Abstract
Myelin loss is a widespread neuropathological hallmark of the atypical parkinsonian disorder multiple system atrophy (MSA). On a cellular level, MSA is characterized by alpha-synuclein (aSyn)-positive glial cytoplasmic inclusions (GCIs) within mature oligodendrocytes leading to demyelination as well as axonal and neuronal loss. Oligodendrocyte progenitor cells (OPCs) represent a proliferative cell population distributed throughout the adult mammalian central nervous system. During remyelination, OPCs are recruited to sites of demyelination, differentiate, and finally replace dysfunctional mature oligodendrocytes. However, comprehensive studies investigating OPCs and remyelination processes in MSA are lacking. In the present study, we therefore investigate the effect of human aSyn (h-aSyn) on early primary rat OPC maturation. Upon lentiviral transduction, h-aSyn expressing OPCs exhibit fewer and shorter primary processes at the initiation of differentiation. Until day 4 of a 6 day differentiation paradigm, h-aSyn expressing OPCs further show a severely delayed maturation evidenced by reduced myelin gene expression and increased levels of the progenitor marker platelet derived growth factor receptor-alpha (PDGFRα). Matching these results, OPCs that take up extracellular recombinant h-aSyn exhibit a similar delayed differentiation. In both experimental setups however, myelin gene expression is restored at day 6 of differentiation paralleled by decreased intracellular h-aSyn levels indicating a reverse correlation of h-aSyn and the differentiation potential of OPCs. Taken together, these findings suggest a tight link between the intracellular level of h-aSyn and maturation capacity of primary OPCs.
Keywords: oligodendrocyte progenitor cells, alpha-synuclein, maturation, multiple system atrophy, myelin basic protein
Introduction
The atypical parkinsonian disorder multiple system atrophy (MSA) is a rare and rapidly progressing neurodegenerative disease. Glial cytoplasmic inclusions (GCIs) present in oligodendrocytes are the neuropathological hallmark of MSA. They consist of high amounts of alpha-synuclein (aSyn) and therefore classify MSA as a synucleinopathy (Spillantini et al., 1998; Tu et al., 1998). On a cellular level, MSA is characterized by a widespread neuronal loss associated with a severe glial pathology including myelin loss (Ubhi et al., 2011). Oligodendrocytic dysfunction with consecutive demyelination is considered as an early event in MSA pathogenesis preceding neuronal degeneration (Song et al., 2007; Wenning et al., 2008). Studies using transgenic mice expressing human aSyn (h-aSyn) under the control of oligodendrocyte-specific promoters support the hypothesis of a myelin-related dysfunction leading to axonal damage and consecutive neuronal loss (Kahle et al., 2002; Yazawa et al., 2005).
During development, myelination is initiated after specification of oligodendrocyte progenitor cells (OPCs) from ventricular zone cells in the brain and spinal cord. Controlled by extrinsic stimuli and an intrinsic, temporally regulated transcription factor network, OPCs differentiate to postmitotic oligodendrocytes finally myelinating responsive axons (Richardson et al., 2006). Although myelination is complete early in life, OPCs persist in the adult mammalian central nervous system (CNS) where they are also referred to as NG2-positive cells, synantocytes or polydendrocytes (Nishiyama et al., 2009). Adult OPCs are distributed within the grey and white matter and maintain their capacity to migrate, proliferate, and differentiate throughout life (Rivers et al., 2008). Not only the typical expression of platelet derived growth factor receptor alpha (PDGFRα) in developmental OPCs is preserved in adult OPCs, but also differentiation mechanisms including signaling pathways and the transcription factor profile are similar for both cell populations (Emery, 2010). Besides recent evidence for a contribution of OPCs to myelin remodeling (Young et al., 2013), OPCs are capable of remyelinating axons under pathological conditions (Groves et al., 1993).
Several neurological disorders including MSA and multiple sclerosis (MS) are characterized by oligodendrocytic pathology with pronounced myelin loss. The widespread demyelination in these diseases suggests that OPCs fail to replace dysfunctional myelinating oligodendrocytes. It is very likely that recruitment, proliferation, or maturation of OPCs is disturbed in MS, an autoimmune disease with pronounced inflammation and focal demyelination (Chang et al., 2002). In contrast to MS, studies on OPCs and their capacity to be recruited and to differentiate in the context of the synucleinopathy MSA are rare. Recent studies demonstrate increased numbers of OPCs in MSA suggesting a similar disturbed OPC maturation in MSA as observed in MS (Ahmed et al., 2013; May et al., 2014). In contrast and given that developmentally produced myelin can be distinguished from thinner myelin laid down during remyelination, thinner myelin sheaths detected in MSA patients implicate that remyelination occurs to a certain extent in MSA (Blakemore, 1974; Song et al., 2007). This implies that the capacity of remyelination is preserved in MSA to some extent, but at insufficient levels. Moreover, oligodendroglial aSyn levels are increased within and around demyelinated lesions in MS where a failure of OPC maturation was recently reported (Kuhlmann et al., 2008; Papadopoulos et al., 2006). In support, we recently demonstrated the presence of aSyn within a fraction of striatal OPCs in a post-mortem analysis of MSA with predominant parkinsonism (MSA-P) patients (May et al., 2014). Therefore, we hypothesize that intraoligodendroglial aSyn impairs early stages of OPC maturation towards pre-myelinating oligodendrocytes.
Up to date, the origin of aSyn in oligodendrocytic cells of MSA patients is still controversial. Studies detecting aSyn mRNA in oligodendrocytes in vitro and in vivo argue for an endogenous oligodendrocytic aSyn expression (Asi et al., 2014; Mori et al., 2002; Richter-Landsberg et al., 2000; Tsuboi et al., 2005), but are in disagreement with reports of undetectable aSyn mRNA in MSA patients (Miller et al., 2005; Solano et al., 2000). There is also an increasing body of evidence that oligodendrocytes are able to take up extracellular or neuronally derived aSyn (Kisos et al., 2012; Konno et al., 2012; Reyes et al., 2014; Rockenstein et al., 2012).
Considering both scenarios, we used a dual approach to analyze the effect of aSyn on OPC maturation. Primary rat OPCs were either transduced with h-aSyn coding lentiviral vectors or exposed to recombinant human wildtype aSyn (rh-aSyn). We analyzed the number and length of primary processes upon transduction as well as progenitor-specific and myelin gene expression during OPC differentiation in the presence of cell-autonomous or non-cell-autonomous h-aSyn, respectively. Furthermore, we investigated the dynamics of intracellular h-aSyn levels during differentiation aiming to determine the link between intracellular h-aSyn and the initiation of OPC maturation.
Results
Characterization of primary OPC maturation using specific markers for differentiation
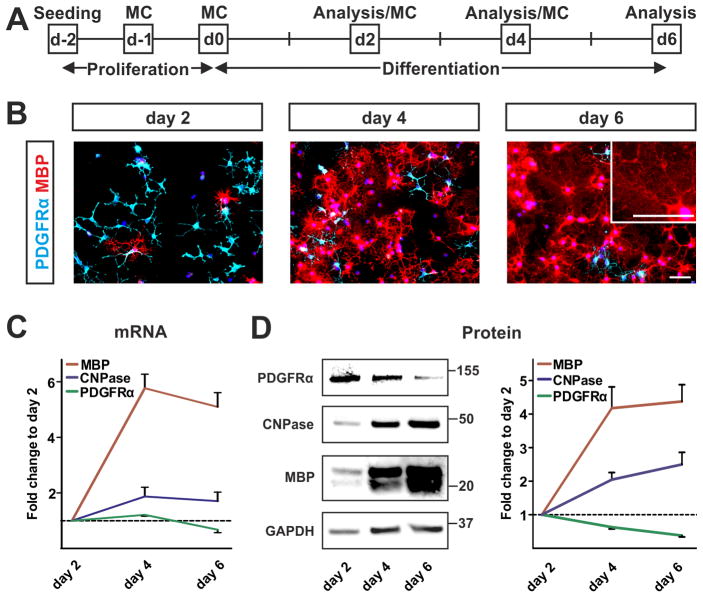
In order to analyze the effect of h-aSyn on OPC maturation, we initially characterized the temporal differentiation dynamics of primary rat OPCs. Previous studies report a high proportion of mature oligodendrocytes after 5–7 days under differentiation promoting conditions, i.e. after withdrawal of growth factors (GFs) and in presence of 1% fetal calf serum (FCS) (Barateiro et al., 2013; Kisos et al., 2012). To dissect the temporal differentiation pattern towards a mature oligodendrocytic phenotype, oligodendrocyte maturation was determined after 2, 4, and 6 days by analyzing stage specific markers (Fig. 1A). Expression of PDGFRα as a marker for an early progenitor status and of the myelin genes 2′,3′-cyclic-nucleotide-phosphodiesterase (CNPase) and myelin basic protein (MBP) as markers of a mature state were analyzed. During differentiation, the number of PDGFRα-positive OPCs gradually decreased whereas the number of MBP-positive mature oligodendrocytes increased (Fig. 1B). Additionally, mature oligodendrocytes with enlarged plasma membrane were observed as a sign for advanced maturation as early as day 6 of differentiation (Fig. 1B, insert). To quantify the process of maturation, mRNA and protein expression of PDGFRα, CNPase, and MBP were analyzed using real time-PCR (RT-PCR) and Western blot. Both PDGFRα mRNA and protein gradually decreased within 6 days of differentiation with the most pronounced decline between day 4 and day 6 (Fig. 1C, D; green line). In contrast, myelin gene expression was stepwise upregulated during differentiation exhibiting the strongest increase between day 2 and day 4. MBP, the later and more abundantly expressed myelin protein, showed a stronger upregulation than the early myelin protein CNPase (Fig. 1C, D; red and blue line, respectively). Taken together, in vitro differentiation of primary OPCs by the applied protocol allows monitoring of early oligodendrocyte maturation.
Fig. 1. Monitoring maturation of primary OPCs.
(A) Experimental paradigm to analyze differentiation of primary OPCs. Maturation of primary OPCs was assessed after 2, 4, and 6 days of differentiation. MC = medium change. (B) Immunocytochemical evaluation (n=3) reveals decreasing numbers of PDGFRα-positive oligodendrocyte precursors (light blue) paralleled by increasing numbers of MBP-positive mature oligodendrocytes (red) during differentiation. Note the advanced maturated morphology of oligodendrocytes with enlarged plasma membranes at day 6 of differentiation (insert). Scale bars: 50μm. (C) mRNA expression profile of oligodendrocytic markers (n=4) confirms progressive maturation of primary oligodendrocytes within 6 days of differentiation. (D) Western blot analysis (n=5) shows the temporal dynamic of oligodendrocyte maturation. Quantification of protein expression patterns reveals a decline in PDGFRα expression while myelin gene expression (CNPase, MBP) increases during differentiation. Analysis of GAPDH expression serves as loading control.
Efficient transgene expression upon lentiviral transduction of primary OPCs
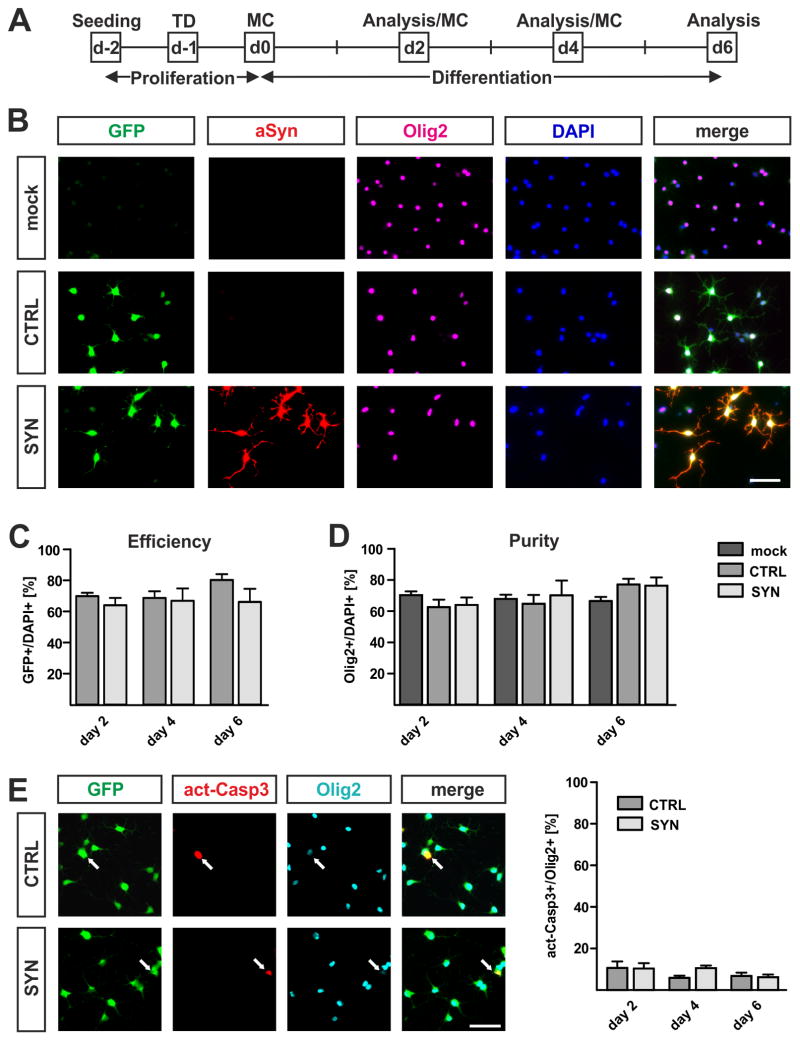
To analyze effects of cell-autonomous h-aSyn on OPC maturation, we used a previously described lentiviral vector system for h-aSyn expression (Winner et al., 2011). One day prior to differentiation, primary OPCs were transduced with lentiviral vectors coding either for aSyn followed by an internal ribosomal entry site (IRES) and the green fluorescent protein (GFP) sequence (further referred to as SYN) or for GFP after the IRES sequence only (further referred to as CTRL). Using a multiplicity of infection (MOI) of 2, we observed constantly high numbers of GFP-positive cells during differentiation for both vectors, i.e. the control vector coding for GFP alone (CTRL, 68.6–80.3% GFP-positive cells) or the expression vector coding for h-aSyn in addition (SYN; 64.0–66.9% GFP-positive cells) (Fig. 2B, C). Furthermore, all GFP expressing cells in the SYN transduced culture also expressed h-aSyn (Fig. 2B, lower panel). By counting the number of Olig2-positive cells as a classical marker for the oligodendrocytic lineage (Ligon et al., 2004), we next analyzed OPC purity and survival (Fig. 2B, D). OPC purity ranged from 66.5 to 70.3% under non-transduced conditions and was not altered by lentiviral transduction as shown by similar proportions of Olig2-positive cells in SYN (64.0–76.3%) and CTRL (62.6–77.2%) cultures indicating no loss of Olig2-positive cells upon h-aSyn expression. In order to confirm the absence of increased cell death in h-aSyn expressing OPCs, we analyzed expression of activated Caspase 3 as a marker for apoptosis upon lentiviral transduction. Proportions of activated Caspase 3-positive cells did not significantly differ between CTRL (day 2: 10.6±3.2%, day 4: 5.9±1.1%, day6: 6.8±1.6% of Olig2-positive cells) and SYN transduced (day 2: 10.4±2.6%, day 4: 10.5±1.3%, day6: 6.2±1.2% of Olig2-positive cells) oligodendrocytes supporting that h-aSyn expression does not lead to increased cell death.
Fig. 2. Lentiviral transduction of primary OPCs.
(A) Experimental paradigm to assess the effect of lentivirally expressed h-aSyn on primary OPC maturation. One day after seeding, transduction (TD) was performed. After another day, differentiation was started. MC = medium change. (B) Representative pictures taken at day 2 of differentiation for the immunocytochemical evaluation of OPC purity and survival as well as lentiviral transduction efficiency. Notice the absence of h-aSyn immunoreactivity (red) in mock as well as CTRL transduced cultures and the completely overlapping expression of GFP (green) and h-aSyn in SYN transduced cells. Note that most DAPI-positive cells (blue) express the oligodendrocyte lineage marker Olig2 (magenta). Scale bar: 50μm. (C) Determination of lentiviral transduction efficiency as percentage of GFP-positive cells of total DAPI-positive cells (n=3). Both lentiviruses result in a high and comparable number of transduced cells (total means: CTRL 72.9%, SYN 65.6%). (D) Quantification of culture purity illustrated as the percentage of Olig2-positive cells of total DAPI-positive cells (n=3). The number of Olig2-positive cells changes neither during differentiation nor by comparing non-transduced and transduced cultures (total means: mock 68.2%, CTRL 68.1%, SYN 70.1%). (E) Immunocytochemistry (n=3) confirms the absence of an increased apoptotic profile in SYN compared to CTRL transduced oligodendrocytes. Arrows in representative pictures taken at day 2 of differentiation depict apoptotic Olig2-positive cells expressing activated Caspase 3 (act-Casp3). Quantification reveals ≤10% act-Casp3-positive oligodendrocytes during differentiation of both CTRL and SYN transduced oligodendrocytes. Scale bar = 50μm.
H-aSyn expressing oligodendrocytes exhibit fewer and shorter primary processes
We next asked whether the morphology of maturating OPCs was affected by h-aSyn expression. Therefore, we quantified the number and length of primary processes and compared SYN and CTRL transduced oligodendrocytes at day 2 of differentiation (Fig. 3). For this purpose, we first outlined vimentin-positive primary processes using the NeuronJ plugin for ImageJ. We used vimentin immunolabelling as this cytoskeletal protein highlights primary processes of OPCs without labeling smaller branches (Behar et al., 1988). Next, we performed a Scholl analysis enabling us to determine the number and length of primary processes (Fig. 3A). When analyzing 50 GFP/Olig2-positive oligodendrocytes per condition, we observed a significant reduction by ~15% in the number of primary processes in SYN (4.4±0.2) compared to CTRL cells (5.2±0.2, p<0.01; Fig. 3B). Moreover, the processes were significantly shorter as evidenced by comparing the numbers of intersections per single Scholl radius (Fig. 3C). A significant reduction in the number of intersections by 24–43% was counted in h-aSyn expressing oligodendrocytes for Scholl radii at a distance of 20 to 36μm from the cell body. In summary, the Scholl analysis revealed reduced numbers and shorter processes in early differentiated h-aSyn expressing primary oligodendrocytes.
Fig. 3. Morphological alterations of h-aSyn expressing OPCs.
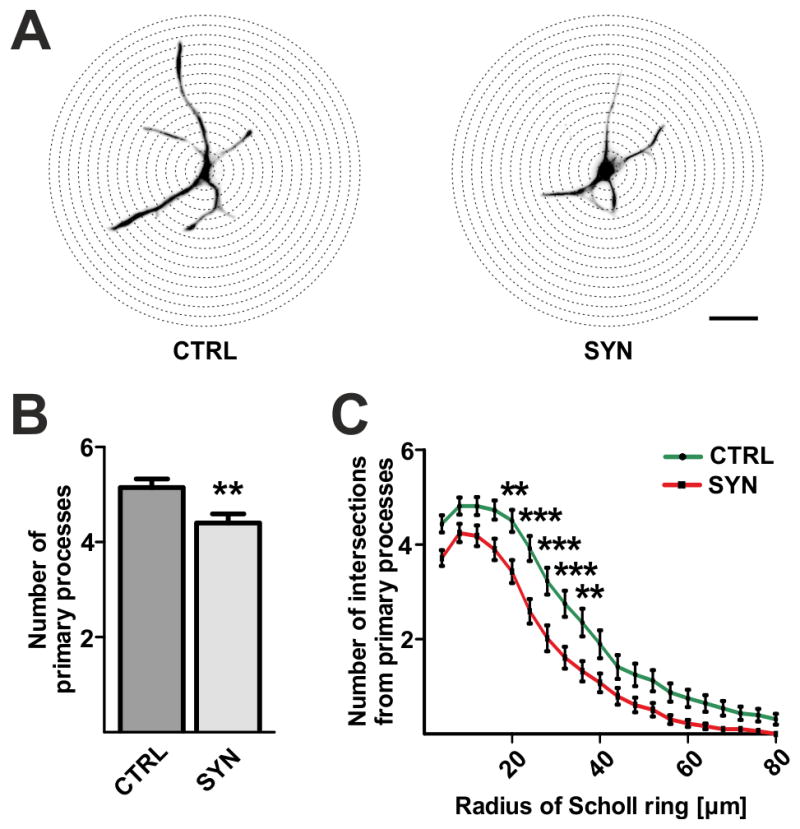
(A) Illustration of Scholl analysis performed to compare the number and length of processes between SYN and CTRL transduced OPCs on the basis of vimentin-positive primary processes. Scale bar: 20μm. (B) Numbers of primary processes are significantly reduced in h-aSyn expressing oligodendrocytes (SYN) compared to controls (CTRL) on day 2 of differentiation. (C) Scholl analysis of 50 GFP/Olig2-positive cells after 2 days of differentiation reveals that h-aSyn expressing oligodendrocytes (SYN) exhibit significant fewer primary processes intersecting Scholl radii between 20 and 36μm compared to controls (CTRL).
Interference of h-aSyn with primary OPC maturation
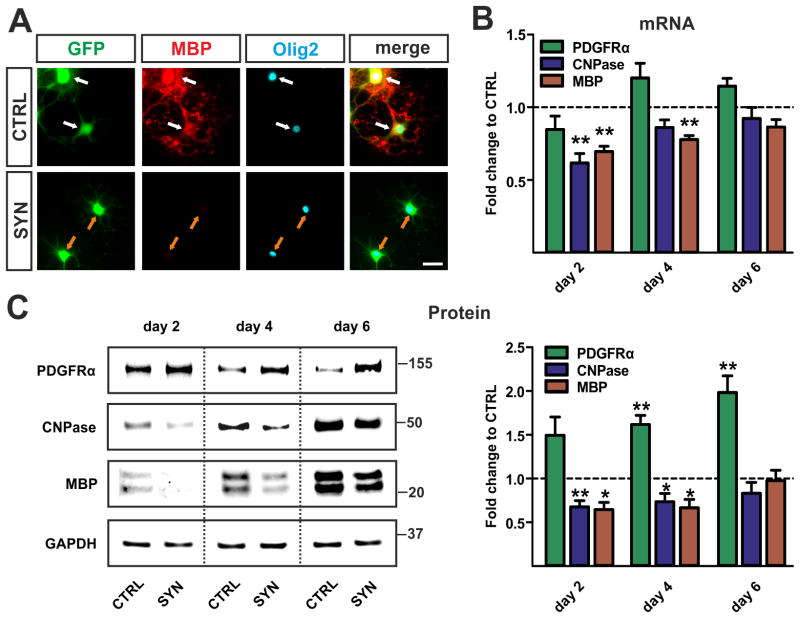
Since we observed altered morphology of h-aSyn expressing OPCs we next analyzed gene expression profiles during OPC maturation. At day 4 of differentiation, CTRL transduced oligodendrocytes showed MBP expression in contrast to SYN transduced oligodendrocytes (Fig. 4A). This observation was further confirmed by mRNA expression analysis: A decreased upregulation of myelin gene expression, i.e. CNPase and MBP, was detected in SYN compared to CTRL expressing cells (Fig. 4B). Transcripts of both myelin genes analyzed were significantly reduced at day 2 (CNPase 0.61±0.07 fold, MBP 0.69±0.04 fold, p<0.01). In addition, MBP mRNA expression levels were significantly decreased at day 4 of differentiation (0.78±0.03 fold, p<0.01). Furthermore, significantly reduced protein levels of CNPase and MBP upon h-aSyn expression were detected at day 2 (CNPase 0.67±0.07 fold, p<0.01; MBP 0.64±0.08 fold, p<0.05) and day 4 (CNPase 0.73±0.10 fold, MBP 0.66±0.10, p<0.05) of differentiation (Fig. 4C). Interestingly, no differences in mRNA and protein levels between SYN and CTRL transduced oligodendrocytes were observed at day 6. To exclude an overall reduction of gene expression by h-aSyn, we asked whether the expression of PDGFRα as a common marker for early OPCs was elevated in h-aSyn expressing cells. PDGFRα protein expression was significantly increased both at day 4 (1.61±0.10 fold, p<0.01) and day 6 (1.98±0.19 fold, p<0.01) of differentiation while mRNA expression was not altered (Fig. 4B, C; green bars). These data suggest that h-aSyn when expressed in a cell-autonomous manner interferes with the induction of primary OPC maturation.
Fig. 4. Impaired maturation of h-aSyn expressing OPCs.
(A) Representative pictures of oligodendrocytes (identified by Olig2 expression) at day 4 of differentiation. Note that CTRL transduced oligodendrocytes already show MBP expression (white arrows) whereas the SYN transduced cells do not (orange arrows). Scale bar: 20 μm. (B) mRNA expression profile (n=4) of oligodendrocyte maturation markers for h-aSyn and GFP expressing cultures (SYN) relative to solely GFP expressing controls (CTRL). Significantly lower levels of myelin gene transcripts (CNPase, MBP) are detected in h-aSyn expressing cells at day 2 and day 4 of differentiation. The difference in myelin gene expression is not present at day 6. (C) Western blots (n=5) comparing expression of progenitor and maturation markers between SYN and CTRL oligodendrocytes. Densitometric analysis reveals significantly increased levels of the progenitor marker PDGFRα in h-aSyn expressing cells reaching the highest difference at day 6. Note the severely reduced levels of the myelin proteins CNPase and MBP in h-aSyn expressing oligodendrocytes at day 2 and day 4, however not present at day 6.
Decline of h-aSyn expression during OPC differentiation
As maturation of SYN transduced oligodendrocytes was particularly impaired at early stages of differentiation, i.e. day 2 and day 4, we asked whether ectopic h-aSyn expression is maintained during the course of differentiation (Fig. 5). mRNA expression of h-aSyn was significantly decreased at day 4 (0.64±0.09 fold, p<0.05) followed by an even more pronounced decline at day 6 of differentiation (0.36±0.05 fold, p<0.001). Furthermore, we detected a reduction of h-aSyn protein level at day 6 of differentiation (0.38±0.06 fold, p<0.01). To address the question whether there is a h-aSyn specific downregulation or a reduction in the activity of the EF1α promoter, we analyzed GFP expression during differentiation of SYN transduced OPCs. Similarly to h-aSyn mRNA, GFP mRNA levels significantly declined during differentiation in both CTRL and SYN transduced OPCs (CTRL: day 4: 0.70±0.08 fold, p<0.05, day 6: 0.47±0.08 fold, p<0.01; SYN: day 4: 0.53±0.06 fold, p<0.01, day 6: 0.34±0.04 fold, p<0.001). GFP protein levels did not significantly change during differentiation of CTRL and SYN OPCs. After an initial upregulation however, GFP expression strongly decreased by day 6 of differentiation (CTRL: day 4: 1.78±0.31 fold, day 6: 0.94±0.37 fold; SYN: day 4: 2.44±0.96 fold, day 6: 1.21±0.61 fold). Furthermore, weaker GFP expression was detected in SYN compared to CTRL OPCs. These results indicate that h-aSyn as well as GFP expression is gradually downregulated during maturation of lentivirally transduced primary OPC.
Fig. 5. Temporal dynamics of GFP and h-aSyn expression in lentivirally transduced OPCs.
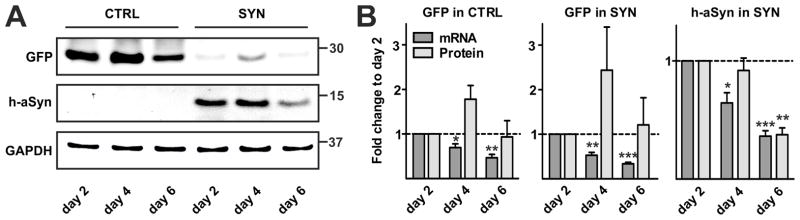
(A) Representative Western blot showing GFP and h-aSyn protein levels in lentivirally transduced primary OPCs. (B) Quantification of GFP as well as h-aSyn mRNA (n=4) and protein (n=4) expression reveals significant declined GFP mRNA levels during differentiation of both CTRL (left panel) and SYN (mid panel) transduced OPCs while GFP protein is not significantly altered. H-aSyn expression in SYN transduced OPCs (right panel) significantly declines both on the mRNA (day 4, day 6) and protein (day 6) level during differentiation.
Uptake of rh-aSyn by primary OPCs
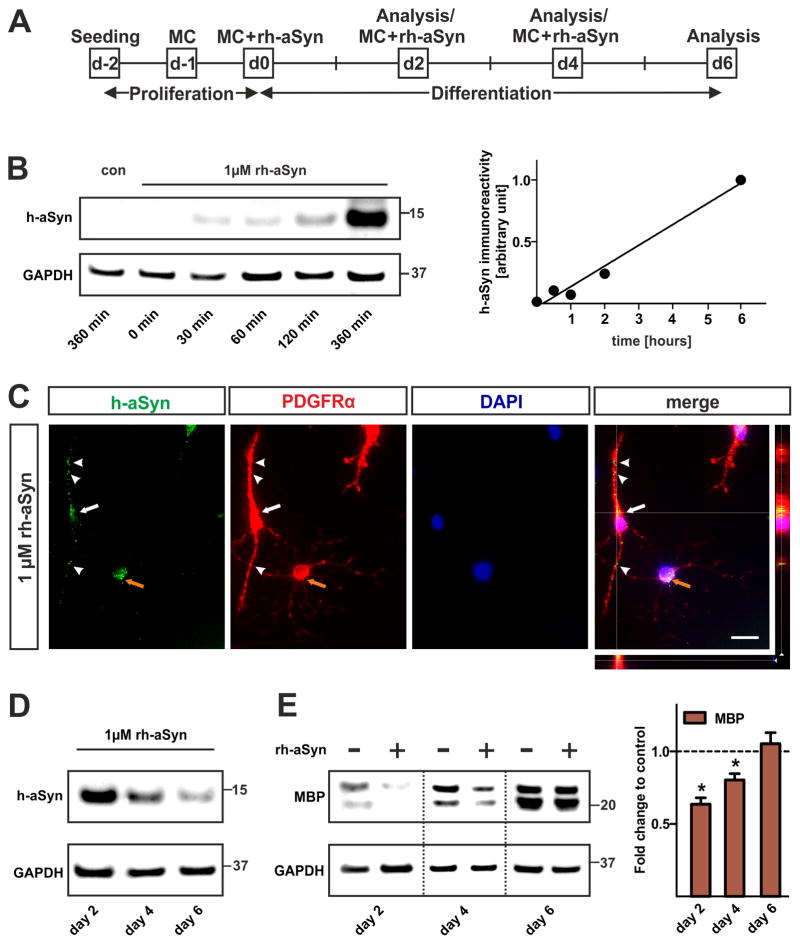
It is still controversial whether aSyn present in GCIs of oligodendrocytes in MSA is of endogenous origin or taken up from the extracellular space. To assess whether primary OPCs are able to take up extracellular aSyn, we performed exposure experiments by adding rh-aSyn to the culture medium for primary OPCs (Fig. 6A). As the physiological concentration of aSyn in the CNS was estimated to be in the range of 1μM, we used this concentration for all exposure experiments (Borghi et al., 2000). We first performed Western blots of OPCs lyzed at different time points within the first 6 hours upon rh-aSyn administration (Fig. 6B). We detected h-aSyn immunoreactivity linearly increasing in a time-dependent manner. To further confirm the intracellular presence of h-aSyn, microscopy was performed using the z-stack module and ApoTome technology (Carl Zeiss). Three-dimensional reconstruction (z-stack parameters: thickness 16.3μm, 27 sections) showed that the h-aSyn immunofluorescent signal was localized within the perinuclear cytoplasm of PDGFRα-positive OPCs (Fig. 6C). An interesting observation was the presence of h-aSyn immunofluorescence in the soma as well as in processes of early, i.e. bipolar and PDGFRαhigh OPCs (Fig. 6C white arrow and arrowheads). In contrast, more mature, i.e. multipolar and PDGFRαlow OPCs exhibit h-aSyn immunofluorescence restricted to the soma (Fig. 6C, orange arrow). To analyze the intracellular h-aSyn level during the applied differentiation paradigm, Western blotting using lysates of rh-aSyn exposed cells was conducted and membranes were probed with antibodies specific for h-aSyn (Fig. 6D). Interestingly, the amount of h-aSyn within oligodendrocytes declined during differentiation. These findings indicate that OPCs take up extracellular soluble rh-aSyn in a time dependent manner and that intracellular h-aSyn levels sequentially decline during differentiation.
Fig. 6. Impaired maturation of primary OPCs upon uptake of rh-aSyn.
(A) Experimental paradigm to assess the effect of rh-aSyn on primary OPC maturation. OPCs were exposed to 1μM rh-aSyn 2 days after seeding prior to differentiation for additional 6 days. MC = medium change. (B) Western blot of primary OPCs lyzed at 0, 30, 60, 120, and 360 minutes upon rh-aSyn administration. Densitometry reveals that primary OPCs take up rh-aSyn in a time dependent manner. (C) Immunocytochemistry demonstrates the presence of intracellular rh-aSyn (green) in PDGFRα-positive primary OPCs (red) at day 2 upon exposure to rh-aSyn. Three-dimensional analysis using the z-stack module (AxioVision, ZEISS) verifies the presence of intracellular rh-aSyn rather than membranous attached rh-aSyn (merged picture). Note that bipolar PDGFRαhigh OPCs show h-aSyn immunoreactivity in the perinuclear cytoplasm (white arrow) as well as in processes (white arrowheads) whereas multipolar PDGFRαlow oligodendrocyte precursors only exhibit perinuclear h-aSyn signals (orange arrow). Scale bar: 20μm. (D) Representative Western blots (n=3) of whole cell lysates show a decline of the intracellular h-aSyn level within the course of differentiation. (E) Representative Western blot (n=3) of primary oligodendrocytes differentiated for 2, 4, and 6 days in the presence of rh-aSyn (1μM) shows the MBP expression pattern. Densitometric analysis reveals a significant reduction in MBP levels restricted to early stages (day 2 and day 4) of oligodendrocyte differentiation upon exposure to rh-aSyn.
OPC maturation is impaired upon uptake of rh-aSyn
We next asked whether the uptake of rh-aSyn impairs OPC maturation to the same extent as observed upon forced h-aSyn expression. Therefore, we exposed differentiating OPCs for up to 6 days with 1μM rh-aSyn (Fig. 6E). We detected significantly lower levels of MBP protein in primary oligodendrocytes exposed to rh-aSyn at day 2 (0.64±0.04 fold, p<0.05) and day 4 (0.80±0.04 fold, p<0.05) of differentiation. MBP expression was similar in rh-aSyn exposed and control cells at day 6 of differentiation (1.05±0.08 fold, p>0.05). OPCs exposed to rh-aSyn did not show significant alterations in PDGFRα as well as CNPase protein expression during differentiation (data not shown). Taken together, uptake of rh-aSyn impairs early primary OPC maturation, albeit in a less pronounced manner than observed after lentiviral transduction.
MBP expression depends on the intracellular h-aSyn level
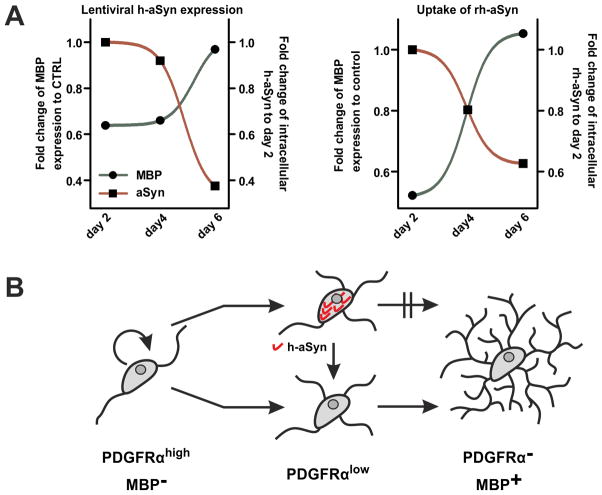
We finally analyzed temporal dynamics of MBP expression in dependence of the intracellular h-aSyn level separately in lentivirally transduced and rh-aSyn exposed OPCs. We observed an inverse dynamic of MBP expression and intracellular h-aSyn levels for both approaches (Fig. 7A). At day 2 and 4 of differentiation, MBP expression (SYN transduced: day 2: 64±8%, day 4: 66±9%; rh-aSyn exposed: day 2: 52±10%; day 4: 80±4% of control cells) was severely reduced compared to control conditions while high levels of intracellular h-aSyn were detected (SYN transduced: day 2: set as 100%; day 4: 92±12%; rh-aSyn exposed: day 2: set as 100%; day 4: 80±10% of day 2). In contrast, h-aSyn levels were drastically reduced at day 6 of differentiation (SYN transduced: 38±6%; rh-aSyn exposed: 63±14% of day 2) paralleled by restored MBP expression (SYN transduced: 97±12%; rh-aSyn exposed: 106±8% of control conditions). These findings suggest that MBP expression is closely linked to the intracellular level of h-aSyn (Fig. 7B).
Fig. 7. Inverse dynamics of MBP expression and intracellular h-aSyn levels.
(A) Intracellular levels of h-aSyn (normalized to h-aSyn signal at day 2 of differentiation) and fold change of MBP expression (compared to control conditions, i.e. to CTRL transduced cultures or without rh-aSyn exposure, respectively) are blotted. MBP expression increases in parallel with declining levels of intracellular h-aSyn using both approaches. (B) Proposed model for the effect of intracellular h-aSyn on the differentiation potential of OPCs. In presence of high h-aSyn levels, maturation towards high MBP-expressing pre-myelinating oligodendrocytes is impaired. After the intracellular h-aSyn level declines, the maturation potential is restored.
Discussion
Analyzing the onset of oligodendrocyte maturation in vitro
To study whether aSyn impairs remyelination in MSA, we analyzed the early phase of OPC differentiation, i.e. the initiation of maturation from PDGFRα-positive OPCs to myelin gene expressing pre-myelinating oligodendrocytes. Therefore, we used primary OPCs derived from neonatal rats which differentiated in absence of mitogenic stimulation within 6 days in a high proportion to mature MBP-positive oligodendrocytes that exhibited advanced phenotypes including enlarged plasma membrane sheaths. To dissect maturation of primary OPCs from a molecular point of view, we chose a set of three dynamically expressed oligodendroglial markers. PDGFRα labels early OPCs, whereas CNPase is long known to be one of the first myelin specific proteins upregulated during differentiation (Noble et al., 1988; Reynolds and Wilkin, 1988). MBP is the most abundantly expressed myelin protein and labels oligodendrocytes at a rather late stage of differentiation (Aggarwal et al., 2011; Knapp et al., 1988). This pattern is recapitulated by our primary OPCs culture during the applied differentiation paradigm which allows the monitoring of early OPC maturation in vitro.
Dual approach to analyze effects of intracellular h-aSyn on OPC maturation
Using lentiviral particles coding for h-aSyn and GFP separated by an IRES sequence (SYN), we obtained a high yield of h-aSyn expressing OPCs. Furthermore, we detected neither a difference in the proportions of Olig2-positive oligodendroglial cells upon transduction nor increased numbers of apoptotic cells upon h-aSyn expression. In contrast, Kragh and colleagues reported the susceptibility of aSyn expressing oligodendrocytes for FAS mediated apoptosis (Kragh et al., 2013). In this study, the authors triggered aSyn aggregation and apoptosis by co-overexpression of p25α. However, we did not apply any further stimuli triggering h-aSyn aggregation and thus, the absence of enhanced apoptosis in our system suggests that aSyn aggregation and GCI formation are prerequisites for oligodendrocytic cell death in MSA. Without influencing survival of Olig2-positive cells during differentiation and being not different from CTRL transduced controls in terms of efficiency, lentiviral transduction is a valid approach to analyze the effect of h-aSyn expression on OPC maturation.
Exogenous addition of rh-aSyn to the culture medium of OPCs represents the second approach to analyze effects of h-aSyn on OPC maturation. Within the first 6 hours upon rh-aSyn administration, primary OPCs take up rh-aSyn in a time-dependent manner. In line with these results obtained in primary OPCs, a time dependent aSyn uptake by OPC-like Oli-neu cells within the first 6 hours upon administration of rh-aSyn was recently demonstrated (Kisos et al., 2012). In addition, we detected h-aSyn-positive immunofluorescence within PDGFRα-positive cells further confirming that OPCs are able to take up rh-aSyn from the supernatant.
Both strategies – lentiviral vector based h-aSyn expression as well as exposure to rh-aSyn –resulted in clearly detectable intracellular h-aSyn. Comparing subcellular distribution, OPCs expressing h-aSyn upon lentiviral transduction show broadly spread h-aSyn immunofluorescence within the entire cell including processes and the nucleus. In contrast, rh-aSyn taken up from the supernatant is rather densely detected in the perinuclear cytoplasm and appears in a more punctate pattern. The latter observation might be attributable to the localization of rh-aSyn within endosomes as recent studies indicate that aSyn is taken up by oligodendrocytes via endocytosis. Kisos and colleagues demonstrated a clathrin-dependent endocytotic aSyn uptake in OPC-like Oli-neu cells (Kisos et al., 2012). In addition, two independent studies recently reported a dynamin-1 dependent uptake of recombinant aSyn monomers by oligodendrocytic cell lines concurring an endocytotic uptake mechanism of aSyn in oligodendrocytes (Konno et al., 2012; Reyes et al., 2014).
Intracellular h-aSyn impairs OPC maturation
In order to study the impact of aSyn on the onset of OPC maturation, we assessed aSyn expressing OPCs for early morphological changes. We analyzed the number and length of primary processes at day 2 of differentiation as cellular homogeneity and dynamics of process extension are very high at early stages of differentiation (Barateiro et al., 2013; Huang et al., 2011). Having less and shorter primary processes, the onset of maturation in SYN transduced oligodendrocytes is severely disrupted. Molecularly, we demonstrated that h-aSyn expression leads to a significantly delayed upregulation of myelin gene expression evidenced on the mRNA and protein levels. We were also able to show an increase in protein levels of PDGFRα in h-aSyn expressing OPCs compared to controls which was still present at day 6 of differentiation. This observation might be due to an inhibition of protein degradation mechanisms by h-aSyn as impaired proteasomal activity leads to accumulation of PDGFRα protein (Matei et al., 2007). Indeed, a direct interaction of h-aSyn with both the ubiquitin-proteasome system and the autophagy-lysosome pathway with consecutive functional impairment of these protein degradation pathways was shown possibly contributing to the observed accumulation of PDGFRα protein (Cuervo et al., 2004; Snyder et al., 2003; Tanaka et al., 2001). In summary, these findings point towards a delayed OPC maturation upon h-aSyn expression and exclude h-aSyn mediated oligodendrocytic cell death as cause for the observed reduction in myelin gene expression. In line with the present study, we previously demonstrated that forced h-aSyn expression in CG4 cells remarkably reduced their differentiation capacity (May et al., 2014). In this earlier study, we used an alternative approach and generated a stable h-aSyn expressing CG4 line using a Sleeping Beauty transposon vector.
In line with the lentiviral approach, maturation of primary OPCs exposed to 1μM rh-aSyn is also disrupted. However, significantly reduced MBP protein at early stages of differentiation is accompanied by unaltered PDGFRα and CNPase protein levels implicating a less pronounced interference of rh-aSyn with OPC differentiation upon uptake than in h-aSyn expressing OPCs. The temporal dynamic of the rh-aSyn uptake suggests that the critical level of intracellular rh-aSyn is reached after induction of CNPase and downregulation of PDGFRα. Thus, we may observe an interference of rh-aSyn at a distinct later developmental status during oligodendrocyte maturation compared to the lentiviral transduction approach. Moreover, lentivirally expressed h-aSyn may undergo post-translational modifications previously reported to exacerbate aSyn dependent effects (Diepenbroek et al., 2014; Kragh et al., 2009).
Reduction of intracellular h-aSyn levels restores myelin gene expression
An interesting observation in both experimental paradigms – lentiviral transductions and exposure experiments – is the almost complete restoration of myelin gene expression at day 6 of differentiation paralleled by a strong reduction of intracellular h-aSyn. GFP mRNA also significantly declines by day 6 of differentiation indicating that EF1α promoter activity is reduced during differentiation. Despite of its decline on the mRNA level, GFP protein levels do not significantly change during OPC differentiation. However, reaching expression maximum at day 4 of differentiation, GFP protein level is strongly decreased by day 6. Although lentiviral vectors are used for long term expression studies, the observed decline of EF1α promoter activity is in line with a recent report analyzing GFP expression driven by the EF1α promoter upon lentiviral transduction during differentiation of mouse embryonic stem (ES) cells (Hong et al., 2007). Therein, GFP mRNA significantly declines during differentiation to less than 10% in fully differentiated neurons compared to proliferative ES cells indicating that the promoter activity is downregulated in vitro upon differentiation. Nevertheless, the observed reduction of aSyn expression during late stage differentiation of oligodendrocytes matches the physiological pattern of aSyn expression since it is downregulated in fully differentiated oligodendrocytes after initial upregulation (Richter-Landsberg et al., 2000).
Although matching the physiological expression dynamics, downregulation of aSyn expression in mature oligodendrocytes is in contradiction to the accumulation of aSyn as GCIs in mature oligodendrocytes of MSA patients. However, we do not observe h-aSyn aggregation in a GCI-like manner most likely due to the lack of further stimuli (e.g. oxidative stress, environmental toxins) required to trigger aSyn aggregation (Nuber et al., 2014; Xiang et al., 2013). As proposed by Ahmed and colleagues (2013), aSyn aggregation might occur in delayed maturating oligodendrocytes and thus, we hypothesize in the light of the present findings that increased aSyn levels in maturating oligodendrocytes of MSA patients cause a delayed maturation of oligodendrocytes and further exogenous stimuli consecutively trigger GCI formation and oligodendrocytic dysfunction.
Conclusion
In summary, the present study shows an interference of h-aSyn with the early maturation of primary OPCs. Analyzing i) OPCs expressing h-aSyn upon lentiviral transduction as well as ii) OPCs that incorporated extracellular rh-aSyn, our findings suggest that the intracellular level of h-aSyn affects the maturation potential of primary OPCs. Upon lentiviral h-aSyn expression, morphology of OPCs at early stages of maturation is severely affected accompanied by altered progenitor and myelin gene expression profiles. Moreover, OPCs take up extracellular added rh-aSyn leading to reduced MBP expression at the onset of maturation. Finally, h-aSyn levels decline during differentiation in both lentivirally transduced and rh-aSyn exposed OPCs restoring myelin gene expression. Taken into account that interventional approaches are very limited in MSA, enhancing maturation and remyelination by lowering the level of aSyn within OPCs may open a novel therapeutical window for MSA treatment. Future studies (i) analyzing the impact of aSyn on OPC maturation in appropriate in vivo models and (ii) defining molecular mediators by which h-aSyn interferes with OPC maturation will contribute to understand the impact of aSyn on OPC maturation and will help to reveal promising interventional targets for MSA therapy.
Experimental Methods
Cell culture
Primary OPCs were derived from mixed glial cultures isolated from P0-P2 neonatal Wistar rats as described elsewhere with minor modifications (McCarthy and de Vellis, 1980). Briefly, mixed glial cultures were kept in Dulbecco’s minimum eagle medium supplemented with 10% FCS and 1% penicillin/streptomycin at 37°C and 5% CO2. After 10–14 days of culture, loosely attached microglia were removed by shaking for 1h at 200rpm and 37°C. OPCs were enriched by additional 17h shaking at 200rpm and 37°C, and seeded at a density of 50,000 cells/mm2 on poly-ornithine coated plates and cultured in SATO medium (Bottenstein and Sato, 1979) supplemented with platelet derived growth factor and fibroblast growth factor-2 at 20ng/ml (SATO/GF). Differentiation of primary OPCs was conducted by replacing growth factors with 1% FCS (SATO/FCS). During differentiation, supernatant was replaced with fresh SATO/FCS every second day.
Lentiviral vectors
For transgene delivery, lentiviral vectors under the control of an elongation factor-1 alpha (EF1α) promoter were used and prepared as described previously (Winner et al., 2011). Briefly, lentiviral vectors coding for h-aSyn followed by an IRES sequence and the GFP coding sequence were used (SYN). Lentiviral vectors coding solely for GFP preceded by an IRES signal served as control (CTRL). Viral particles were produced by transfecting HEK293T cells with the coding vector and the following three packaging vectors: pMDL, pREV, and pVSVG.
Transduction procedures
One day after seeding, transduction was conducted by replacing the supernatant with fresh SATO/GF containing viral particles. For all experiments, a MOI of 2 was used. After one day of incubation, supernatant was aspirated and cells were washed once with PBS prior to starting differentiation using SATO/FCS.
Exposure to rh-aSyn
Rh-aSyn was prepared as described previously (Xiang et al., 2013). After shake off procedure and 2 days of recovery, differentiation of primary OPCs was started in presence of 1μM (14.4μg/ml) rh-aSyn. Each second day during differentiation, OPCs were either harvested or supernatant was replaced with fresh SATO/FCS including rh-aSyn.
Immunocytochemistry
For immunocytochemical analyses, cells were fixed using 4% paraformaldehyde for 15 minutes. After blocking in PBS supplemented with 3% donkey serum and 0.1% triton-X100 (blocking buffer), cells incubated with primary antibodies diluted in blocking buffer overnight at 4°C. The following primary antibodies were used: rat anti MBP (AbD serotec; MCA409S; 1:500), goat anti PDGFRα (R&D systems; AF1062; 1:100), rabbit anti Olig2 (Millipore; AB9610; 1:1000), mouse anti Olig2 (clone 211F1.1; Millipore; MABN50; 1:250), rabbit anti activated Caspase 3 (Asp175; Cell Signaling; #9661; 1:500), rat anti human alpha-synuclein (15G7; ENZO; ALX-804-258; 1:200), rat anti GFP (GERBU; GF090R; 1:1000), and mouse anti vimentin (Millipore; MAB3400; 1:1000). After subsequent washing steps, incubation with fluorescent secondary antibodies in blocking buffer was conducted for 2h at RT: donkey anti rat 488 (Invitrogen; 1:3000), donkey anti rat Rhodamine Red™-X (Dianova; 1:3000), donkey anti goat 647 (Invitrogen; 1:3000), donkey anti rabbit 568 (Invitrogen; 1:3000), and donkey anti mouse 647 (Dianova; 1:3000). Prior to mounting with Prolong Gold Antifade, nuclei were counterstained with DAPI (Sigma; 1:10,000). Microscopy was performed using an Axio Imager.M2 fluorescence microscope with ApoTome technology (Carl Zeiss) and the AxioVision Software. Cell counting was performed semi-automatically using the AutMess module for AxioVision.
Analysis of morphology
In order to analyze morphology, a total of 50 cells per condition derived from two different OPC preparations and transductions was subjected to Scholl analysis as previously described (Barateiro et al., 2013). Briefly, primary processes of GFP/Olig2-positive cells after 2 days of differentiation were outlined in accordance to vimentin expression using the NeuronJ plugin for ImageJ. Scholl analysis was performed by adopting the following parameters: start radius 4μm, step size 4μm, end radius 80μm.
Protein isolation and Western blotting
For Western blotting, total protein of primary cultures was extracted using radioimmunoprecipitation assay buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, protease inhibitor cocktail (Roche), 1% NP-40, 0.1% SDS, 0.5% S-DOC). Protein concentrations were determined using BCA protein assay kit (Thermo Scientific) prior to loading 7.5μg of total protein on 4–12% bis-tris gels (Invitrogen). Proteins were blotted on polyvinylidene fluoride membranes optimized for fluorescence detection systems (Millipore). After blocking for 1h in 1% bovine serum albumin diluted in PBS-T (PBS supplemented with 0.1% Tween-20), membranes were probed with primary antibodies overnight at 4°C: rat anti MBP (AbD serotec; MCA409S; 1:500), goat anti PDGFRα (R&D systems; AF1062; 1:250), mouse anti CNPase (clone 11-5B; Millipore; MAB326; 1:1000), rat anti h-aSyn (15G7; ENZO; ALX-804-258; 1:30), rabbit anti GFP (E385; Abcam; ab32146; 1:1000), mouse anti h-aSyn (Syn211; Thermo Scientific; MA1-12874; 1:500), and mouse anti GAPDH (Millipore; MAB374; 1:100,000). Consecutively, membranes were incubated with fluorescence-conjugated secondary antibodies: donkey anti rat 488 (Invitrogen; 1:3000), donkey anti goat 568 (Invitrogen; 1:3000), and donkey anti mouse 647 (Dianova; 1:3000). Signal capture was conducted using a Fusion FX7 detection system (PeqLab). Densitometric quantifications were performed using the Bio1D software (Vilber Lourmat) by normalizing signals to GAPDH expression.
Real time polymerase chain reaction (RT-PCR)
Total RNA was isolated using the RNeasy Mini Kit (Qiagen) following manufacturer’s instructions. Briefly, cells were homogenized in 350μl RLT buffer supplemented with 0.1 M 2-mercaptoethanol. Genomic DNA was removed by subjecting lysates to gDNA elimination columns prior loading on silica gel membrane columns to extract total RNA. After sequential washing steps, RNA was eluted using deionized, RNase-free water. RNA concentration was determined by spectometry using Nano-Drop technology (PeqLab). Reverse transcription of total RNA was performed using GoScript™ Reverse Transcription System (Promega) following the kit’s manual for first-strand cDNA synthesis. Random primers were annealed to 50ng of total RNA. RT-PCRs were conducted on a Light Cycler 480 system (Roche) with the SSO Fast EvaGreen Supermix (Biorad) using the volume corresponding to 150pg of the input RNA. Expression levels of target genes were normalized to GAPDH expression. The following sequences were used as primers: gapdh forward (5′-CACAGTCAAGGC-TGAGAATGGGAAG-3′) and gapdh reverse (5′-GTGGTTCACACCCATCACAAACATG-3′); pdgfra forward (5′-CTGCACCAAGTCAGGCCCCA-3′) and pdgfra reverse (5′-ATAGGAGGCCGGCCGATCGT-3′); cnpase forward (5′-GCCCAACAGGATGTGG-TGAGGAG-3′) and cnpase reverse (5′-CTTCCGGCTGCCGTGTACGG-3′); mbp forward (5′-ACTACGGCTCCCTGCCCCAG-3′) and mbp reverse (5′-GGGATGGAGGGGGTGT-ACGAGG-3′); gfp forward (5′-CACTACCTGAGCACCCAGTC-3′) and gfp reverse (5′-TTGTACAGCTCGTCCATGCC-3′). Primers specific for h-aSyn were described elsewhere (Ebrahimi-Fakhari et al., 2011).
Data analysis
Graphical and statistical data analyses were performed using GraphPad Prism® 5.0 (GraphPad Software). Data are presented as mean ± standard error of mean (SEM). Statistics were conducted on the basis of either ANOVA (in the case of the Scholl analysis and MBP/h-aSyn ratio comparison), two-tailed student’s t-test or one sample t-test when reference values were fixed and considered to be significantly different when p-values were lower than 0.05 (* = p < 0.05, ** = p < 0.01, *** = p < 0.001).
Acknowledgments
This work was supported by the Interdisciplinary Center of Clinical Research (IZKF, TP E18: “Assessing developmental potential and differentiation capabilities of NG2-positive cells in the healthy and diseased central nervous system”), the Bavarian State Ministry of Education and Culture, Science and Arts in the framework of the Bavarian Molecular Biosystems Research Network, and the Bavarian Research Network Induced Pluripotent Stem Cells (ForIPS). We thank Nada Ben Abdallah and Jochen Klucken for discussion and helpful comments. Holger Wend, Daniela Graef, and Holger Meixner are gratefully acknowledged for excellent technical support.
Abbreviations
- act-Casp3
activated Caspase3
- aSyn
alpha-synuclein
- CG4
central glia-4
- CNPase
2′,3′-cyclic-nucleotide-phosphodiesterase
- CNS
central nervous system
- DLB
Dementia with Lewy bodies
- EF1α
elongation factor 1-alpha
- ES
embryonic stem
- FCS
fetal calf serum
- GCI
glial cytoplasmic inclusion
- GF
growth factor
- GFP
green fluorescent protein
- h-aSyn
human alpha-synuclein
- IRES
internal ribosomal entry site
- MBP
myelin basic protein
- MC
medium change
- MOI
multiplicity of infection
- MS
multiple sclerosis
- MSA
multiple system atrophy
- MSA-P
multiple system atrophy with predominant parkinsonism
- OPC
oligodendrocyte progenitor cell
- PD
Parkinson’s disease
- PDGFRα
platelet derived growth factor receptor-alpha
- rh-aSyn
recombinant human wildtype alpha-synuclein
- RT-PCR
real-time PCR
- TD
transduction
Footnotes
The authors declare no conflicts of interest.
References
- Aggarwal S, Yurlova L, Simons M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 2011;21:585–593. doi: 10.1016/j.tcb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Asi YT, Lees AJ, Revesz T, Holton JL. Identification and quantification of oligodendrocyte precursor cells in multiple system atrophy, progressive supranuclear palsy and Parkinson’s disease. Brain Pathol. 2013;23:263–273. doi: 10.1111/j.1750-3639.2012.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asi YT, Simpson JE, Heath PR, Wharton SB, Lees AJ, Revesz T, Houlden H, Holton JL. Alpha-synuclein mRNA expression in oligodendrocytes in MSA. Glia. 2014;62:964–970. doi: 10.1002/glia.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barateiro A, Miron VE, Santos SD, Relvas JB, Fernandes A, Ffrench-Constant C, Brites D. Unconjugated bilirubin restricts oligodendrocyte differentiation and axonal myelination. Mol Neurobiol. 2013;47:632–644. doi: 10.1007/s12035-012-8364-8. [DOI] [PubMed] [Google Scholar]
- Behar T, McMorris FA, Novotny EA, Barker JL, Dubois-Dalcq M. Growth and differentiation properties of O-2A progenitors purified from rat cerebral hemispheres. J Neurosci Res. 1988;21:168–180. doi: 10.1002/jnr.490210209. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. Pattern of remyelination in the CNS. Nature. 1974;249:577–578. doi: 10.1038/249577a0. [DOI] [PubMed] [Google Scholar]
- Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D, Abbruzzese G, Tabaton M. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson’s disease and normal subjects. Neurosci Lett. 2000;287:65–67. doi: 10.1016/s0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Diepenbroek M, Casadei N, Esmer H, Saido TC, Takano J, Kahle PJ, Nixon RA, Rao MV, Melki R, Pieri L, Helling S, Marcus K, Krueger R, Masliah E, Riess O, Nuber S. Overexpression of the calpain-specific inhibitor calpastatin reduces human alpha-Synuclein processing, aggregation and synaptic impairment in [A30P]alphaSyn transgenic mice. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci. 2011;31:14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Groves AK, Barnett SC, Franklin RJ, Crang AJ, Mayer M, Blakemore WF, Noble M. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 1993;362:453–455. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- Hong S, Hwang DY, Yoon S, Isacson O, Ramezani A, Hawley RG, Kim KS. Functional analysis of various promoters in lentiviral vectors at different stages of in vitro differentiation of mouse embryonic stem cells. Mol Ther. 2007;15:1630–1639. doi: 10.1038/sj.mt.6300251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Baron-Van Evercooren A, Chambon P, Ffrench-Constant C, Franklin RJ. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011;14:45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H, Hasegawa M, Iwatsubo T, Kretzschmar HA, Haass C. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisos H, Pukass K, Ben-Hur T, Richter-Landsberg C, Sharon R. Increased neuronal alpha-synuclein pathology associates with its accumulation in oligodendrocytes in mice modeling alpha-synucleinopathies. PLoS One. 2012;7:e46817. doi: 10.1371/journal.pone.0046817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp PE, Skoff RP, Sprinkle TJ. Differential expression of galactocerebroside, myelin basic protein, and 2′,3′-cyclic nucleotide 3′-phosphohydrolase during development of oligodendrocytes in vitro. J Neurosci Res. 1988;21:249–259. doi: 10.1002/jnr.490210217. [DOI] [PubMed] [Google Scholar]
- Konno M, Hasegawa T, Baba T, Miura E, Sugeno N, Kikuchi A, Fiesel FC, Sasaki T, Aoki M, Itoyama Y, Takeda A. Suppression of dynamin GTPase decreases alpha-synuclein uptake by neuronal and oligodendroglial cells: a potent therapeutic target for synucleinopathy. Mol Neurodegener. 2012;7:38. doi: 10.1186/1750-1326-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh CL, Fillon G, Gysbers A, Hansen HD, Neumann M, Richter-Landsberg C, Haass C, Zalc B, Lubetzki C, Gai WP, Halliday GM, Kahle PJ, Jensen PH. FAS-dependent cell death in alpha-synuclein transgenic oligodendrocyte models of multiple system atrophy. PLoS One. 2013;8:e55243. doi: 10.1371/journal.pone.0055243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh CL, Lund LB, Febbraro F, Hansen HD, Gai WP, El-Agnaf O, Richter-Landsberg C, Jensen PH. Alpha-synuclein aggregation and Ser-129 phosphorylation-dependent cell death in oligodendroglial cells. J Biol Chem. 2009;284:10211–10222. doi: 10.1074/jbc.M809671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, Rowitch DH. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63:499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- Matei D, Satpathy M, Cao L, Lai YC, Nakshatri H, Donner DB. The platelet-derived growth factor receptor alpha is destabilized by geldanamycins in cancer cells. J Biol Chem. 2007;282:445–453. doi: 10.1074/jbc.M607012200. [DOI] [PubMed] [Google Scholar]
- May VEL, Ettle B, Poehler AM, Nuber S, Ubhi K, Rockenstein E, Winner B, Wegner M, Masliah E, Winkler J. α-Synuclein impairs oligodendrocyte progenitor maturation in multiple system atrophy. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.02.028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DW, Johnson JM, Solano SM, Hollingsworth ZR, Standaert DG, Young AB. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm. 2005;112:1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Nuber S, Tadros D, Fields J, Overk CR, Ettle B, Kosberg K, Mante M, Rockenstein E, Trejo M, Masliah E. Environmental neurotoxic challenge of conditional alpha-synuclein transgenic mice predicts a dopaminergic olfactory-striatal interplay in early PD. Acta Neuropathol. 2014;127:477–494. doi: 10.1007/s00401-014-1255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos D, Ewans L, Pham-Dinh D, Knott J, Reynolds R. Upregulation of alpha-synuclein in neurons and glia in inflammatory demyelinating disease. Mol Cell Neurosci. 2006;31:597–612. doi: 10.1016/j.mcn.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Reyes JF, Rey NL, Bousset L, Melki R, Brundin P, Angot E. Alpha-synuclein transfers from neurons to oligodendrocytes. Glia. 2014;62:387–398. doi: 10.1002/glia.22611. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Wilkin GP. Development of macroglial cells in rat cerebellum. II. An in situ immunohistochemical study of oligodendroglial lineage from precursor to mature myelinating cell. Development. 1988;102:409–425. doi: 10.1242/dev.102.2.409. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM. alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res. 2000;62:9–14. doi: 10.1002/1097-4547(20001001)62:1<9::AID-JNR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Masliah E. Neuronal to oligodendroglial alpha-synuclein redistribution in a double transgenic model of multiple system atrophy. Neuroreport. 2012;23:259–264. doi: 10.1097/WNR.0b013e3283509842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B. Aggregated and monomeric alpha-synuclein bind to the S6′ proteasomal protein and inhibit proteasomal function. J Biol Chem. 2003;278:11753–11759. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- Solano SM, Miller DW, Augood SJ, Young AB, Penney JB., Jr Expression of alpha-synuclein, parkin, and ubiquitin carboxy-terminal hydrolase L1 mRNA in human brain: genes associated with familial Parkinson’s disease. Ann Neurol. 2000;47:201–210. [PubMed] [Google Scholar]
- Song YJ, Lundvig DM, Huang Y, Gai WP, Blumbergs PC, Hojrup P, Otzen D, Halliday GM, Jensen PH. p25alpha relocalizes in oligodendroglia from myelin to cytoplasmic inclusions in multiple system atrophy. Am J Pathol. 2007;171:1291–1303. doi: 10.2353/ajpath.2007.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Engelender S, Igarashi S, Rao RK, Wanner T, Tanzi RE, Sawa A, VLD, Dawson TM, Ross CA. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum Mol Genet. 2001;10:919–926. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Grzesiak JJ, Bouvet M, Hashimoto M, Masliah E, Shults CW. Alpha-synuclein overexpression in oligodendrocytic cells results in impaired adhesion to fibronectin and cell death. Mol Cell Neurosci. 2005;29:259–268. doi: 10.1016/j.mcn.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Ubhi K, Low P, Masliah E. Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci. 2011;34:581–590. doi: 10.1016/j.tins.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG. Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol. 2008;64:239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang W, Schlachetzki JC, Helling S, Bussmann JC, Berlinghof M, Schaffer TE, Marcus K, Winkler J, Klucken J, Becker CM. Oxidative stress-induced posttranslational modifications of alpha-synuclein: specific modification of alpha-synuclein by 4-hydroxy-2-nonenal increases dopaminergic toxicity. Mol Cell Neurosci. 2013;54:71–83. doi: 10.1016/j.mcn.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Yazawa I, Giasson BI, Sasaki R, Zhang B, Joyce S, Uryu K, Trojanowski JQ, Lee VM. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]