Abstract
Cannabinoids affect immune responses in ways that may be beneficial for autoimmune diseases. We sought to determine whether chronic Cannabis use differentially modulates a select number of immune parameters in healthy controls and individuals with multiple sclerosis (MS cases).
Subjects were enrolled and consented to a single blood draw, matched for age and BMI. We measured monocyte migration isolated from each subject, as well as plasma levels of endocannabinoids and cytokines. Cases met definition of MS by international diagnostic criteria.
Monocyte cell migration measured in control subjects and individuals with MS were similarly inhibited by a set ratio of phytocannabinoids. The plasma levels of CCL2 and IL17 were reduced in non-naïve cannabis users irrespective of the cohorts. We detected a significant increase in the endocannabinoid arachidonoylethanolamine (AEA) in serum from individuals with MS compared to control subjects, and no significant difference in levels of other endocannabinoids and signaling lipids irrespective of Cannabis use. Chronic Cannabis use may affect the immune response to similar extent in individuals with MS and control subjects through the ability of phytocannabinoids to reduce both monocyte migration and cytokine levels in serum. From a panel of signaling lipids, only the levels of AEA are increased in individuals with MS, irrespective from Cannabis use or not. Our results suggest that both MS cases and controls respond similarly to chronic Cannabis use with respect to the immune parameters measured in this study.
Keywords: multiple sclerosis, endocannabinoids, inflammation, monocytes, cytokines, Cannabis, case control study
Introduction
The plant Cannabis (CB), known since antiquity for its medicinal qualities, synthesizes over 400 metabolites that collectively participate in producing its’ biological and therapeutic effects (ElSohly 2007, Russo 2007). The most abundant phytocannabinoid (pCB) is Δ9-tetrahydrocannabinol (THC), known for the psychoactive, analgesic and anti-inflammatory effects that it produces. The studies of the molecular mechanisms underlying effects produced by THC led to the discovery of the cannabinoid CB1 and CB2 G protein-coupled receptors, endocannabinoid (eCBs) ligands and their modulatory function in many cell types (Pertwee 1997, Felder and Glass 1998, Stella 2010, Tanasescu and Constantinescu 2010, Downer 2011, Pini, Mannaioni et al. 2012). CB1 receptors are expressed at a high level by neural cells while CB2 receptors are predominately expressed by hematopoietic cells. Many other protein targets and receptors are modulated by pCBs and eCBs. The extent and action by which these non-CB1/CB2 targets mediate some of the biological and therapeutic properties carried by Cannabis have been described in detail elsewhere (Howlett, Barth et al. 2002, Mackie and Stella 2006, Pertwee, Howlett et al. 2010, Zhao and Abood 2012, Abood 2013).
It is well known that regimented use of pCB may induce prolonged immunosuppressive and anti-inflammatory effects (Klein 2003, Klein 2005). Clinical studies show that heavy use of smoked Cannabis decreased lymphocyte proliferation and suppressed cell-mediated immunity (Gaoni 1964, Nahas, Suciu-Foca et al. 1974, Nahas, Morishima et al. 1977). Peripheral blood mononuclear cells from chronic smokers have altered basal levels of the ratio of CB1:CB2 cannabinoid receptors compared to controls (Nong, Newton et al. 2002). Furthermore, THC acting on T cells induces a shift toward a TH2 phenotype in animal models of infection (Newton, Klein et al. 1994, Massi, Fuzio et al. 2000, Smith, Terminelli et al. 2000). Accordingly, long-term Cannabis use decreases T cell proliferation and IL-2 levels (a TH1 cytokine), and increases IL-10 levels (a TH2 cytokine) in human blood (Pacifici, Zuccaro et al. 2003, Pacifici, Zuccaro et al. 2007). Thus, chronic use of Cannabis might bias immune effector mechanisms thereby affecting autoimmune reactions such as in MS.
The phenotype of circulating monocytes is relatively ‘plastic’, evidenced by cell-surface protein expression profiles, migratory potentials and differential cytokine secretion in response to specific environmental cues (Murray and Wynn 2011). Accordingly, phenotypic variability in circulating monocytes will influence the phenotype of the ensuing tissue macrophages. Evidence shows that pCB are likely to modulate the phenotype of circulating monocytes. A single low-dose of THC in mice induced recruitment of bone marrow monocytes (myeloid-derived suppressor cells, MDSC) into blood and alter cytokine release (also using cannabidiol (CBD, the second most abundant pCB) (Hegde, Nagarkatti et al. 2011). This direct modulation of the phenotype of MDSCs induced a four-fold increase in CD11bGR1+ cells (Hegde, Nagarkatti et al. 2011). MDSC are known to inhibit T-cell proliferation in humans after viral illness (Simmons 2001, McCoy, Tsunoda et al. 2006, De Santo, Salio et al. 2008, Hegde, Nagarkatti et al. 2010, Mameli, Poddighe et al. 2012). Together, these studies provide a mechanistic framework indicating that regimented use of pCB might affect the phenotype of circulating monocytes.
Prevalence of medicinal Cannabis use by individuals with MS is estimated at 14% in Nova Scotia, 17% in Spain and 22% in the UK (Clark, Ware et al. 2004, Ware, Adams et al. 2005, Martinez-Rodriguez, Munteis et al. 2008). Given the critical role of circulating immune cells in MS and the immuno-modulatory actions of pCB on these cells, we sought to determine if Cannabis use differentially modulates three parameters: ex-vivo migration of circulating monocytes (Sexton 2012), quantity of cytokines and eCB released in serum.
Methods and Materials
Subjects and Study Design
Subjects were recruited from 2009–2011 from the MS Center at the University of Washington (UW), the MS Center at Swedish Hospital in Seattle, and the MS Society of Greater Seattle and from medical, nursing and research staff at UW with approval from the UW Human Subjects Committee. Subjects were screened by telephone for inclusion/exclusion criteria prior to enrollment. Written informed consent was obtained prior to fasting, ante-cubital venipuncture using an approved protocol. Individuals with stable MS either were or were not currently using Disease-modifying therapy (DMT) and an we controlled for an equal number in each arm as a control for potential effects on our outcome measures. In addition, all subjects were asked not to use ibuprofen on the day prior to their blood draw, as part of the protocol. Subjects were stratified based on current Cannabis exposure.
All subjects were ≥ 21 and ≤ 50. MS Diagnostic criteria: positive diagnosis in the last 10 years of relapsing-remitting MS (made by a neurologist, according to international guidelines); an EDSS score between 3–5; not currently in exacerbation and no exacerbation less than 30 prior to the blood draw. Exclusion criteria: BMI ≤ 19 and ≥ 27; diagnosis of diabetes; diagnosis of other chronic inflammatory or autoimmune disease; current pregnancy; previous DSM IV diagnosis; tobacco use; or currently a performance athlete. These criteria were to minimize variables influencing the eCS or immune function. Subjects were stratified and matched by Cannabis exposure: non-naïve is current Cannabis use at least 2–3 times per week; and naïve, is ‘never used’ or no history of use in the last three years.
Monocyte Isolation and Migration
The buffy coat was collected from centrifuged whole blood using BD Vacutainer® CPT Cell Preparation Tubes with sodium citrate (VWR Scientific, San Francisco, CA). The buffy coat was washed with PBS (30 ml, centrifuged at 3000 rpm × 15 min). The resulting pellet was re-suspended in buffer and monocytes isolated using an ex-vivo deletion using the Midi Macs™ cocktail kit (indirect cell labeling by negative selection). The cells retained on a magnetized column are positive for CD3, CD7, CD16, CD19, CD56, CD123 and CD235a; unlabeled cells passing through the magnet are CD14+. Serum was flash frozen at −80C for cytokine and eCB analysis.
The modified Boyden chamber cell migration assay was previously described (Miller and Stella 2009, Sexton 2012). Briefly, filters (pore diameter = 5 µm) were coated with human fibronectin (10 µg/ml in PBS for 30 min). Isolated CD14+ monocytes were fluorescently labeled with DRAQ-5 (700 nM, 10 min at 37°C in RPMI 1640 supplemented with 0.1% BSA). Cells were rinsed in RPMI (0.1% BSA) and resuspended for a final density of 104 cells per upper well (390 µl). Lower wells were loaded with media (82 µl) containing vehicle (0.1% DMSO for basal migration) or the chemoattractant. Detection was by Odyssey® Imaging system (Li-COR Biosciences, Lincoln, NE). pCB ‘mix’ was a set ratio of 5.2 : consisting of 1µM THC /100 nM CBN / 300nM CBD (Broseus, Anglada et al. 2010).
Cytokine Analysis
Levels of 10 human cytokines/chemokines (CCL2, IFN-γ, IL-2, IL-4, IL-6, IL-10, IL-13, IL-17 and TNF-α) from human plasma were determined using a custom multiplex micro-bead based immunoassay kit (Millipore, Billercia, MA, USA). Undiluted human plasma samples were assayed according to the manufacturer’s protocol and analyzed using Luminex (Liquichip, Qiagen, Valencia, CA). TH1 cytokines were: IFN-γ, IL-2, IL-10; TH2 cytokines were: IL-4, IL-6 and IL-13.
Endocannabinoid analysis
Serum samples were analyzed by liquid chromatography mass spectrometry (LC-MS). Two milliliters of serum was collected within 30 min after centrifugation of whole blood (at room temperature). Proteins were precipitated using 2 volumes of ice-cold methanol and centrifuged. Samples were completely dried down under a stream of nitrogen and flash frozen until the time of analysis. Plasma samples were reconstituted in 1ml chloroform:methanol (2:1 containing 34.8 mg PMSF/ml) from which 200 ul aliquots were mixed with 50 ul of each of deuterated internal standards (Cayman Chemicals, USA) and mixed with 2.8 ml chloroform: methanol (2:1 containing 34.8 mg PMSF/ml). Samples were vortexed and 0.6 ml of 0.73 % w/v NaCl was added to each sample, vortexed again and then centrifuged for 10 min at 3220*g 4°C. The aqueous phase plus debris were collected and extracted again twice with 1.6 ml chloroform. The organic phases from the three extractions were pooled and evaporated under nitrogen gas. The dried samples were reconstituted with 0.1 ml chloroform and mixed with 1 ml cold acetone. The mixtures were then centrifuged for 5 min at 1811*g and 4 °C to precipitate the proteins. The upper layer of each sample was collected and evaporated under nitrogen. Dried samples were reconstituted with 0.1 ml methanol and placed in auto sample vials for analysis.
The LC-MS method has been previously described (Ramesh, Ross et al. 2011). Briefly, LC-MS/MS was used to quantify anandamide (AEA), 2-Arachidonyl Glycerol (2-AG) palmitoyethanolamide (PEA), oleoylethanolamide (OEA) and arachidonic acid (AA). The mobile phase consisted of (10:90) water/methanol with 0.1% ammonium acetate and 0.1% formic acid. The column used was a Discovery HS C18, 2.1×150 cm, 3 micron (Supelco, PA). A calibration curve was constructed based on linear regression using the peak area ratios of the calibrators. The extracted standard curves ranged from 0.039 to 40 pmol for AEA, from 0.0625 to 64 nmol for 2-AG, from 0.039 nm to 1.25 nm for PEA and OEA and from 1 nm to 32 nm for AA. (Deuterated compound concentrations: 2 pmole AEA −d8, 1 nmole 2-AG-d8, 3.3 nmole PEA-d4, 3 nmole OEA-d4 and 1 nmole AA-d8).
Materials
Miltenyi LD Midi Macs® separation unit and monocyte isolation kit II (Miltenyi Biotech Inc., Auburn, CA USA). Gibco® RPMI1640 (Invitrogen, Carlsbad, CA). DRAQ-5™ (Axxora, San Diego, CA). Multiplex microbead-based immunoassay kit (Millipore, Billercia, MA, USA). Luminex instrument (Liquichip, Qiagen, Valencia, CA). AEA, 2-AG, PEA, OEA and AA (Cayman Chemical, Ann Arbor, MI USA).
Study Design
This case control, cross-sectional study was designed to take a ‘snapshot’ of potential immunological effects of chronic Cannabis use. To control for immunomodulating effects of DMT’s, consistent numbers of DMT-naïve (n=4) and non-naïve (n=3) MS cases were using interferon beta-1a (Avonex® or Rebif®). Subjects were not administered any Cannabis, but were self-accessing this botanical medicine under a law passed in Washington State in 1998 allowing for its’ medical use. Healthy controls were recreational Cannabis users. Here we compare outcome measures between individuals with MS to healthy subjects assessing whether Cannabis exposure is an effect modifier of quantitative measurements in defined immune parameters.
Statistical Analysis
Data for monocyte migration and cytokine analysis were analyzed with Prism® 4.0 software (Graphpad, San Diego, CA) using 2-way ANOVA followed by Bonferroni’s post-test. Data for eCB were analyzed with and STATA IC 11.0 (StataCorp, College Station, TX) using two-tailed, paired t-tests. Results are expressed as mean +/− SEM. Point estimates for the odds ratio and 95% confidence interval (CI) were calculated using STATA IC (StataCorp, College Station, TX).
RESULTS
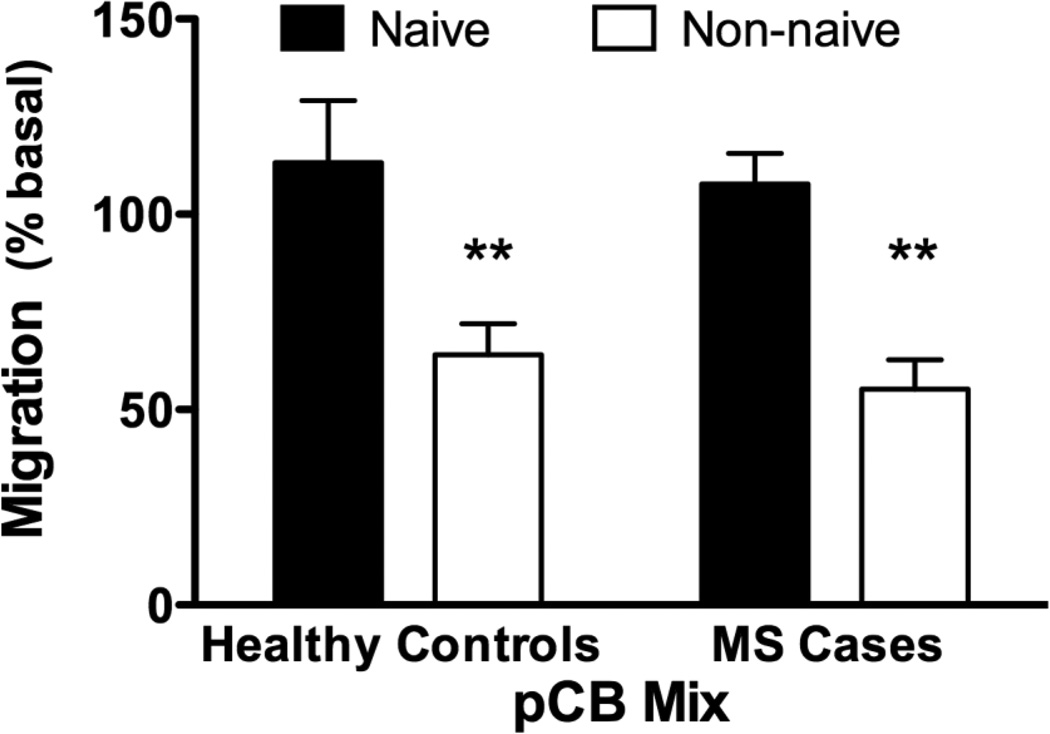
Eleven healthy subjects and ten individuals with MS were enrolled (Table 1). On the day of blood draw, subjects were matched by age, BMI and cannabinoid use (i.e. naïve versus non-naive). Isolated monocytes were tested to evaluate whether pCBs regulate the migration of these cells (Miller and Stella 2009, Sexton 2012). In vitro monocyte migration was insensitive to acute treatment with a set ratio of three pCBs when cells were isolated from either healthy subjects or from individuals with MS also naïve to cannabis (Figure 1). By contrast, acute treatment with pCBs significantly inhibited the migration of monocytes isolated from both healthy subjects non-naïve and individuals with MS non-naïve to cannabis.
Table 1. Demographic and descriptive data of subjects.
In patients with MS, two non-naïve subjects were on no disease-modifying therapy (DMT) Of the naïve, subjects, 3 were on DMTs (Rebif, Avonex.)
| Subject | Healthy Controls | MS Cases |
|---|---|---|
| Male Female |
5 6 |
3 7 |
| Total | 11 | 10 |
| Average age (+/− s.d.) | 37.6 (+/− 7.1) | 32.8 (+/− 3.4) |
| Cannabis Non-naïve | 4 | 5 |
| Cannabis Naïve | 7 | 5 |
| BMI | 23.8 (+/− 2.4) | 23.6 (+/− 2.25) |
Fig. 1.
Freshly isolated monocytes respond differentially to pCB stimulation. Cell migration of freshly isolated human monocytes was significantly reduced by addition of phytocannabinoids (pCB Mix) to the lower wells of the migration chamber. There was a 50% reduction in monocyte migration in Cannabis users, both in healthy controls and MS cases. (2-way ANOVA followed by Bonferroni’s post-test).
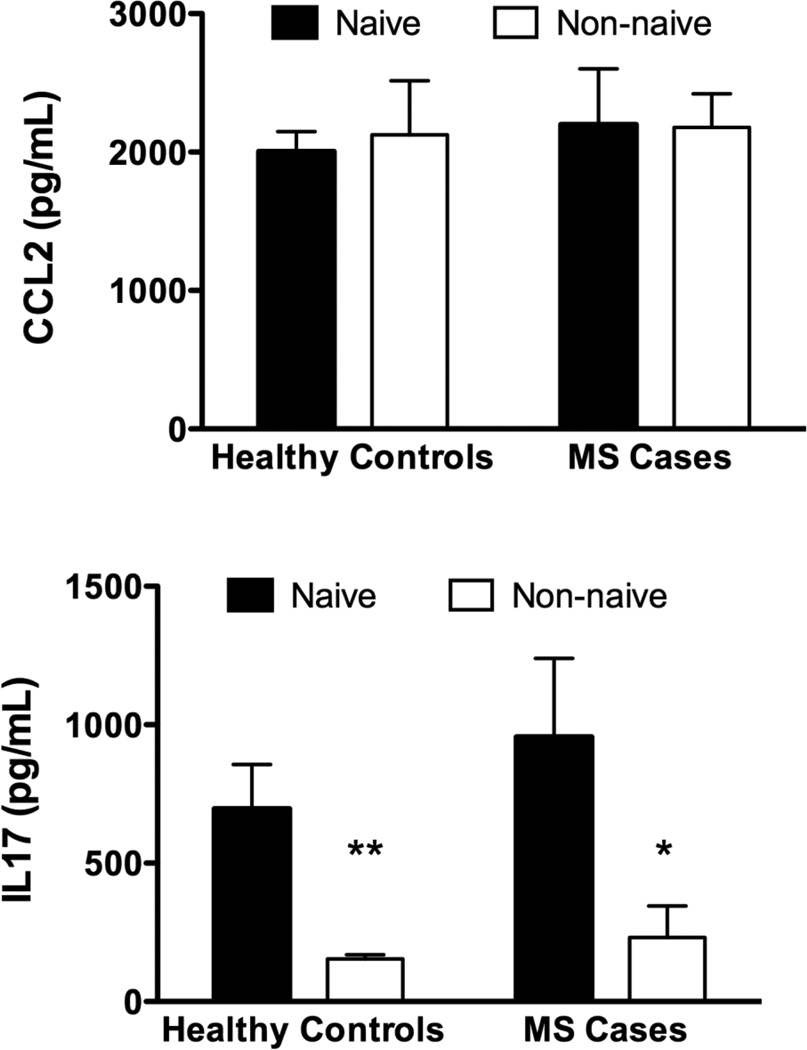
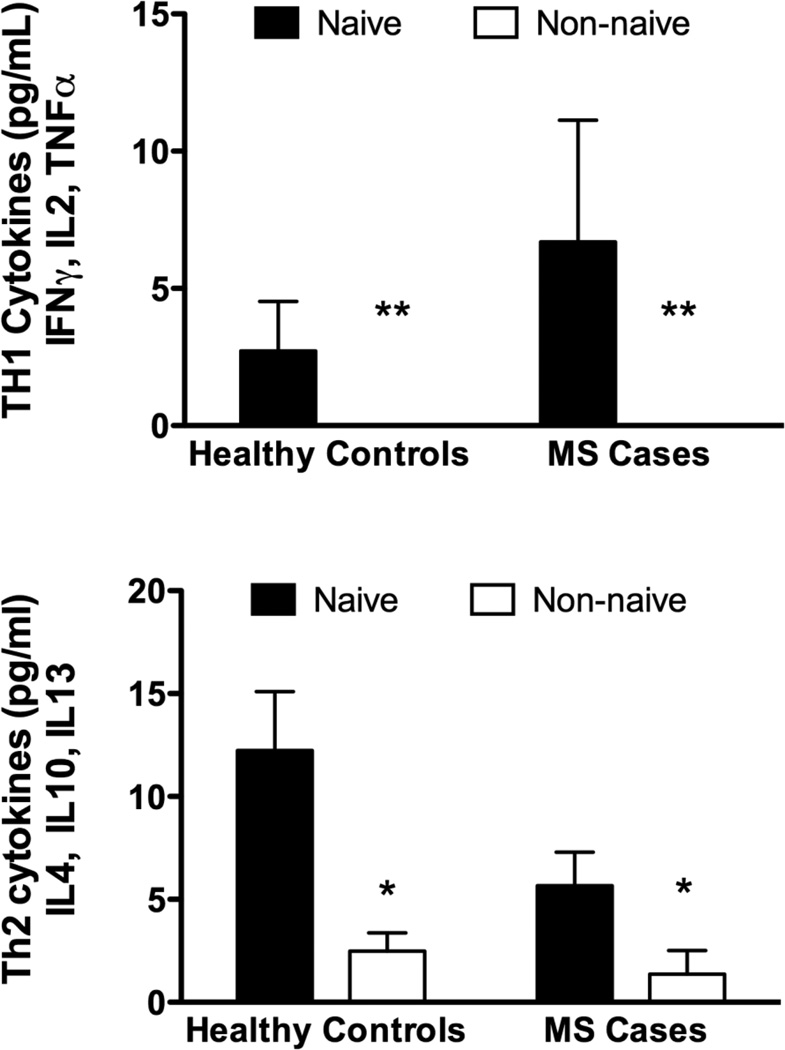
There were no significant differences in serum levels of CCL2 across cohorts (Figure 2a). Serum levels of IL17 were significantly reduced in non-naïve subjects, whether cases or controls. (Figure 2b) Cases and controls with Cannabis exposure had reduced levels of both TH1 and TH2 cytokines compared to naïve subjects (Figure 2c,d : TH1 cytokines were below the limit of detection of the assay for the non-naïve subjects).
Fig. 2.
Serum Cytokine Levels: Using a bead array assay we measured CCL2, IL17 and panels of Th1 and Th2 cytokines. a) There was no significant difference in the level of CCL2 across cohorts. b) Levels of IL17 were significantly reduced in both cohorts who were current Cannabis users (non-naïve), compared to naïve subjects c,d) Levels of both Th1 and Th2 cytokines were significantly suppressed (undetectable in some cases) in current Cannabis users compared to naïve subjects (2-way ANOVA followed by Bonferroni’s post-test).
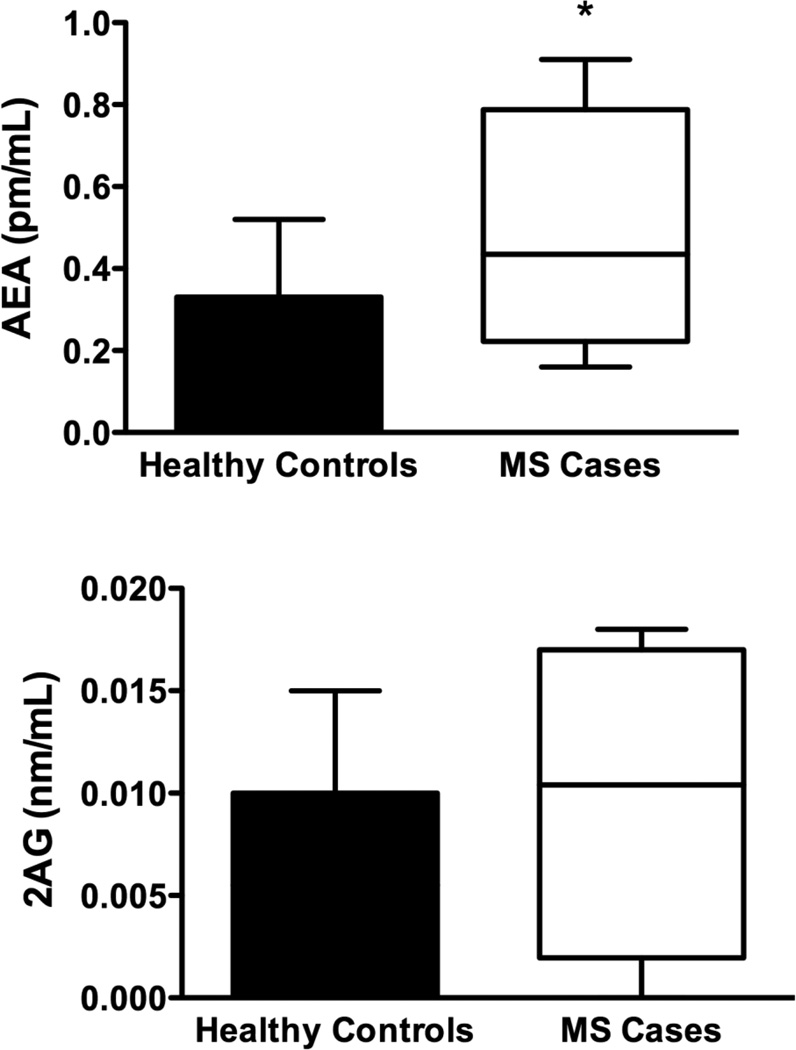
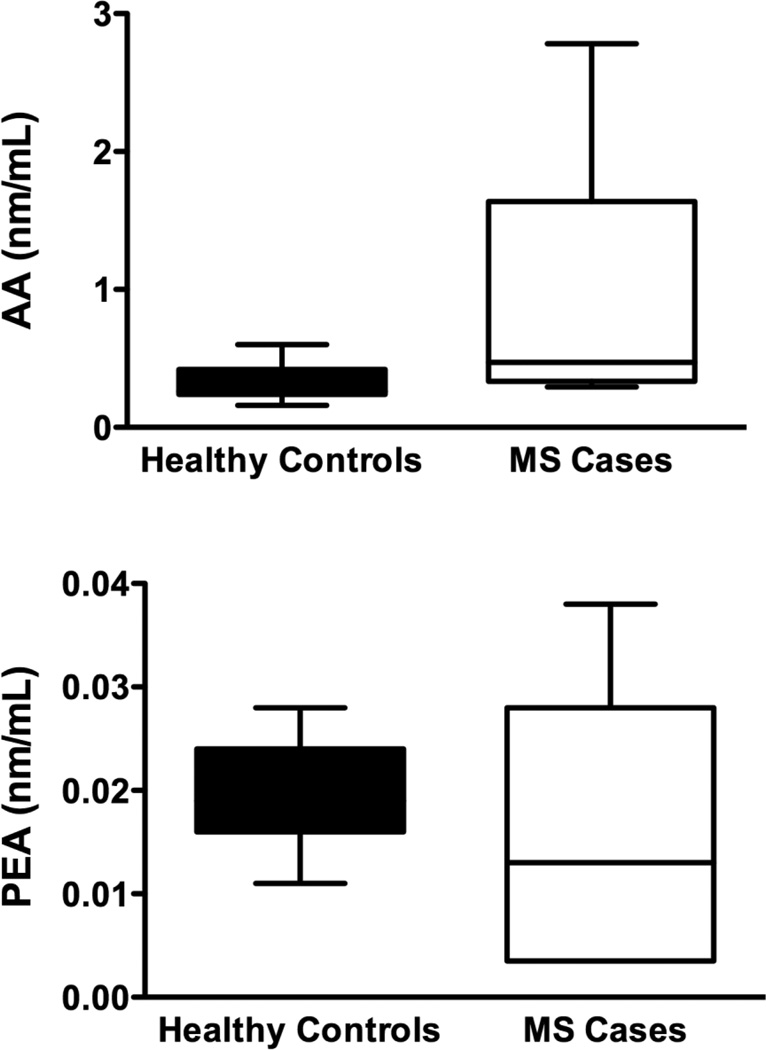
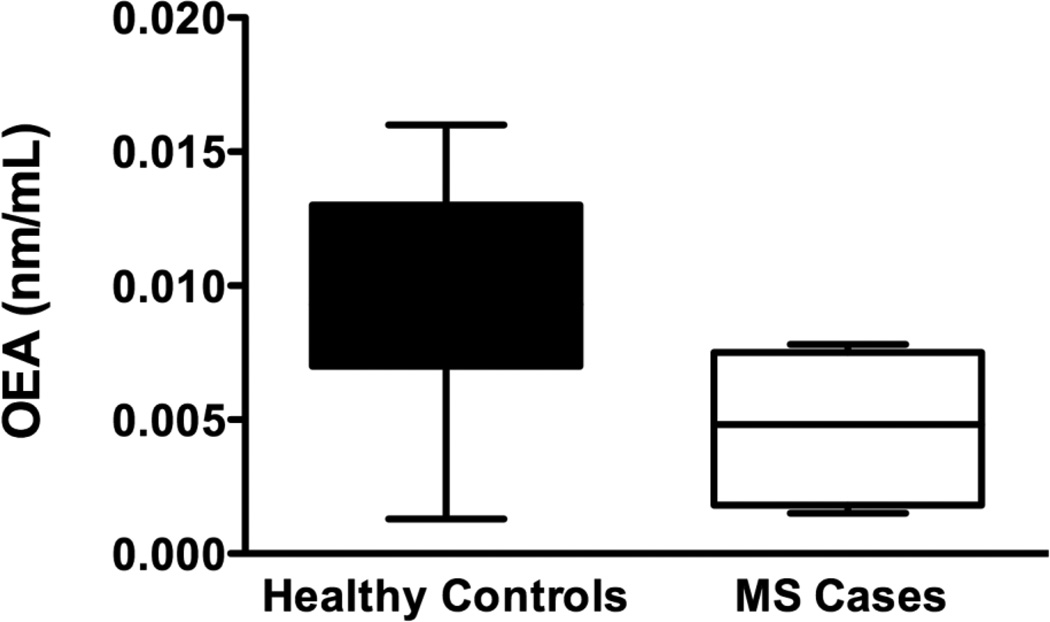
There was a statistically significant increase in levels of AEA (p=0.04) in cases compared to controls (Figure 3a). Serum levels of 2AG (p=0.14), PEA (p=0.99) OEA (p=0.08) and AA (p=0.09), were not significantly different between healthy controls and MS cases. (Figure 3b,c,d,e)
Fig. 3.
Serum levels of endocannabinoids (eCB): Using HPLC/MS/MS we measured serum levels of 5 eCBs. a) Serum AEA levels were elevated in patients with MS compared to controls. b–e) We found no significant differences in the levels of 2AG, AA, PEA or, OEA between healthy controls and MS cases. However there was a trend for elevated 2AG and AA in patients with MS compared to controls. These data are not presented as stratified for Cannabis exposure as there were no significant differences in that regard (two-tailed, paired t-tests).
Discussion
Current strategies for MS attempt to reduce or stop the migration of T cells towards the brain. Despite a wealth of evidence pointing to the role of the eCB system in the pathogenesis of MS, there is little data on the migratory potential of monocytes isolated from human subjects and individuals with MS. (Pertwee 2002) Since brain macrophages and microglia accumulate in MS lesions, changes in circulating monocytes are likely to influence overall disease progression. (Kouwenhoven, Teleshova et al. 2001, Shechter and Schwartz 2012) Thus, specifically targeting circulating monocytes might influence brain repair by modifying the phenotype of these cells as they become brain macrophages.
Since Cannabis therapy may be for symptom palliation by individuals with MS (Boven, Van Meurs et al. 2006, Mikita, Dubourdieu-Cassagno et al. 2011), we addressed the question: “Does chronic Cannabis use induce a phenotypic change in circulating monocytes?” Monocyte cell migration was significantly inhibited by a mixture of pCBs in non-naïve subjects irrespective of whether these individuals are cases or controls. We previously reported that subjects naïve to Cannabis have an increased migratory response to CCL2. (Sexton 2012) CCL2 (monocyte chemo-attractant protein 1, MCP1) provides a strong chemotactic cue for monocytes, guiding these cells to inflammatory lesion sites throughout the body, including the brain. (Conductier, Blondeau et al. 2010) Our results suggest that while cannabis use affects the cell migration properties of circulating monocytes, it does not affect the serum levels of CCL2, a major chemotaxic cue for these cells (Fig 2A).
Our results suggest that chronic CB use may lead to global reduction of cytokines (Fig 2B,C) in both healthy controls and individuals with MS. Previous clinical reports have measured either no change in levels of IFNγ, IL-10 or IL-12 with THC administration, or saw promotion of pro-inflammatory cytokines with THC or nabiximols (Sativex®, G W Pharmaceuticals) administration (Killestein, Hoogervorst et al. 2003, Katona, Kaminski et al. 2005). Differences between this study and other trials include the fact that these were prospective studies where a protocol included known and standardized doses of cannabinoids, administered regularly. Here we had an observational approach to probe patients were self-titrating at an unknown dose and rate. Additionally, differences in administration, such as oral vs. inhaled, and administration of products having various cannabinoid ratios may yield different results. A balance in TH1 and TH2 cytokines is important in controlling the progression of both EAE and MS pathogenesis. Specifically, EAE and MS are TH1-driven and there is compelling data that a TH2 shift would be beneficial for achieving a desired anti-inflammatory response by the immune system. (Kennedy and Karpus 1999, Oreja-Guevara, Ramos-Cejudo et al. 2012). However, our results extend previous studies showing that Cannabis use may globally suppress cytokine production. IL17-producing T-cells (TH17) are important players in a number of autoimmune processes and IL17 is considered to be a key player in the cytokine milieu in patients with MS, a putative biomarker for disease activity, and predictor of drug response in MS (Axtell, de Jong et al. 2010, Hecker, Paap et al. 2011, Li, Wang et al. 2011, Chen, Wang et al. 2012) (Frisullo, Nociti et al. 2008, Kallaur, Oliveira et al. 2013, Kozela, Juknat et al. 2013). Notable in this study is the global suppression of IL17 in subjects who are exposed to Cannabis, as cannabinoids were shown elsewhere to decrease the Th17-associated autoimmune phenotype (Kozela, Juknat et al. 2013). Our cytokine results suggest general immunosuppression instead of immune biasing, an environment likely to regulate the phenotype of circulating monocytes. These innate effector cells might be targets for immunotherapeutic strategies that guide the innate immune reaction. (Downer 2011) Further studies are required to extend our results.
eCB levels in human serum change in disease states such as obesity, Alzheimer’s disease, schizophrenia, depression and liver cirrhosis. (De Marchi, De Petrocellis et al. 2003, Hill, Miller et al. 2009, Koppel, Bradshaw et al. 2009, Caraceni, Viola et al. 2010, Matias, Gatta-Cherifi et al. 2012) Little is known about eCBs in MS and previously published data on serum eCBs levels in individuals with MS were inconclusive. (Jean-Gilles, Feng et al. 2009) Our results suggest that AEA levels may be increased in individuals with MS. A change in eCB signaling is not apparently sufficient to affect disease process in MS, but may participate in MS progression. (Pryce 2012) Specifically, since AEA has been suggested to be neuro-protective and have an anti-inflammatory profile in CNS parenchyma (Correa, Mestre et al. 2009) (Eljaschewitsch, Witting et al. 2006), eCB changes likely influence both immune response and resulting cell damage in brain tissue.
Drugs that boost eCB signaling system are thought to induce both palliative and neuro-protective responses by tempering the immune response, promoting oligodendrocyte survival, reducing demyelinated lesions and attenuating neuronal loss. These results were obtained by studying the EAE mouse model, a T cell-mediated autoimmune disease often used to study MS. (Jackson, Pryce et al. 2005, Pryce and Baker 2007, Rog, Nurmikko et al. 2007, Kuerten 2011, Notcutt, Langford et al. 2012) With regard to individuals with MS, an oral-mucosal spray that delivers a standardized 1:1 ratio of THC:CBD has been shown to be effective for treating refractory spasticity in MS with few side-effects and low potential for addiction. (Wade, Collin et al. 2010, Sastre-Garriga, Vila et al. 2011)
The odds ratio (OR) that Cannabis use is more likely in cases versus controls is 0.36 (95% CI: 0.40 to 2.89, Fisher’s exact p=0.39). The interpretation of the OR is that the odds for exposure to Cannabis are 64% (non-significant) lower in MS cases than in controls in our study population. We also acknowledge the small sample size may not be adequate for firm conclusions as our original enrollment powered for eCB measurement was 18 subjects for each cohort.
In summary, our results suggest a model wherein pCB (in a whole plant context), are affecting monocyte phenotype, cytokine production and eCB signaling molecules. (Palazuelos, Davoust et al. 2008) The combined effects of eCB modulation are: a) skewing the phenotype of select immune cells toward a potentially anti-inflammatory phenotype; and b) contributing to global immune suppression by a decrease in cytokine production. Our results provide the foundation for conducting prospective human trials to investigate the effects of supplementing individuals with MS with regimented intake of pCB and measuring the long-term effects on inflammatory response and disease outcome in MS.
Supplementary Material
Dietary intake levels of fat-soluble vitamins and dietary fats. There were no significant differences in selected fats and fat soluble vitamins that have been shown to have associations with inflammation between controls and cases. Both cohorts consumed well over the recommended daily allowance (RDA) for vitamin A and less than the RDA for vitamins D and E. Total fat intake for both cohorts’ was between 30–33% of total calories.
Nutrient Supplementation for controls and cases. A significantly greater number of patients with MS used vitamin D supplementation than healthy controls. There were no other significant differences between controls and subjects with regard to the number of subjects who were supplementing their diets with selected fat-soluble vitamins and essential fatty acids that have been shown to have associations with immune function.
Supplemental Figure 1. This graph shows a representative sample analysis of Cannabis flower that is being accessed by patients in the Greater Seattle area by provision of State law. Average THC content was 12.7% and average CBD content was 1% of total weight. (Analysis provided by Phytalytics LLC, Seattle, WA using high pressure liquid chromatography (HPLC) with UV detection. These data were reported at the 2012 International Cannabinoid Research Society Meeting in Freiburg, Germany, in July 2012)
Acknowledgments
Conflicts of Interest and Source of Funding: The authors declare that no support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. There was no sponsor providing any of the medication or medical Cannabis in this study. Funding to: NS (DA014486); MS (F32AT005046); MR (T32AT00815).
References
- Abood ME, Sorenson RG, Stella N. Endocannabinoids: Actions at non CB1/CB2 receptors. New York: Springer; 2013. [Google Scholar]
- Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16(4):406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG, Aerts JM, Amor S, Nieuwenhuis EE, Laman JD. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006;129(Pt 2):517–526. doi: 10.1093/brain/awh707. [DOI] [PubMed] [Google Scholar]
- Broseus J, Anglada F, Esseiva P. The differentiation of fibre- and drug type Cannabis seedlings by gas chromatography/mass spectrometry and chemometric tools. Forensic Sci Int. 2010;200(1–3):87–92. doi: 10.1016/j.forsciint.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Caraceni P, Viola A, Piscitelli F, Giannone F, Berzigotti A, Cescon M, Domenicali M, Petrosino S, Giampalma E, Riili A, Grazi G, Golfieri R, Zoli M, Bernardi M, Di Marzo V. Circulating and hepatic endocannabinoids and endocannabinoid-related molecules in patients with cirrhosis. Liver Int. 2010;30(6):816–825. doi: 10.1111/j.1478-3231.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Wang YL, Fan HC, Lo WT, Wang CC, Sytwu HK. Current status of the immunomodulation and immunomediated therapeutic strategies for multiple sclerosis. Clin Dev Immunol. 2012;2012:970789. doi: 10.1155/2012/970789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology. 2004;62(11):2098–2100. doi: 10.1212/01.wnl.0000127707.07621.72. [DOI] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010 doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Correa FG, Mestre L, Docagne F, Borrell J, Guaza C. The endocannabinoid anandamide from immunomodulation to neuroprotection. Implications for multiple sclerosis. Vitam Horm. 2009;81:207–230. doi: 10.1016/S0083-6729(09)81009-1. [DOI] [PubMed] [Google Scholar]
- De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:5. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, Grone HJ, Platt FM, Zambon M, Cerundolo V. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118(12):4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer EJ. Cannabinoids and innate immunity: taking a toll on neuroinflammation. ScientificWorldJournal. 2011;11:855–865. doi: 10.1100/tsw.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49(1):67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, editor. Forensic Science and Medicine: Marijuana and the Cannabinoids. Forensic Science and Medicine. Totowa, NJ: Humana Press, Inc; 2007. [Google Scholar]
- Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- Frisullo G, Nociti V, Iorio R, Patanella AK, Marti A, Caggiula M, Mirabella M, Tonali PA, Batocchi AP. IL17 and IFNgamma production by peripheral blood mononuclear cells from clinically isolated syndrome to secondary progressive multiple sclerosis. Cytokine. 2008;44(1):22–25. doi: 10.1016/j.cyto.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Gaoni YMR. Isolation, Structure and Partial Synthesis of an Active Constituent of Hashish. Journal of the American Chemical Society. 1964;86(8):1646–1647. [Google Scholar]
- Hecker M, Paap BK, Goertsches RH, Kandulski O, Fatum C, Koczan D, Hartung HP, Thiesen HJ, Zettl UK. Reassessment of blood gene expression markers for the prognosis of relapsing-remitting multiple sclerosis. PLoS One. 2011;6(12):e29648. doi: 10.1371/journal.pone.0029648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur J Immunol. 2010;40(12):3358–3371. doi: 10.1002/eji.201040667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Nagarkatti PS, Nagarkatti M. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6(4):e18281. doi: 10.1371/journal.pone.0018281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34(8):1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Jackson SJ, Pryce G, Diemel LT, Cuzner ML, Baker D. Cannabinoid-receptor 1 null mice are susceptible to neurofilament damage and caspase 3 activation. Neuroscience. 2005;134(1):261–268. doi: 10.1016/j.neuroscience.2005.02.045. [DOI] [PubMed] [Google Scholar]
- Jean-Gilles L, Feng S, Tench CR, Chapman V, Kendall DA, Barrett DA, Constantinescu CS. Plasma endocannabinoid levels in multiple sclerosis. J Neurol Sci. 2009;287(1–2):212–215. doi: 10.1016/j.jns.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Kallaur AP, Oliveira SR, Colado Simao AN, Delicato de Almeida ER, Kaminami Morimoto H, Lopes J, de Carvalho Jennings Pereira WL, Marques Andrade R, Muliterno Pelegrino L, Donizete Borelli S, Kaimen-Maciel DR, Reiche EM. Cytokine profile in relapsing remitting multiple sclerosis patients and the association between progression and activity of the disease. Mol Med Rep. 2013;7(3):1010–1020. doi: 10.3892/mmr.2013.1256. [DOI] [PubMed] [Google Scholar]
- Katona S, Kaminski E, Sanders H, Zajicek J. Cannabinoid influence on cytokine profile in multiple sclerosis. Clin Exp Immunol. 2005;140(3):580–585. doi: 10.1111/j.1365-2249.2005.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KJ, Karpus WJ. Role of chemokines in the regulation of Th1/Th2 and autoimmune encephalomyelitis. J Clin Immunol. 1999;19(5):273–279. doi: 10.1023/a:1020535423465. [DOI] [PubMed] [Google Scholar]
- Killestein J, Hoogervorst EL, Reif M, Blauw B, Smits M, Uitdehaag BM, Nagelkerken L, Polman CH. Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. J Neuroimmunol. 2003;137(1–2):140–143. doi: 10.1016/s0165-5728(03)00045-6. [DOI] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5(5):400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Klein TW, Cathy N, Larsen Kelly, Lu Lily, Perkins Izabella, Nong Liang, Friedman Herman. The Cannabinoid System and Immune Modulation. Journal of Leukocyte Biology. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- Koppel J, Bradshaw H, Goldberg TE, Khalili H, Marambaud P, Walker MJ, Pazos M, Gordon ML, Christen E, Davies P. Endocannabinoids in Alzheimer's disease and their impact on normative cognitive performance: a case-control and cohort study. Lipids Health Dis. 2009;8:2. doi: 10.1186/1476-511X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwenhoven M, Teleshova N, Ozenci V, Press R, Link H. Monocytes in multiple sclerosis: phenotype and cytokine profile. J Neuroimmunol. 2001;112(1–2):197–205. doi: 10.1016/s0165-5728(00)00396-9. [DOI] [PubMed] [Google Scholar]
- Kozela E, Juknat A, Kaushansky N, Rimmerman N, Ben-Nun A, Vogel Z. Cannabinoids Decrease the Th17 Inflammatory Autoimmune Phenotype. J Neuroimmune Pharmacol. 2013 doi: 10.1007/s11481-013-9493-1. [DOI] [PubMed] [Google Scholar]
- Kozela E, Juknat A, Kaushansky N, Rimmerman N, Ben-Nun A, Vogel Z. Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J Neuroimmune Pharmacol. 2013;8(5):1265–1276. doi: 10.1007/s11481-013-9493-1. [DOI] [PubMed] [Google Scholar]
- Kuerten SL, PV The immune pathogenesis of experimental autoimmune enchephalitis: lessons learned for multiple sclerosis? Jounal of Interferon and Cytokine Research. 2011;12(31):907–916. doi: 10.1089/jir.2011.0072. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang H, Long Y, Lu Z, Hu X. Increased memory Th17 cells in patients with neuromyelitis optica and multiple sclerosis. J Neuroimmunol. 2011;234(1–2):155–160. doi: 10.1016/j.jneuroim.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. AAPS J. 2006;8(2):E298–E306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli G, Poddighe L, Mei A, Uleri E, Sotgiu S, Serra C, Manetti R, Dolei A. Expression and activation by epstein barr virus of human endogenous retroviruses-w in blood cells and astrocytes: inference for multiple sclerosis. PLoS One. 2012;7(9):e44991. doi: 10.1371/journal.pone.0044991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rodriguez JE, Munteis E, Carreno M, Blanco Y, Roquer J, Abanades S, Graus F, Saiz A. Cannabis use in Spanish patients with multiple sclerosis: fulfilment of patients' expectations? J Neurol Sci. 2008;273(1–2):103–107. doi: 10.1016/j.jns.2008.06.037. [DOI] [PubMed] [Google Scholar]
- Massi P, Fuzio D, Vigano D, Sacerdote P, Parolaro D. Relative involvement of cannabinoid CB(1) and CB(2) receptors in the Delta(9)-tetrahydrocannabinol-induced inhibition of natural killer activity. Eur J Pharmacol. 2000;387(3):343–347. doi: 10.1016/s0014-2999(99)00860-2. [DOI] [PubMed] [Google Scholar]
- Matias I, Gatta-Cherifi B, Tabarin A, Clark S, Leste-Lasserre T, Marsicano G, Piazza PV, Cota D. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS One. 2012;7(7):e42399. doi: 10.1371/journal.pone.0042399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy L, Tsunoda I, Fujinami RS. Multiple sclerosis and virus induced immune responses: autoimmunity can be primed by molecular mimicry and augmented by bystander activation. Autoimmunity. 2006;39(1):9–19. doi: 10.1080/08916930500484799. [DOI] [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C, Petry KG. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17(1):2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- Miller AM, Stella N. Microglial cell migration stimulated by ATP and C5a involve distinct molecular mechanisms: quantification of migration by a novel near-infrared method. Glia. 2009;57(8):875–883. doi: 10.1002/glia.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahas GG, Morishima A, Desoize B. Effects of cannabinoids on macromolecular synthesis and replication of cultured lymphocytes. Fed Proc. 1977;36(5):1748–1752. [PubMed] [Google Scholar]
- Nahas GG, Suciu-Foca N, Armand JP, Morishima A. Inhibition of cellular mediated immunity in marihuana smokers. Science. 1974;183(4123):419–420. doi: 10.1126/science.183.4123.419. [DOI] [PubMed] [Google Scholar]
- Newton CA, Klein TW, Friedman H. Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect Immun. 1994;62(9):4015–4020. doi: 10.1128/iai.62.9.4015-4020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong L, Newton C, Cheng Q, Friedman H, Roth MD, Klein TW. Altered cannabinoid receptor mRNA expression in peripheral blood mononuclear cells from marijuana smokers. J Neuroimmunol. 2002;127(1–2):169–176. doi: 10.1016/s0165-5728(02)00113-3. [DOI] [PubMed] [Google Scholar]
- Notcutt W, Langford R, Davies P, Ratcliffe S, Potts R. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex(R) (nabiximols) Mult Scler. 2012;18(2):219–228. doi: 10.1177/1352458511419700. [DOI] [PubMed] [Google Scholar]
- Oreja-Guevara C, Ramos-Cejudo J, Stark Aroeira L, Chamorro B, Diez-Tejedor E. TH1/TH2 Cytokine profile in relapsing-remitting multiple sclerosis patients treated with Glatiramer acetate or Natalizumab. BMC Neurol. 2012;12(1):95. doi: 10.1186/1471-2377-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R, Zuccaro P, Farre M, Poudevida S, Abanades S, Pichini S, Langohr K, Segura J, de la Torre R. Combined immunomodulating properties of 3,4-methylenedioxymethamphetamine (MDMA) and cannabis in humans. Addiction. 2007;102(6):931–936. doi: 10.1111/j.1360-0443.2007.01805.x. [DOI] [PubMed] [Google Scholar]
- Pacifici R, Zuccaro P, Pichini S, Roset PN, Poudevida S, Farre M, Segura J, De la Torre R. Modulation of the immune system in cannabis users. JAMA. 2003;289(15):1929–1931. doi: 10.1001/jama.289.15.1929-b. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Davoust N, Julien B, Hatterer E, Aguado T, Mechoulam R, Benito C, Romero J, Silva A, Guzman M, Nataf S, Galve-Roperh I. The CB(2) cannabinoid receptor controls myeloid progenitor trafficking: involvement in the pathogenesis of an animal model of multiple sclerosis. J Biol Chem. 2008;283(19):13320–13329. doi: 10.1074/jbc.M707960200. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoids and multiple sclerosis. Pharmacol Ther. 2002;95(2):165–174. doi: 10.1016/s0163-7258(02)00255-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2) Pharmacol Rev. 2010;62(4):588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini A, Mannaioni G, Pellegrini-Giampietro D, Passani MB, Mastroianni R, Bani D, Masini E. The role of cannabinoids in inflammatory modulation of allergic respiratory disorders, inflammatory pain and ischemic stroke. Curr Drug Targets. 2012;13(7):984–993. doi: 10.2174/138945012800675786. [DOI] [PubMed] [Google Scholar]
- Pryce D, Baker G. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol. 2007;150(4):519–525. doi: 10.1038/sj.bjp.0707003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce GB, D Potential Control of Multiple Sclerosis by Cannabis and the Endocannabinoid System. CNS and neurological Disorders-Drug Targets. 2012;11:624–621. doi: 10.2174/187152712801661310. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, Akbarali HI, Lichtman AH. Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther. 2011;339(1):173–185. doi: 10.1124/jpet.111.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther. 2007;29(9):2068–2079. doi: 10.1016/j.clinthera.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. Chem Biodivers. 2007;4(8):1614–1648. doi: 10.1002/cbdv.200790144. [DOI] [PubMed] [Google Scholar]
- Sastre-Garriga J, Vila C, Clissold S, Montalban X. THC and CBD oromucosal spray (Sativex(R)) in the management of spasticity associated with multiple sclerosis. Expert Rev Neurother. 2011;11(5):627–637. doi: 10.1586/ern.11.47. [DOI] [PubMed] [Google Scholar]
- Sexton MS, Moller A, Stella T, N Differential migratory Properties of Monocytes isolated from human subjects naive and non-naive to Cannabis. Inflammopharmacology. 2012 doi: 10.1007/s10787-012-0133-9. (ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: No longer 'if' but 'how'. J Pathol. 2012 doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- Simmons A. Herpesvirus and multiple sclerosis. Herpes. 2001;8(3):60–63. [PubMed] [Google Scholar]
- Smith SR, Terminelli C, Denhardt G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J Pharmacol Exp Ther. 2000;293(1):136–150. [PubMed] [Google Scholar]
- Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58(9):1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an overview. Immunobiology. 2010;215(8):588–597. doi: 10.1016/j.imbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Wade DT, Collin C, Stott C, Duncombe P. Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler. 2010;16(6):707–714. doi: 10.1177/1352458510367462. [DOI] [PubMed] [Google Scholar]
- Ware MA, Adams H, Guy GW. The medicinal use of cannabis in the UK: results of a nationwide survey. Int J Clin Pract. 2005;59(3):291–295. doi: 10.1111/j.1742-1241.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- Zhao P, Abood ME. GPR55 and GPR35 and their relationship to cannabinoid and lysophospholipid receptors. Life Sci. 2012 doi: 10.1016/j.lfs.2012.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dietary intake levels of fat-soluble vitamins and dietary fats. There were no significant differences in selected fats and fat soluble vitamins that have been shown to have associations with inflammation between controls and cases. Both cohorts consumed well over the recommended daily allowance (RDA) for vitamin A and less than the RDA for vitamins D and E. Total fat intake for both cohorts’ was between 30–33% of total calories.
Nutrient Supplementation for controls and cases. A significantly greater number of patients with MS used vitamin D supplementation than healthy controls. There were no other significant differences between controls and subjects with regard to the number of subjects who were supplementing their diets with selected fat-soluble vitamins and essential fatty acids that have been shown to have associations with immune function.
Supplemental Figure 1. This graph shows a representative sample analysis of Cannabis flower that is being accessed by patients in the Greater Seattle area by provision of State law. Average THC content was 12.7% and average CBD content was 1% of total weight. (Analysis provided by Phytalytics LLC, Seattle, WA using high pressure liquid chromatography (HPLC) with UV detection. These data were reported at the 2012 International Cannabinoid Research Society Meeting in Freiburg, Germany, in July 2012)