Abstract
Ghrelin regulates homeostatic food intake, hedonic eating, and is a mediator in the stress response. In addition, ghrelin has metabolic, cardiovascular, and anti-aging effects. This cross-sectional study examined associations between total plasma ghrelin, caloric intake based on 3 day diet diaries, hedonic eating attitudes, stress-related and metabolic factors, and leukocyte telomere length in overweight (n=25) and obese women (n=22). We hypothesized associations between total plasma ghrelin and eating behaviors, stress, metabolic, cardiovascular, and cell aging factors among overweight women, but not among obese women due to lower circulating ghrelin levels and/or central resistance to ghrelin. Confirming previous studies demonstrating lowered plasma ghrelin in obesity, ghrelin levels were lower in the obese compared with overweight women. Among the overweight, ghrelin was positively correlated with caloric intake, giving in to cravings for highly palatable foods, and a flatter diurnal cortisol slope across 3 days. These relationships were non-significant among the obese group. Among overweight women, ghrelin was negatively correlated with insulin resistance, systolic blood pressure, and heart rate, and positively correlated with telomere length. Among the obese subjects, plasma ghrelin concentrations were negatively correlated with insulin resistance, but were not significantly correlated with blood pressure, heart rate or telomere length. Total plasma ghrelin and its associations with food intake, hedonic eating, and stress are decreased in obesity, providing evidence consistent with the theory that central resistance to ghrelin develops in obesity and ghrelin’s function in appetite regulation may have evolved to prevent starvation in food scarcity rather than cope with modern food excess. Furthermore, ghrelin is associated with metabolic and cardiovascular health, and may have anti-aging effects, but these effects may be attenuated in obesity.
Ghrelin, the only known appetite-stimulating hormone in humans, is a 28 amino acid protein produced principally in the stomach. Ghrelin and its receptor, growth hormone secret agogue receptor (GHS-R), are found extensively throughout the body, indicating widespread central and peripheral functions (Gnanapavan et al., 2002; Korbonits, Goldstone, Gueorguiev, & Grossman, 2004; Muccioli, Baragli, Granata, Papotti, & Ghigo, 2007). When energy supply is low, ghrelin is secreted from gut mucosa and acts centrally by signaling to the hypothalamic arcuate nucleus and also stimulates the vagal afferent nerves (Date & Kangawa, 2012) to increase appetite and food intake. After food ingestion, plasma levels decrease (Couce et al., 2006).
In addition to the regulation of homeostatic food intake ghrelin appears to be involved in hedonic eating, and maybe necessary for the experience of food-induced reward (Diz-Chaves, 2011; King, Isaacs, O’Farrell, & Abizaid, 2011; Kirsz & Zieba, 2011; Skibicka, Hansson, Alvarez-Crespo, Friberg, & Dickson, 2011). There is evidence that ghrelin increases preference for sweet taste (Disse et al., 2010; Malik, McGlone, Bedrossian, & Dagher, 2008). Moreover, ghrelin was found to increase, rather than decrease, in response to palatable food intake under conditions of satiety, suggesting that ghrelin’s central signaling may drive hedonic food consumption in the absence of caloric need (Monteleone et al., 2013).
Furthermore, accumulating evidence suggests that ghrelin signaling is important in the neurological response to stressors (Asakawa et al., 2001; Chuang et al., 2011; Raspopow, Abizaid, Matheson, & Anisman, 2010; Rouach et al., 2007) and stress increases ghrelin in humans (Harrold, Dovey, Blundell, & Halford, 2012; Lowe & Butryn, 2007). As stress induces eating and the motivation to eat comfort foods (Adam & Epel, 2007; Dallman, 2010), increased ghrelin may mediate the motivation to eat under stress.
Studies of diet-induced obese mice have demonstrated hypothalamic resistance to ghrelin, in which ghrelin no longer stimulates activation of neuropeptide Y(NPY) and agouti-related peptide (AgRP) neurons, which trigger hunger (Briggs, Enriori, Lemus, Cowley, & Andrews, 2010; Briggs et al., 2013; Finger, Dinan, & Cryan, 2012). Diet-induced obesity in mice suppresses the neuroendocrine ghrelin system by decreasing mRNA of ghrelin and the enzyme which converts ghrelin to its active acylated form (ghrelin o-acyltransferase) in the stomach, expression of GHS-R in the hypothalamus, and acylated and total plasma ghrelin (Briggs et al., 2010). In humans, plasma ghrelin levels have been found to be substantially lower among obese compared to lean adults (Druce et al., 2005; McLaughlin, Abbasi, Lamendola, Frayo, & Cummings, 2004; Ozkan et al., 2009; Tschop et al., 2001). These findings have led to the speculation that obesity may induce central ghrelin resistance in humans, and relationships between ghrelin and eating behavior observed in lean adults may not apply to obese populations (Andrews, 2011). However, little research has examined the central resistance theory in obese humans. Furthermore, it is unclear whether evidence of central ghrelin resistance would be observed in an overweight population, presumably where there is also decreased plasma ghrelin, but perhaps not as low as levels found in obese populations (David E. Cummings, 2006; Sumithran et al., 2011; Tschop et al., 2001).
In terms of other actions of ghrelin, ghrelin has been associated with increased plasma glucose levels, decreased insulin and insulin resistance, and with lower blood pressure and heart rate (Broglio et al., 2001; Eizadi, Afsharmand, Behbudi, & Sohaily, 2011; Freeman, Carmo, Adi, & da Silva, 2013; Garcia & Korbonits, 2006; Okumura et al., 2002; Tong et al., 2010; Verhulst & Depoortere, 2012). Ghrelin may also have anti-aging effects as it reduces inflammation (Dixit et al., 2004) and regulates growth hormone secretion (Veldhuis & Keenan, 2012), which controls secretion of insulin-like growth factor-1 (IGF-1). Lower levels of IGF-1 are related to shorter telomere length, which is a marker of cell aging (Barbieri et al., 2009; Kaplan et al., 2009; Moverare-Skrtic et al., 2009), and which has been linked to diabetes risk factors (Demissie et al., 2006; Gardner et al., 2005) and earlier mortality (Blackburn, Greider, & Szostak, 2006; Cawthon, Smith, O’Brien, Sivatchenko, & Kerber, 2003; Epel et al., 2009; Fitzpatrick et al., 2011). Epidemiological studies have also shown that telomere length is moderately correlated with chronological age (for a review, see Sanders & Newman, 2013). Telomere length is thought to represent biological aging of the cell in that the longer the telomere length, the greater the cell’s ability to keep dividing, conversely, the shorter the telomere length, the greater the cell’s replicative senescence (Blackburn, 2000). As peripherally circulating ghrelin appears to exert some beneficial metabolic, cardiovascular and anti-aging effects (Aoki et al., 2013; Garcia & Korbonits, 2006; Granado, Priego, Martin, Villanua, & Lopez-Calderon, 2005), it is possible that the lowered circulating levels found in obesity may negatively impact ghrelin’s favorable physiological effects.
Indeed, there are many putative roles for ghrelin beyond homeostatic appetite control, and research is needed to describe and understand the multiplicity of ghrelin’s functions and any differential functions of ghrelin among overweight and obese adults. In this preliminary cross-sectional analysis, we examined associations of ghrelin with caloric intake, hedonic eating, psychological and physiological indicators of stress, metabolic and aging-related factors among overweight and obese women. First, we hypothesized that circulating plasma ghrelin levels would be lower among obese compared to overweight women. Second, we hypothesized that ghrelin maybe positively related to food intake, hedonic eating, and stress among overweight women, but that such correlations would be attenuated among obese women. We also aimed to determine whether ghrelin’s previously established associations with glucose and insulin levels, blood pressure, and heart rate would be observed among the overweight and obese samples. Finally, we explored the relationship in both groups between ghrelin and leukocyte telomere length, an association that has not yet been examined in human or animal research.
Methods
This study is a cross-sectional, secondary analysis of baseline data collected from a randomized controlled pilot study of a mindfulness intervention to reduce stress eating (Daubenmier et al., 2011). As previously reported in the parent study, overweight and obese women were recruited from the San Francisco Bay Area community using flyers and local media. Exclusion criteria included: weight over 300lbs, diabetes, taking medication that could affect weight loss, taking pain steroids or antipsychotic medication, post-menopausal, history of bilateral oophorectomy, total hysterectomy, polycystic ovary syndrome, active endocrine disorder, pregnancy, less than 1 year postpartum or breastfeeding, current eating disorder, alcohol addiction, drug addiction, positive urine test for diabetes and opiate use, and English illiteracy. A total of 322 women were screened, and 47 women with Body Mass Index (BMI) of 25–40 were enrolled into the study and completed 2 baseline assessments by staff at the UCSF General Clinical Research Center (GCRC) (Daubenmier et al., 2011). This study was performed at the University of California, San Francisco (UCSF) with approval from the institutional review board.
Biological Measures
At the first baseline visit, a digital scale (Wheelchair Scale 6002, Scale-Tronix, Carol Stream, IL) was used to measure weight in kg to the nearest 0.10kg and a standard stadiometer (Perspective Enterprises, Portage, Mich, USA) was used to measure height to the nearest 1/8 inch to calculate BMI. Blood pressure and heart rate were also measured (CritikonDinamap 1846SX Non Invasive Vital Signs Monitor, GE Healthcare, Milwaukee, WI). To assess blood pressure, participants rested for five minutes and three measurements were taken, each one minute apart. The three measurements were averaged to determine systolic and diastolic blood pressure values.
At the second baseline visit, nurses confirmed with participants that they completed a 12 hour fast the night before. Morning blood plasma samples were taken from an indwelling forearm catheter for total plasma ghrelin levels, insulin, glucose, and telomere length. Blood samples were drawn into tubes on ice containing ethylenediaminetetraacetic acid (EDTA) as anticoagulant and kept on ice at all times. The tubes were centrifuged for 10 min at 3000g at 4°C, transferred to aliquot vials, and frozen at −70°C until assaying.
Total plasma ghrelin was measured without an extraction step using a commercial RIA (Phoenix Peptide, Phoenix, AZ), as described previously (D. E. Cummings, Clement, et al., 2002; D. E. Cummings et al., 2001). Insulin was assayed with a radioimmunoassay kit using an I125-Iodinated insulin tracer, anti-Human Insulin Specific antibody, and human insulin standards from Linco Research, Inc. (St. Charles, MO). The intra- and inter-assay variation are 5.8 and 10.2% respectively. Glucose was measured enzymatically (glucose oxidase) with an automated YSI 2300 Analyzer from YSI Life Sciences (Yellow Spring, Ohio). Instrument precision is within 2%. Insulin resistance was measured by homeostatic model assessment (HOMA) from values obtained from plasma glucose and insulin assays (Wallace, Levy, & Matthews, 2004).
Salivary cortisol was measured by participants at home. Participants were instructed on how to collect saliva samples by salivating into a straw in 2 ml “SaliCaps” tubes. They were instructed to collect the first sample before getting out of bed, and not to eat, drink or brush their teeth or engage in vigorous activity between the morning samples or for 20 minutes before the evening sample. Participants collected salivary cortisol samples upon awakening, 30 minutes later, and prior to bedtime. Samples for the cortisol awakening response (CAR) were collected each day over 4 days, and samples for the cortisol slope was collected each day over 3 days. The CAR was calculated by subtracting the 30-minute post awakening sample from the waking sample. Higher CARs are related to greater perceptions of life stress (Chida & Steptoe, 2009). Cortisol slope was calculated by subtracting the morning value from the bedtime value such that higher values indicated steeper slopes. Flatter cortisol slopes are associated with threatening, traumatic, and uncontrollable experiences of stress (Schulkin, 2004). Values were averaged over the days collected. Hormone analysis was performed at Dresden Lab Service, overseen by Dr. Clemens Kirschbaum, at the Dresden University of Technology (Germany) using a commercial chemiluminescence immunoassay (CLIA, IBL, Hamburg, Germany). Values greater than 100 mg/dl were excluded as it is probable that these values are physiologically abnormal, possibly due to blood contamination or a medical or assay issue. They are also statistical outliers.
Mean telomere length was measured quantitatively in peripheral blood nuclear cells. Telomere length was measured from DNA by a quantitative polymerase chain reaction (PCR) assay that determines the relative ratio of telomere repeat copy number to single-copy gene copy number (T/S ratio) in experimental samples as compared with a reference DNA sample, as described previously (Kiecolt-Glaser et al., 2013). All PCRs were carried out on a Roche Lightcycler 480 real-time PCR machine with 384-tube capacity (Roche Diagnostics Corporation, Indianapolis, IN) (J. Lin et al., 2010).
Self-Report Measures
Three Day Diet Diaries
Participants were given paper forms to complete food diaries over a three day period. As the participants consumed food, they were instructed to record the types of food they ate and portion sizes. The diaries were used to assess total daily caloric intake, and percentages of dietary calories from carbohydrates, fats, proteins, and sugar. The Food Processor SQL software was used for nutritional analysis.
Hedonic Eating Attitude
Participants completed on-line questionnaires at home
Hedonic eating attitudes were measured using the Food Craving Inventory, which measures specific cravings and giving in to cravings for foods high in fat, sugar, carbohydrates, and high fat fast-food over the previous month. A craving was defined as “an intense desire to consume a particular food (or food type) that is difficult to resist,” and the subscales ask participants to rate the frequency of cravings on a 5-point Likert scale (1 = never, 2 = rarely, 3 =sometimes, 4 = often, and 5= always/almost every day) (White, Whisenhunt, Williamson, Greenway, & Netemeyer, 2002). Examples of foods in the high fat category included fried fish, fried chicken, hot dog; the sweet category included brownies, cookies, cakes; and the fast food fats category included hamburgers, French fries, pizza. To provide a robust measure of highly palatable food cravings, two composite scores were created. For the composite score of craving highly palatable foods, we averaged the scores for high fat, sweet, and fast-food fats. To measure “giving in” to cravings for highly palatable foods, we averaged the scores for giving in to cravings for high fat, sweet, and fast food fats. Cronbach’s alpha for these composite measures of the Food Craving Inventory in this sample was 0.86 for craving highly palatable foods, and 0.91 for giving in to cravings.
Psychological Stress
Psychological stress was measured using the 10-item version of the Perceived Stress Scale. This measure utilizes a 5-point Likert scale to evaluate the degree to which individuals consider their lives to be stressful over the previous month (from 0 = never, to 4 = very often). Scores range from 0 to 40, with higher scores associated with higher perceived stress (S. Cohen & Janicki-Deverts, 2012; S. Cohen, Kamarck, & Mermelstein, 1983). Cronbach’s alpha for the Perceived Stress Scale in this study was 0.84.
Emotional Eating
Emotional eating is an individual’s trait behavior of eating in response to negative emotions
Emotional eating was measured with the Emotional Eating subscale of the Dutch Eating Behavior Questionnaire. The scale asks participants to rate their desire to eat in response to clearly labeled and diffuse emotions, such as boredom and anger using a 5-point Likert scale (ranging from 1 = never to 5 = very often). It has been widely used to assess emotional eating in a variety of populations including obese women (Pinaquy, Chabrol, Simon, Louvet, & Barbe, 2003; van Strien, Frijters, Bergers, & Defares, 1986). Cronbach’s alpha for the Dutch Eating Behaviors Questionnaire in this sample was 0.78.
Statistical Analysis
Distributions and descriptive statistics of all variables were reviewed, including means and standard deviations for continuous variables. Non-normal, skewed distributions were natural log transformed. Student’s t-tests and chi-square tests were performed to compare baseline characteristics between the overweight and obese groups. The associations between ghrelin and the measured variables were examined using Pearson correlations. One overweight participant, and three obese participants were taking anti-hypertensive medications at the time of the study, and their data were excluded from the correlations of plasma ghrelin with blood pressure. All data were analyzed using Stata 12.0 (College Station, TX, USA).
Results
Sample characteristics
The sample characteristics are described in detail in the report on the mindfulness intervention for stress eating (Daubenmier et al., 2011). Out of 47 participants measured at baseline, 25 women were overweight, and 22 obese. As described previously, the sample identified as 62% white, 15% Hispanic/Latino, 15% Asian/Pacific Islander, and 9% other. The groups were similar in regards to white/non-white status by BMI group (p=0.34). Participants were found to have higher mean levels of perceived stress, compared to are presentative U.S. population sample, and higher mean levels of emotional eating compared to a representative Dutch sample (Daubenmier et al., 2011).
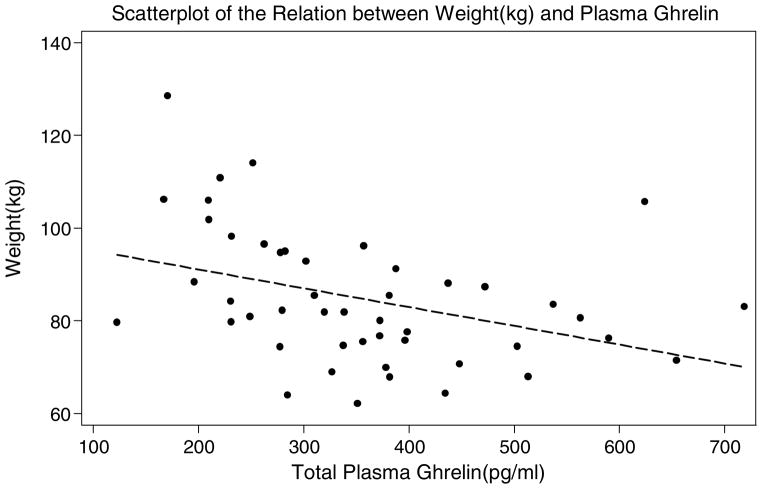
The characteristics of the overweight and obese women are described in Table 1. As expected, total fasting plasma ghrelin was lower in the obese compared to the overweight women (see Figures 1 and 2 for scatterplots). There were no statistically significant differences in total daily calorie intake between the groups. However, there were differences in percentage of calorie intake from fat and from carbohydrates between the two groups, with the obese women consuming a greater percentage of calories from fat, and lower percentage from carbohydrates compared to the overweight women. No statistically significant differences between groups were found in perceived stress, emotional eating, or hedonic eating attitudes. Among the metabolic factors, the obese women had higher mean insulin levels, and higher mean insulin resistance measured by HOMA compared to the overweight women. The groups were similar in regards to blood pressure and heart rate. There was no evidence of a significant difference in telomere length between the groups, nor after controlling for age (p = .21).
Table 1.
Baseline Characteristics of Overweight and Obese Women
| Overweight (n=25) | Obese (n=22) | p value | |||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | ||
| Age (years) | 25 | 41.76 (7.2) | 22 | 39.9 (7.5) | 0.39 |
| Weight (kg) | 25 | 75.17 (7.1) | 22 | 95.7(12.6) | <0.01 |
| Total plasma ghrelin (pg/ml) | 25 | 409.27(128.9) | 22 | 294.45 (116.2) | <0.01 |
| Food intake - Diet Diaries | |||||
| Average daily calories | 22 | 2086.59 (542.6) | 20 | 2127.48 (412.0) | 0.78 |
| % of calories from fat | 22 | 32.21 (4.4) | 20 | 35.67 (6.4) | 0.05 |
| % of calories from protein | 22 | 17.78 (6.2) | 20 | 17.62 (4.0) | 0.92 |
| % of calories from carbohydrates | 22 | 50.41 (5.8) | 20 | 45.53 (7.2) | 0.02 |
| % of calories from sugar | 22 | 17.32 (6.2) | 20 | 15.48 (4.3) | 0.27 |
| Hedonic Eating Attitudes | |||||
| Craving palatable foods | 24 | 51.67 (10.0) | 22 | 48 (12.6) | 0.28 |
| Giving into palatable foods | 23 | 43.95 (15.3) | 21 | 42.76 (15.7) | 0.80 |
| Stress-Related Factors | |||||
| Perceived stress | 25 | 19.56(6.6) | 22 | 18.45 (5.0) | 0.69 |
| Emotional Eating | 24 | 3.29 (0.7) | 22 | 3.56(0.8) | 0.23 |
| Cortisol slope (mg/dL) | 19 | 15.23 (6.6) | 23 | 13.83 (4.1) | 0.41 |
| Cortisol Awakening Response (mg/dL) | 23 | 7.06 (7.8) | 22 | 6.90 (8.1) | 0.95 |
| Metabolic, Cardiovascular, and Cell Aging Factors | |||||
| Glucose (mg/dl) | 25 | 91.94 (5.5) | 22 | 92.58 (9.7) | 0.79 |
| Insulin (μU/ml) | 25 | 10.86 (5.7) | 22 | 16.5(9.6) | 0.02 |
| Insulin Resistance (HOMA) | 25 | 2.48 (1.3) | 22 | 3.86(2.6) | 0.03 |
| Systolic blood pressure (mmHg) | 25 | 117.27 (14.0) | 22 | 123.3 (11.9) | 0.12 |
| Diastolic blood pressure (mmHg) | 25 | 72.21 (11.1) | 22 | 71.70 (6.4) | 0.84 |
| Heart rate (bpm) | 25 | 73 (10) | 22 | 74 (14) | 0.83 |
| Telomere length (t/s ratio) | 25 | 1.11 (0.16) | 20 | 1.08 | 0.18 |
Figure 1.
Scatterplot of the Relation between Weight (kg) and Plasma Ghrelin
Figure 2.
Scatterplots of the Relation between Weight (kg) and Plasma Ghrelin by BMI Group
Correlations with plasma ghrelin and other variables
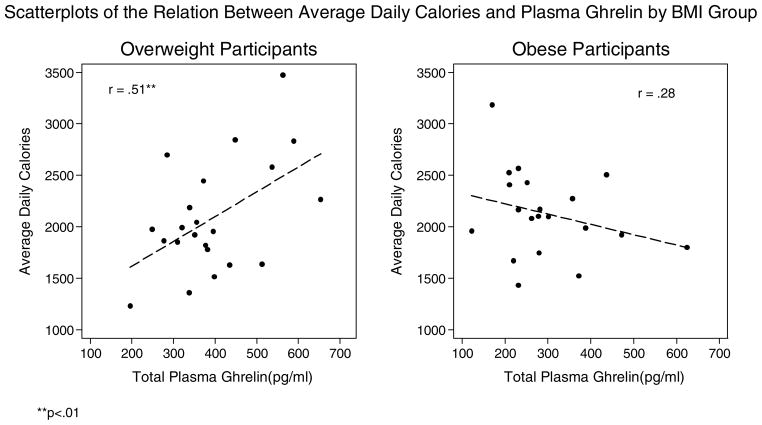
Correlations of total plasma ghrelin with food intake, hedonic eating attitudes, stress related factors, metabolic factors, and telomere length by weight status are shown in Table 2 and Figures 3–9. Among the overweight, ghrelin was positively associated with average daily caloric intake and negatively correlated with percentage of calories from protein. Ghrelin appeared to be positively related to percentage of calories from sugar, but this did not reach statistical significance (p = .058). These correlations were non-significant among the obese group. Ghrelin was not significantly related to percentage of calories from fat or carbohydrate in either group.
Table 2.
Pearson Correlations of Ghrelin with Eating, Stress, Metabolic, and Telomere Length by Group
| Overweight | Obese | |||
|---|---|---|---|---|
| n | r | n | r | |
| 3 Day Diet Diaries | ||||
| Average daily calories | 22 | .51** | 20 | −.28 |
| % of calories from fat | 22 | .14 | 20 | −.27 |
| % of calories from protein | 22 | −.43* | 20 | .19 |
| % of calories from carbohydrate | 22 | .25 | 20 | .13 |
| % of calories from sugar | 22 | .41^ | 20 | .08 |
| Hedonic Eating Attitudes | ||||
| Craving palatable foods | 24 | .24 | 22 | .10 |
| Giving in to palatable foods | 23 | .44* | 21 | .05 |
| Stress-Related Factors | ||||
| Perceived stress | 25 | .12 | 22 | .05 |
| Emotional eating | 24 | .25 | 22 | .29 |
| Cortisol Awakening Response (mg/dL) | 23 | −.01 | 22 | .11 |
| Cortisol Slope (mg/dL) | 19 | .48* | 23 | .27 |
| Metabolic, Cardiovascular, and Cell Aging Factors | ||||
| Glucose (mg/dl) | 25 | −.16 | 22 | .02 |
| Insulin (μU/ml) | 25 | −.57** | 22 | −.43* |
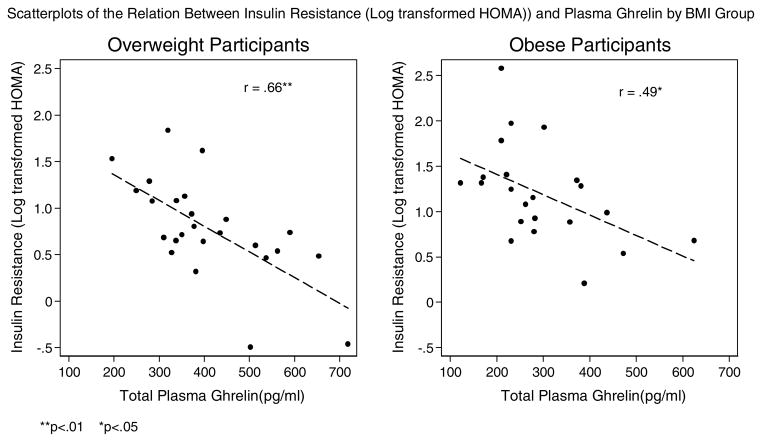
| Insulin Resistance (HOMA) (ln) | 25 | −.66** | 22 | −.49* |
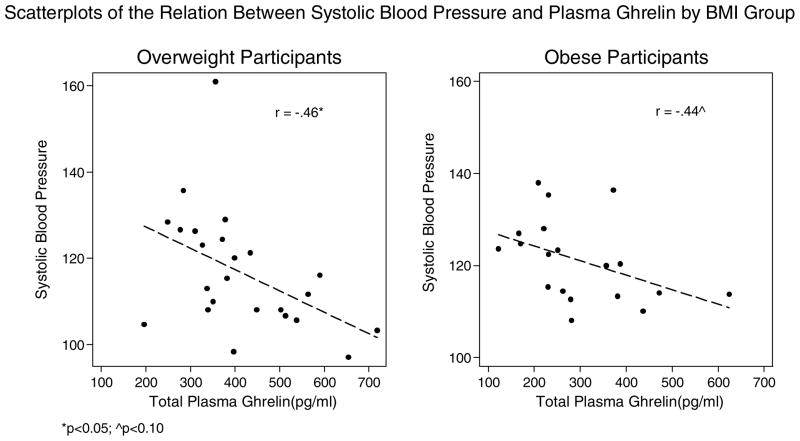
| Systolic blood pressure (mmHg)a | 24 | −.46* | 19 | −.44^ |
| Diastolic blood pressure (mmHg)a | 24 | −.38^ | 19 | −.25 |
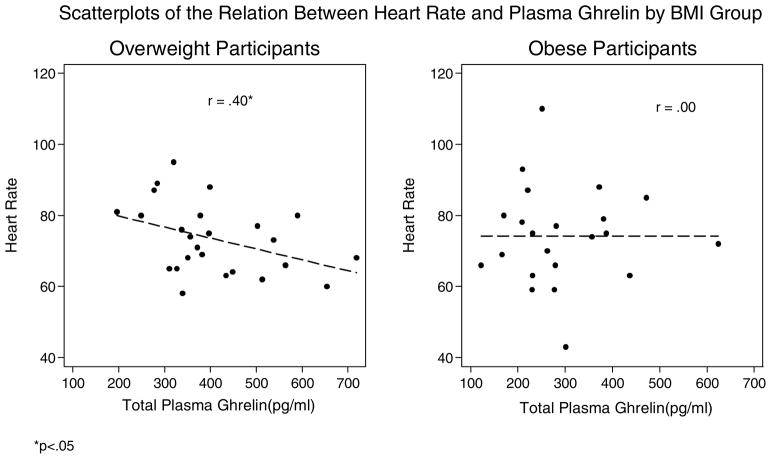
| Heart rate (bpm) | 25 | −.40* | 22 | .00 |
| Telomere length (t/s ratio) | 25 | .41* | 20 | −.28 |
Excluding participants taking anti-hypertensive medication.
p<.10;
p < .05;
p<0.01.
Figure 3.
Scatterplots of the Relation Between Average Daily Calories and Plasma Ghrelin by BMI Group
**p<.01
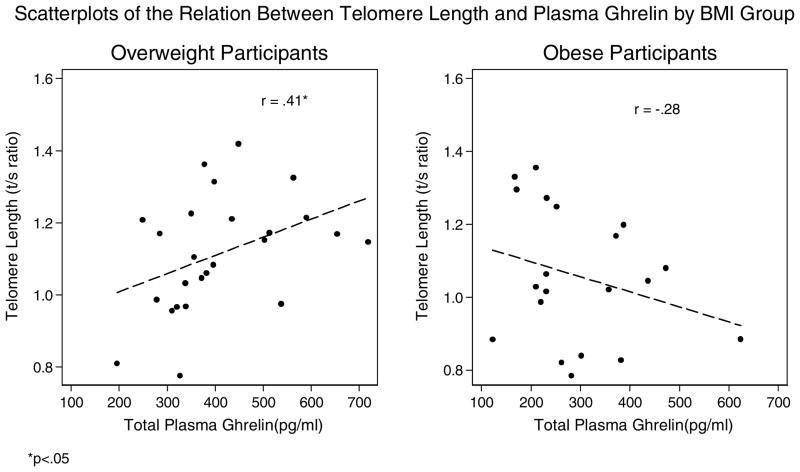
Figure 9.
Scatterplots of the Relation Between Telomere Length and Plasma Ghrelin by BMI Group
*p<.05
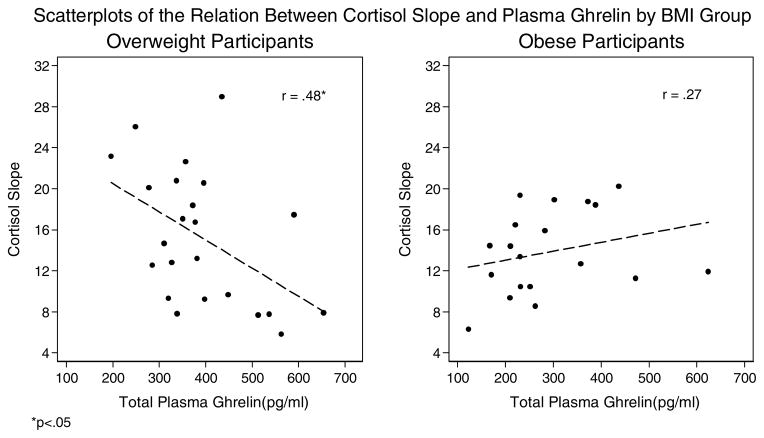
In terms of hedonic eating attitudes, ghrelin was positively associated with giving in to palatable foods in the overweight, but not in the obese group. Ghrelin was not associated with cravings for palatable foods in either group. Correlations of plasma ghrelin with perceived stress, emotional eating, and the CAR were negligible in both groups. However, ghrelin was significantly negatively related to average cortisol slope in the overweight group, but the relationship was non-significant in the obese group.
Ghrelin was not related to glucose, but was significantly negatively correlated with insulin and insulin resistance in both the overweight and obese groups. Excluding data from four participants who were taking anti-hypertensive medications, ghrelin was negatively correlated with systolic blood pressure in both groups, although the correlation did not reach statistical significance in the obese group (p = 06). There was some evidence to indicate that ghrelin was negatively correlated with diastolic blood pressure in the overweight group, although not statistically significant (p = 0.07), but we did not find any evidence of a strong correlation in the obese group.
Age was not significantly correlated with telomere length among the overweight (r = 0.08, p = 0.70), although it was among the obese group (r = −0.53, p <0.05). Telomere length was significantly positively correlated with ghrelin bivariately among the overweight women, but not among the obese group, nor after controlling for age (partial r = −0.04, p = 0.88). In order to further understand the association between ghrelin and telomere length among the overweight group, we conducted exploratory analyses correlating telomere length with dietary (total daily caloric intake, percentages of dietary calories from carbohydrates, fats, proteins, and sugar) and metabolic (glucose, insulin, cortisol, insulin resistance, blood pressure and heart rate) factors to identify potential biological mediators. We found no significant correlations with telomere length except for percentage of calories from protein, which was negatively related to telomere length (r= −0.59, p <.05).
Discussion
Our results are consistent with those of previous studies showing that plasma ghrelin decreases with weight gain, as the obese women had significantly lower levels of ghrelin than the overweight women. In addition, our overweight sample had lower levels of ghrelin compared to lean adults from other studies (Kroemer et al., 2012; Liddle, 2013). Further research is needed to elucidate the relationship between body weight and circulating ghrelin to address whether weight gain is a necessary factor for depleting circulating ghrelin levels, or if it is a marker for other underlying metabolic changes affecting total plasma ghrelin levels.
Despite the fact that the overweight women had lower plasma ghrelin levels compared to normal weight women from other studies, we found positive associations between ghrelin and eating behaviors in the overweight, consistent with findings from studies of normal weight adults and animals (Dickson et al., 2011; Disse et al., 2010; Malik et al., 2008; Skibicka et al., 2011). Specifically, we found that ghrelin was positively associated with daily caloric intake. There was also some evidence in support of ghrelin’s role in hedonic eating as overweight women reported giving in more to eating palatable foods and ghrelin was also positively associated with sugar intake, although the correlation did not quite reach statistical significance (p = 0.058). These findings provide support for the notion that ghrelin may promote hedonic eating in the overweight, as described in prior studies of lean adults and animals (Chuang et al., 2011; Monteleone et al., 2012; Perello & Zigman, 2012; Schellekens, Dinan, & Cryan, 2013). However, this pattern of results was not observed in the obese group. Correlations of ghrelin with food intake and hedonic eating attitudes were weak or tended to be negatively correlated.
One explanation for the differential pattern of findings among the overweight and obese groups is that obesity produces resistance to central signaling of ghrelin in the hypothalamus (Briggs et al., 2010). It is not yet known whether resistance also occurs in parts of the brain circuitry that signal food reward, such as the ventral tegmental area. Of note, counter to the theory of central ghrelin resistance, are the results of one study in which administration of intravenous ghrelin to overweight and obese adults increased both intake and palatability of food (Druce et al., 2005). Therefore, lack of association of ghrelin with homeostatic and hedonic eating behaviors in obesity may be attributable to lowered circulating plasma ghrelin levels or central resistance to ghrelin. Future work replicating many of the paradigms examining the role of ghrelin in hedonic eating behaviors should be conducted among obese individuals.
Even though plasma ghrelin is reduced and may not impact eating behavior in obesity, ghrelin levels increase after weight loss (D. E. Cummings, Weigle, et al., 2002; Hansen et al., 2002; Sumithran et al., 2011). Researchers have suggested that the increase of circulating ghrelin, particularly after calorie-restricted weight loss, may make individuals more vulnerable to weight regain as the NPY and AgRP neurons in the hypothalamus become re-sensitized to ghrelin’s signaling (Briggs et al., 2013). Further longitudinal study in humans is needed to observe if weight loss and rebound in plasma ghrelin restores sensitivity to ghrelin signaling and if weight re-gain occurs as a result of ghrelin re-sensitization. It has been suggested that decreased ghrelin in obesity represents a physiological adaptation to positive energy balance - rather than having a purpose in diet-induced obesity, ghrelin’s key role is to maintain homeostasis in times of negative energy balance. In other words, ghrelin’s function in appetite regulation may have evolved to prevent starvation in food scarcity rather than cope with food excess (Andrews, 2011; Briggs & Andrews, 2011).
Reduced circulating ghrelin or central resistance in obesity may impair ghrelin’s regulation of the HPA axis, as ghrelin stimulates corticotropin-releasing hormone (CRH) secretion in mice (Asakawa et al., 2001). Studies have also shown that ghrelin is responsive to stress and may play a mediating role in stress-related eating (Chuang et al., 2011; Rouach et al., 2007). In support, we found that a flatter diurnal cortisol slope, suggesting greater cortisol concentrations in circulation throughout the day, was associated with higher ghrelin levels among overweight women (Anders, 1982; Desir et al., 1980). However, we did not find this relationship in the obese group. We did not find that ghrelin was related to self-reported psychological stress or emotional eating in either weight category. These null findings may be due to the restricted range of these variables as our sample reported significantly higher levels of stress and emotional eating compared to representative samples. In addition, as cortisol may be responsive to other factors aside from psychological stress and we did not observe a significant relationship between ghrelin and the cortisol awakening response (Fries, Dettenborn, & Kirschbaum, 2009), future research could examine associations with ghrelin in laboratory controlled studies of stress to better determine the relation between ghrelin and stress as a function of weight status.
While we found evidence supporting the view that ghrelin’s actions may be impaired in obesity, we found evidence that some of ghrelin’s metabolic and cardiovascular-related actions may remain intact while others may be impaired. Specifically, in both groups of women we found that ghrelin was negatively related to insulin resistance, as found in previous research (Amini et al., 2012; Poykko et al., 2003). As plasma ghrelin decreases in the shift towards obesity, there may be increases of insulin levels and insulin resistance, which affect normal glucose homeostasis, and may increase risk for metabolic disease. Ghrelin’s action in insulin resistance may be due to the effect of higher plasma acylated ghrelin in obesity. Plasma ghrelin consists of acylated (A-GHR) and non-acylated (NA-GHR). NA-GHR accounts for 80–90% of circulating ghrelin; however, in obesity the ratio of A-GHR to NA-GHR may be higher, and higher A-GHR is associated with insulin resistance (Barazzoni et al., 2007; Pacifico et al., 2009). In addition, we found ghrelin to be negatively correlated with systolic blood pressure among the overweight and negatively correlated, although not statistically significant, in the obese group. Ghrelin is associated with lower blood pressure in healthy, normal weight adults (Garcia & Korbonits, 2006; Okumura et al., 2002). The mechanism by which ghrelin lowers blood pressure is not yet fully described; there is evidence to suggest it may be regulated through central mechanisms (Y. Lin et al., 2004) and peripheral mechanisms by increasing diuretic action through increased renal nitric-oxide production thereby decreasing salt-induced hypertension (Aoki et al., 2013). Heart rate was also negatively correlated with ghrelin among the overweight women, but not in the obese group. Central mechanisms may regulate heart rate as ghrelin modulates baroreflex-regulation of sympathetic vasomotor tone (Krapalis et al., 2012). It may be possible that as circulating ghrelin decreases or central ghrelin resistance increases in obesity some beneficial cardiovascular effects may be reduced and this could potentially explain the null association of ghrelin with heart rate and attenuated associations between ghrelin and blood pressure among the obese group.
Finally, growing research suggests that ghrelin may have anti-aging effects (Dixit et al., 2004; Granado et al., 2005). We explored the relationship between ghrelin and leukocyte telomere length, a marker of aging (Sanders & Newman, 2013). Longer telomeres have been related to less inflammation and higher levels of IGF-1, which, in part, are regulated by ghrelin (Barbieri et al., 2009; Dixit et al., 2004; Kaplan et al., 2009; Moverare-Skrtic et al., 2009). We found that ghrelin was positively associated with telomere length among the overweight but not among the obese women. We did not assess inflammatory markers or IGF-1 in this study so could not examine these factors as potential mechanisms. However, we found that ghrelin was negatively associated with daily percentage of calories from protein, and, in exploratory analyses, less dietary protein intake was associated with greater telomere length among the overweight women. Animal studies have shown that decreased protein intake is associated with longer telomeres (O’Callaghan et al., 2012; Tanrikulu-Kucuk & Ademoglu, 2012). Therefore, lower dietary protein intake might have a beneficial effect on telomere length. Overweight women with higher plasma ghrelin levels may eat foods higher in carbohydrates and fats, and as percent calories from macronutrients are dependent on one another, protein consumption may decrease. Alternatively, there is also evidence to suggest that protein intake suppresses ghrelin levels, at least acutely (Blom et al., 2006; Bowen, Noakes, & Clifton, 2006; Brennan et al., 2012; Erdmann, Topsch, Lippl, Gussmann, & Schusdziarra, 2004; Foster-Schubert et al., 2008; Moran et al., 2005; Tannous dit El Khoury, Obeid, Azar, & Hwalla, 2006). Further research is needed to elucidate the pathways by which ghrelin may affect cellular aging mechanisms, such as telomere length, and whether these pathways are attenuated in obesity.
Limitations
Our analysis is cross-sectional and therefore we are unable to determine causality. The study sample did not include men, therefore we cannot know if there are gender differences in the associations that we found. Our results should also be considered preliminary due to a small sample size. Further research is needed to examine and replicate the associations we described, in particular, by including normal weight adults. We measured total plasma ghrelin, but circulating ghrelin is found as acylated and non-acylated, both with different roles in promoting appetite and feeding (Adams, Greenway, & Brantley, 2011). Although total plasma ghrelin is lower in obesity, the ratio of acylated to non-acylated may increase in obesity (Pacifico et al., 2009). Future work should examine both acylated and non-acylated ghrelin in obesity.
Central ghrelin sensitivity may be affected by circulating leptin concentrations (Hewson, Tung, Connell, Tookman, & Dickson, 2002). Moreover, as ghrelin promotes feeding and leptin suppresses feeding, there is evidence to suggest that plasma ghrelin levels are negatively associated with plasma leptin levels and their actions on hypothalamic neurons may be oppositional, at least in normal weight adults and animals (Lockie & Andrews, 2013). However, diet-induced obese individuals are resistant to the effects of leptin (Mantzoros et al., 2011). Further research is needed to examine the relation between ghrelin and leptin in obesity.
Some studies (Al-Massadi et al., 2010; Kellokoski et al., 2005; Matsubara et al., 2004; Shibata et al., 2004), but not all (Dafopoulos, Sourlas, Kallitsaris, Pournaras, & Messinis, 2009; Gualillo et al., 2001) have found associations with ghrelin levels and ovarian hormones, so it is possible that the findings in this study are affected by participants’ menstrual cycle or use of oral contraception. Postmenopausal, and pregnant and breastfeeding women were excluded from this study, as were those with a history of polycystic ovary syndrome, oophorectomy, and hysterectomy.
Hedonic eating, diet diaries and psychological stress were measured using self-report instruments. Self-report data may introduce error as individuals may not recall information with accuracy, give socially desirable responses, or choose not to respond. Hedonic eating and psychological stress measures were collected on-line when participants were at home. We did not record or control for the time of day or other situational factors that may have influenced participant self-reports.
Conclusion
In conclusion, our results indicate a complex picture of the potential health impact of circulating ghrelin concentrations in overweight and obesity. Ghrelin in overweight people may contribute to hedonic eating and eventual weight gain. However, lowered circulating ghrelin and/or central resistance to ghrelin signaling in obesity may reduce the impact of ghrelin on homeostatic energy regulation and hedonic eating. Ghrelin may have beneficial metabolic, cardiovascular and anti-aging effects and these effects may be attenuated in obesity. Future work is needed to examine the role of ghrelin in obesity, especially as regards eating behavior.
Figure 4.
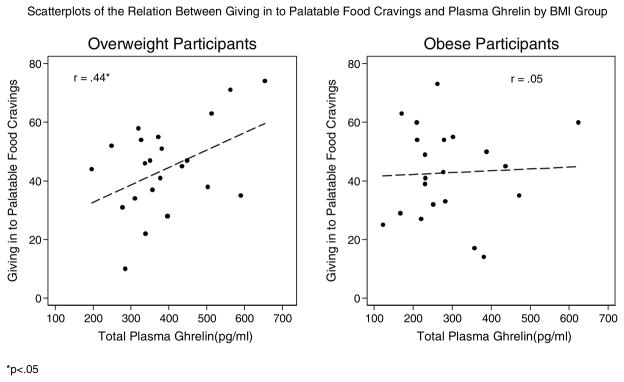
Scatterplots of the Relation Between Giving in to Palatable Food Cravings and Plasma Ghrelin by BMI Group
*p<.05
Figure 5.
Scatterplots of the Relation Between Cortisol Slope and Plasma Ghrelin by BMI Group
*p<.05
Figure 6.
Scatterplots of the Relation Between Insulin Resistance (Log transformed HOMA)) and Plasma Ghrelin by BMI Group
**p<.01 *p<.05
Figure 7.
Scatterplots of the Relation Between Systolic Blood Pressure and Plasma Ghrelin by BMI Group
*p<0.05; ^p<0.10
Excluding participants taking anti-hypertensive medication.
Figure 8.
Scatterplots of the Relation Between Heart Rate and Plasma Ghrelin by BMI Group
*p<.05
Highlights.
Ghrelin is positively associated with caloric intake and stress in overweight women.
Ghrelin is positively associated with hedonic eating in overweight women.
Ghrelin is associated with metabolic, cardiac, and cell aging factors in overweight.
In obesity, associations with eating, cardiac and cell aging factors are attenuated.
Acknowledgments
Funding
This research was supported by the Mt Zion Health Fund; The William Bowes, Jr., Fund; the Robert Deidrick Fund; and NIH grant K01AT004199 awarded to JD from the National Center For Complementary & Alternative Medicine; and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR000144. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Complementary & Alternative Medicine or the National Institutes of Health. Dr. Havel’s research program receives funding from NIH grants HL-091333, HL-107256, DK-095980 and U24DK092993, Drs. Epel and Havel also receive support from a Multi-campus grant from the University of California Office of the President (Award #142691).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. Epub 2007 Apr 2014. [DOI] [PubMed] [Google Scholar]
- Adams CE, Greenway FL, Brantley PJ. Lifestyle factors and ghrelin: critical review and implications for weight loss maintenance. Obes Rev. 2011;12(5):e211–218. doi: 10.1111/j.1467-789X.2010.00776.x. [DOI] [PubMed] [Google Scholar]
- Al-Massadi O, Crujeiras AB, Gonzalez RC, Pardo M, Dieguez C, Casanueva FF, Seoane LM. Age, sex, and lactating status regulate ghrelin secretion and GOAT mRNA levels from isolated rat stomach. Am J Physiol Endocrinol Metab. 2010;299(3):E341–350. doi: 10.1152/ajpendo.00057.2010. [DOI] [PubMed] [Google Scholar]
- Amini P, Wadden D, Cahill F, Randell E, Vasdev S, Chen XH, Sun G. Serum Acylated Ghrelin Is Negatively Correlated with the Insulin Resistance In the CODING study. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0045657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders TF. Biological rhythms in development. Psychosom Med. 1982;44(1):61–72. doi: 10.1097/00006842-198203000-00008. [DOI] [PubMed] [Google Scholar]
- Andrews ZB. Central mechanisms involved in the orexigenic actions of ghrelin. Peptides. 2011;32(11):2248–2255. doi: 10.1016/j.peptides.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Aoki H, Nakata M, Dezaki K, Lu M, Gantulga D, Yamamoto K, Yada T. Ghrelin counteracts salt-induced hypertension via promoting diuresis and renal nitric oxide production in Dahl rats. Endocr J. 2013;16:16. doi: 10.1507/endocrj.ej12-0371. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, Kasuga M. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74(3):143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, Guarnieri G. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. Journal of Clinical Endocrinology & Metabolism. 2007;92(10):3935–3940. doi: 10.1210/jc.2006-2527. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Paolisso G, Kimura M, Gardner JP, Boccardi V, Papa M, Aviv A. Higher circulating levels of IGF-1 are associated with longer leukocyte telomere length in healthy subjects. Mech Ageing Dev. 2009;130(11–12):771–776. doi: 10.1016/j.mad.2009.10.002. doi:710.1016/j.mad.2009.1010.1002. Epub. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408(6808):53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12(10):1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- Blom WA, Lluch A, Stafleu A, Vinoy S, Holst JJ, Schaafsma G, Hendriks HF. Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr. 2006;83(2):211–220. doi: 10.1093/ajcn/83.2.211. [DOI] [PubMed] [Google Scholar]
- Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91(8):2913–2919. doi: 10.1210/jc.2006-0609. Epub 2006 May 2930. [DOI] [PubMed] [Google Scholar]
- Brennan IM, Luscombe-Marsh ND, Seimon RV, Otto B, Horowitz M, Wishart JM, Feinle-Bisset C. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am J Physiol Gastrointest Liver Physiol. 2012;303(1):G129–140. doi: 10.1152/ajpgi.00478.2011. Epub 02012 May 00473. [DOI] [PubMed] [Google Scholar]
- Briggs DI, Andrews ZB. Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology. 2011;93(1):48–57. doi: 10.1159/000322589. [DOI] [PubMed] [Google Scholar]
- Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151(10):4745–4755. doi: 10.1210/en.2010-0556. [DOI] [PubMed] [Google Scholar]
- Briggs DI, Lockie SH, Wu Q, Lemus MB, Stark R, Andrews ZB. Calorie-Restricted Weight Loss Reverses High-Fat Diet-Induced Ghrelin Resistance, Which Contributes to Rebound Weight Gain in a Ghrelin-Dependent Manner. Endocrinology. 2013;10:10. doi: 10.1210/en.2012-1421. [DOI] [PubMed] [Google Scholar]
- Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86(10):5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121(7):2684–2692. doi: 10.1172/JCI57660. Epub 52011 Jun 57623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Who’s Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 2009. Journal of Applied Social Psychology. 2012;42(6):1320–1334. doi:0.1111/j.1559-1816.2012.00900.x. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Couce ME, Cottam D, Esplen J, Teijeiro R, Schauer P, Burguera B. Potential role of hypothalamic ghrelin in the pathogenesis of human obesity. J Endocrinol Invest. 2006;29(7):599–605. doi: 10.1007/BF03344158. [DOI] [PubMed] [Google Scholar]
- Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89(1):71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Weigle DS. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8(7):643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- Dafopoulos K, Sourlas D, Kallitsaris A, Pournaras S, Messinis IE. Blood ghrelin, resistin, and adiponectin concentrations during the normal menstrual cycle. Fertil Steril. 2009;92(4):1389–1394. doi: 10.1016/j.fertnstert.2008.07.1773. doi:1310.1016/j.fertnstert.2008.1307.1773. Epub 2008 Sep 1330. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress-induced obesity and the emotional nervous system. Trends in endocrinology and metabolism: TEM. 2010;21(3):159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Kangawa K. Ghrelin as a starvation signal. Obesity Research & Clinical Practice. 2012;6(4):E263–E269. doi: 10.1016/j.orcp.2012.08.195. [DOI] [PubMed] [Google Scholar]
- Daubenmier J, Kristeller J, Hecht F, Maninger N, Kuwata M, Jhaveri K, Epel E. Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: an exploratory randomized controlled study. Journal of obesity. 2011;2011:13. doi: 10.1155/2011/651936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Desir D, van Cauter E, Golstein J, Fang VS, Leclercq R, Refetoff S, Copinschi G. Circadian and ultradian variations of ACTH and cortisol secretion. Horm Res. 1980;13(4–5):302–316. doi: 10.1159/000179297. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 2011;340(1):80–87. doi: 10.1016/j.mce.2011.02.017. Epub 2011 Feb 1024. [DOI] [PubMed] [Google Scholar]
- Disse E, Bussier AL, Veyrat-Durebex C, Deblon N, Pfluger PT, Tschop MH, Rohner-Jeanrenaud F. Peripheral ghrelin enhances sweet taste food consumption and preference, regardless of its caloric content. Physiol Behav. 2010;101(2):277–281. doi: 10.1016/j.physbeh.2010.05.017. Epub 2010 Jun 1011. [DOI] [PubMed] [Google Scholar]
- Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114(1):57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Chaves Y. Ghrelin, appetite regulation, and food reward: interaction with chronic stress. International journal of peptides. 2011;2011:898450. doi: 10.1155/2011/898450. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, Bloom SR. Ghrelin increases food intake in obese as well as lean subjects. International journal of obesity. 2005;29(9):1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- Eizadi M, Afsharmand Z, Behbudi L, Sohaily S. Serum Ghrelin, Insulin and Glucose Levels Are Correlated in Patients with Type 2 Diabetes Mellitus. Acta Endocrinologica- Bucharest. 2011;7(4):441–450. [Google Scholar]
- Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 2009;1(1):81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann J, Topsch R, Lippl F, Gussmann P, Schusdziarra V. Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J Clin Endocrinol Metab. 2004;89(6):3048–3054. doi: 10.1210/jc.2003-031610. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. Diet-induced obesity blunts the behavioural effects of ghrelin: studies in a mouse-progressive ratio task. Psychopharmacology (Berl) 2012;220(1):173–181. doi: 10.1007/s00213-011-2468-0. doi:110.1007/s00213-00011-02468-00210. Epub 02011 Sep 00213. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Aviv A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66(4):421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Cummings DE. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93(5):1971–1979. doi: 10.1210/jc.2007-2289. doi:1910.1210/jc.2007-2289. Epub 2008 Jan 1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JN, Carmo JM, Adi AH, da Silva AA. Chronic central ghrelin infusion reduces blood pressure and heart rate despite increasing appetite and promoting weight gain in normotensive and hypertensive rats. Peptides. 2013;42C:35–42. doi: 10.1016/j.peptides.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Garcia EA, Korbonits M. Ghrelin and cardiovascular health. Current Opinion in Pharmacology. 2006;6(2):142–147. doi: 10.1016/j.coph.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111(17):2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. Epub 2005 Apr 2125. [DOI] [PubMed] [Google Scholar]
- Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87(6):2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab. 2005;288(3):E486–492. doi: 10.1152/ajpendo.00196.2004. Epub 2004 Oct 2026. [DOI] [PubMed] [Google Scholar]
- Gualillo O, Caminos JE, Kojima M, Kangawa K, Arvat E, Ghigo E, Dieguez C. Gender and gonadal influences on ghrelin mRNA levels in rat stomach. Eur J Endocrinol. 2001;144(6):687–690. doi: 10.1530/eje.0.1440687. [DOI] [PubMed] [Google Scholar]
- Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, Jorgensen JO. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 2002;56(2):203–206. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- Harrold JA, Dovey TM, Blundell JE, Halford JC. CNS regulation of appetite. Neuropharmacology. 2012;63(1):3–17. doi: 10.1016/j.neuropharm.2012.01.007. Epub 2012 Jan 1030. [DOI] [PubMed] [Google Scholar]
- Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL. The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes. 2002;51(12):3412–3419. doi: 10.2337/diabetes.51.12.3412. [DOI] [PubMed] [Google Scholar]
- Kaplan RC, Fitzpatrick AL, Pollak MN, Gardner JP, Jenny NS, McGinn AP, Aviv A. Insulin-like growth factors and leukocyte telomere length: the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2009;64(11):1103–1106. doi: 10.1093/gerona/glp036. Epub 2009 Apr 1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellokoski E, Poykko SM, Karjalainen AH, Ukkola O, Heikkinen J, Kesaniemi YA, Horkko S. Estrogen replacement therapy increases plasma ghrelin levels. J Clin Endocrinol Metab. 2005;90(5):2954–2963. doi: 10.1210/jc.2004-2016. Epub 2005 Feb 2915. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, Blackburn E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain, behavior, and immunity. 2013;28(0):16–24. doi: 10.1016/j.bbi.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Isaacs AM, O’Farrell E, Abizaid A. Motivation to obtain preferred foods is enhanced by ghrelin in the ventral tegmental area. Hormones and behavior. 2011;60(5):572–580. doi: 10.1016/j.yhbeh.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Kirsz K, Zieba DA. Ghrelin-mediated appetite regulation in the central nervous system. Peptides. 2011;32(11):2256–2264. doi: 10.1016/j.peptides.2011.04.010. Epub 2011 Apr 2215. [DOI] [PubMed] [Google Scholar]
- Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin--a hormone with multiple functions. Front Neuroendocrinol. 2004;25(1):27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Krapalis AF, Reiter J, Machleidt F, Iwen KA, Dodt C, Lehnert H, Sayk F. Ghrelin modulates baroreflex-regulation of sympathetic vasomotor tone in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2012;302(11):R1305–1312. doi: 10.1152/ajpregu.00663.2011. Epub 02012 Apr 00664. [DOI] [PubMed] [Google Scholar]
- Kroemer NB, Krebs L, Kobiella A, Grimm O, Pilhatsch M, Bidlingmaier M, Smolka MN. Fasting levels of ghrelin covary with the brain response to food pictures. Addict Biol. 2012;13(10):1369–1600. doi: 10.1111/j.1369-1600.2012.00489.x. [DOI] [PubMed] [Google Scholar]
- Liddle RA. Ghrelin. UpToDate. 2013 Retrieved 2/03/2013, 2013, from www.uptodate.com.
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Blackburn E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1–2):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Matsumura K, Fukuhara M, Kagiyama S, Fujii K, Iida M. Ghrelin acts at the nucleus of the solitary tract to decrease arterial pressure in rats. Hypertension. 2004;43(5):977–982. doi: 10.1161/01.HYP.0000122803.91559.55. Epub 2004 Mar 2001. [DOI] [PubMed] [Google Scholar]
- Lockie SH, Andrews ZB. The hormonal signature of energy deficit: Increasing the value of food reward. Molecular Metabolism. 2013;2(4):329–336. doi: 10.1016/j.molmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav. 2007;91(4):432–439. doi: 10.1016/j.physbeh.2007.04.006. Epub 2007 Apr 2012. [DOI] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell metabolism. 2008;7(5):400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Koniaris A. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301(4):E567–584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara M, Sakata I, Wada R, Yamazaki M, Inoue K, Sakai T. Estrogen modulates ghrelin expression in the female rat stomach. Peptides. 2004;25(2):289–297. doi: 10.1016/j.peptides.2003.12.020. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab. 2004;89(4):1630–1635. doi: 10.1210/jc.2003-031572. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Piscitelli F, Scognamiglio P, Monteleone AM, Canestrelli B, Di Marzo V, Maj M. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study. J Clin Endocrinol Metab. 2012;97(6):E917–924. doi: 10.1210/jc.2011-3018. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Scognamiglio P, Monteleone AM, Perillo D, Canestrelli B, Maj M. Gastroenteric hormone responses to hedonic eating in healthy humans. Psychoneuroendocrinology. 2013;8(12):00422–00422. doi: 10.1016/j.psyneuen.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Luscombe-Marsh ND, Noakes M, Wittert GA, Keogh JB, Clifton PM. The satiating effect of dietary protein is unrelated to postprandial ghrelin secretion. J Clin Endocrinol Metab. 2005;90(9):5205–5211. doi: 10.1210/jc.2005-0701. Epub 2005 Jul 5212. [DOI] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Svensson J, Karlsson MK, Orwoll E, Ljunggren O, Mellstrom D, Ohlsson C. Serum insulin-like growth factor-I concentration is associated with leukocyte telomere length in a population-based cohort of elderly men. J Clin Endocrinol Metab. 2009;94(12):5078–5084. doi: 10.1210/jc.2009-1450. doi:5010.1210/jc.2009-1450. Epub 2009 Oct 5021. [DOI] [PubMed] [Google Scholar]
- Muccioli G, Baragli A, Granata R, Papotti M, Ghigo E. Heterogeneity of ghrelin/growth hormone secretagogue receptors. Toward the understanding of the molecular identity of novel ghrelin/GHS receptors. Neuroendocrinology. 2007;86(3):147–164. doi: 10.1159/000105141. Epub 2007 Jul 2002. [DOI] [PubMed] [Google Scholar]
- O’Callaghan NJ, Toden S, Bird AR, Topping DL, Fenech M, Conlon MA. Colonocyte telomere shortening is greater with dietary red meat than white meat and is attenuated by resistant starch. Clin Nutr. 2012;31(1):60–64. doi: 10.1016/j.clnu.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Okumura H, Nagaya N, Enomoto M, Nakagawa E, Oya H, Kangawa K. Vasodilatory effect of ghrelin, an endogenous peptide from the stomach. J Cardiovasc Pharmacol. 2002;39(6):779–783. doi: 10.1097/00005344-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Ozkan Y, Aydin S, Donder E, Koca SS, Aydin S, Ozkan B, Sahin I. Effect of orlistat on the total ghrelin and leptin levels in obese patients. J Physiol Biochem. 2009;65(3):215–223. doi: 10.1007/BF03180574. doi:210.1007/BF03180574. [DOI] [PubMed] [Google Scholar]
- Pacifico L, Poggiogalle E, Costantino F, Anama C, Ferraro F, Chiarelli F, Chiesa C. Acylated and nonacylated ghrelin levels and their associations with insulin resistance in obese and normal weight children with metabolic syndrome. European Journal of Endocrinology. 2009;161(6):861–870. doi: 10.1530/EJE-09-0375. [DOI] [PubMed] [Google Scholar]
- Perello M, Zigman JM. The Role of Ghrelin in Reward-Based Eating. Biol Psychiatry. 2012;72(5):347–353. doi: 10.1016/j.biopsych.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaquy S, Chabrol H, Simon C, Louvet JP, Barbe P. Emotional eating, alexithymia, and binge-eating disorder in obese women. Obes Res. 2003;11(2):195–201. doi: 10.1038/oby.2003.31. [DOI] [PubMed] [Google Scholar]
- Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003;52(10):2546–2553. doi: 10.2337/diabetes.52.10.2546. [DOI] [PubMed] [Google Scholar]
- Raspopow K, Abizaid A, Matheson K, Anisman H. Psychosocial stressor effects on cortisol and ghrelin in emotional and non-emotional eaters: influence of anger and shame. Hormones and Behavior. 2010;58(4):677–684. doi: 10.1016/j.yhbeh.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N, Greenman Y. The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology. 2007;32(6):693–702. doi: 10.1016/j.psyneuen.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Newman AB. Telomere Length in Epidemiology: A Biomarker of Aging, Age-Related Disease, Both, or Neither? Epidemiol Rev. 2013 doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens H, Dinan TG, Cryan JF. Ghrelin at the interface of obesity and reward. Vitam Horm. 2013;91:285–323. doi: 10.1016/B978-0-12-407766-9.00013-4. [DOI] [PubMed] [Google Scholar]
- Schulkin J. Introduction. In: Schulkin J, editor. Allostasis, Homeostasis, and the Costs of Physical Adaptation. New York: Cambridge University Press; 2004. [Google Scholar]
- Shibata K, Hosoda H, Kojima M, Kangawa K, Makino Y, Makino I, Gomita Y. Regulation of ghrelin secretion during pregnancy and lactation in the rat: possible involvement of hypothalamus. Peptides. 2004;25(2):279–287. doi: 10.1016/j.peptides.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- Tannous dit El Khoury D, Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann Nutr Metab. 2006;50(3):260–269. doi: 10.1159/000091684. Epub 2006 Feb 2023. [DOI] [PubMed] [Google Scholar]
- Tanrikulu-Kucuk S, Ademoglu E. Dietary restriction of amino acids other than methionine prevents oxidative damage during aging: involvement of telomerase activity and telomere length. Life Sci. 2012;90(23–24):924–928. doi: 10.1016/j.lfs.2012.04.024. Epub 2012 Apr 1027. [DOI] [PubMed] [Google Scholar]
- Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59(9):2145–2151. doi: 10.2337/db10-0504. Epub 2010 Jun 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5(2):295–315. [Google Scholar]
- Veldhuis JD, Keenan DM. Model-based evaluation of growth hormone secretion. Methods Enzymol. 2012;514:231–48. doi: 10.1016/B978-0-12-381272-8.00014-3. [DOI] [PubMed] [Google Scholar]
- Verhulst PJ, Depoortere I. Ghrelin’s second life: From appetite stimulator to glucose regulator. World J Gastroenterol. 2012;18(25):3183–3195. doi: 10.3748/wjg.v18.i25.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10(2):107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]