhibitor finasteride with oral TU SEDDS, similar to the formulation evaluated in the present study (Roth et al, 2011), had no significant effect on serum DHT, probably because of the differing routes of absorption (ie, TU via intestinal lymphatics and finasteride via the portal circulation).
In summary, single oral dosing with the new TU SEDDS formulation in hypogonadal male participants was well tolerated, and no safety concerns were identified. Dietary fat content modulated serum T and DHT levels. However, only in the absence of food or at a dietary fat level <10% was the food effect associated with significantly lower serum T concentrations compared to a normal diet observed. Coadministration of TU with food is appropriate to enhance the likelihood of achieving sufficient TU absorption and physiologic levels of serum T. The results indicate that an appropriate twice-a-day dose of the new oral TU formulation can maintain serum T concentrations within the normal reference range when administered over a spectrum of diets containing relatively low (20%) to high (50%) amounts of fat. However, the failure of the SEDDS formulation approach to completely overcome the effect of food is noteworthy and indicates that for very lipophilic T-esters, oral dosing with food probably will be required to achieve desired serum T concentrations. Finally, consistent with all other forms of T replacement, oral TU does not yield a serum T profile that mirrors the circadian pattern in eugonadal men. The physiological role of circadian rhythm of serum T is not known and this should not be of concern, particularly given the fact that the circadian pattern becomes significantly blunted in middle-aged and older men (Bremner et al, 1983; Diver et al, 2003). The clinical efficacy goal in T-replacement therapy is attainment of an average serum T concentration (ie, Cavg) in the adult male reference range. The oral TU formulation tested in this study and in our prior study (Yin et al, 2011) indicates this is feasible.
Acknowledgments
We are grateful to the nurses and laboratory technicians of the UCLA-CTSI and Endocrine and Metabolic Research Laboratory at Harbor-UCLA Medical Center and the Los Angeles Biomedical Research Center (UL1RR033176/ UL1TR000124) for their skilled assistance.
Supported by grants from Clams Therapeutics Inc, to R.S. and C.W. and by T32DK007571 Endocrinology Training Grant and UL1RR033176/ UL1TR000124 to the UCLA-CTSI at Los Angeles Biomedical Research Institute and Harbor-UCLA Medical Center. R.D. and S.F. are employees of Clarus Therapeutics Inc; J.L. is a consultant to Clarus Therapeutics Inc. Clinical trial No. CLAR-09008.
References
- Attardi BJ, Hild SA, Reel JR. Dimethandrolone undecanoate: a new potent orally active androgen with progestational activity. Endocrinology. 2006;147:3016–3026. doi: 10.1210/en.2005-1524. [DOI] [PubMed] [Google Scholar]
- Bagchus WM, Hust R, Maris F, Schnabel PG, Houwing NS. Important effect of food on the bioavailability of oral testosterone undecanoate. Pharmacotherapy. 2003;23:319–325. doi: 10.1592/phco.23.3.319.32104. [DOI] [PubMed] [Google Scholar]
- Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol (Oxf) 2003;58:710–717. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. J Am Med Assoc. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- Gooren LJ. A ten-year safety study of the oral androgen testosterone undecanoate. 1 Androl. 1994;15:212–215. [PubMed] [Google Scholar]
- Hirschhauser C, Hopkinson CR, Sturm G, Coert A. Testosterone undecanoate: a new orally active androgen. Acta Endocrinol (Cph) 1975;80:179–187. doi: 10.1530/acta.0.0800179. [DOI] [PubMed] [Google Scholar]
- Horst HJ, Holtje WJ, Dennis M, Coert A, Geelen J, Voigt KD. Lymphatic absorption and metabolism of orally administered testosterone undecanoate in man. Klin Wochenschr. 1976;54:875–879. doi: 10.1007/BF01483589. [DOI] [PubMed] [Google Scholar]
- Legros JJ, Meuleman EJ, Elbers JM, Geurts TB, Kaspers MJ, Bouloux PM. Oral testosterone replacement in symptomatic late-onset hypogonadism: effects on rating scales and general safety in a randomized, placebo-controlled study. Eur J Endocrinol. 2009;160:821–831. doi: 10.1530/EJE-08-0634. [DOI] [PubMed] [Google Scholar]
- Roth MY, Dudley RE, Hull L, Leung A, Christenson P, Wang C, Swerdloff R, Amory JK. Steady-state pharmacokinetics of oral testosterone undecanoate with concomitant inhibition of 5α-reductase by finasteride. Int J Androl. 2011;34:541–547. doi: 10.1111/j.1365-2605.2010.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel PG, Bagchus W, Lass H, Thomsen T, Geurts TB. The effect of food composition on serum testosterone levels after oral administration of Andriol Testocaps. Clin Endocrinol (Oxf) 2007;66:579–585. doi: 10.1111/j.1365-2265.2007.02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS, Wang C. Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry. Clin Chem. 2008;54:1855–1863. doi: 10.1373/clinchem.2008.103846. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Bancroft J, Davidson DW, Warner P. Androgen replacement with oral testosterone undecanoate in hypogonadal men: a double blind controlled study. Clin Endocrinol (Oxf) 1981;14:49–61. doi: 10.1111/j.1365-2265.1981.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Yin AY, Htun M, Swerdloff RS, Diaz-Arjonilla M, Dudley RE, Faulkner S, Bross R, Leung A, Baravarian S, Hull L, Longstreth JA, Kulback S, Flippo G, Wang C. Re-examination of pharmacokinetics of oral testosterone undecanoate in hypogonadal men with a new self-emulsifying formulation. J Androl. 2011;33:190–201. doi: 10.2164/jandrol.111.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]