Abstract
The effect of opioids on the immunopathology of sepsis models in mice has been controversial. In previous work, we showed that mortality and various inflammatory parameters did not differ between female mice given saline or buprenorphine after cecal ligation and puncture. To investigate further, we hypothesized that buprenorphine would not affect outcomes of sepsis at any stage of estrous. Female mice were allocated into 4 groups (n = 20 per group) according to stage of estrous. Mice then underwent cecal ligation and puncture and received either buprenorphine or saline. In 3-wk survival studies, overall survival did not differ between buprenorphine- and saline-treated mice. When mice were stratified according to stage of estrous, survival did not vary among saline-treated groups but was lower in buprenorphine-treated mice in metestrus compared with proestrus. To investigate inflammation as a potential mechanism for survival, we measured cell counts and cytokine levels in the peripheral blood and peritoneal lavage fluid at 12 and 24 h after cecal ligation and puncture. At 24 h, buprenorphine-treated mice in proestrus had more circulating neutrophils and monocytes than did saline-treated mice in proestrus and more circulating WBC than did mice in any other stage with or without buprenorphine. Our current results suggest that the effects of buprenorphine on a 50% survival model of sepsis in BALB/c female mice are minimal overall but that the stage of estrous has various effects in this model. Investigators should consider the effects of buprenorphine and estrous cycle when using female mice in sepsis research.
Abbreviations: CLP, cecal ligation and puncture
Sepsis is a complex, multifactorial disease that remains a common cause of morbidity and mortality after events such as surgery, trauma, and burns.2,27 Sepsis is difficult to investigate with a single animal model, but many researchers consider cecal ligation and puncture (CLP) in rodents the ‘gold standard’ in creating polymicrobial peritonitis.5,18 However, several innate and environmental factors cause variability in CLP studies.
One variable is the use of analgesia in rodents undergoing CLP. The use of perioperative analgesia for studies using major surgery is the standard of veterinary care. The 2011 Guide also reminds us that the appropriate use of analgesics in research animals is an “ethical and moral imperative.”20 However, sepsis research focuses on the immune response during the course of the disease, and some analgesics, including nonsteroidal antiinflammatory drugs and opioids such as morphine and fentanyl, are known to have immunomodulatory effects.31,37
Buprenorphine is a safe and effective opioid analgesic16 for rodents and is considered to be minimally immunosuppressive.31 Our laboratory has shown that a specific dosing regimen of buprenorphine did not significantly affect survival or immune parameters in female ICR and C57BL/6 mice undergoing CLP,7,19 whereas male C57BL/6 mice showed a dose-responsive decrease in survival. There were no obvious changes in immune parameters to explain the differences between male and female mice. However, there are known sex-associated differences in responses to opioids. Several reports show that opioids have greater potency, efficacy, and overall analgesia in male rodents than in female, although results looking specifically at buprenorphine are mixed.8,35
In addition to differences in response to opioids, gender may also play a role in the response to sepsis. In humans, several studies have shown that women are less susceptible to sepsis than men.24,38 In contrast, a different group showed that women with sepsis have a worse survival rate than do men.26 Similarly, contradictory literature in mice has shown either a higher or lower survival in female mice as compared with male in sepsis studies.23,41 One possible factor for these contradictory results in both rodent and human studies is that female subjects were not grouped by phase of the estrous or menstrual cycle. In both mice and humans, fluctuations of estrogen and progesterone occur regularly through their respective cycles. Other hormonal factors such as prolactin, follicle stimulating hormone, and luteinizing hormone vary as well.14 Studies show that estrogen, prolactin, and other hormones can affect the immune system with and without sepsis,1,22,42 leading to the concern that the changing hormonal status of female subjects may affect the responses to opioids and to sepsis. Although several sepsis and trauma studies in rodents look at specific stages of estrous34,41 or the effects of exogenous estrogen,9,10,33,39 few target CLP specifically, and none look at this issue in conjunction with buprenorphine.
The purpose of our study was to investigate the effects of buprenorphine and the estrous cycle on a CLP model of sepsis. For these investigations, we chose to use female BALB/c mice to assess survival and immune responses. In addition, we performed survival experiments in male mice for comparison. Coupled with our previous studies, these findings will give a comprehensive profile of the major mouse strains used in sepsis research.
Materials and Methods
Study design.
Female mice underwent vaginal cytology and CLP, after which they were randomized to receive either buprenorphine or an equal volume of saline. To examine survival, mice were observed for 3 wk after CLP (n = 12 to 14 mice per stage of estrous per treatment). Behavioral scores were recorded for 5 d after CLP. Mice were removed from the study when they met criteria for end-stage illness, including inability to ambulate, inability to right themselves, unconsciousness, or severe dyspnea.29 A group of male mice underwent CLP as well and were randomized to receive buprenorphine or an equal volume of saline (n = 10 mice per treatment); 3-wk survival and 5-d behavioral observations were repeated in the male mice.
To investigate immune parameters, a second cohort of female mice was euthanized at 12 or 24 h after CLP (n = 8 to 12 mice per stage of estrous per treatment per time point). Peripheral blood and peritoneal lavage fluids were collected for cell counts and cytokine levels (IL6, IL10, CXCL1/KC, and CXCL2/MIP2α). For all studies with female mice, CLP was performed over multiple days in a week to achieve our desired number of 10 mice per stage of estrous per treatment group. Groups of mice were housed as mixed populations so that multiple stages and treatments were present in each cage. Mice were grouped by estrous stage according to the vaginal cytology in the morning just prior to CLP. A total of 104 female mice were used for the survival study, and 80 female mice were used for each immune parameter time point (12 and 24 h).
Experimental animals.
Female and male BALB/c mice (age, 12 wk) were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed 4 or 5 per cage in ventilated microisolation cages in an SPF barrier facility. Male mice were housed only with the same cage mates. Mice were SPF for viruses, bacteria, and parasites including mouse hepatitis virus, minute virus of mice, mouse parvovirus, enzootic diarrhea of infant mice virus, ectromelia virus, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus, lymphocytic choriomeningitis virus, mouse adenovirus, polyomavirus, Mycoplasma pulmonis, and pinworms. Mouse norovirus, Helicobacter spp., and other bacterial pathogens are not tested routinely at our institution. Mice had ad libitum access to food (Laboratory Rodent Diet 5001, PMI Lab Diet, St Louis, MO) and water. The animal housing room was maintained on a 12:12-h light:dark cycle and constant temperature (72 ± 2 °F [22.2 ± 1.1 °C]). Mice were acclimated for at least 5 d before experimental use. University of Michigan's Animal Care and Use Committee approved all experimental procedures.
Determination of estrous stage.
To confirm the stage of estrous, vaginal cytology was performed at a standardized time each morning for 1 to 2 d before surgery and on the day of surgery. A well-described technique6 was used to flush the vagina with 20 µL of sterile saline and create a wet mount on a microscope slide. The stage of estrous was determined by direct visualization of the cells by using light microscopy at 10× magnification. A previously published criterion was used to describe the 4 stages of estrous differentiated by vaginal cytology.6 Based on these criteria, proestrus and estrus were defined as having high estrogen and metestrus and diestrus with low estrogen. Proestrus was defined by nucleated epithelial cells as the main population seen on cytology. Estrus was described as mainly cornified epithelial cells with a characteristic ‘cornflake’ appearance. Metestrus was defined as having 3 different cell types on cytology: nucleated epithelial cells, cornified epithelial cells, and leukocytes. Diestrus consisted of mainly leukocytes and was characterized by a very dense population of these cells.
Surgical procedures.
All mice underwent CLP surgery under isoflurane anesthesia (Vetone, Boise, ID). A single ligature of 4-0 silk was made at the halfway point of the cecum distal to the ileocecal junction. The cecum was punctured twice by using a 21-gauge needle and then gently expressed to ensure patency of the punctures. The abdominal musculature was closed with sutures followed by closure of the skin with tissue glue. Lactated Ringers solution (1 mL) was given subcutaneously immediately after surgery and mice recovered while receiving heat support before being placed back into their home cage. Mice then received nutritional support in the form of a gel diet (76A formulation, Clear H2O, Portland, ME) for 5 d after surgery.
Analgesic dosing.
The treatment group received 0.1 mg/kg buprenorphine (Bedford Labs, Bedford, OH) immediately before the CLP surgery and again at 12 h after surgery for a total of 2 doses. Dosing was determined by institutional recommendations, which were set by doses previously published in the literature. Control mice received an equal volume of saline only.
Behavior analysis.
All mice in the survival studies were evaluated once daily according to specific behavioral parameters starting 24 h after surgery for 5 d. Evaluations were performed at the same time each day, and mice were left undisturbed in their home cage. An evaluator, blinded to treatment group, performed independent scoring to quantify abnormal behaviors in each mouse. Mice were scored based on previously published indices that included activity, coat condition, posture, breathing, and relation to other mice.7,25 Scores of 0 (normal) or 1 (abnormal) were given; the maximal total behavioral score was 5.
Blood collection and processing.
Mice were deeply anesthetized with isoflurane, and approximately 500 µL blood was collected from the retroorbital sinus into 1.5-mL tubes containing 50 µL of 160 mmol EDTA. A 50-µL aliquot of blood was used for automated CBC analysis (Hemavet Veterinary Multispecies Hematology System, Drew Scientific, Waterbury, CT). The remainder of the blood was centrifuged (2000 × g, 5 min) and the plasma stored at −20 °C for later cytokine analysis. Mice then were euthanized by cervical dislocation and bilateral pneumothorax.
Peritoneal lavage collection and cell counts.
After euthanasia, 10 mL of Hanks Balanced Salt Solution (Invitrogen, Grand Island, NY) containing 1:100 heparin sodium (1000 USP U/mL; Abraxis, Schaumberg, IL) was injected into the peritoneal cavity and then 8 mL of the solution was drawn back into the syringe. The peritoneal lavage fluid was centrifuged (600 × g, 10 min), and the supernatant saved at −20 °C for later cytokine analysis. The pellet was reconstituted in 200 µL RPMI 1640 (Invitrogen) containing 0.1% heat-inactivated fetal bovine serum (Invitrogen). Cells were counted (model Z1, Coulter Counter, Coulter, Miami, FL) after RBC lysis (Zap-O-globin II (Coulter). Slides were centrifuged (109 × g, 5 min), loaded with 1 × 105 cells, and stained with Diff-Quick (Baxter, Detroit, MI). Differentials (300 cells) were counted under light microscopy.
Cytokine ELISA.
Cytokines were measured in plasma (dilution, 1:10) and peritoneal lavage fluid (dilution, 1:2) by using sandwich ELISA. Matched pairs (biotinylated and nonbiotinylated) of antimurine antibodies against IL10, IL6, CXCL1/KC, and CXCL2/MIP2α with their recombinant proteins (R and D Systems, Minneapolis, MN) were used in methods previously described by this laboratory.28 Peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA), and the color reagent tetramethylbenzidine were used as the detection system. The reaction was stopped by using 1.5 N sulfuric acid, and absorbance was read at 465 and 490 nm.
Statistical analysis.
All analyses were performed by using Prism (GraphPad, LaJolla, CA) and SAS version 9.1 (SAS Institute, Cary, NC).
For the survival study, Kaplan–Meier survival curves were calculated for each group, and differences between groups were measured by using log-rank tests. The relative risk of mortality and corresponding 2-sided 95% CI were calculated to compare groups. For the survival analyses considering both analgesic treatment and stage of estrous, a Cox proportional hazards regression was calculated to investigate the potential interaction between buprenorphine and estrous stage.
For the immune parameters, normality was analyzed by using D'Agostino–Pearson omnibus normality tests or Kolmogorov–Smirnov tests, depending on sample size. Parametric or nonparametric analyses then were performed depending on the distribution of the data. Treatment group means and SD or medians and interquartile ranges were calculated for each parameter of interest. Differences between the experimental means or medians were assessed by using one-way ANOVA or Kruskal–Wallis tests with post hoc Tukey or Dunn tests. Significant pairwise comparisons were confirmed using Student t tests or Wilcoxon–Mann–Whitney U tests.
Results
Effect of buprenorphine administration.
For this evaluation, all of the mice were included without consideration of the stage of estrous and stratified by analgesic treatment.
Survival study.
Survival was 47% for saline-treated, female mice. Most of the mice were euthanized within the first 3 d after CLP, and no deaths occurred after 5 d. Buprenorphine-treated mice had a similar survival rate (53%), with no significant difference from the saline group (Figure 1 A). Survival in a group of male BALB/c mice treated with saline and buprenorphine also showed no overall significant difference, with survival rates of 40% and 50%, respectively (Figure 1 B).
Figure 1.
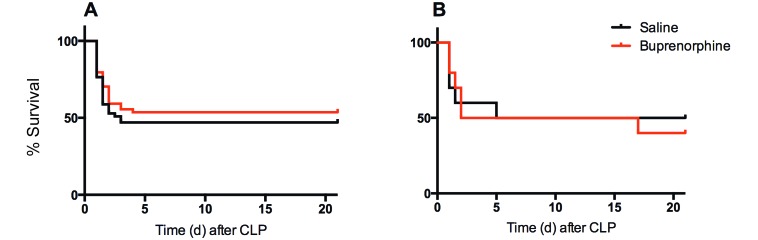
Kaplan–Meier survival curves for (A) female (n = 52 per group) and (B) male (n = 10 per group) BALB/c mice after CLP and treatment with either saline or buprenorphine. No significant difference in survival was seen after buprenorphine treatment in either female or male mice.
Behavior analysis.
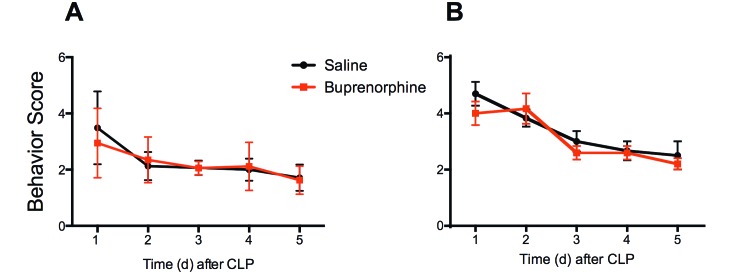
The difference in behavior scores between saline- and buprenorphine-treated mice was not significant for either sex on any day. In female mice, the greatest range in scores between saline-and buprenorphine-treated mice was at 24 h (3.5 ± 1.3 and 2.9 ± 1.2, respectively). Similarly, male mice showed the greatest range at 24 h as well, with saline-treated mice scoring 4.7 ± 1.3 and buprenorphine mice 4.0 ± 1.3 (Figure 2). On the following 4 d, differences between the groups were minimal.
Figure 2.
Behavioral scores (mean ± 1 SD) over 5 d after CLP for (A) female and (B) male mice divided by treatment. Five behavioral criteria (coat condition, breathing, activity, posture, and relation to other mice) were scored for 5 d after CLP and treatment with either saline or buprenorphine. Higher scores indicate more abnormal behaviors. No significant differences were seen in behavioral scores between saline- and buprenorphine-treated mice in either female or male BALB/c mice. Number varied by day due to mortality in both groups (day 1: female, n = 40 per group; male n = 10 per group; day 2: female, n = 17 to 21 per group; male, n = 6 per group; day 3: female, n = 17 to 21 per group; male, n = 5 or 6 per group; day 4: female, n = 15 to 18 per group; male n = 5 or 6 per group; day 5: female, n = 15 to 17 per group; male, n = 5 or 6 per group).
Immune parameters.
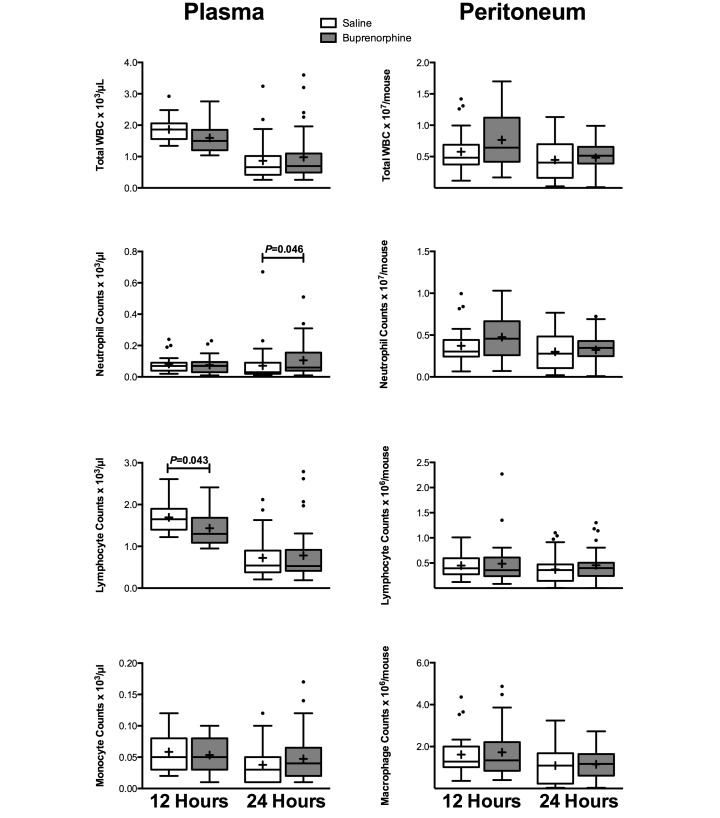
The lack of difference in survival was accompanied by an overall absence of differences in immune parameters between treatment groups. All mice showed typical patterns of decreased peripheral white blood cell counts after CLP.3,12,13 In plasma, buprenorphine-treated mice had significantly (P = 0.043) fewer lymphocytes at 12 h than did saline-treated mice and significantly (P = 0.046) more neutrophils at 24 h, but these differences did not occur at other time points (Figure 3). No significant differences were seen in plasma cytokine levels (Table 1). In the peritoneal fluid, no significant differences between saline- and buprenorphine-treated mice were present in peritoneal cell differentials (Figure 3) or cytokine levels (Table 1).
Figure 3.
Plasma and peritoneal cell counts and differentials for female BALB/c mice divided by treatment (plasma: 12 h, n = 19 to 21 per group; 24 h, n = 37 to 39 per group; peritoneum: 12 h, n = 29 per group; 24 h, n = 34 to 38 per group). Mice underwent CLP, and whole blood and peritoneal fluid were collected for cell counts at 12 and 24 h after CLP. Box indicates interquartile range; horizontal line and cross represent the median and mean, respectively. Whiskers represent the smaller of either 1.5 × interquartile range or range of the data. Dots represent outliers. For significance levels below 0.05, P values are presented.
Table 1.
Cytokine levels (ng/mL, mean ± 1 SD) according to treatment in female BALB/c mice after CLP
| 12 h |
24 h |
|||
| Control | Buprenorphine | Control | Buprenorphine | |
| Plasma | ||||
| IL6 | 10.51 ± 14.39 | 10.92 ± 18.99 | 3.15 ± 6.22 | 1.73 ± 2.47 |
| IL10 | 5.00 ± 7.20 | 4.80 ± 7.92 | 1.97 ± 2.97 | 0.96 ± 1.29 |
| CXCL1/KC | 0.24 ± 0.31 | 0.21 ± 0.33 | 39.28 ± 65.11 | 26.44 ± 44.29 |
| CXCL2/MIP2α | 0.33 ± 0.33 | 0.29 ± 0.28 | 3.04 ± 3.93 | 2.10 ± 3.33 |
| Peritoneum | ||||
| IL6 | 8.39 ± 7.49 | 8.64 ± 8.89 | 1.11 ± 2.42 | 1.05 ± 1.99 |
| IL10 | 0.96 ± 1.10 | 0.83 ± 0.93 | 0.64 ± 0.74 | 0.47 ± 0.54 |
| CXCL1/KC | 0.03 ± 0.03 | 0.03 ± 0.04 | 3.74 ± 6.12 | 5.35 ± 11.82 |
| CXCL2/MIP2α | 0.08 ± 0.04 | 0.07 ± 0.05 | 0.57 ± 0.60 | 0.76 ± 1.50 |
Effect of stage of estrous.
For this evaluation, the female mice were placed in groups according to stage of estrous at time of surgery, regardless of analgesic treatment.
Survival study.
When grouped according to stage of estrous, female mice in estrus, proestrus, diestrus, and metestrus had survival rates of 62.5%, 56%, 50%, and 34.6%, respectively. Although survival during proestrus and estrus (high estrogen groups) seemed higher than that during diestrus and metestrus (lower estrogen groups), there were no significant differences in survival between any groups (Figure 4).
Figure 4.
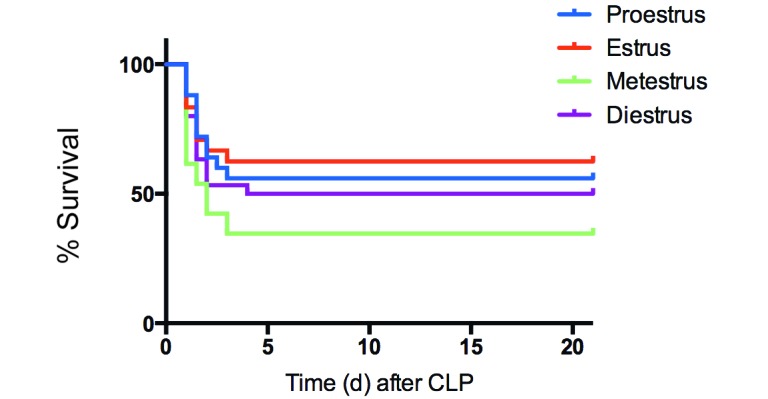
Kaplan–Meier survival curve of female BALB/c mice after CLP divided by stage of estrous (n = 24 to 30 per group). No significant difference in survival was seen between stages of estrous.
Immune parameters.
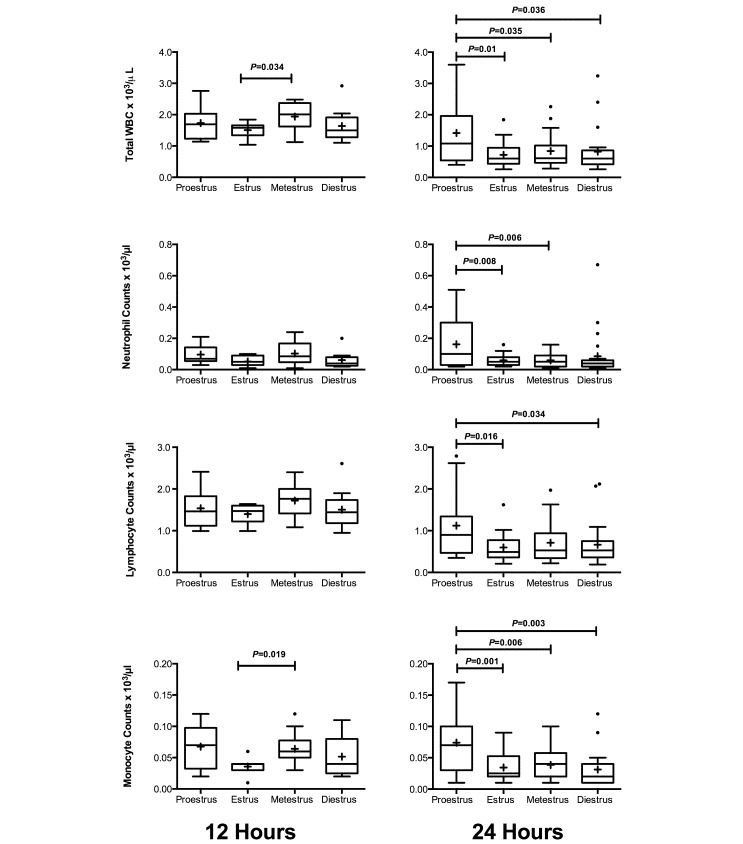
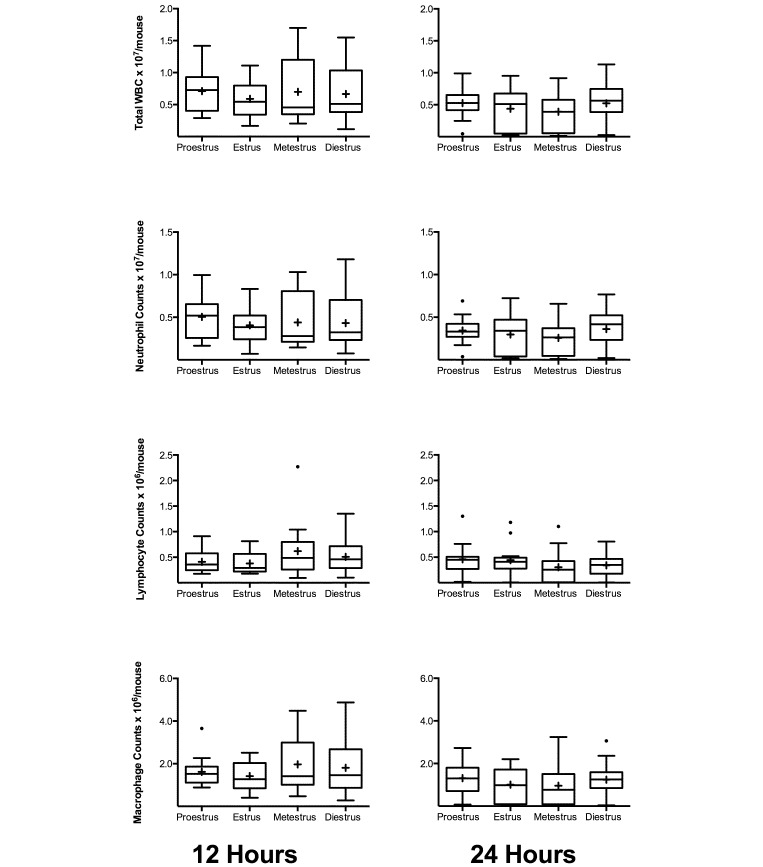
Several significant changes in immune parameters became apparent when mice were grouped according to estrous stage. At 12 h after surgery, total WBC and monocyte counts were significantly higher in mice in metestrus as compared with mice in estrus (P = 0.034 and P = 0.019, respectively; Figure 5). This difference did not persist to 24 h after surgery. At 24 h after surgery, counts in all WBC parameters were higher (P < 0.05) in mice in proestrus compared with estrus. Similarly, counts in total WBC, neutrophils, and monocytes were higher (P < 0.05) during proestrus than metestrus. In addition, total WBC, lymphocyte, and monocyte counts were higher (P < 0.05) during proestrous than diestrus (Figure 5). There were no significant differences between any stages of estrous for any parameter in the peritoneum (Figure 6).
Figure 5.
Plasma cell counts for female BALB/c mice divided by estrous stage (12 h, n = 7 to 13 per group; 24 h, n = 15 to 23 per group). Mice underwent CLP, and whole blood was collected for automated cell counts at 12 and 24 h after CLP. Box indicates interquartile range; horizontal line and cross represent the median and mean, respectively. Whiskers represent the smaller of either 1.5 × interquartile range or range of the data. Dots represent outliers. For significance levels below 0.05, P values are presented.
Figure 6.
Peritoneal cell counts for female BALB/c mice divided by estrous stage (12 h, n = 10 to 20 per group; 24 h, n = 13 to 20 per group). Mice underwent CLP, and whole blood was collected for cell counts at 12 and 24 h after CLP. Box indicates interquartile range; horizontal line and cross represent the median and mean, respectively. Whiskers represent the smaller of either 1.5 × interquartile range or range of the data. Dots represent outliers. No comparisons were significant at the less than 0.05 level.
In addition, differences in cytokine concentrations in the blood and peritoneal fluid occurred. At 24 h, plasma concentrations of IL10 and CXCL2/MIP2α were higher for mice in diestrus as compared with mice in estrus (P = 0.026 and P = 0.043, respectively). At the same time point, peritoneal lavage fluid concentrations of IL6 and CXCL1/KC were higher in mice in diestrus than estrus (P = 0.016 and P = 0.013, respectively; Table 2).
Table 2.
Cytokine levels (ng/mL, mean ± 1 SD) according to estrous stage in female BALB/c mice after CLP
| 12 h |
24 h |
|||||||
| Proestrus | Estrus | Metestrus | Diestrus | Proestrus | Estrus | Metestrus | Diestrus | |
| Plasma | ||||||||
| IL6 | 13.53 ± 19.5 | 5.29 ± 8.22 | 13.97 ± 18.12 | 9.43 ± 17.27 | 1.94 ± 2.49 | 0.94 ± 0.87 | 2.01 ± 3.27 | 4.19 ± 7.41 |
| IL10 | 6.25 ± 10.19 | 2.95 ± 4.32 | 6.04 ± 7.85 | 4.15 ± 6.17 | 0.92 ± 1.06 | 0.73 ± 1.03a | 1.47 ± 1.88 | 2.32 ± 3.45a |
| CXCL1/KC | 0.22 ± 0.25 | 0.18 ± 0.30 | 0.31 ± 0.44 | 0.20 ± 0.31 | 33.83 ± 56.07 | 18.67 ± 28.21 | 33.53 ± 65.80 | 42.79 ± 62.58 |
| CXCL2/MIP2α | 0.33 ± 0.23 | 0.30 ± 0.38 | 0.38 ± 0.41 | 0.25 ± 0.21 | 1.78 ± 1.87 | 1.31 ± 1.06b | 2.87 ± 3.94 | 3.76 ± 5.02b |
| Peritoneum | ||||||||
| IL6 | 9.24 ± 5.93 | 7.20 ± 6.74 | 8.92 ± 11.15 | 8.67 ± 8.21 | 1.42 ± 2.57 | 0.40 ± 0.61c | 1.15 ± 3.07 | 1.34 ± 1.87c |
| IL10 | 1.00 ± 1.03 | 0.94 ± 1.30 | 0.82 ± 0.99 | 0.85 ± 0.86 | 0.40 ± 0.36 | 0.44 ± 0.58 | 0.49 ± 0.67 | 0.79 ± 0.77 |
| CXCL1/KC | 0.02 ± 0.02 | 0.02 ± 0.04 | 0.03 ± 0.03 | 0.03 ± 0.04 | 6.47 ± 11.80 | 1.94 ± 5.52d | 3.55 ± 7.13 | 6.50 ± 11.89d |
| CXCL2/MIP2α | 0.08 ± 0.05 | 0.07 ± 0.04 | 0.07 ± 0.05 | 0.08 ± 0.06 | 0.73 ± 0.94 | 0.33 ± 0.22 | 0.71 ± 1.13 | 0.92 ± 1.71 |
P < 0.05 for each pairwise comparison
Effect of stage of estrous combined with analgesic treatment.
For this evaluation, mice were grouped according to stage of estrous at time of surgery and analgesic treatment.
Survival study.
When mice that received saline were evaluated, female mice had variable survival rates in proestrus, estrus, metestrus, and diestrus (41.7%, 58.3%, 38.5, and 50%, respectively), with no significant differences between groups (Figure 7 A). Interestingly, when the mice that received buprenorphine were evaluated, survival in proestrus (69.2%) was higher than that metestrus (30.8%; P = 0.045; Figure 7 B). Survival in mice in estrus (66.7%) was similar to that in proestrus (69.2%) but did not significantly (P = 0.064) differ from that of mice in metestrus. In mice treated with buprenorphine, death was 2.3 times less likely to occur in proestrus mice as compared with mice in metestrus (r2, 2.25; 95% CI, 0.92 to 5.49) and 2.2 times less likely in mice in estrus (r2, 2.17; 95% CI, 0.87 to 5.38). When proestrus and estrus were grouped as high-estrogen states and compared with metestrus and diestrus as a single low-estrogen group, a Cox proportional hazards regression analysis showed no significant interaction between the effect of buprenorphine and estrogen (P = 0.51).
Figure 7.
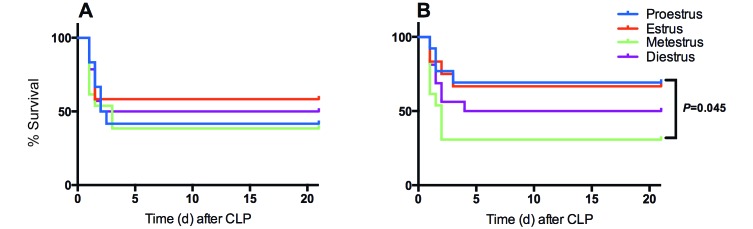
Kaplan–Meier survival curves after CLP for (A) saline- (n = 12 to 14 per group) and (B) buprenorphine- (n = 12 to 16 per group) treated female BALB/c mice divided by estrous stage. No significant difference in survival was seen in control mice based on estrous stage, but survival was significantly increased in buprenorphine-treated mice in proestrus as compared with buprenorphine-treated mice in metestrus. P values less than 0.05 are shown.
Immune parameters.
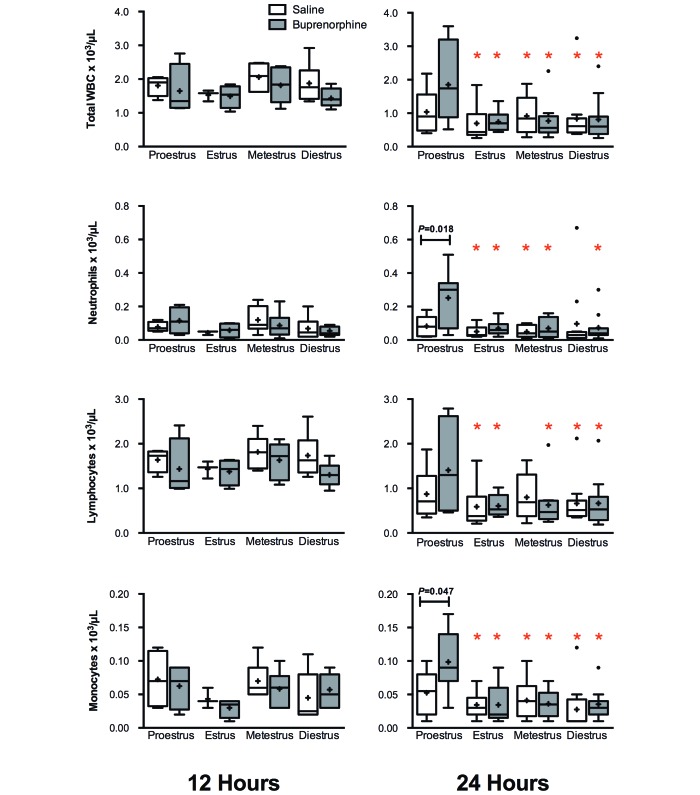
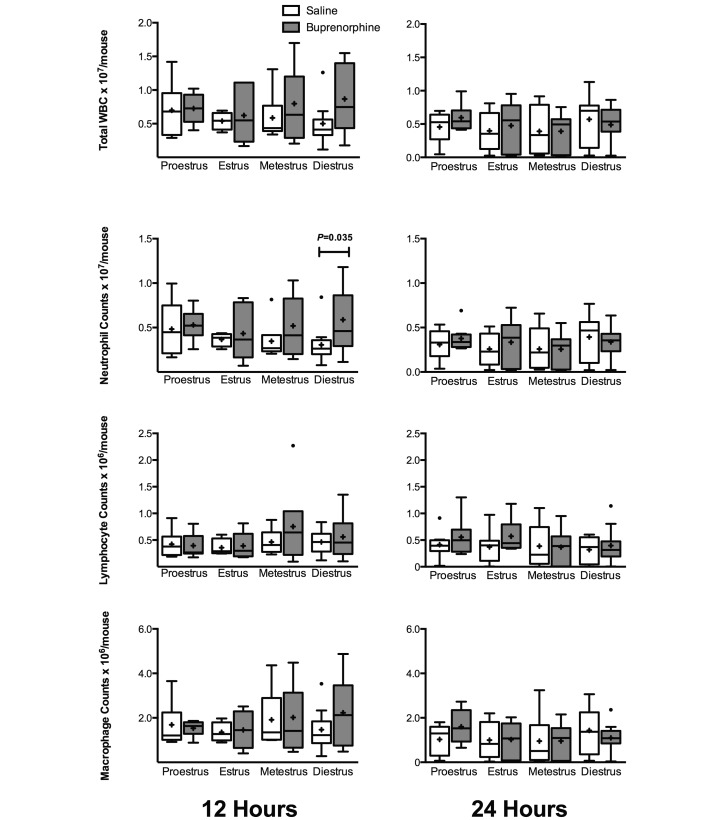
At 24 h, significant differences in peripheral cell counts were seen between buprenorphine, but not saline-treated, mice. Compared with most of the other groups, buprenorphine-treated proestrus mice showed a significant increase (P < 0.05) in peripheral counts (Figure 8). Between groups of mice in proestrus, saline-treated mice had lower neutrophil and monocyte counts than did buprenorphine-treated mice (P = 0.018 and P = 0.047, respectively). For almost all groups, peripheral cell counts decreased from 12 h to 24 h after CLP. However, buprenorphine-treated proestrus mice trended the opposite way, with an increase in neutrophils at 24 h (mean, 251 cells/µL; SE, 63 cells/µL) from 12 h (mean, 115 cells/µL; SE, 40 cells/µL) as well as monocytes (24 h: mean, 99 cells/µL; SE, 18 cells/µL; 12 h: mean, 62 cells/µL; SE: 17 cells/µL). These differences, however, were not significant. In the peritoneum, cell differential counts showed few differences in regard to stage of estrous with or without treatment. At 12 h after surgery, mice in diestrus that received buprenorphine had a significantly (P = 0.035) higher neutrophil count (mean, 5.88 × 106 cells/mouse; SE, 1.17 × 106 cells/mouse) than did those receiving saline (mean, 3.05 × 106 cells/mouse; SE, 0.06 × 106 cells/mouse); however, this difference was no longer seen at 24 h (Figure 9).
Figure 8.
Plasma cell counts for female mice divided by estrous stage and analgesic treatment (12 h, n = 4 to 7 per group; 24 h, n = 8 to 12 per group). Mice underwent CLP and were treated with either saline or buprenorphine. Whole blood was collected for automated cell counts at 12 and 24 h after CLP. Box indicates interquartile range; horizontal line and cross represent the median and mean, respectively. Whiskers represent the smaller of either 1.5 × interquartile range or range of the data. Dots represent outliers. P values less than 0.05 are shown. The red star denotes significant difference at the 0.05 level when compared with the proestrus + buprenorphine group.
Figure 9.
Peritoneal cell counts for female mice divided by estrous stage and analgesic treatment (12 h, n = 4 to 11 per group; 24 h, n = 8 to 11 per group). Mice underwent CLP and were treated with either saline or buprenorphine. Peritoneal lavage fluid was collected for cell differential counts at 12 and 24 h after CLP. Box indicates interquartile range; horizontal line and cross represent the median and mean, respectively. Whiskers represent the smaller of either 1.5 × interquartile range or range of the data. Dots represent outliers. For significant difference below the 0.05 level, P values are presented.
Regarding cytokines, concentrations for all plasma cytokines once again showed few differences between buprenorphine-treated and saline-treated mice at both 12 and 24 h after surgery. The only significant difference seen was in IL10 at 24 h, in which the concentration of IL10 was lower (P = 0.046) in buprenorphine-treated mice in proestrus as compared with buprenorphine-treated mice in diestrus (Table 3). The differences seen in peripheral IL10 and CXCL2/MIP2α at 12 h when mice were grouped according to estrous stage only (Table 2) did not appear to be associated with buprenorphine treatment.
Table 3.
Cytokine Levels (ng/mL, mean ± 1 SD) according to estrous stage and treatment in female BALB/c mice after CLP
| Proestrus |
Estrus |
Metestrus |
Diestrus |
|||||
| Control | Buprenorphine | Control | Buprenorphine | Control | Buprenorphine | Control | Buprenorphine | |
| Plasma, 12 h | ||||||||
| IL6 | 12.32 ± 17.57 | 14.62 ± 21.90 | 7.62 ± 9.94 | 2.96 ± 5.80 | 17.1 ± 20.09 | 10.84 ± 16.48 | 5.98 ± 6.25 | 12.88 ± 23.64 |
| IL10 | 6.37 ± 10.60 | 6.14 ± 10.33 | 4.56 ± 5.67 | 1.34 ± 1.40 | 7.19 ± 8.28 | 4.9 ± 7.71 | 2.51 ± 2.03 | 5.79 ± 8.34 |
| CXCL1/KC | 0.23 ± 0.26 | 0.22 ± 0.25 | 0.28 ± 0.37 | 0.05 ± 0.05 | 0.29 ± 0.46 | 0.32 ± 0.45 | 0.19 ± 0.21 | 0.21 ± 0.39 |
| CXCL2/MIP2α | 0.30 ± 0.22 | 0.36 ± 0.25 | 0.40 ± 0.49 | 0.18 ± 0.14 | 0.36 ± 0.47 | 0.39 ± 0.40 | 0.27 ± 0.16 | 0.23 ± 0.26 |
| Plasma, 24 h | ||||||||
| IL6 | 1.87 ± 1.54 | 2.01 ± 3.30 | 1.01 ± 1.07 | 0.88 ± 0.70 | 2.73 ± 4.16 | 1.28 ± 2.03 | 5.96 ± 10.17 | 2.55 ± 3.00 |
| IL10 | 1.32 ± 0.83 | 0.52 ± 1.17a | 0.75 ± 0.76 | 0.72 ± 1.27 | 1.99 ± 2.46 | 0.95 ± 0.88 | 3.29 ± 4.6 | 1.42 ± 1.60a |
| CXCL1/KC | 56.38 ± 70.53 | 7.52 ± 5.63 | 9.29 ± 9.76 | 26.18 ± 35.89 | 54.07 ± 89.80 | 12.99 ± 13.68 | 37.67 ± 59.94 | 46.19 ± 66.68 |
| CXCL2/MIP2α | 1.93 ± 1.90 | 1.64 ± 1.95 | 1.31 ± 0.85 | 1.32 ± 1.26 | 3.90 ± 5.16 | 1.84 ± 1.94 | 4.37 ± 4.78 | 3.19 ± 5.36 |
| Peritoneum, 12 h | ||||||||
| IL6 | 6.94 ± 5.13 | 11.54 ± 6.20 | 8.84 ± 7.89 | 5.78 ± 5.83 | 8.98 ± 11.58 | 8.86 ± 11.63 | 8.58 ± 5.83 | 8.76 ± 10.41 |
| IL10 | 1.08 ± 1.25 | 0.92 ± 0.86 | 1.29 ± 1.80 | 0.63 ± 0.67 | 0.89 ± 0.82 | 0.76 ± 1.21 | 0.75 ± 0.71 | 0.95 ± 1.01 |
| CXCL1/KC | 0.01 ± 0.01 | 0.03 ± 0.03 | 0.04 ± 0.05 | 0.01 ± 0.01 | 0.03 ± 0.03 | 0.03 ± 0.04 | 0.02 ± 0.02 | 0.03 ± 0.05 |
| CXCL2/MIP2α | 0.07 ± 0.05 | 0.08 ± 0.06 | 0.06 ± 0.05 | 0.07 ± 0.03 | 0.11 ± 0.03b | 0.04 ± 0.04b | 0.08 ± 0.04 | 0.08 ± 0.07 |
| Peritoneum, 24 h | ||||||||
| IL6 | 0.82 ± 0.82 | 2.11 ± 3.69 | 0.26 ± 0.22c | 0.53 ± 0.81 | 1.72 ± 4.20 | 0.57 ± 1.25 | 1.45 ± 2.04c | 1.25 ± 1.80 |
| IL10 | 0.45 ± 0.34 | 0.33 ± 0.40 | 0.29 ± 0.36d | 0.58 ± 0.71 | 0.71 ± 0.85 | 0.28 ± 0.33 | 0.98 ± 0.92d | 0.61 ± 0.58 |
| CXCL1/KC | 2.91 ± 3.50 | 11.21 ± 17.25 | 1.04 ± 1.77 | 2.75 ± 7.52 | 5.88 ± 9.66 | 1.45 ± 2.92 | 4.90 ± 6.04 | 7.84 ± 15.35 |
| CXCL2/MIP2α | 0.74 ± 0.68 | 0.71 ± 1.28 | 0.33 ± 0.20 | 0.34 ± 0.26 | 0.58 ± 0.64 | 0.80 ± 1.44 | 0.67 ± 0.75 | 1.12 ± 2.24 |
P < 0.05 for each pairwise comparison
Overall, the patterns of peritoneal cytokines were very similar to those seen in the peripheral blood. At 12 h, metestrus mice had a significantly (P = 0.003) decreased CXCL2/MIP2α concentration after treatment with buprenorphine as compared with saline; however, this difference was not seen at 24 h. Unlike other parameters (survival, peripheral cell counts), in which significant differences were seen in buprenorphine- but not saline-treated mice, several differences in peritoneal cytokines occurred in saline- but not buprenorphine- treated mice. In the peritoneum, saline-treated mice in estrus had significantly lower concentrations of both IL6 and IL10 than mice in diestrus (P = 0.033 and P = 0.046, respectively).
Discussion
In this study, we found that there were no significant differences in survival between saline- and buprenorphine-treated BALB/c female or male mice overall. The few differences seen in immune parameters did not persist across time points. However, when the estrous cycle in female mice was investigated, there were effects on survival and immune parameters in our CLP model when the model included buprenorphine. Other significant changes were seen in the circulating WBC, where proestrus mice treated with buprenorphine had higher cell numbers than almost every other group at 24 h. The remaining differences seen in cell counts and cytokines were few and did not persist across time points.
Our results showed that the estrous cycle had minimal effects on our study until buprenorphine treatment was added. In the literature, studies have suggested that estrogen may be protective against sepsis and that female subjects are less susceptible to sepsis than are male.24,38 In addition, estrogen causes increases in IL6, IL8, IgG/IgM, and nitric oxide synthase production as well as various effects on IFNγ, IL2, and TNFα secretion in humans.30 All of these effects are important for the progression and resolution of the systemic inflammatory response syndrome and the compensatory antiinflammatory response syndrome.5 Our data did not show an increased survival for female as compared with male mice or for female mice in higher estrogen stages and so did not support the conclusions of previous sepsis studies.23,41 However, those sepsis studies were not performed in a CLP model, and there is very little in the literature specifically looking at sex-associated differences in the CLP model overall. Even more importantly, the studies that do exist focus on differences between male and female subjects, whereas the question of whether the stage of the estrous cycle in intact female subjects has an effect in sepsis remains unanswered.
There were transient significant changes in cytokine concentrations in the plasma and peritoneal fluid between estrous stages. However, the cytokine data overall showed a wide range of responses in both plasma and peritoneal fluid. This result is expected for a 50% survival model, with the animals that did not survive representing the higher results seen.11 The cytokines we investigated represent important changes in the progression of sepsis. IL6 is a proinflammatory cytokine that increases significantly very early in sepsis and modulates the antiinflammatory cytokine IL10. Both IL6 and IL10 have been used to predict outcome in the past, but we did not see a predictive effect for survival between our groups according to IL6 or IL10.32 CXCL1 and 2 are neutrophil attractants that increased greatly at 24 h in both plasma and peritoneal fluid, as is expected in the normal course of sepsis.11 We also found significant changes in circulating WBC counts due to estrous cycle alone; however the differences in both circulating WBC and cytokines did not seem to be clinically relevant (as evidenced by survival rates) and may be easily controlled for by taking into account the short cycle.
We grouped mice by stage of estrous immediately prior to surgery. It is possible that by the 24 h time point, the mice may have progressed to the next stage of estrous. However, sepsis is a severely dysfunctional physiologic state, and it is more likely that the mice did not continue to cycle normally after CLP. Regardless, the results of this study suggest that the status at the time of surgery will affect the outcome of that procedure. The mouse estrous cycle is only 4 to 5 d long; therefore, it is likely that studies that perform procedures on multiple, consecutive days end up using mice in every stage. Even though we did not find that estrous was synchronized within a cage of mice, we suggest that doing procedures on multiple days in a week will result in less bias due to a specific stage. However, the only definitive way to know whether the estrous cycle is a factor in a study is to perform vaginal cytology and subsequently stage estrous.
The question that must still be answered is what role buprenorphine is playing to enhance the survival during high-estrogen stages. In our search for factors to explain these survival differences, the accompanying changes to the blood cell counts seemed like a potential factor. However, an increase in these parameters has not been linked to increased survival in the CLP model.12 As stated earlier, IL6 levels can be predictive of survival.32 It has also been shown that 17β-estradiol suppresses the IL6 gene;4 however, our data did not show significant differences in IL6 levels between buprenorphine-treated groups in different stages of estrous.
Another possible explanation for the buprenorphine-associated effects is the previous suggestion that this drug may have negative effects on behavior and recovery in mice that undergo abdominal procedures.17 We did not find this possibility to be supported by behavioral analysis in C57BL/6 mice previously, and this study in BALB/c had similar findings. We did not see significant differences between saline- and buprenorphine-treated mice. One challenge in this particular model is that septic mice display many of the same behaviors as do mice in pain, such as hunched posture, scruffy coat condition, abnormal activity levels, and loss of interest in socialization.15 However, our goal in assessing abnormal behavior was to see whether buprenorphine had an effect on long-term (days) recovery from the procedure, for example whether mice treated with buprenorphine had differences in time to normal coat condition, posture, and activity. We did not find differences in long-term recovery in buprenorphine- as compared with saline-treated mice. Another point to consider is that behavioral observation could only be completed on surviving mice, such that the mice with the highest abnormal scores dropped off when they met their endpoints and were euthanized. That said, the survivors had similar scores no matter which group they were in. Importantly, the score was not significantly different between treatment groups for either male or female groups at 24 h, a time point at which all animals were alive.
Without taking estrous cycle into account, our results in BALB/c female mice are consistent with previous studies from our lab showing that buprenorphine did not have an overall effect on the CLP model in female C57BL/6 or ICR mice.7,19 One notable difference from our previous studies was in the male survival curve. Our previous work in C57BL/6 male mice showed that a buprenorphine dose of 0.05 mg/kg had no effect on survival but that it decreased when mice were given 0.1 mg/kg.7 That higher dose had no effect on survival in BALB/c male mice. Strain-associated variation has been reported in multiple CLP studies,5 with BALB/c mice reportedly less sensitive to the model overall, according to a significantly higher survival rate than that of C57BL/6 mice undergoing an identical CLP procedure.43 Therefore strain variations in both sensitivity to buprenorphine and susceptibility to CLP could be contributing to our findings here. Our choice to dose buprenorphine every 12 h was based on the most consistent dosing regimen found in an informal literature search, as well as our desire to provide consistency with our previous studies. However, pharmacokinetic data shows, that by 12 h after administration, very little buprenorphine remains in the serum, and it has been suggested that dosing mice every 6 to 8 h is a more appropriate regimen. That said, other reports have shown analgesic effect for as long as 12 h in mice.21,36,40 This factor could play a role in the few differences we see with buprenorphine treatment, and other dosing frequencies could be a target for future studies.
Combined with our previous data, we have shown consistently that buprenorphine does not have an overall effect on a CLP model in female mice of 3 commonly used laboratory strains. However, in the current study, estrous stage did seem to affect the model that potentially was enhanced by buprenorphine. This effect can be avoided entirely by using male mice, ovariectomized female mice, or female mice all in one stage of estrous; however, doing so is not always an option. When intact female mice are used, with or without buprenorphine, the estrous cycle factor might be controlled by doing surgeries on multiple days in a week.
Requiring analgesics for surgical procedures is becoming the standard of care in research institutions, and buprenorphine appears to be an appropriate option for the CLP model, with minimal changes to survival and immune parameters in most situations. However, the combination of buprenorphine and stage of estrous may affect outcomes in the model, and investigators should consider this possible effect when designing sepsis studies.
Acknowledgments
We thank Dr Gerry Hish and Christopher Fry for their assistance with this project. We also thank the ACLAM Foundation for funding this work.
References
- 1.Angele MK, Schwacha MG, Ayala A, Chaudry IH. 2000. Effect of gender and sex hormones on immune responses following shock. Shock 14:81–90 [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 3.Benjamim CF, Ferreira SH, Cunha FQ. 2000. Role of nitric oxide in the failure of neutrophil migration in sepsis. J Infect Dis 182:214–223 [DOI] [PubMed] [Google Scholar]
- 4.Bjornstrom L, Sjoberg M. 2005. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19:833–842 [DOI] [PubMed] [Google Scholar]
- 5.Buras JA, Holzmann B, Sitkovsky M. 2005. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4:854–865 [DOI] [PubMed] [Google Scholar]
- 6.Caligioni CS. 2009. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4:Appendix 4I [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotroneo TM, Hugunin KM, Shuster KA, Hwang HJ, Kakaraparthi BN, Nemzek-Hamlin JA. 2012. Effects of buprenorphine on a cecal ligation and puncture model in C57BL/6 mice. J Am Assoc Lab Anim Sci 51:357–365 [PMC free article] [PubMed] [Google Scholar]
- 8.Dahan A, Kest B, Waxman AR, Sarton E. 2008. Sex-specific responses to opiates: animal and human studies. Anesth Analg 107:83–95 [DOI] [PubMed] [Google Scholar]
- 9.Dienstknecht T, Schwacha MG, Kang SC, Rue LW, Bland KI, Chaudry IH. 2004. Sex steroid-mediated regulation of macrophage/monocyte function in a 2-hit model of trauma–hemorrhage and sepsis. Cytokine 25:110–118 [DOI] [PubMed] [Google Scholar]
- 10.Doucet D, Badami C, Palange D, Bonitz RP, Lu Q, Xu DZ, Kannan KB, Colorado I, Feinman R, Deitch EA. 2010. Estrogen receptor hormone agonists limit trauma hemorrhage shock-induced gut and lung injury in rats. PLoS ONE 5:e9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. 1999. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun 67:6603–6610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebong SJ, Call DR, Bolgos G, Newcomb DE, Granger JI, O'Reilly M, Remick DG. 1999. Immunopathologic responses to nonlethal sepsis. Shock 12:118–126 [DOI] [PubMed] [Google Scholar]
- 13.Fox JG, Anderson LC, Loew FM, Quimby FW. 2002. Laboratory animal medicine, 2nd ed. New York (NY): Academic Press [Google Scholar]
- 14.Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL. 2007. The mouse in biomedical research, 2nd ed. New York (NY): Academic Press [Google Scholar]
- 15.Granger JI, Ratti PL, Datta SC, Raymond RM, Opp MR. 2013. Sepsis-induced morbidity in mice: effects on body temperature, body weight, cage activity, social behavior, and cytokines in brain. Psychoneuroendocrinology 38:1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guarnieri M, Brayton C, DeTolla L, Forbes-McBean N, Sarabia-Estrada R, Zadnik P. 2012. Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Anim (NY) 41:337–343 [DOI] [PubMed] [Google Scholar]
- 17.Hayes KE, Raucci JA, Jr, Gades NM, Toth LA. 2000. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci 39:18–23 [PubMed] [Google Scholar]
- 18.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. 2005. Cecal ligation and puncture. Shock 24 Suppl 1:52–57 [DOI] [PubMed] [Google Scholar]
- 19.Hugunin KM, Fry C, Shuster K, Nemzek JA. 2010. Effects of tramadol and buprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock 34:250–260 [DOI] [PubMed] [Google Scholar]
- 20.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 21.Kalliokoski O, Jacobsen KR, Hau J, Abelson KS. 2011. Serum concentrations of buprenorphine after oral and parenteral administration in male mice. Vet J 187:251–254 [DOI] [PubMed] [Google Scholar]
- 22.Krzych U, Strausser HR, Bressler JP, Goldstein AL. 1978. Quantitative differences in immune responses during the various stages of the estrous cycle in female BALB/c mice. J Immunol 121:1603–1605 [PubMed] [Google Scholar]
- 23.Leon LR, White AA, Kluger MJ. 1998. Role of IL6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol 275:R269–R277 [DOI] [PubMed] [Google Scholar]
- 24.Martin GS, Mannino DM, Eaton S, Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 25.Morton DB, Griffiths PH. 1985. Guidelines on the recognition of pain, distress, and discomfort in experimental animals and an hypothesis for assessment. Vet Rec 116:431–436 [DOI] [PubMed] [Google Scholar]
- 26.Nachtigall I, Tafelski S, Rothbart A, Kaufner L, Schmidt M, Tamarkin A, Kartachov M, Zebedies D, Trefzer T, Wernecke KD, Spies C. 2011. Gender-related outcome difference is related to course of sepsis on mixed ICUs: a prospective, observational clinical study. Crit Care 15:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemzek JA, Hugunin KM, Opp MR. 2008. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comp Med 58:120–128 [PMC free article] [PubMed] [Google Scholar]
- 28.Nemzek JA, Siddiqui J, Remick DG. 2001. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods 255:149–157 [DOI] [PubMed] [Google Scholar]
- 29.Nemzek JA, Xiao HY, Minard AE, Bolgos GL, Remick DG. 2004. Humane endpoints in shock research. Shock 21:17–25 [DOI] [PubMed] [Google Scholar]
- 30.Oertelt-Prigione S. 2012. The influence of sex and gender on the immune response. Autoimmun Rev 11:A479–A485 [DOI] [PubMed] [Google Scholar]
- 31.Pergolizzi J, Aloisi AM, Dahan A, Filitz J, Langford R, Likar R, Mercadante S, Morlion B, Raffa RB, Sabatowski R, Sacerdote P, Torres LM, Weinbroum AA. 2010. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract 10:428–450 [DOI] [PubMed] [Google Scholar]
- 32.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. 2002. Six at six: interleukin 6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17:463–467 [DOI] [PubMed] [Google Scholar]
- 33.Saia RS, Anselmo-Franci JA, Carnio EC. 2008. Hypothermia during endotoxemic shock in female mice lacking inducible nitric oxide synthase. Shock 29:119–126 [DOI] [PubMed] [Google Scholar]
- 34.Samy TS, Zheng R, Matsutani T, Rue LW, 3rd, Bland KI, Chaudry IH. 2003. Mechanism for normal splenic T lymphocyte functions in proestrus females after trauma: enhanced local synthesis of 17beta-estradiol. Am J Physiol Cell Physiol 285:C139–C149 [DOI] [PubMed] [Google Scholar]
- 35.Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM. 2005. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain 6:261–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tubbs JT, Kissling GE, Travlos GS, Goulding DR, Clark JA, King-Herbert AP, Blankenship-Paris TL. 2011. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci 50:185–191 [PMC free article] [PubMed] [Google Scholar]
- 37.Vallejo R, de Leon-Casasola O, Benyamin R. 2004. Opioid therapy and immunosuppression: a review. Am J Ther 11:354–365 [DOI] [PubMed] [Google Scholar]
- 38.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. 2000. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med 26:167–172 [DOI] [PubMed] [Google Scholar]
- 39.Yu HP, Chaudry IH. 2009. The role of estrogen and receptor agonists in maintaining organ function after trauma-hemorrhage. Shock 31:227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu S, Zhang X, Sun Y, Peng Y, Johnson J, Mandrell T, Shukla AJ, Laizure SC. 2006. Pharmacokinetics of buprenorphine after intravenous administration in the mouse. J Am Assoc Lab Anim Sci 45:12–16 [PubMed] [Google Scholar]
- 41.Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. 1997. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med 25:106–110 [DOI] [PubMed] [Google Scholar]
- 42.Zellweger R, Zhu XH, Wichmann MW, Ayala A, DeMaso CM, Chaudry IH. 1996. Prolactin administration following hemorrhagic shock improves macrophage cytokine-release capacity and decreases mortality from subsequent sepsis. J Immunol 157:5748–5754 [PubMed] [Google Scholar]
- 43.Zhang J, Liu ZG, Luo YW, He Y, Gu DS, Wang M, Zheng YT, Sun EW. 2010 [Differences in the response to sepsis between C57BL/6 and BALB/c mice] Nan Fang Yi Ke Da Xue Xue Bao 30:973–975 [Article in Chinese] [Google Scholar]