Abstract
Physical inactivity contributes to the frailty and the decline in function that develops over time among patients with end-stage renal disease. We assessed physical activity among 1547 ambulatory patients new to dialysis in the United States Renal Data System Comprehensive Dialysis Study. We used a self-reporting Human Activity Profile that included Maximal and Adjusted Activity Scores and compared results to established norms by age and gender. Physical activity was found to be extremely low with scores for all age and gender categories below the 5th percentile of healthy individuals and 95% of patients had scores consonant with low fitness. Older age, female gender, diabetes, atherosclerotic disease, and a low level of education were associated with lower activity scores assessed by univariate and multivariable linear regression analysis. Higher serum albumin, creatinine, and lower body mass index, but not hemoglobin levels, were associated with greater physical activity. By multivariable analysis, patients on hemodialysis using a catheter reported lower levels of physical activity compared to those on peritoneal dialysis, hemodialysis using an arteriovenous fistula, or with a graft. Lower Maximal and Adjusted Activity Scores were associated with poor physical function and mental health. Hence, physical activity is distressingly low among patients new to dialysis. Thus, strategies to enhance activity in these patients should be explored.
Keywords: dialysis, end-stage renal disease (ESRD), Human Activity Profile (HAP), physical activity
Patients with end-stage renal disease (ESRD) report limitations in physical functioning that have an adverse effect on overall health status and have been associated with reduced survival.1–3 It has recently been appreciated that low levels of physical activity are associated with poor functioning in this population,4–6 and it is possible that physical inactivity contributes to the decline in functioning and frailty that develops over time in this group. Although there is no direct evidence that increasing physical activity can improve functioning or increase survival in the dialysis population, physical activity is a potentially modifiable risk factor for frailty, debility, and mortality.
To improve our understanding of physical activity and functioning in incident dialysis patients, self-reported physical activity was assessed in the USRDS (United States Renal Data Systems) Comprehensive Dialysis Study (CDS), a cohort study of patients starting dialysis in 2005–2007, sampled from a geographically stratified national random sample of dialysis units. The aim of the current study is to describe ambulatory CDS patients’ self-reported physical activity compared with normative data and to examine the clinical and demographic correlates of physical activity participation in this cohort.
RESULTS
Of the 1640 CDS participants who participated in the interview, 1625 completed the Human Activity Profile (HAP), 1547 of whom had not had an amputation, were able to walk and transfer, and were included in our analyses. The characteristics of the study participants are shown in Table 1. CDS participants were slightly younger (60.3 ± 14.2) than the average age of patients initiating dialysis in 2005 (63.2 ± 16.0), and the fraction of CDS participants receiving peritoneal dialysis (10.4%) was higher than in the 2005 incident dialysis population (7%).7,8 There were several differences between women and men in the cohort. Specifically, women were more likely to be non-white and to have atherosclerotic disease. They also had higher body mass index (BMI) and lower serum creatinine concentrations than men, likely reflective of their expected greater fat mass and lower muscle mass. In addition, women were more likely to dialyze via a catheter and less likely to have an arteriovenous (AV) fistula than men.
Table 1.
Characteristics of CDS participants with physical activity data (N=1547)
| Variable | Men (n=855) |
Women (n=692) |
P-value |
|---|---|---|---|
| Age | 60.7 ± 14.6 | 59.8 ± 13.8 | 0.32 |
| Race, White | 617 (72%) | 442 (64%) | 0.0005 |
| BMI (kg/m2) | 28.4 ± 6.5 | 31.2 ± 9.1 | <0.0001 |
| Serum albumin (g/dl) | 3.2 ± 0.7 | 3.2 ± 0.7 | 0.60 |
| Serum creatinine (mg/dl) | 7.5 ± 3.8 | 6.2 ± 3.0 | <0.0001 |
| Hemoglobin concentration (g/dl) | 10.2 ± 1.8 | 9.9 ± 1.7 | 0.0006 |
| Peritoneal dialysis | 79 (9%) | 82 (12%) | 0.10 |
| Vascular access type | <0.0001 | ||
| Catheter | 404 (52%) | 361 (60%) | |
| AV graft | 72 (9%) | 82 (14%) | |
| AV fistula | 296 (38%) | 161 (27%) | |
| Comorbidity | |||
| Diabetes | 469 (55%) | 401 (58%) | 0.22 |
| Congestive heart failure | 600 (70%) | 487 (70%) | 0.93 |
| Atherosclerotic disease | 559 (65%) | 494 (71%) | 0.01 |
| Some college education or more | 396 (46%) | 295 (43%) | 0.16 |
| Current smoker | 145 (17%) | 93 (13%) | 0.06 |
| HAP | |||
| MAS | 65.2 ± 14.9 | 57.2 ± 15.1 | <0.0001 |
| AAS | 47.9 ± 18.1 | 39.1 ± 17.4 | <0.0001 |
Abbreviations: AAS, Adjusted Activity Score; AV, arteriovenous; BMI, body mass index; CDS, Comprehensive Dialysis Study; HAP, Human Activity Profile; MAS, Maximum Activity Score.
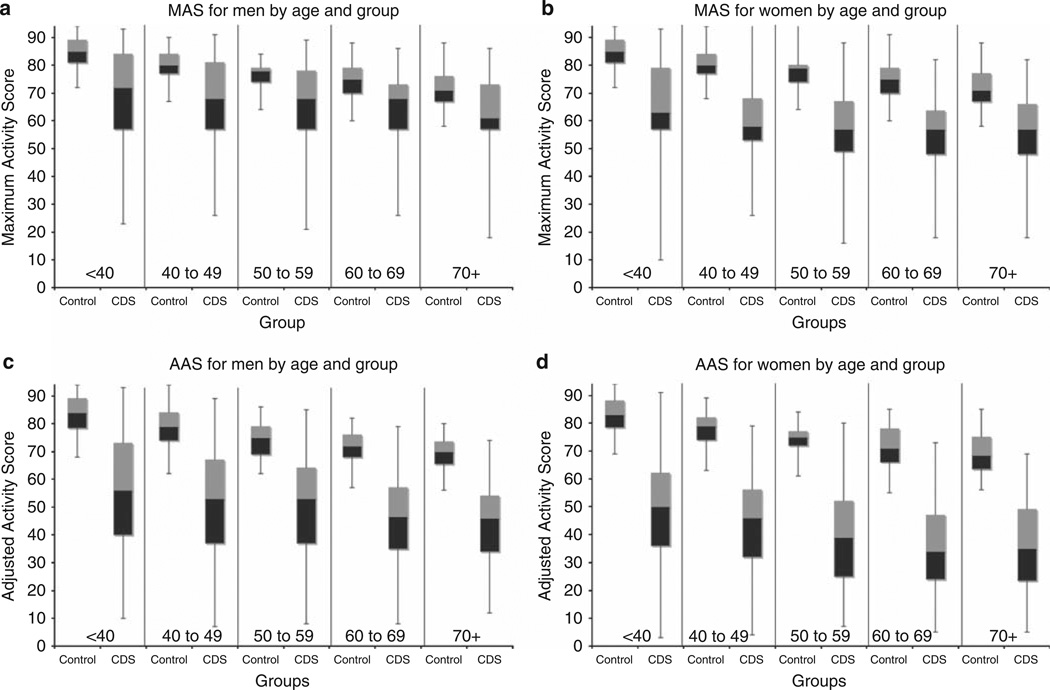
Self-reported physical activity was extremely low when compared with healthy individuals. The median Maximum Activity Score (MAS) for men in all age groups in the CDS was considerably below the 25th percentile for healthy men (Figure 1). For women, the median MAS for CDS participants was consistently below the first percentile for healthy individuals in all age groups. The Adjusted Activity Score (AAS) of CDS participants was even lower relative to normative data, with the 75th percentile for men in all age groups below the 25th percentile for the general population and the 75th percentile for women in all groups below the 1st percentile (Figure 1). Median MAS scores translated into an activity age of 70+ for all age groups, meaning that even the younger participants’ activity level was at or below the 50th percentile for healthy individuals >70 years of age. The AAS scores of all groups would be expected to correspond to a low level of fitness (low peak or maximal oxygen consumption). There was no age group for which the average AAS was above the threshold of low fitness; however, there were some individuals whose fitness levels were fair or average or above. Among the 1547 participants, 1420 (92%) were between the ages of 20 and 79 years, allowing for estimation of fitness level. Only 74 (5.2%) patients between 20 and 79 years of age had AAS above the threshold for low fitness. To put these data into perspective, we examined a few specific activities that would be expected to be important for daily living, including climbing 12 steps without stopping, walking one block without stopping, climbing 24 steps, and walking one mile (Table 2). Less than half of all respondents reported that they climb 12 steps (one flight of stairs) without stopping, and less than one-quarter climb two flights of stairs even with the possibility of resting. Subjects were somewhat more likely to report walking one block (56.4%) than climbing stairs, but only 18.5% reported walking one mile. Reported performance of each activity was lower for women and for older participants (Table 2).
Figure 1. Human Activity Profile (HAP) scores among men and women in the Comprehensive Dialysis Study (CDS) compared with norms for healthy individuals.
The black boxes represent the 25th to 50th percentile; gray boxes represent the 50th to 75th percentile; lines above and below extend to the 99th and 1st percentile, respectively. In each figure, scores are shown by age group, beginning with age <40 and progressing by decade to age 70+ years. Within each age group, normative data are represented on the left and CDS participants’ data are plotted on the right. (a, b) Maximum Activity Scores (MAS) and (c, d) Adjusted Activity Scores (AAS) for men and women are shown.
Table 2.
Percentage of Comprehensive Dialysis Study (CDS) respondents reporting that they still perform selected activities overall and by age and sex categories
| Activity | ||||
|---|---|---|---|---|
| Participants | Climb 12 steps (nonstop) | Walk 1 block (nonstop) | Climb 24 steps | Walk 1 mile |
| All (n=1547) | 43.8 | 56.4 | 23.8 | 18.5 |
| Men (n=855) | 53.3 | 65.0 | 29.8 | 23.8 |
| <40 (n=73) | 65.8 | 68.5 | 38.4 | 41.1 |
| 40–49 (n=119) | 56.3 | 68.9 | 40.3 | 30.3 |
| 50–59 (n= 232) | 56.0 | 70.3 | 34.1 | 29.3 |
| 60–69 (n=186) | 50.5 | 58.1 | 22.6 | 19.4 |
| 70+ (n=245) | 47.8 | 62.4 | 23.7 | 15.5 |
| Women (n=692) | 32.1 | 45.7 | 16.3 | 11.3 |
| <40 (n=57) | 49.1 | 59.6 | 33.3 | 22.8 |
| 40–49 (n=105) | 41.0 | 56.2 | 19.0 | 18.1 |
| 50–59 (n=174) | 28.7 | 43.7 | 17.2 | 10.9 |
| 60–69 (n=188) | 26.1 | 39.9 | 11.7 | 7.4 |
| 70+ (n=168) | 31.0 | 42.9 | 13.1 | 7.7 |
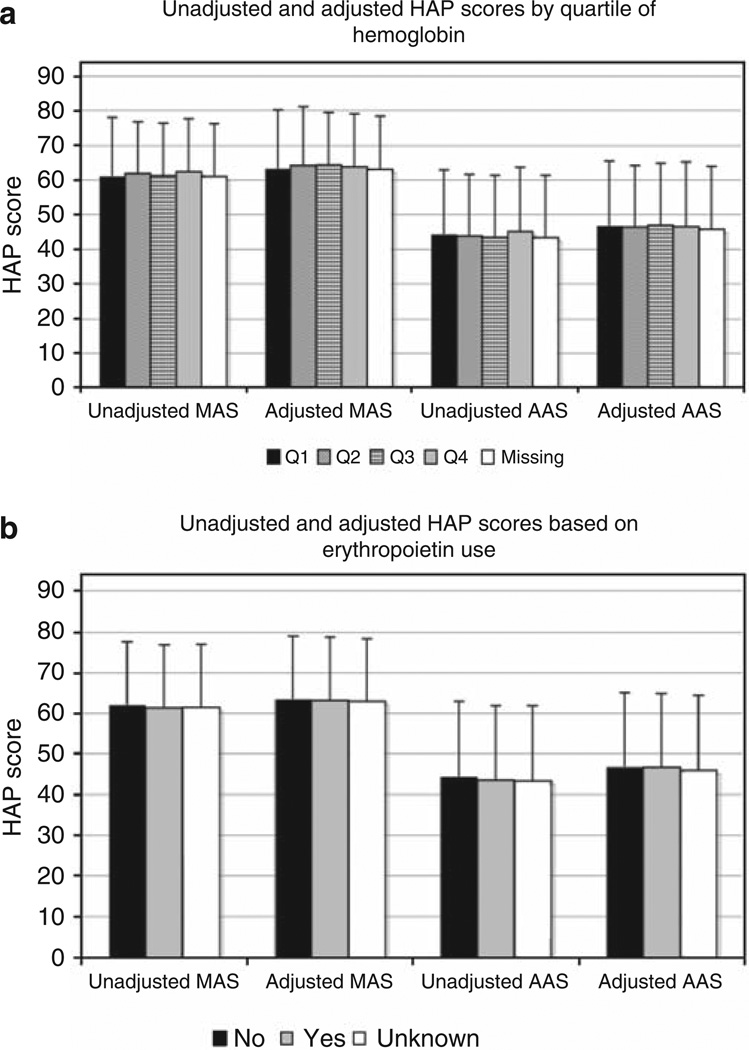
The correlates of self-reported physical activity are shown in Table 3. Generally, correlates of MAS and AAS were similar in magnitude and direction but slightly stronger for AAS. Self-reported physical activity was lower among women, older participants, and participants with less education, as has been described for healthy populations. In addition, higher BMI was associated with lower self-reported physical activity. On the other hand, higher serum albumin and creatinine concentrations were associated with greater physical activity. There was no significant association between hemoglobin concentration and physical activity. Figure 2 shows that there was no gradient of unadjusted or adjusted HAP scores by quartile of hematocrit. We also examined HAP scores according to whether erythropoietin was prescribed before starting dialysis and found no significant differences (Figure 2). Patients with diabetes, atherosclerotic disease, and congestive heart failure were all less active than their counterparts without these diagnoses. Peritoneal dialysis patients had MAS and AAS scores that were similar to hemodialysis patients dialyzing via an AV fistula, but hemodialysis patients dialyzing using an AV graft or catheter were less active. Patients reporting lower levels of physical activity also reported lower physical and mental composite scores on the SF-12 (Medical Outcomes Study short form 12-item survey), although the estimated association with activity was stronger for physical than for mental health.
Table 3.
Univariate associations of patient characteristics with physical activity
| MAS | AAS | |||
|---|---|---|---|---|
| Variable | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value |
| Age, per 10 years | −2.04 (−2.57, −1.51) | <0.0001 | −2.82 (−3.44, −2.20) | <0.0001 |
| Sex (female) | −8.12 (−9.60, −6.63) | <0.0001 | −9.18 (−10.93, −7.43) | <0.0001 |
| Race (White vs other) | 0.27 (−1.38, 1.93) | 0.75 | −0.22 (−2.16, 1.73) | 0.83 |
| BMI, per 10 kg/m2 | −2.30 (−3.25, −1.34) | <0.0001 | −3.42 (−4.54, −2.30) | <0.0001 |
| Serum albumin, g/dl | ||||
| 1st quartile (≤2.7) | 0 (Reference) | 0 (Reference) | ||
| 2nd quartile (2.8–3.2) | 2.66 (0.23, 5.10) | 0.03 | 3.95 (1.08, 6.82) | 0.007 |
| 3rd quartile (3.3–3.7) | 3.98 (1.62, 6.33) | 0.001 | 5.62 (2.84, 8.40) | <0.0001 |
| 4th quartile (≥3.8) | 7.15 (4.65, 9.64) | <0.0001 | 9.10 (6.16, 12.04) | <0.0001 |
| Serum creatinine, mg/dl | ||||
| 1st quartile (≤4.7) | 0 (Reference) | 0 (Reference) | ||
| 2nd quartile (4.8–6.1) | 3.63 (1.54, 5.71) | 0.0007 | 5.47 (3.05, 7.89) | <0.0001 |
| 3rd quartile (6.2–8.0) | 7.36 (5.27, 9.46) | <0.0001 | 10.46 (8.03, 12.88) | <0.0001 |
| 4th quartile (≥8.1) | 9.29 (7.22, 11.36) | <0.0001 | 13.74 (11.35, 16.14) | <0.0001 |
| Hemoglobin, g/dl | ||||
| 1st quartile (≤8.9) | 0 (Reference) | 0 (Reference) | ||
| 2nd quartile (9.0–10.1) | 0.58 (−1.70, 2.85) | 0.62 | −0.61 (−3.28, 2.07) | 0.66 |
| 3rd quartile (10.2–11.2) | 0.04 (−2.23, 2.32) | 0.92 | −0.71 (−3.39, 1.96) | 0.60 |
| 4th quartile (≥11.3) | 1.66 (−0.61, 3.92) | 0.14 | 0.88 (−1.79, 3.54) | 0.52 |
| Dialysis modality, access | ||||
| Hemodialysis via AVF | 0 (Reference) | 0 (Reference) | ||
| Hemodialysis via AVG | −4.20 (−6.95, −1.45) | 0.003 | −5.12 (−8.36, −1.88) | 0.002 |
| Hemodialysis via catheter | −5.24 (−7.00, −3.47) | <0.0001 | −6.26 (−8.34, −4.18) | <0.0001 |
| Peritoneal dialysis | 0.15 (−2.59, 2.88) | 0.92 | 2.30 (−0.91, 5.51) | 0.16 |
| Comorbidity | ||||
| Diabetes | −9.26 (−11.02, −7.49) | <0.0001 | −8.62 (−10.37, −6.86) | <0.0001 |
| Atherosclerosis | −4.58 (−6.18, −2.98) | <0.0001 | −6.49 (−8.36, −4.62) | <0.0001 |
| Congestive heart failure | −4.00 (−5.65, −2.35) | <0.0001 | −6.15 (−8.08, −4.22) | <0.0001 |
| Education (no college) | −3.54 (−5.08, −2.01) | <0.0001 | −5.31 (−7.10, −3.51) | <0.0001 |
| Smoker | 1.01 (−1.12, 3.14) | 0.35 | 1.67 (−0.83, 4.17) | 0.19 |
| SF-12 PCS, per 10 units | 6.78 (6.22, 7.35) | <0.0001 | 9.81 (9.21, 10.42) | <0.0001 |
| SF-12 MCS, per 10 units | 2.46 (1.47, 3.45) | <0.0001 | 3.13 (1.97, 4.30) | <0.0001 |
Abbreviations: AAS, Adjusted Activity Score; AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; CI, confidence interval; MAS, Maximum Activity Score; MCS, Mental Component Summary score; PCS, Physical Component Summary score; SF-12, Medical Outcomes Study 12-item short form health survey.
Figure 2. Unadjusted and adjusted Human Activity Profile (HAP) scores according to quartiles of hemoglobin and erythropoietin use.
(a) In HAP scores according to quartiles of hemoglobin, the black bars represent the first quartile of hemoglobin (≤8.9 g/dl), diagonally striped bars the second quartile (9.0–10.1 g/dl), horizontally striped bars the third quartile (10.2–11.2 g/dl), gray bars the fourth quartile (≥11.2 g/dl), and white bars those with missing hemoglobin values. (b) In HAP scores based on erythropoietin use, the black bars represent those who did not receive erythropoietin before starting dialysis, gray bars represent those receiving erythropoietin, and white bars those whose receipt of erythropoietin was unknown. AAS, Adjusted Activity Score; MAS, Maximum Activity Score.
Multivariable analyses (Table 4) showed that most of the crude correlates of physical activity remained significantly associated after adjustment for other predictors. Age and BMI remained inversely associated with physical activity, and serum albumin and creatinine remained directly associated with physical activity. Although diabetes and atherosclerosis were independently associated with HAP scores, congestive heart failure was not. After adjustment for the other correlates of physical activity, only hemodialysis via catheter was associated with lower HAP scores; patients dialyzing through AV grafts did not have scores that were significantly different from patients on peritoneal dialysis or patients on hemodialysis with AV fistulae. Multivariable analysis excluding participants with missing serum albumin values (n = 369) yielded results that were similar to the main model (data not shown).
Table 4.
Multivariable associations among patient characteristics and physical activity
| MAS | AAS | |||
|---|---|---|---|---|
| Variable | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value |
| Age | ||||
| <40 | 4.12 (1.62, 7.58) | 0.002 | 7.43 (4.01, 10.93) | <0.0001 |
| 40 to <50 | 2.60 (0.34, 5.23) | 0.03 | 4.53 (1.69, 7.34) | 0.002 |
| 50 to <60 | 0.35 (−1.59, 2.43) | 0.68 | 2.28 (−0.07, 4.64) | 0.06 |
| 60 to <70 | 0 (Reference) | 0 (Reference) | ||
| ≥70 | −2.84 (−5.02, −0.89) | 0.005 | −1.87 (−4.31, 0.54) | 0.13 |
| Sex, female | −6.60 (−8.11, −5.12) | <0.0001 | −6.78 (−8.51, −5.02) | <0.0001 |
| Race, White | 1.47 (−0.25, 2.99) | 0.10 | 1.68 (−0.19, 3.66) | 0.08 |
| BMI, per 10 kg/m2 | −1.10 (−2.09, −0.21) | 0.02 | −1.75 (−2.90, −0.63) | 0.002 |
| Serum albumin, g/dl | 0.0009 | |||
| 1st quartile (≤2.7) | 0 (Reference) | 0 (Reference) | ||
| 2nd quartile (2.8–3.2) | 1.40 (−0.92, 3.52) | 0.25 | 2.12 (−0.40, 4.54) | 0.10 |
| 3rd quartile (3.3–3.7) | 2.79 (0.32, 4.72) | 0.02 | 4.02 (1.18, 6.87) | 0.006 |
| 4th quartile (≥3.8) | 3.42 (1.14, 5.58) | 0.004 | 4.32 (1.33, 6.48) | 0.003 |
| Serum creatinine, mg/dl | ||||
| 1st quartile (≤4.7) | 0 (Reference) | 0 (Reference) | ||
| 2nd quartile (4.8–6.1) | 1.00 (−0.61, 3.41) | 0.17 | 2.36 (0.04, 4.74) | 0.05 |
| 3rd quartile (6.2–8.0) | 3.18 (1.42, 5.57) | 0.001 | 5.77 (3.36, 8.18) | <0.0001 |
| 4th quartile (≥8.1) | 4.24 (2.27, 6.62) | <0.0001 | 7.41 (4.80, 9.97) | <0.0001 |
| Diabetes | −3.21 (−4.97, −1.79) | <0.0001 | −4.88 (−6.75, −3.05) | <0.0001 |
| Congestive heart failure | −0.49 (−1.75, 1.57) | 0.75 | −1.14 (−3.08, 0.82) | 0.26 |
| Atherosclerosis | −2.74 (−4.46, −1.25) | 0.0007 | −3.40 (−5.28, −1.44) | 0.0006 |
| Modality/access | <0.0001 | |||
| HD via fistula | 0 (Reference) | 0 (Reference) | ||
| HD via graft | −1.62 (−4.16, 1.00) | 0.23 | −1.86 (−4.95, 1.10) | 0.21 |
| HD via catheter | −3.70 (−5.60, −2.20) | <0.0001 | −4.20 (−6.24, −2.33) | <0.0001 |
| Peritoneal dialysis | −0.12 (−2.97, 2.20) | 0.77 | 1.94 (−1.17, 4.93) | 0.23 |
| No college | −2.43 (−4.12, −1.24) | 0.0003 | −3.88 (−5.59, −2.22) | <0.0001 |
| Smoker | −0.84 (−2.76, 1.32) | 0.49 | −0.93 (−3.29, 1.44) | 0.44 |
Abbreviations: AAS, Adjusted Activity Score; CI, confidence interval; HD, hemodialysis; MAS, Maximum Activity Score.
In multivariable analyses, self-reported physical functioning remained strongly associated with MAS (coefficient 6.0 per 10 points on Physical Component Summary score, 95% confidence interval 5.3–6.6) and AAS (8.6 per 10 points, CI 8.0–9.4). Mental health also remained directly associated with physical activity (3.4 per 10 points on Mental Component Summary score, confidence interval 2.4–4.5 for MAS; 4.2 per 10 points, confidence interval 3.0–5.4 for AAS) but to a lesser extent than physical functioning.
DISCUSSION
It has been previously reported that patients on dialysis are less physically active than sedentary controls without kidney disease,9 and that many incident dialysis patients report no participation in leisure time physical activity.10,11 However, this study expands considerably on previous work. First, use of the HAP to quantify the level of physical activity in a relatively large cohort of patients new to dialysis, selected from a geographically stratified random sample of dialysis facilities, is a major strength of the study. The range of activities covered by the HAP is larger than that of most activity questionnaires, and the availability of normative data allows for comparison with healthy individuals of the same sex and similar age.
The relatively large sample size of the CDS allowed us to test a number of potential correlates of physical activity, as well as to begin to explore the associations among physical activity and physical and mental health. The results of this study show that the level of self-reported physical activity is extremely low among ambulatory incident dialysis patients, the median falling below the first percentile for most subgroups defined by age and sex. Although normative data are not available for specific activities, the low reported rates of walking and stair climbing were remarkable and suggest that a large proportion of dialysis patients are not performing routine activities such as shopping or other errands. Similar to most instruments designed to measure physical activity or the range of activities performed, the HAP does not provide information about the reasons for limited physical activity (that is, it does not distinguish between unable vs unwilling). However, patients who were unable to walk or transfer or who had previous amputations were excluded from analysis, making the low rates of walking and stair climbing all the more remarkable, and suggesting that inability to be active does not account for all of the reduced physical activity observed in this population.
Patient characteristics associated with self-reported physical activity paralleled those described in the general population, with higher levels of physical activity among those who were male, younger, and better educated. Although lower physical activity among women is consistent with data for the general population, the finding that women in the CDS were more sedentary than men relative to sex-specific norms was somewhat surprising. This may be explained, in part, by the observed differences between men and women in this cohort (for example, higher BMI, lower creatinine, and more catheters among women), all of which would be expected to correlate with lower levels of physical activity among the women.
Several health- or dialysis-related factors were associated with physical activity, including the presence of diabetes, lower serum albumin and creatinine concentrations, and use of a catheter for hemodialysis, all of which were associated with lower reported physical activity. In contrast, hemoglobin concentrations and previous use of erythropoietin were not associated with self-reported physical activity in this cohort. The lack of association with hemoglobin and erythropoietin is consistent with recent evidence suggesting that although severe anemia may limit exercise capacity and physical functioning, there is little, if any, benefit to correction of anemia with erythropoietin above currently recommended targets in either CKD or end-stage renal disease.12,13
We showed strikingly similar associations among albumin, creatinine, and BMI with HAP scores as we had described with physical activity measured by three-dimensional accelerometry in a small, single-center study published nearly a decade ago.9 Although serum albumin and creatinine are generally considered to reflect nutritional status, they may also reflect acute and/or chronic inflammation. Interestingly, higher BMI, despite being associated with longevity in multiple end-stage renal disease cohorts,14–17 was associated with lower MAS and AAS in this cohort.
Patients who reported less self-reported physical activity also reported poorer health-related quality of life in both the physical and mental domains, suggesting the possibility that physical inactivity could lead to impaired functioning. This possibility is biologically plausible as sedentary behavior can result in reduced physical fitness and thus in poor performance and function in the physical domain. In addition, exercise training has been shown to improve physical functioning among dialysis patients.18–20 The association of lower levels of physical activity with lower self-reported mental health is also consistent with associations observed in the general population,21–23 and is potentially important as physical activity has the potential to relieve symptoms of depression and anxiety and improve mood.22,24 However, the cross-sectional nature of this study precludes determination of causality. It is also plausible that limitations in physical function or depression could lead to reduced participation in physical activity.
Although the CDS was designed to produce a nationally representative sample of incident dialysis patients, enrollment rates were low, raising the possibility that the final sample may not be fully representative of the target population.8 In fact, when compared with all patients beginning dialysis in 2005 and to the Dialysis Morbidity and Mortality Wave 2 study, CDS participants were slightly younger, more likely to initiate peritoneal dialysis, and better educated.8 As all of these characteristics were associated with relatively higher levels of physical activity in this cohort, it is possible that our results reflect a ‘best estimate’ of physical activity performed by incident dialysis patients, which would tend to obscure differences between patients on dialysis and healthy individuals rather than to accentuate them. In addition, it is not likely that selection of a cohort that is slightly more active than the population as a whole would have influenced the associations of patient characteristics with physical activity. Moreover, it is possible (arguably probable) that levels of physical activity decline with advancing dialysis vintage. In other words, compared with age- and sex-matched healthy individuals, patients new to dialysis are likely to be significantly more active than patients established on dialysis for ≥1 year. Thus, the mean or median levels of physical activity and levels of fitness of the overall dialysis program are likely to be far worse than what we have described here.
In summary, ambulatory participants in the CDS reported extremely low physical activity within all age and sex subgroups compared with the general population. Approximately 95% of patients had AAS scores that corresponded to physical fitness levels below the 20th percentile for the general population. Although numerous correlates of physical activity were identified in this population, most are not readily modifiable. Thus, risk factor modification is unlikely to ameliorate the problem. Rather, strategies aimed to enhance participation in physical activity among patients on dialysis, such as education, counseling, and physical therapy, have the potential to improve fitness and self-reported functioning and should be tested prospectively.
MATERIALS AND METHODS
Study subjects
The CDS is a prospective study of adult dialysis patients who started regular dialysis within centers throughout the United States between June 2005 and June 2007.8 A total of 1677 adult dialysis patients from 296 dialysis clinics within 18 End-Stage Renal Disease Networks participated. Study information, a consent form, and participation availability form were sent to eligible participants. Patients willing to participate completed and returned a signed consent form or provided verbal consent by telephone, and 1640 subsequently completed a telephone interview conducted by Data Banque Research Services (Pittsburgh, PA), a data management organization contracted by the CDS. Because a major goal of this study was to describe physical activity among patients who could potentially be counseled to increase activity if it were low, we restricted our analyses to CDS participants who had not had an amputation and could walk and transfer (n = 1547).
Self-reported physical activity
CDS participants were asked about physical activity using the 94-item HAP, which includes assessment of activities across a broad range of energy requirements. We selected the HAP as the measure of physical activity in the CDS because we had previously tested several questionnaires of physical activity participation among a single-center cohort of dialysis patients and found the HAP to be most strongly associated with 7-day physical activity measured by accelerometry.6 Also, unlike some other assessments, the HAP could be administered by telephone, making it suitable for the design and implementation of the CDS.
On the HAP, activity items are ranked based on estimated energy expenditure, with item 1 being the activity requiring the least energy expenditure and item 94 requiring the greatest energy expenditure. For each item, the respondent indicates whether he or she is still doing, has stopped doing, or never did the activity in question. The MAS is the highest oxygen-demanding activity that the respondent still performs. The AAS is determined by subtracting from the MAS the total number of activities the respondent has stopped doing that have numbers lower than the MAS. The AAS is interpreted as a measure of usual physical activity level.25
Explanatory variables
Potential correlates of physical activity were obtained from the CDS or from the Medical Evidence Form (Center for Medicare and Medicaid Services (CMS) form 2728). Vascular access type was ascertained directly from CDS participants. The SF-12 was administered in its entirety as part of the CDS patient questionnaire. Self-reported physical functioning was assessed using the Physical Component Summary score of the SF-12. Age, sex, race, and comorbidity information was obtained from the 2728 form. Age was divided into categories as follows: <40, 40 to <49 years, 50 to <60 years, 60 to <70 years, and ≥70 years to match normative categories reported in the HAP manual.25 Specific comorbidities considered were diabetes, congestive heart failure, and atherosclerotic disease, the latter defined as one or more of the following diagnoses: peripheral vascular disease, atherosclerotic heart disease, or stroke. Quetélet’s index (BMI, kg/m2) was calculated from height and weight data from the 2728 form. Serum albumin, creatinine, and hemoglobin concentrations before dialysis initiation were obtained from the 2728 and categorized into quartiles. Missing values for serum creatinine concentration (n = 10) were replaced with the mean value. Missing values for serum albumin concentration (24.1%) and hemoglobin (10.8%) were ignored in univariate analyses. For multivariable analyses, hemoglobin was not included because it was not associated with physical activity on univariate analysis. For serum albumin, multiple imputation using the regression method was used to impute missing values.
Statistical analysis
Patient characteristics, including demographic information, clinical characteristics, laboratory data, and self-reported physical activity, were described using mean and s.d. for continuous variables and proportions for categorical variables. HAP scores by age and sex categories were compared with norms that were derived from a sample of 447 individuals aged 20–79 without significant medical problems.25 Normative comparisons included percentile scores for MAS and AAS, activity age for MAS, and fitness classification for AAS. Activity age is defined as the age at which 50% of healthy adult respondents continue to engage in an activity. Fitness classification was assigned using the linear relationship between AAS and estimated maximal oxygen consumption described in the HAP manual25 and comparing with norms available for individuals aged 20–79 from the American College of Sports Medicine.26 Participants were classified as having low fitness if their estimated maximal oxygen consumption was below the 20th percentile for age and sex.
Univariate and multivariable linear regression analysis was used to assess associations among demographic, clinical, and laboratory parameters and the MAS and AAS of the HAP. Multivariable models were selected based on the fraction of variation explained and the Mallow’s Cp. Patients’ dialysis facility was included in the model as a random effect to account for clustering. As a sensitivity analysis, multivariable analysis was also conducted excluding subjects with missing data for serum albumin concentration. The two-tailed P-values of <0.05 were considered to indicate statistical significance. Analyses were performed using SAS 9.2 (Cary, NC).
ACKNOWLEDGMENTS
This work was supported through contracts N01-DK-7-0005 and N01-DK-7-5004 from the National Institute of Diabetes and Digestive and Kidney Diseases and was presented in oral form at the 42nd Annual Meeting and Scientific Exposition of the American Society of Nephrology in San Diego, CA. We acknowledge David Daughton for permission to include the Human Activity Profile in the CDS.
Footnotes
DISCLOSURE
The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the United States government. All the authors declared no competing interests
REFERENCES
- 1.Churchill D, Torrance G, Taylor D, et al. Measurement of quality of life in end-stage renal disease: the time trade-off approach. Clin Invest Med. 1987;10:14–20. [PubMed] [Google Scholar]
- 2.DeOreo P. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30:204–212. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 3.Lowrie E, Curtin R, LePain N, et al. Medical outcomes study short form-36: a consistent and powerful predictor of mortality in dialysis patients. Am J Kidney Dis. 2003;41:1286–1292. doi: 10.1016/s0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 4.Brenner I, Brohart K. Weekly energy expenditure and quality of life in hemodialysis patients. CANNT J. 2008;18:36–40. [PubMed] [Google Scholar]
- 5.Kutner N, Zhang R, McClellan W. Patient-reported quality of life early in dialysis treatment: effects associated with usual exercise activity. Nephrol Nurs J. 2000;27:357–367. [PubMed] [Google Scholar]
- 6.Johansen K, Painter P, Kent-Braun J, et al. Validation of questionnaires to estimate physical activity and functioning in ESRD. Kidney Int. 2001;59:1121–1127. doi: 10.1046/j.1523-1755.2001.0590031121.x. [DOI] [PubMed] [Google Scholar]
- 7.United States Renal Data System. USRDS 2007 Annual Data Report. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 8.Kutner N, Johansen K, Kaysen G, et al. The comprehensive dialysis study (CDS): a USRDS special study. Clin J Am Soc Nephrol. 2009;4:645–650. doi: 10.2215/CJN.05721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansen K, Chertow G, Ng A, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57:2564–2570. doi: 10.1046/j.1523-1755.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 10.O’Hare A, Tawney K, Bacchetti P, et al. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41:447–454. doi: 10.1053/ajkd.2003.50055. [DOI] [PubMed] [Google Scholar]
- 11.Gutman R, Stead W, Robinson R. Physical activity and employment status of patients on maintenance dialysis. N Engl J Med. 1981;304:309–313. doi: 10.1056/NEJM198102053040601. [DOI] [PubMed] [Google Scholar]
- 12.Johansen K, Finkelstein F, Revicki D, et al. Systematic review and meta-analysis of exercise tolerance and physical functioning in dialysis patients treated with erythropoiesis-stimulating agents. Am J Kidney Dis. 2010;55:423–425. doi: 10.1053/j.ajkd.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Gandra S, Finkelstein F, Bennett A, et al. Impact of erythropoiesis-stimulating agents on energy and physical function in nondialysis CKD patients with anemia: a systematic review. Am J Kidney Dis. 2010;55:519–534. doi: 10.1053/j.ajkd.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Johansen K, Young B, Kaysen G, et al. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nut. 2004;80:324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 15.Fleischmann E, Teal N, Dudley J, et al. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55:1560–1567. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 16.Kopple J, Zhu X, Lew N, et al. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 17.Port F, Ashby V, Dhingra R, et al. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol. 2002;13:1061–1066. doi: 10.1681/ASN.V1341061. [DOI] [PubMed] [Google Scholar]
- 18.Painter P, Carlson L, Carey S, et al. Physical functioning and health-related quality-of-life changes with exercise training in hemodialysis patients. Am J Kidney Dis. 2000;35:482–492. doi: 10.1016/s0272-6386(00)70202-2. [DOI] [PubMed] [Google Scholar]
- 19.Painter P, Moore G, Carlson L, et al. Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis. 2002;39:257–265. doi: 10.1053/ajkd.2002.30544. [DOI] [PubMed] [Google Scholar]
- 20.Ouzouni S, Kouidi E, Sioulis A, et al. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil. 2009;23:53–63. doi: 10.1177/0269215508096760. [DOI] [PubMed] [Google Scholar]
- 21.Roshanaei-Moghaddam B, Katon W, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. 2009;31:306–315. doi: 10.1016/j.genhosppsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Weyerer S, Kupfer B. Physical exercise and psychological health. Sports Med. 1994;17:108–116. doi: 10.2165/00007256-199417020-00003. [DOI] [PubMed] [Google Scholar]
- 23.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med. 2008;46:397–411. doi: 10.1016/j.ypmed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Office of the US Surgeon General. Physical Activity and Health: A Report of the Surgeon General. Washington, DC: US Department of Health and Human Services, National Center for Chronic Disease Prevention and Health Promotion; 1996. p. 278. [Google Scholar]
- 25.Fix A, Daughton D. Human Activity Profile Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc.; 1988. [Google Scholar]
- 26.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8th edn. Baltimore, MD: Lippincott Williams & Wilkins; 2009. [Google Scholar]