Abstract
Background
Methamphetamine use has been previously associated with poor medication adherence, but, to date, there have been no studies that have conducted event-level analyses on correlates of medication adherence in studies of pharmacologic agents for methamphetamine dependence.
Methods
We pooled data from two previous, randomized controlled trials (using bupropion and mirtazapine, respectively) for methamphetamine dependence and used a mixed effects logistic model to examine correlates of daily opening of the medication event monitoring system (MEMS) cap as a repeated measure. We explored whether periods of observed methamphetamine use via urine testing were associated with study medication adherence based on MEMS cap openings.
Results
We found a significant negative association between methamphetamine-urine positivity and event-level study medication adherence as measured by MEMS cap openings (AOR: 0.69; 95% CI: 0.49–0.98). In addition, age (AOR: 1.07; 95% CI: 1.02–1.11) and depressive symptoms (AOR: 0.78; 95% CI: 0.64–0.90) were significantly associated with adherence. Finally, participants were more likely to open their study medication bottles on days when they presented for in-person urine testing.
Conclusions
Our event-level analysis shows that methamphetamine use can be associated with reduced medication adherence as measured by MEMS cap openings in pharmacologic trials, which corroborates prior research. These findings may suggest that medication adherence support in pharmacologic trials among methamphetamine users may be needed to improve study compliance and could be targeted towards periods of time when there are more likely to not open their study medication pill bottles.
Keywords: methamphetamine, medication adherence, men who have sex with men
1. INTRODUCTION
Methamphetamine is a psychostimulant with significant physical and psychological consequences (Darke et al., 2008) that has been linked with high-risk sexual behavior (Rawson et al., 2002) and HIV and STI incidence for men who have sex with men (MSM; Koblin et al., 2006; Colfax et al., 2011). Researchers have evaluated various medications in clinical trials to treat methamphetamine dependence, but there is currently no pharmacotherapy approved by the Food and Drug Administration (FDA) for this use (Elkashef et al., 2007; Colfax et al., 2011). Many trials among methamphetamine-dependent populations have been inconclusive and complicated by challenges including low levels of medication adherence (Longo et al., 2009; Colfax et al., 2011; Anderson et al., 2012).
Methamphetamine use is broadly associated with decreased medication adherence, particularly for HIV antiretroviral therapy (Hinkin et al., 2004, 2006; Marquez et al., 2009, Reback et al., 2010). Previous studies have described how methamphetamine use can negatively affect medication adherence via disrupted sleep, altered eating behavior, difficulty maintaining a schedule, and planned medication holidays during methamphetamine binges (Reback et al., 2003; Hinkin et al., 2006). In addition, methamphetamine use can lead to psychiatric symptoms such as depression and neurocognitive changes that may also affect adherence (Hinkin et al., 2006; Zweben et al., 2004). Studies of interventions for methamphetamine dependence have had difficulty retaining participants (Elkashef et al., 2007; Shoptaw et al., 2008), but even among those studies with good retention, adherence to pharmacologic therapies has made results difficult to interpret. A trial of modafinil showed no effect, but adherence was at most 50% (Anderson et al., 2012). We documented a significant reduction in methamphetamine use with mirtazapine, although adherence by MEMS caps was only 48% overall; the effect of mirtazapine was positively associated with adherence (Colfax et al., 2011). Self-report has been observed to overestimate medication adherence (Das et al., 2010); using more complex methods such as medication labeling, which is limited by preparation and analysis costs, and electronic medication event monitoring systems (MEMS) based on pill bottle opening may provide data that is more objective than self-report despite several MEMS measurement limitations (e.g., if a person is using an additional, external device to store pills or has removed several capsules during a pill bottle opening; Liu et al., 2001; Colfax et al., 2011). Nevertheless, the direct effect of methamphetamine on medication adherence in these trials is poorly understood. We were unable to identify any studies that conducted an event-level analysis on the correlates of medication adherence in pharmacologic trials for methamphetamine dependence using MEMS as a primary measurement outcome. We evaluated the predictors of events or observed periods of medication adherence within-person and hypothesized that days of weekly urine collection and periods of methamphetamine use would be associated with periods of adherence to study medications.
2. METHODS
2.1 Study Design
A total of ninety participants were pooled from two previously published placebo-controlled medication trials that evaluated bupropion (n = 30) and mirtazapine (n = 60). Full details of each study were previously described (Das et al., 2008; Colfax et al., 2011). Briefly, participants were MSM aged 18–60 years with methamphetamine dependence (verified by the Structured Clinical Interview for DSM-IV) who were interested in reducing their methamphetamine use or abstaining completely. To be eligible for the study, participants needed at least one methamphetamine-positive urine toxicology test during the initial screening period. Both trials excluded men with any acute medical or psychiatric conditions, abnormal baseline laboratory studies, a history of major depression, or men with HIV infection and a CD4 count less than 200 cells/µL.
2.2 Measures and Study Procedures
Participants presented for weekly urine collection and received substance use counseling based on a manualized treatment using both motivation interviewing and cognitive behavioral therapy techniques. At 4-week intervals, subjects had a repeat physical exam, laboratory testing, and behavioral assessments. We used audio computer-assisted self-interview (ACASI) to collect information about various participant characteristics including drug use (including frequency, type of drug, and route of administration), substance use treatment, and sexual risk behavior. We assessed depression symptoms based on the Center for Epidemiologic Studies-Depression Scale (CES-D; Andresen et al., 1994). Based on previous literature, we deemed participants with a score higher than 16 as having significant depressive symptoms (Andresen et al., 1994). Participants who also reported use of one or more illicit substances (e.g., marijuana, poppers, crack-cocaine, powdered cocaine, heroin, GHB, MDMA, ketamine, or hallucinogens) in addition to methamphetamine were classified as polysubstance users.
2.3 Primary Outcome
Our primary outcome of interest was adherence to study medications as recorded by the daily opening of the MEMS cap as a repeated measure. For each participant, days with a record of a MEMS cap opening were classified as an adherent day.
2.4 Statistical Analysis
To assess whether there were significant differences in demographic characteristics in the two trials pooled for this study, we performed Wilcoxon-Mann-Whitney or Fisher’s exact tests, depending on whether the demographic variable of interest was continuous or categorical, respectively. We fitted a mixed effects logistic regression model that examined the relationship between daily opening of the MEMS cap as a repeated measure and predictors of interest, including methamphetamine use (defined as the last collected methamphetamine-positive urine test result carried forward for the days between urine tests), age, treatment group (placebo vs. active arm), presence of significant depressive symptoms (i.e., CES-D score was greater than16), HIV status, number of days since the last urine result, polysubstance use, and whether the participant was in the bupropion or mirtazapine study. In our mixed effects logistic regression model, we accounted for repeated measures of our main predictor of interest and covariates whose values could change across the course of the study periods (e.g., CES-D score, polysubstance use). In order to assess for differential medication adherence in the two studies, we included an interaction term examining the association between the last collected urine result and the specific study sample. We also flexibly modeled trends in the baseline rate of bottle opening using a cubic spline in days since randomization.
3. RESULTS
3.1 Sample characteristics
Participants enrolled in the two trials were similar with respect to age, race, education level, and employment status. The mean age for the pooled participants was 39 years (standard deviation = 9.2), approximately 12% reported no income, and 60% were unemployed. Most participants (93%) had at least a high school education and 66% had a regular healthcare provider. White, Caucasian, or European Americans made up 59% of the sample followed by people who were Asian-American or Pacific Islander (16%), Latino (14%), mixed or multiracial (6%), African-American (3%), or Native-American (2%). Most subjects (92%) reported using other substances in addition to methamphetamine. The mean CES-D score at baseline was 18.12 (standard deviation = 11.01). Over 57% of the study sample used methamphetamine at least three times per week, and over 78% of the participants had tried to stop or reduce their methamphetamine use in the past.
3.2 Event-Level Analyses
Results of the mixed effects logistic model revealed a significant, negative association between a methamphetamine-positive urine test and event-level medication adherence (AOR: 0.69; 95% CI: 0.49–0.98). There was a significant, positive association between age and event-level medication adherence (AOR: 1.07; 95% CI: 1.02–1.11). Having a significant level of depressive symptoms based on the CES-D was associated with decreased odds of event-level medication adherence (AOR: 0.78; 95% CI: 0.64–0.90). Being in the mirtazapine study was also associated with lower adherence when compared to the bupropion study participants (AOR: 0.22; 95% CI: 0.10–0.50) and the interaction term between study type and having a methamphetamine-positive test had a p-value < 0.05.
In addition, results indicate a significant negative association between days prior to urine-testing and event-level medication adherence; AORs for three days prior, two days prior, and one day prior to testing were 0.63 (95% CI: 0.51–0.79), 0.74 (95% CI: 0.59–0.93), and 0.61 (95% CI: 0.48–0.76), respectively, when compared to the day of the study visit. Similarly, results indicate a significant negative association between days after urine-testing and event-level medication adherence; AORs for one day after, two days after, and three days after testing were 0.78 (95% CI: 0.62–0.98), 0.61 (95% CI: 0.49–0.77), and 0.66 (95% CI: 0.53–0.83). Participants were more likely to be non-adherent on non-urine testing days (Figure 1). We later performed subset analyses in which we limited the data sample to urine testing windows (either 48 hours or 72 hours prior to or after the date of the urine test). The positive association between age and medication adherence noted in the main analysis persisted in the subset analyses, and the negative association between having significant depressive symptoms and medication adherence remained significant in the pre-urine test windows but not in the post-urine test windows. However, the negative association between having a methamphetamine-positive urine test and medication adherence lost statistical significant in these separate analyses.
Figure 1.
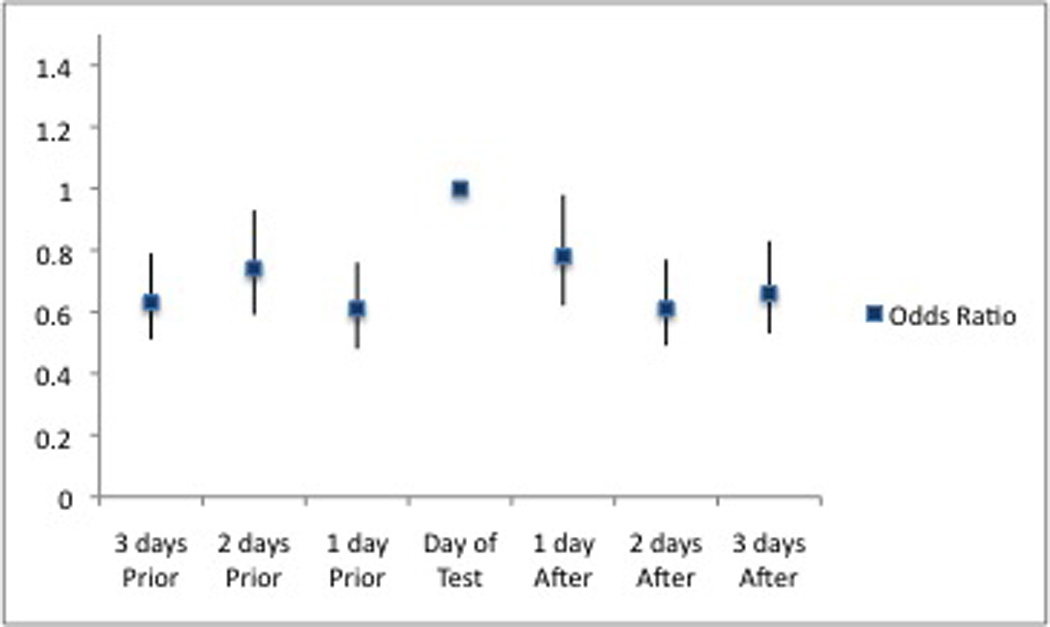
The relationship between likelihood of being medication adherence vs. day of urine testing (72-hour pre- and post-test windows).
4. DISCUSSION
In our study sample, we found a significant negative association with methamphetamine-positive urine tests and using MEMS cap opening as a measure of medication adherence. Previous researchers have shown a similar association between methamphetamine use and medication adherence using measures including self-reported methamphetamine use (Marquez et al., 2009), urine toxicology studies (Hinkin et al., 2006), and diagnostic criteria for substance abuse or dependence (Hinkin et al., 2004). Past research using semi-structured interviews among HIV-positive methamphetamine-using MSM revealed that the effects of methamphetamine can lead to disruptions in eating and sleeping schedules, which in turn led to unplanned non-adherence to antiretroviral medications (Reback et al., 2003). It is possible participants in our studies also experienced methamphetamine-related schedule disruptions that led to less pill bottle opening and subsequent non-adherence to their study medications.
In addition, we found a negative association with symptomatic depression as represented by repeated measures of the total CES-D score and MEMS cap opening in our sample, which appears consistent with earlier literature. Previous authors have hypothesized that depression can affect medication adherence via cognitive deficits, social isolation, and hopelessness (DiMatteo et al., 2002). Co-occurring psychosocial problems such as substance use and depression have been linked to increased HIV risk behavior in men who have sex with men (Stall et al., 2003), and our results may further highlight the need for multilevel interventions that can address syndemics in this population.
The positive association between increasing age and MEMS cap opening is also consistent with prior research. Previous authors have discussed explanations for this association including the possibility of less medication-related lifestyle disruption for older participants and a more extensive lifetime history of taking medications (Hinkin et al., 2002, 2004; Barclay et al., 2007). Our findings indicate that researchers should consider age as one of the many factors that might influence adherence in future medication trials.
Participants in our sample were less likely to open medication pill bottles when they were not providing urine samples for the study, which may suggest that additional efforts are needed to support adherence on non-visit days. For examples, previous studies have examined the use of text messages or mobile phone applications as a way to support improved adherence (Park et al., 2014). An alternative approach would be to have more frequent study visits, although this has been associated with retention difficulties in prior studies (Elkashef et al., 2007; Shoptaw et al., 2008). Our findings may support using strategies that target times when we would expect worse pill adherence without overburdening participants with frequent reminders or visits.
Our study had limitations. We pooled data from two studies, which would obscure any contextual differences such as the years the studies were conducted, the different medication adherence rates and treatment effects in the bupropion and mirtazapine trials, and the slight trend towards statistical significance (p-value = 0.052) when comparing the mean ages of the two study groups. We also relied on MEMS caps for adherence rather than a biologic measure. A MEMS cap could be removed by participants without taking the pills, and while we considered that any measurement bias should be within-person, we could not confirm whether trial participants actually took medications. Future studies could bolster our findings by using biological markers that monitor medication consumption. Finally, our findings linking depressive symptoms to reduced pill bottle opening should be understood in the context that both the bupropion and mirtazapine trials excluded people with major depression.
We did find a significant association between recent methamphetamine use and study drug adherence as measured by MEMS cap opening, and participants were more likely to open their pill bottles on study visit days, suggesting a need to support adherence on non-visit days. In addition, we found that age and significant depressive symptoms are two of several factors to consider when assessing a participant’s likelihood to be adherent in a pharmacologic study. Future studies that include multiple strategies to target these potential barriers to taking medications consistently may result in improved adherence measures and superior data to support the development of pharmacologic therapies for methamphetamine dependence.
Table 1.
Demographic characteristics of the bupropion (n = 30) and mirtazapine (n = 60) participants in the pooled study
| Demographic variables | Bupropion | Mirtazapine | P-values | |
|---|---|---|---|---|
| Age | 36.5 (+/−9.34) | 40.5(+/− 8.90) | 0.052 | |
| Education level | 0.372 | |||
| Less than high school graduate | 3 (10.00%) | 3 (5.00%) | ||
| High school graduate or GED | 7 (23.33%) | 12 (20.00%) | ||
| Some college, 2-year college degree, or Associate's Degree | 14 (46.67%) | 22 (36.67%) | ||
| Bachelor's Degree | 4 (13.33%) | 19 (31.67%) | ||
| Master's degree or higher | 2 (6.67%) | 4 (6.67%) | ||
| Racial/Ethnic Background | ||||
| African American or Black | 3 (10.00) | 11 (18.33) | 0.481 | |
| Asian American or Pacific Islander | 1 (10.00) | 2 (3.33) | ||
| Latino, Hispanic, or Chicano | 6 (20.00) | 7 (11.67) | ||
| Native American, American Indian, or Alaskan Native | 1 (3.33) | 1 (1.67) | ||
| White, Caucasian, or European American | 16 (53.33) | 37 (61.67) | ||
| Mixed or Multi-racial | 3 (10.00) | 2 (3.33) | ||
| Employment Status | 0.503 | |||
| Not employed | 20 (66.67) | 34 (56.67) | ||
| Employed full-time | 4 (13.33) | 11 (18.33) | ||
| Employed part-time | 5 (16.67) | 13 (21.67) | ||
| Currently a student, not employed | 0 | 2 (3.33) | ||
| Currently a student, employed full or part-time | 1 (3.33) | 0 | ||
P-values represent test statistics for Wilcoxon-Mann-Whitney or Fisher Exact tests depending on whether the variable of interest was continuous or categorical, respectively.
Highlights.
We used data from two medication trials that targeted methamphetamine dependence.
We examined whether methamphetamine use was associated with medication adherence.
There was a negative association between methamphetamine use and MEMS cap openings.
People were more likely to open pill bottles on in-person study visit days.
Acknowledgements
Dr. Hermanstyne was supported by the National Institutes of Mental Health Research Education Programs Supporting Psychiatric Residents (R25 MH060482; Corresponding PI Carol Mathews) and the UCLA-Robert Wood Johnson Foundation Clinical Scholars Program. The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The remaining authors have no other acknowledgements.
Role of funding source
Nothing declared
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Keith Hermanstyne contributed to study design, data analysis, and drafted the first draft of the manuscript. Glenn-Milo Santos contributed to study design, data analysis, and editing of the manuscript. Eric Vittinghoff contributed to data analysis, helped create the statistical model, and conceptualized the design of the original studies. Deirdre Santos supervised enrollment of participants and conceptualized the design of the original studies. Grant Colfax conceptualized the design and secured funding for the original studies. Phillip Coffin contributed to study design and editing of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
Contributor Information
Keith A. Hermanstyne, Email: khermanstyne@mednet.ucla.edu.
Glenn-Milo Santos, Email: glenn-milo.santos@sfgov.org.
Eric Vittinghoff, Email: eric@biostat.ucsf.edu.
Deirdre Santos, Email: Deirdre.Santos@sfdph.org.
Grant Colfax, Email: grant_colfax@yahoo.com.
Phillip Coffin, Email: phillip.coffin@sfdph.org.
REFERENCES
- Anderson AL, Li S-H, Biswas K, McSherry F, Holmes T, Iturriaga E, Kahn R, Chiang N, Beresford T, Campbell J, Haning W, Mawhinney J, McCann M, Rawson R, Stock C, Weis D, Yu E, Elkashef AM. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120:135–141. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am. J. Prev. Med. 1994;20:77–84. [PubMed] [Google Scholar]
- Barclay TR, Hinkin CH, Castellon SA, Mason KI, Reinhard MJ, Marion SD, Levine AJ, Durvasula RS. Age-associated predictors of medication adherence in HIV-positive adults: health beliefs, self-efficacy, and neurocognitive status. Health Psychol. 2007;26:40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, Santos G-M, Das M, Santos DM, Huffaker S, Matheson T, Gasper J, Vittinghoff E, Colfax GN. Aripiprazole for the treatment of methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:751–761. doi: 10.1111/add.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colfax GN, Santos G-M, Das M, Santos DM, Matheson T, Gasper J, Shoptaw S, Vittinghoff E. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch. Gen. Psychiatry. 2011;68:1168–1175. doi: 10.1001/archgenpsychiatry.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Das M, Santos D, Matheson T, Santos G-M, Chu P, Vittinghoff E, Shoptaw S, Colfax GN. Feasibility and acceptability of a phase II randomized pharmacologic intervention for methamphetamine dependence in high-risk men who have sex with men. AIDS. 2010;24:991–1000. doi: 10.1097/qad.0b013e328336e98b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Magidson JF, Schuster RM, Safren SA. ACT HEALTHY: A combined cognitive-behavioral depression and medication adherence treatment for HIV-Infected substance users. Cogn. Behav. Pract. 2010;17:309–321. doi: 10.1016/j.cbpra.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave SS, Copas A, Richens J, White RG, Kosambiya JK, Desai VK, Stephenson JM. HIV and STI prevalence and determinants among male migrant workers in India. PLoS ONE. 2012;7:e43576. doi: 10.1371/journal.pone.0043576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med. Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li S-H, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2007;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Glasner-Edwards S, Marinelli-Casey P, Hillhouse M, Ang A, Mooney LJ, Rawson R. Depression among methamphetamine users. J. Nerv. Ment. Dis. 2009;197:225–231. doi: 10.1097/NMD.0b013e31819db6fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, Myers HF, Longshore D. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2006;11:185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Thrasher MB, Goetz MB, Stefaniak M. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl. 1):S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24:1448–1452. doi: 10.1080/09540121.2012.687816. [DOI] [PubMed] [Google Scholar]
- Krousel-Wood M, Islam T, Muntner P, Holt E, Joyce C, Morisky DE, Webber LS, Frohlich ED. Association of depression with antihypertensive medication adherence in older adults: cross-sectional and longitudinal findings from CoSMO. Ann. Behav. Med. 2010;40:248–257. doi: 10.1007/s12160-010-9217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, Christian J, Maldonado T, Duran D, Kaplan AH, Wenger NS. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann. Intern. Med. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- Longo M, Wickes W, Smout M, Harrison S, Cahill S, White JM. Randomized controlled trial of dexamphetamine maintenance for the treatment of methamphetamine dependence. Addiction. 2009;105:146–154. doi: 10.1111/j.1360-0443.2009.02717.x. [DOI] [PubMed] [Google Scholar]
- Marquez C, Mitchell SJ, Hare CB, John M, Klausner JD. Methamphetamine use, sexual activity, patient–provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006. AIDS Care. 2009;21:575–582. doi: 10.1080/09540120802385579. [DOI] [PubMed] [Google Scholar]
- McCann D, Li S-H. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci. Ther. 2011;18:414–418. doi: 10.1111/j.1755-5949.2011.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DL, Sarafian F, Silvestre A, Brown T, Jacobson L, Badri S, Detels R. Evaluation of adherence and factors affecting adherence to combination antiretroviral therapy among white, hispanic, and black men in the MACS Cohort. J. Acquir. Immune Defic. Syndr. 2009;52:290–293. doi: 10.1097/QAI.0b013e3181ab6d48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park LG, Howie-Esquivel J, Dracup K. A quantitative systematic review of the efficacy of mobile phone interventions to improve medication adherence. J. Adv. Nurs. 2014;0:1–22. doi: 10.1111/jan.12400. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Kowalczyk WJ, Botsko M, Tomassilli J, Golub SA. Aggregate versus day level association between methamphetamine use and HIV medication non-adherence among gay and bisexual Men. AIDS Behav. 2013;17:1478–1487. doi: 10.1007/s10461-013-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reback CJ, Larkins S, Shoptaw S. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. AIDS Care. 2003;15:775–785. doi: 10.1080/09540120310001618621. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Amaro H, Strathdee SA, Zians J, Patterson TL. Ethnic differences in substance use, sexual risk behaviors, and psychosocial factors in a sample of heterosexual methamphetamine users. Subst. Use Misuse. 2009;44:1101–1120. doi: 10.1080/10826080802490055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson A-N, La Garza De R, Newton T, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall R, Mills T, Williamson J, Hart T, Greenwood G, Paul J, Pollack L, Binson D, Osmond D, Catania JA. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am. J. Public Health. 2003;93:939–942. doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Weiser SD, Petersen ML, Ragland K, Kushel MB, Bangsberg DR. A marginal structural model to estimate the causal effect of antidepressant medication treatment on viral suppression among homeless and marginally housed persons with HIV. Arch. Gen. Psychiatry. 2010;67:1282–1290. doi: 10.1001/archgenpsychiatry.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Simpson CA, Huang J, Roth DL, Stewart KE. Utility of an interactive voice response system to assess antiretroviral pharmacotherapy adherence among substance users living with HIV/AIDS in the rural South. AIDS Patient Care STDS. 2013;27:280–286. doi: 10.1089/apc.2012.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M Methamphetamine Treatment Project. Psychiatric symptoms in methamphetamine users. Am. J. Addict. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]


