Abstract
Background
The average dietary sodium intake of Koreans is 2.6 times higher than the World Health Organization's recommended amount. The effect of a diet high in sodium on the skeletal system, especially osteoporosis, has not previously been examined in Korean postmenopausal women with low bone mass. We assessed the daily sodium intake, and determined the impact of sodium intake on urinary calcium excretion and bone resorption marker.
Methods
A retrospective review of medical records was performed for 86 postmenopausal subjects who were initially diagnosed with osteopenia or osteoporosis at the health promotion center. They were subsequently referred to the Division of Endocrinology and Metabolism between 2010 and 2013. All subjects completed a modified food frequency questionnaire. Twenty-four hour urine collection for sodium, calcium and creatinine excretion, and serum C-terminal telopeptides of type I collagen (CTX-I) were also obtained.
Results
The average amount of daily sodium and calcium intake were 3,466 mg and 813 mg, respectively. Average dietary sodium intake and 24-hour urinary sodium excretion showed significant positive linear correlation (r=0.29, P=0.006). There was also a significant positive linear correlation between 24-hour urine sodium and calcium excretion (r=0.42, P<0.001); CTX-I and 24-hour urinary calcium excretion (r=0.29, P=0.007).
Conclusions
Excessive sodium intake assessed by 24-hour urine specimen is associated with high calcium excretion in urine. High calcium excretion is also related to increasing bone resorption marker.
Keywords: Bone resorption marker, Dietary sodium intake, Urinary calcium excretion, Urinary sodium excretion
INTRODUCTION
The prevalence of osteoporosis among Korean women over the age of 50 has been reported at 38.7%;[1] this rate is substantively higher than that about 10% prevalence rate reported for women over 50 years in the United States according to the 2010, 2012 National Center for Health Statistics (NCHS).[2,3] High sodium intake is recognized as a risk factor for osteoporosis because: it alters calcium metabolism by increasing urinary calcium excretion.[4,5] Therefore, one of the important factors leading to a high prevalence of osteoporosis in Korean women could be excessive dietary sodium intake. In 2011, the Korea National Health and Nutrition Examination Survey (KNHANES V-2) reported that the average daily sodium intake was 6,172 mg for males and 4,172 mg per day for females in Korea.[6] The amount of daily sodium intake is much greater than that of the Americans mean consumption of approximately 3,400 mg of sodium per day,[7] and it exceeds the World Health Organization (WHO)'s recommendation for adult of sodium intake less than 2,000 mg per day.[8] Salt consumption, that which exceeds physiologic need has been associated with a raft of adverse clinical outcomes in various organ systems;[9] however, there is no published data showing sodium intake with bone metabolism for the population of Korean women. The authors assessed daily sodium intake in postmenopausal Korean women with low bone mass and analyzed the association with urinary calcium excretion and bone resorption marker.
METHODS
1. Subjects
Subjects included postmenopausal women who were at least one year past menopause and were first diagnosed with osteopenia or osteoporosis by WHO criteria through the health promotion center at the Samsung Medical Center (Seoul, Korea). The subjects initially went in for a medical checkup in general population; they were subsequently referred to the Division of Endocrinology and Metabolism between March 2010 and March 2013. A total of three hundred four subjects initially included those who completed the survey on dietary sodium and calcium intake by modified food frequency questionnaire (FFQ) on the basis of the FFQ used in the KNHANES. Subjects were excluded if she used glucocorticoids, estrogen, supplemental calcium, vitamin D, anticonvulsant agents, diuretics. An estimated glomerular filtration rate less than 60 mL/min/1.73 m2 (by Modification of Diet in Renal Disease study equation) were also excluded from the study. After exclusion, 86 subjects who collected 24-hour urine specimen for sodium and calcium excretion at the outpatient department of Endocrinology and Metabolism were reviewed and analyzed for our study. This study was approved by the Institutional Review Board of Samsung Medical Center (2013-07-179-001).
2. Measurements
Measurements of biomarkers of bone resorption and baseline serum electrolyte, calcium and phosphorus, creatinine profile were retrospectively obtained from electronic medical records. All patients had collected 24-hour urine samples to estimate urine sodium, calcium, and creatinine excretion. Blood samples were obtained at their first visit at the outpatient clinic of Division of Endocrinology and Metabolism. Subjects were sampled in the morning after an overnight fast exceeding 8 hours. C-terminal crosslinking telopeptides of type I collagen (CTX-I), a marker of bone resorption, was measured in the serum by electrochemiluminescence immunoassay (ECLIA) (Roche Modular Analytics E170 [Roche Diagnostics, Mannheim, Germany]). The interassay coefficient of variation for CTX-I ranged between 2.6% and 2.9% depending on CTX-I level. All patients underwent baseline bone mineral density (BMD) using dual energy X-ray absorptiometry (DXA; GE Healthcare Lunar, Madison, WI, USA). In accordance with the WHO criteria, osteopenia was defined as a T-score for the lumbar spine, total hip of between -1 and -2.5, and osteoporosis was defined as T-score of less than -2.5.
1) Assessment of dietary sodium and calcium intake
All subjects completed the modified FFQ based on the KNHANES (2008)[10] during a health care examination at the health promotion center. The modified FFQ is composed of 71 questions; the questions assess the frequency of servings included in 11 food groups consisting of a total of 63 food items. The detailed contents of food groups and frequency of servings were the same as FFQ used in the KNHANES. "Modified FFQ" is the questionnaire format, based on FFQ, utilized in the KNHANES and modified in Samsung Medical Center to promote better understanding and easy to recall for patients of food groups and consumption frequency. Subjects were asked to complete the survey on dietary intake for the previous three months. Nutritional intake was analyzed using CAN-Pro 4.0 (Korean Nutrition Society, Seoul, Korea).
Subjects were stratified into two different groups based on sodium intake: 1) low sodium intake group (<2 g/day); and 2) high sodium intake group (≥2 g/day), based on the WHO's recommended daily sodium intake guidelines. As urinary sodium excretion is the gold standard to evaluate actual dietary sodium intake, we also applied the same cut-off point to evaluation of the subjects' 24-hour urinary sodium excretion amount.
3. Statistical analysis
Statistical analyses were performed using SPSS statistics 21.0 (SPSS, Inc. Chicago, IL, USA). Pearson's correlation coefficients were obtained between dietary sodium intake and 24-hour urinary sodium excretion, 24-hour urinary sodium excretion and calcium excretion, and 24-hour urinary calcium excretion and serum CTX-I. The association of the dietary sodium intake assessed by modified FFQ and 24-hour urinary sodium excretion was analyzed using a paired t-test. The differences of the 24-hour urinary calcium excretion and serum CTX-I in the sodium intake or sodium excretion group (<2 g/day vs. ≥2 g/day) were analyzed by t-tests. A P value less than 0.05 was considered to indicate statistical significance.
RESULTS
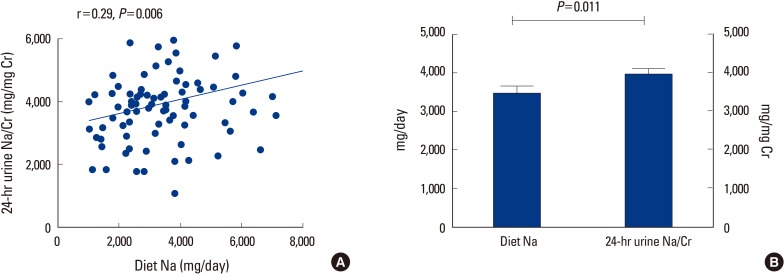
Table 1 showed the baseline characteristics of the study population based on sodium intake assessed with the modified FFQ. The mean age of our study group was 58.4 years old; the average years since menopause was 9.6 years. No statistically significant differences between groups were observed in the baseline parameters, except for dietary sodium, calcium intake and 24-hour urinary sodium excretion. Among the 86 subjects, the average amount of daily sodium intake and calcium intake were 3,466 mg and 813 mg, respectively. Subjects' calcium intake, as assessed by modified FFQ was significantly higher than the low sodium intake group (<2 g/day, n=15) in high sodium intake group (≥2 g/day, n=71) (Table 1). A significant positive correlation was found between the average dietary sodium intake (mg/day) and 24-hour urinary sodium excretion (mg/mg Cr) (Fig. 1A), before and after adjusted by dietary calcium intake (mg/day). Sodium excretion assessed by 24-hour urine collection (3,949±1,224 mg) was significantly greater than dietary sodium intake amount assessed by the modified FFQ used with KNHANES (3,466±1,614 mg) (Fig. 1B).
Table 1.
Baseline characteristics of the low (<2 g/day) and high (≥2 g/day) sodium intake group (n=86) based on modified food frequency questionnaire
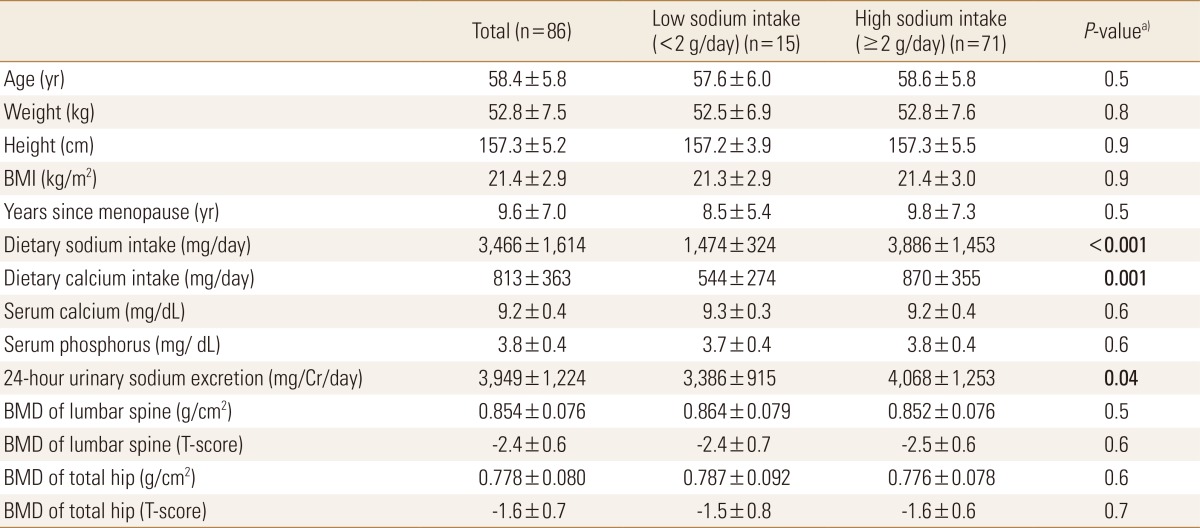
Data presents as means±SD, statistical analysis by t-tests. Bold entries is a significant values of P<0.05.
a)P values for differences in baseline characteristics between sodium excretion groups.
BMI, body mass index; BMD, bone mineral density.
Fig. 1.
Association of the estimated sodium intake assessed by modified food frequency questionnaire (FFQ) and 24-hour urinary sodium excretion. (A) Correlation between daily sodium intake (mg/day) and 24-hour urinary sodium excretion (mg/mg Cr). (B) Differences between average amount of daily sodium intake assessed by the FFQ and the 24-hour urinary sodium excretion. Na, sodium; Cr, creatinine.
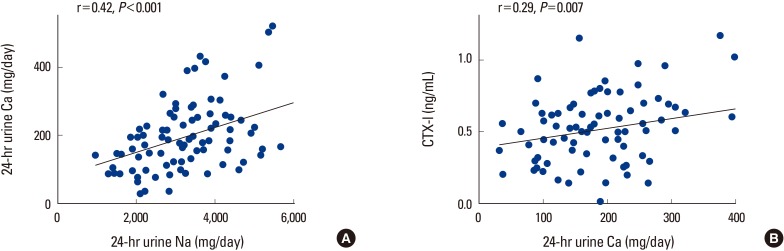
The 86 patients were stratified into two groups based on self-reported daily sodium intake: as low sodium intake group (<2 g/day) and high sodium intake group (≥2 g/day). The sodium level group cut-off points were based on the recommended daily sodium amount suggested by the WHO.[8] We also applied the same cut-off point to evaluation of 24-hour urinary sodium excretion amount, as dietary sodium intake and 24-hour urinary sodium excretion amount showed a significant positive correlation (P=0.006). Based on the amounts of dietary sodium intake assessed by the modified FFQ, low sodium intake group (<2 g/day) included 15 subjects; the high sodium intake group (≥2 g/day) included 71 subjects. However, the number of subjects included in the high sodium intake group (≥2 g/day) were greater (n=86) when stratified based on the 24-hour urinary sodium excretion. There were no significant differences in average urinary calcium excretion according to the sodium intake group stratified by self-reported dietary sodium intake (P=0.48). However, 24-hour urinary calcium excretion was significantly higher in the high sodium intake group based on 24-hour urinary sodium excretion (P=0.016) (Table 2). There was also a significant linear correlation between 24-hour urinary sodium excretion and 24-hour urinary calcium excretion at the level of P<0.001 (r=0.421) (Fig. 2A). CTX-I, a biochemical marker of bone resorption, measured in serum were significantly associated with 24-hour urinary calcium excretion (P=0.007) (Fig. 2B). There were no statistically significant correlation found between 24-hour urinary sodium excretion and CTX-I (P=0.3, data not shown). Serum CTX-I showed a higher tendency in the high urinary sodium excretion group (≥2 g/day); however, the relationship was not statistically significant (Table 2). There were also no statistically significant correlation between 24-hour urinary sodium excretion and BMD (lumbar spine, P=0.6; total hip, P=0.1; data not shown).
Table 2.
Average amount of 24-hour urinary calcium excretion and serum C-terminal telopeptides of type I collagen in low (<2 g/day) and high (≥2 g/day) sodium intake group based on the modified food frequency questionnaire and 24-hour urinary sodium collection

Data presents as means±SD or median (interquartile range).
a)P<0.05.
CTX-I, C-terminal crosslinking telopeptides of type I collagen; FFQ, food frequency questionnaire.
Fig. 2.
Correlation of (A) 24-hour urinary sodium excretion and calcium excretion; (B) 24-hour urinary calcium excretion and serum C-terminal telopeptides of type I collagen. CTX-I, C-terminal crosslinking telopeptides of type I collagen; Ca, calcium.
DISCUSSION
According to the 2011 KNHANES V-2,[6] the average Korean adult consumes 5,158 mg sodium per day (12 g of salt), which is 2.6 times higher than the recommended amount from the WHO.[8] The health implications of a high sodium diet on an individual's health have been highlighted and recently re-emphasized in terms of clinical impact on cardiovascular system.[9] The skeletal system is also deleteriously affected by the level of dietary salt levels that are commonly consumed.[4,11]
We assessed subjects' sodium intake by both self-reported sodium intake via modified FFQ and directly estimated 24-hour urine specimen for sodium excretion. These two measures were significantly and positively correlated, which has been proven as the quantity of sodium in a 24-hour urine specimen is almost the same as the quantity of sodium ingested in the absence of hydration disorders or large change of volume load.[12] Evidence suggested sodium is nearly absorbed due to the great solubility of its salts in the aqueous environment of the digestive tract. Sodium is excreted mainly with urine and sweat, and to a much smaller extent through body secretions, skin, and via feces.[13] Excessive sodium intake increases blood osmolality and thus osmosensors in the hypothalamus sense and the increased water intake in response to ensuing thirst leads to the dilution of plasma sodium and provides the water volume for renal sodium excretion.[13]
The coefficient of correlation calculated between dietary sodium and 24-hour urinary sodium excretion in our study (r=0.29, P=0.006) was similar to a previous study of sodium and calcium intake in postmenopausal women (r=0.31, P<0.001).[11] However, the average sodium excretion assessed by 24-hour urine collection was significantly greater than the self-reported dietary sodium intake amount assessed by the modified FFQ. Indeed, measuring dietary sodium intake has some difficulties as seen in the weak correlation coefficient with urinary sodium excretion observed in our study and previous study.[11] Discretionary use of sodium in food preparation or cooking may vary from 25% to 45% among individuals,[14] and subjects may find reporting discretionary sodium use difficult to levels of variation. Furthermore, the estimated sodium intake in FFQ, estimated by personal recall, also has notable limitations: the sodium content of processed foods may vary considerably depending on preparation, processing, and various cooking techniques.[11] Thus, the calculation of sodium intake amount by nutritional databases based on reported surveys is not likely to be accurate of actual intake. Therefore, urinary sodium excretion is used as the gold standard for estimate actual sodium intake in clinical research.
Our data showed a correlation between high sodium intake, measured as urinary sodium excretion, and high levels of calcium excretion via urine. In addition, there was a significant correlation between 24-hour urinary calcium excretion and the bone resorption marker, CTX-I. Although there was no statistical significance, serum CTX-I was higher in the high sodium intake group based on 24-hour urine sodium collection. The small number of individuals in the low sodium excretion group could introduce bias for comparing two groups. CTX-I has previously been deemed as one of the best bone markers for fracture prediction and skeletal bone loss.[15,16] The physiologic mechanism is: higher urinary sodium excretion induces hypercalciuria and is responsible for a significant negative change in calcium balance.[17,18,19] Moreover, increased bone turnover due to bone resorption is associated with a loss of calcium in the urine; that is, sodium takes out calcium in the urine that, in turn, stimulates bone resorption activities. Although the overall evidence on the relationship between sodium and osteoporosis is inconsistent,[4,11,20] considering the effects on bone metabolism as described above, one would expect a negative effect of sodium excretion on bone loss.
There were a number of limitations to this study. First, the study only required one 24-hour urine sample to estimate sodium and calcium excretion. Indeed, it is well known that in order to accurately estimate sodium intake, multiple specimens of 24-hour urine sodium is needed to reflect their usual diet.[21] Our study population showed that the average amount of daily sodium and calcium intake were 3,465 mg and 814 mg, respectively. However, that of Korean women reported in KNHANES V-2[6] showed clear distinction that 4,172 mg and 451 mg; which were higher in sodium intake and lower in calcium intake. The subjects' characteristics that healthy selected person who underwent regular health care could be attributed to these differences.
In conclusion, excessive sodium intake assessed by 24-hour urine specimen is associated with high calcium excretion in the urine and high calcium excretion is related to the increasing bone resorption marker. Large prospective trials, including outcomes with serial BMDs with osteoporotic fracture incidences, are needed to demonstrate the effect of high sodium diet on bone health.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ministry of Health & Welfare, Korea Centers for Disease Control and Prevention. Korea health statistics 2009: Korea national health and nutrition examination survey (KNHANES IV-3) Seoul: Ministry of Health & Welfare; 2010. [Google Scholar]
- 2.Looker AC, Borrud LG, Dawson-Hughes B, et al. Osteoporosis or low bone mass at the femur neck or lumbar spine in older adults: United States, 2005-2008. 2012. [cited by 2014 May 10]. Available from: http://www.cdc.gov/nchs/data/databriefs/db93.pdf. [PubMed]
- 3.Centers for Disease Control and Prevention. Healthy people 2010: Arthritis, osteoporosis, and chronic back conditions. 2010. [cited by 2010 May 10]. Available from: http://www.cdc.gov/nchs/data/hpdata2010/hp2010_final_review_focus_area_02.pdf.
- 4.Nordin BE, Need AG, Morris HA, et al. The nature and significance of the relationship between urinary sodium and urinary calcium in women. J Nutr. 1993;123:1615–1622. doi: 10.1093/jn/123.9.1615. [DOI] [PubMed] [Google Scholar]
- 5.Zarkadas M, Gougeon-Reyburn R, Marliss EB, et al. Sodium chloride supplementation and urinary calcium excretion in postmenopausal women. Am J Clin Nutr. 1989;50:1088–1094. doi: 10.1093/ajcn/50.5.1088. [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health & Welfare, Korea Centers for Disease Control and Prevention. Korea health statistics 2011: Korea national health and nutrition examination survey (KNHANES V-2) Seoul: Ministry of Health & Welfare; 2012. [Google Scholar]
- 7.Centers for Disease Control and Prevention. Sodium intake among adults - United States, 2005-2006. MMWR Morb Mortal Wkly Rep. 2010;59:746–749. [PubMed] [Google Scholar]
- 8.World Health Organization. Guideline: Sodium intake for adults and children. Geneva, CH: World Health Organization; 2012. [PubMed] [Google Scholar]
- 9.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease-a delicate balance. N Engl J Med. 2013;368:1229–1237. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Health & Welfare, Korea Centers for Disease Control and Prevention. Korea health statistics 2007: Korea national health and nutrition examination survey (KNHANES IV-1) Seoul: Ministry of Health & Welfare; 2008. [Google Scholar]
- 11.Devine A, Criddle RA, Dick IM, et al. A longitudinal study of the effect of sodium and calcium intakes on regional bone density in postmenopausal women. Am J Clin Nutr. 1995;62:740–745. doi: 10.1093/ajcn/62.4.740. [DOI] [PubMed] [Google Scholar]
- 12.Audran M, Legrand E. Hypercalciuria. Joint Bone Spine. 2000;67:509–515. doi: 10.1016/s1297-319x(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 13.Kohlmeier M. Nutrient metabolism. 2nd ed. Amsterdam, NL: Academic Press; 2006. [Google Scholar]
- 14.National Health and Medical Research Council. Report of the working party on sodium in the Australian diet. Canberra, AU: Australian Government Publishing Service; 1984. [Google Scholar]
- 15.Garnero P. Markers of bone turnover for the prediction of fracture risk. Osteoporos Int. 2000;11(Suppl 6):S55–S65. doi: 10.1007/s001980070006. [DOI] [PubMed] [Google Scholar]
- 16.Johnell O, Odén A, De Laet C, et al. Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int. 2002;13:523–526. doi: 10.1007/s001980200068. [DOI] [PubMed] [Google Scholar]
- 17.Sabto J, Powell MJ, Breidahl MJ, et al. Influence of urinary sodium on calcium excretion in normal individuals. A redefinition of hypercalciuria. Med J Aust. 1984;140:354–356. [PubMed] [Google Scholar]
- 18.McParland BE, Goulding A, Campbell AJ. Dietary salt affects biochemical markers of resorption and formation of bone in elderly women. BMJ. 1989;299:834–835. doi: 10.1136/bmj.299.6703.834-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teucher B, Dainty JR, Spinks CA, et al. Sodium and bone health: impact of moderately high and low salt intakes on calcium metabolism in postmenopausal women. J Bone Miner Res. 2008;23:1477–1485. doi: 10.1359/jbmr.080408. [DOI] [PubMed] [Google Scholar]
- 20.Dawson-Hughes B, Fowler SE, Dalsky G, et al. Sodium excretion influences calcium homeostasis in elderly men and women. J Nutr. 1996;126:2107–2112. doi: 10.1093/jn/126.9.2107. [DOI] [PubMed] [Google Scholar]
- 21.Massey LK, Whiting SJ. Dietary salt, urinary calcium, and bone loss. J Bone Miner Res. 1996;11:731–736. doi: 10.1002/jbmr.5650110603. [DOI] [PubMed] [Google Scholar]