Abstract
OBJECTIVE
To determine whether an intensive lifestyle intervention (ILI) designed to sustain weight loss and improve physical fitness in overweight or obese persons with type 2 diabetes was associated with bone loss after 4 years of follow-up.
RESEARCH DESIGN AND METHODS
This randomized controlled trial of intensive weight loss compared an ILI with a diabetes support and education (DSE) group among 1,309 overweight or obese subjects. Bone mineral density was assessed at baseline and after 1 year and 4 years of intervention.
RESULTS
ILI was effective in producing significant weight loss (5.3% vs. 1.8% in ILI and DSE, respectively; P < 0.01) and increased fitness (6.4% vs. −0.8%) at year 4. In men, ILI participants had a greater rate of bone loss during the first year (−1.66% vs. −0.09% per year in ILI and DSE, respectively). Differences between groups were diminished by one-half after 4 years (−0.88% vs. −0.05% per year in ILI and DSE, respectively) but remained significant (P < 0.01). The difference in rate of hip bone loss between groups over 4 years was related to increased weight loss in ILI. Among women, the rate of bone loss did not differ between ILI and DSE after 4 years.
CONCLUSIONS
A 4-year weight loss intervention was significantly associated with a modest increase in bone loss at the hip in men but not in women.
Introduction
Type 2 diabetes affects a significant proportion of the population in North America, with an incidence directly proportional to the development of obesity (1). Weight loss has been shown to be effective in improving cardiovascular disease risk factors and slowing progression to type 2 diabetes among high-risk obese adults (2). However, weight loss has also been associated with diminished bone mineral density (BMD) (3–5).
The impact of type 2 diabetes on bone has been reviewed (6,7), but only two publications could be identified that characterized bone loss with weight loss and exercise in adults with type 2 diabetes (8,9). One study showed a reduction in bone loss with resistance training in addition to caloric restriction compared with caloric restriction alone (8). The Look AHEAD (Action in Health for Diabetes) Trial presents a unique opportunity to examine the longitudinal impact of lifestyle modification on bone mass in a large cohort of overweight and obese subjects with type 2 diabetes.
Data from the 1-year follow-up of the Look AHEAD Trial documented significant loss of bone at the total hip and femoral neck, which was proportional to weight loss (9). The intensive lifestyle modification was designed to promote substantial weight loss during the first year and to maintain weight in subsequent years. The impact of this intensive lifestyle modification, which sought to maintain the initial weight losses over time, on long-term changes in BMD remains unknown but may be of significance for the pathogenesis of fractures. The studies of Armamento-Villareal and colleagues (3–5) documented a protective effect of physical activity on hip bone loss resulting from loss of weight in an elderly nondiabetic obese cohort. This seminal work still leaves open whether the same effect may be characteristic of weight loss in overweight or obese people with type 2 diabetes.
This study examined whether individuals who participated in the intensive lifestyle intervention (ILI) for 4 years in the Look AHEAD Trial have a greater loss of BMD at the hip than participants in the standard diabetes support and education (DSE) (control) treatment arm. The BMD of the hip was chosen as the primary outcome variable because it is less susceptible to artifacts from degenerative changes that occur frequently in individuals >60 years of age (10). We also examined whether this change in hip BMD reflects that of the lumbar spine, femoral neck, and total body BMD and whether changes in BMD at the hip and lumbar spine were proportional to change in weight and fitness.
Research Design and Methods
Subject Selection, DXA, Physical Activity, Fitness, and Dietary Calcium Intake
Subjects were recruited into the Look AHEAD Trial as previously described (9,11). Participation was limited to people with type 2 diabetes from 16 clinical centers in the U.S., those 45–76 years old, and those with a BMI ≥25 kg/m2 (or ≥27 kg/m2 if taking insulin). Subjects were randomized to either ILI, including a weight loss diet and increased physical activity, or DSE. The goal of ILI was a sustained 7% loss of weight during the first year, with weight maintenance thereafter. A substudy was conducted at five clinical sites to measure longitudinal changes in bone density and body composition by DXA scanning as previously described (9). All randomized subjects at these five sites (1,479 of 5,145 total) were approached to participate in the DXA substudy with the exception of those who weighed over the weight limit for the scanner (300 lb). A total of 1,373 subjects consented to and completed baseline assessments of body composition, including total body BMD (including the head), BMD of the lumbar spine (L1–L4), total hip, and the femoral neck of the hip, using a Hologic fan beam densitometer. Of these, 1,274 subjects had a scan 1 year after randomization (Y1) and 1,158 subjects after 4 years (Y4); 1,123 of the 1,158 Y4 subjects undergoing DXA scans also had a Y1 scan. A total of 1,309 subjects within the current database had a DXA scan at baseline and at least one follow-up assessment. The common reasons for missing data were loss to follow-up and weight >300 lb. A complete accounting of the subjects is detailed in the Supplementary Data.
At baseline, Y1 and Y4 clinic visits, weight, height, prescription medication use, demographic characteristics, smoking history, alcohol use, calcium intake in the diet (but not supplement use), and A1C were obtained as described (9). Fitness, expressed in METs, was quantified from a submaximal graded exercise test (12). The MET level presented is that at 80% of the subject’s maximal heart rate. Graded exercise test measures were done at the same visit as the DXA measures or within 1 week of each other if not on the same day. Physical activity was quantified in a subgroup of Look AHEAD participants in kilocalories per week at baseline, 1-year, and 4-year follow-up from a self-reported questionnaire developed by Paffenbarger et al. (13) for assessment of leisure-time physical activity. Of 1,309 participants in the data set for this article, 564 have leisure-time physical activity data (43%). Calcium intake from food was quantified from a food frequency questionnaire in a subgroup of 50% of the subjects seen at each clinic site and accounted for 54% of the current cohort (14). Data related to bone fractures, which was considered a final outcome measure for the larger trial, were not accessible for this article.
Statistics
Mean and SD were computed for continuous baseline measures, and frequencies and percentages were computed for categorical baseline measures. Baseline data were analyzed to assess the effectiveness of the randomization and differences by sex. All analyses were conducted by sex.
BMD outcome measures were expressed as absolute differences in areal density (g/cm2), an accepted measure of bone mass per a two-dimensional space, which is the actual quantity obtained from a DXA scan, and as percent change from baseline [(Y4 BMD – baseline BMD) / (baseline BMD) × 100]. Additionally, mean and SD were calculated by treatment arm on the Y4 change in the BMD of lumbar spine and total hip and the Y4 change in continuous measures of interest: weight, fitness, physical activity, and A1C. These means were tested by treatment arm for differences using t tests. To examine the relationship between Y4 and Y1 changes in lumbar spine and total hip BMD, the Y4 changes were examined as a function of the Y1 changes. The within-arm mean baseline-to-Y4 change was tested for equality to zero using t tests for the outcome measures. An ANOVA was used to test for differences in change between the ILI and DSE arms. The categorical measures (smoking status, alcohol use in the past year, menopausal status, proteinuria) and arm of the study completed the list of independent variables of interest.
Generalized linear modeling (GLM) procedures, adjusting for site, were used to examine the relationship between Y4 BMD change (response variables) and predictor variables identified as correlated with the outcome measures. As noted, fewer paired Y1 scans were available than for the Y4 cohort, and to take this into account, an available data method as opposed to a complete case method was used. No imputed data (e.g., last observation carried forward) were used.
We examined correlations to identify potential confounding or colinear variables. The variables change in weight and change in fitness were identified as predictor variables based on a visual examination of the bivariable scatterplots, biological plausibility, and significant change over the 4-year interval. Change in A1C was added to the list because of its clinical significance as a marker of diabetes disease control. All models were adjusted for age, diagnosed diabetes duration, baseline BMI, site, race, baseline insulin, thiazolidinedione (TZD) use, menopausal status (for women), proteinuria, smoking status, and baseline level of the response and predictor measures. Repeated-measures (mixed) modeling was used to assess the relationship between time and change in BMD and was more fully adjusted for Y1 changes in weight and BMD. For the GLM and the repeated-measures modeling, the by-arm interaction was evaluated for all significant main effects among the independent measures. Rate of change (β) estimates are reported for continuous measures, and adjusted means are reported for categorical measures. Significance was determined using a fixed α= 0.05. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC) software. The significance of the total hip BMD results was also assessed using a calculation of Cohen's d for estimating the effect size (15) for the ILI arm compared with the DSE arm.
Sensitivity analyses were performed to examine the impact of age and glucocorticoid and TZD use because they have been shown to be deleterious to bone (16–21). Additional analyses examined the impact of age, bariatric surgery, and insulin use versus nonuse on the current results.
Results
Subject Characteristics
The baseline characteristics of subjects in the two study groups are summarized in Table 1, as are the variables describing the DXA subsample and the full Look AHEAD cohort (Supplementary Data). Randomization was generally successful in distributing subjects equally for demographic variables in the overall trial; however, weight differed significantly across treatment groups (P = 0.049). There was a very low prevalence of osteoporosis (defined as a T-score <−2.5) at any site of measurement at baseline and no difference in prevalence between groups.
Table 1.
Baseline data for subjects from the Look AHEAD trial used for longitudinal assessment of BMD
| DSE |
ILI |
||||
|---|---|---|---|---|---|
| Variable | Men | Women | Men | Women | P value^ |
| Number | 252 | 403 | 239 (100) | 415 | |
| Age (years) | 60.0 (6.4) | 57.8 (6.4) | 60.4 (6.4) | 57.0 (6.5) | 0.390 |
| Ethnicity (number, %) | |||||
| Black | 17 (7) | 56 (14) | 9 (4) | 55 (13) | 0.585 |
| White | 182 (72) | 196 (49) | 169 (71) | 212 (51) | |
| Other | 53 (21) | 151 (37) | 61 (26) | 148 (36) | |
| Height (cm) | 175.4 (6.7) | 160.4 (6.7) | 174.3 (6.9) | 160.2 (6.9) | 0.157 |
| Weight (kg) | 104.9 (14.4) | 93.5 (16.0) | 103.0 (15.3) | 91.9 (16.7) | 0.049 |
| L/S spine BMD (g/cm2) | 1.15 (0.17) | 1.08 (0.16) | 1.12 (0.14) | 1.07 (0.17) | 0.017 |
| Mean T-score L/S spine | 0.49 (1.57) | 0.15 (1.40) | 0.19 (1.31) | 0.06 (1.47) | 0.027* |
| T-score <−2.5 for L/S spine | 2 (0.8) | 10 (2.5) | 2 (0.8) | 13 (3.2) | 0.567 |
| Total hip BMD (g/cm2) | 1.10 (0.14) | 1.03 (0.14) | 1.08 (0.12) | 1.03 (0.14) | 0.390 |
| Mean T-score total hip | 0.30 (0.96) | 0.51 (1.10) | 0.18 (0.85) | 0.53 (1.09) | 0.562 |
| T-score <−2.5 for total hip | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | N/A |
| BMI (kg/m2) | 34.1 (4.3) | 36.3 (5.5) | 33.9 (4.6) | 35.7 (5.7) | 0.142 |
| Diabetes duration (years) | 7.2 (6.2) | 6.5 (6.1) | 7.3 (6.8) | 5.8 (5.5) | 0.244 |
| Calcium intake (mg/day)# | 850.2 (589.8) | 844.3 (530.5) | 822.3 (508.7) | 838.3 (437.1) | 0.732* |
| Insulin use | 34 (13) | 66 (16) | 46 (19) | 61 (15) | 0.601* |
| TZD use | 66 (26) | 68 (17) | 56 (23) | 63 (15) | 0.355 |
| Steroid use | 7 (2.8) | 86 (21.4) | 9 (3.8) | 79 (19.1) | 0.564 |
| Bisphosphonate use | 0 (0) | 7 (1.7) | 1 (0.4) | 10 (2.4) | 0.349 |
| Fitness (METs) | 6.0 (1.7) | 5.0 (1.4) | 5.9 (1.6) | 5.0 (1.3) | 0.710 |
| Leisure-time physical activity (kcal/week) | 1,067.8 (1,608.5) | 594.0 (1,549.4) | 819.9 (1,108.0) | 520.8 (824.2) | 0.196 |
| Smoking status | |||||
| Never | 98 (39) | 258 (64) | 94 (39) | 267 (64) | 0.898 |
| Past | 143 (57) | 127 (32) | 129 (54) | 132 (32) | |
| Present | 11 (4) | 17 (4) | 16 (7) | 16 (4) | |
| Alcohol in past year | 165 (65) | 187 (47) | 172 (72) | 200 (48) | 0.160 |
| Menopausal status | |||||
| Postmenopausal | — | 311 (77) | — | 297 (72) | 0.054 |
| Premenopausal | — | 43 (11) | — | 50 (12) | |
| Unknown | — | 49 (12) | — | 68 (16) | |
| Proteinuria (mg albumin/mg creatinine ≥0.3 on spot urine) | 5 (2) | 9 (2) | 4 (2) | 14 (3) | 0.499* |
| A1C (%) | 7.3 (1.2) | 7.4 (1.2) | 7.2 (1.2) | 7.3 (1.2) | 0.101* |
| A1C (mmol/mol) | 55 (13) | 56 (13) | 55 (13) | 55 (13) | |
Data are mean (SD) or n (%) unless otherwise indicated. L/S, lumbar spine.
^P value for randomization arm adjusted for sex and clinic.
*Sex effect not significant at the 0.05 α level.
#Diet data were only collected on a subset of the Look AHEAD cohort. Numbers for calcium intake were 708 for total cohort, 258 for men, and 450 for women.
The demographic variables of the subsample of the Look AHEAD cohort participating in the bone substudy differed from the total Look AHEAD cohort (Supplementary Data), reflecting the Latino population at these participating centers. The DXA cohort was shorter, weighed less, had a lower BMI, was more fit, was more likely never to have smoked, was treated less frequently with TZDs and steroids, and comprised fewer blacks and non-Latino whites.
Changes in BMD Comparing ILI and DSE
Men randomized to ILI had a twofold greater rate of bone loss at the hip (−0.78% per year) than those randomized to DSE (−0.36% per year, P < 0.01) at Y4, with an effect size of 0.42. However, no intervention effect on lumbar spine BMD (0.34% vs. 0.33% per year, P = 0.99) was found. The changes in male total hip BMD were also reflected in similar changes at the femoral neck. Women in both treatment arms had decreases in all measures of BMD (0.26–1.31% per year), and in contrast to men, there were no significant differences (P ≥ 0.24) in mean BMD loss between treatment groups by Y4 at any site.
Effects of Treatment Arm on Alcohol Intake, Smoking Status, Dietary Calcium Intake, Weight, and Fitness at Y4
Primary explanatory variables believed to affect change in BMD from baseline are summarized in Table 2, stratified by sex and intervention arm. There was a statistically significant effect of ILI to reduce body weight and BMI and increase fitness in both groups, but physical activity was only increased in men. No statistically significant effect of treatment arm on alcohol intake in the past year, smoking status, or dietary calcium intake in either men or women was found.
Table 2.
Summary results for changes in BMD and explanatory (predictor) variables that may affect change in BMD, stratified by study arm and sex
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Variable | ILI (n = 208) | DSE (n = 218) | P value | ILI (n = 368) | DSE (n = 364) | P value |
| BMD measure | ||||||
| L/S spine BMD change (g/cm2) | 0.01 ± 0.05 | 0.02 ± 0.06 | 0.72 | −0.03 ± 0.05 | −0.03 ± 0.06 | 0.38 |
| % change in L/S spine BMD/year | 0.3 ± 1.1 | 0.3 ± 1.2 | 0.99 | −0.7 ± 1.2 | −0.6 ± 1.4 | 0.24 |
| Total hip BMD change (g/cm2) | −0.03 ± 0.05 | −0.02 ± 0.04 | <0.01 | −0.04 ± 0.05 | −0.04 ± 0.05 | 0.44 |
| % change in hip BMD/year | −0.8 ± 1.0 | −0.4 ± 0.9 | <0.01 | −1.1 ± 1.3 | −1.0 ± 1.3 | 0.46 |
| Explanatory variable | ||||||
| Weight change (kg) | −5.9 ± 7.8 | −1.7 ± 7.0 | <0.01 | −4.7 ± 7.4 | −2.1 ± 8.1 | <0.01 |
| % weight change | −5.7 ± 7.1 | −1.6 ± 6.6 | <0.01 | −5.0 ± 7.9 | −2.0 ± 7.9 | <0.01 |
| BMI change (kg/m2) | −1.9 ± 2.5 | −0.6 ± 2.3 | <0.01 | −1.8 ± 2.9 | −0.7 ± 3.1 | <0.01 |
| % BMI change | −5.5 ± 7.0 | −1.6 ± 6.6 | <0.01 | −4.9 ± 7.9 | −1.8 ± 8.0 | <0.01 |
| Change in fitness (METs) | 0.3 ± 1.5 | −0.1 ± 1.5 | <0.01 | 0.2 ± 1.3 | −0.2 ± 1.3 | <0.01 |
| % change in fitness | 7.3 ± 26.1 | −0.23 ± 25.0 | <0.01 | 5.8 ± 26.5 | −1.4 ± 24.8 | <0.01 |
| Change in dietary calcium (mg/day) | −51.1 ± 608 | −47.4 ± 656 | 0.97 | −81.8 ± 431 | −164.7 ± 534 | 0.10 |
| % change in dietary calcium | 18.0 ± 82.4 | 16.7 ± 92.8 | 0.91 | 7.3 ± 60.5 | −3.7 ± 64.4 | 0.09 |
| Change in leisure-time physical activity (kcal/week) | 466.2 ± 1,612 | −62.7 ± 1,659 | 0.03 | 204.5 ± 1,105 | 8.8 ± 1,653 | 0.21 |
| % change in leisure-time physical activity | 361 ± 1,053 | 192 ± 739 | 0.26 | 286 ± 921 | 266 ± 978 | 0.87 |
| Change in A1C (%) | −0.4 ± 1.0 | 0.05 ± 1.2 | <0.01 | −0.1 ± 1.5 | 0.0 ± 1.6 | 0.51 |
| Change in A1C (mmol/mol) | −4 ± 11 | 0.5 ± 13 | −1 ± 17 | 0 ± 18 | ||
| Change in fat mass (g) | −2,601 ± 5,467 | 166 ± 4,842 | <0.01 | −1,900 ± 5,588 | −416 ± 5,959 | <0.01 |
| Alcohol use in past year | 153 (73.6) | 148 (67.9) | 0.20 | 177 (48.1) | 174 (47.9) | 0.96 |
| Menopause | ||||||
| Post | N/A | N/A | N/A | 260 (70.7) | 282 (77.5) | 0.08 |
| Pre | — | — | — | 45 (12.2) | 39 (10.7) | — |
| Unknown | — | — | — | 63 (17.1) | 43 (11.8) | — |
| Smoking status | ||||||
| Never | 81 (38.9) | 87 (39.9) | 0.33 | 231 (62.8) | 234 (64.5) | 0.73 |
| Past | 114 (54.8) | 124 (56.9) | 121 (32.9) | 117 (32.2) | — | |
| Present | 13 (6.3) | 7 (3.2) | 16 (4.4) | 12 (3.3) | — | |
Data are mean (SD) or n (%). All differences are Y4 – baseline. Continuous and categorical measures were tested with t test and χ2 test, respectively. N/A, not applicable.
Effect of Sex, Weight Loss, and Glucose Control on Bone Loss to Y4 Adjusted for Weight
Among men, the magnitude of weight loss was significantly correlated with bone loss at the hip (β = 0.03, P < 0.001) but with some gain in spine BMD (β = −0.02, P = 0.005) (Table 3). In women, the magnitude of weight loss was also significantly correlated with bone loss at the hip (β = 0.05, P < 0.001) but not at the spine (β = −0.008, P = 0.251). Hip bone loss did not correlate with change in fitness in either sex. Improved glycemic control, as reflected by lower A1C levels, correlated with loss of bone in the lumbar spine in men and hip bone loss in women. A statistically significant −0.4% (4 mmol/mol, P < 0.01) decrease in HbA1c in the ILI arm compared with the DSE arm was observed in men only in this DXA cohort, which can be estimated to account for 0.04% per year spine BMD loss.
Table 3.
Modeling results: GLM estimated change at Y4 in lumbar spine and total hip BMD regression coefficients for predictor variables
| By arm
mean (SE) |
Predictor variable |
|||||
|---|---|---|---|---|---|---|
| Response variable | DSE | ILI | Change in fitness (METs) β (SE) | Change in weight (kg) β (SE) | Change in HbA1c (%) β (SE) | Y1 change in outcome β (SE) |
| Men | ||||||
| L/S BMD % change/year baseline–Y4 | 0.96 (0.19) | 0.98 (0.19) | 0.005 (0.04) | −0.02 (0.007) | 0.09 (0.05) | 15.4 (1.4) |
| P value | 0.835 | 0.884 | 0.005 | 0.042 | <0.001 | |
| Total hip BMD % change/year baseline–Y4 | −0.53 (0.16) | −0.53 (0.16) | −0.02 (0.03) | 0.03 (0.006) | 0.01 (0.04) | 15.4 (1.4) |
| P value | 0.931 | 0.475 | <0.001 | 0.738 | <0.001 | |
| Women | ||||||
| L/S BMD % change/year baseline–Y4 | −0.65 (0.20) | −0.68 (0.19) | -0.04 (0.04) | −0.008 (0.007) | 0.05 (0.03) | 16.5 (1.3) |
| P value | 0.759 | 0.426 | 0.254 | 0.087 | <0.001 | |
| Total hip BMD % change/year baseline–Y4 | −1.27 (0.19) | −1.01 (0.18) | −0.02 (0.04) | 0.05 (0.006) | 0.09 (0.03) | 16.7 (1.4) |
| P value | 0.005 | 0.595 | <0.001 | 0.005 | <0.001 | |
Dependent variables were L/S and hip BMD. Independent variables were intervention arm (DSE vs. ILI). Predictor variables were change in fitness (METs), weight change, change in HbA1c, Y1 change in outcome. Also adjusted for were the following covariates: TZD and insulin use at baseline, age, ethnicity, study center (%), menstrual status (for female models only), duration of diabetes, baseline BMI, proteinuria (%), smoking status, baseline fitness, baseline A1C, and baseline level of outcome measure. L/S, lumbar spine.
For change in total hip BMD in men, there was no longer a significant difference by Y4 between the ILI and DSE arms (rate of BMD loss −0.53%, P = 0.93) after adjustment for postrandomization changes in weight. In women, after adjusting for these changes, ILI was associated with less (−1.01% per year) Y4 hip bone loss compared with DSE (−1.27% per year, P = 0.005).
In a sensitivity analysis that excluded subjects using any steroid, TZD, or bisphosphonate at baseline, the results were similar. Only 12.8% of the sample was using inhaled or topical steroids not generally believed to be detrimental to bone; 5.4% was using oral steroids known to cause bone loss; and 1.5% was using bisphosphonates, a medication known to increase BMD measures. The use of steroids was similar in the ILI and DSE groups. There also was no significant difference (P = 0.355) in TZD use across arms of the DXA substudy or by sex. Adjusting for age did not significantly change the results. For example, the rates of bone loss at the hip at Y4 in women for the ILI and DSE groups were −1.38 and −1.31% per year with adjustment for age vs. −1.33 and −1.27% per year after removing the age adjustment. Of the 1,309 participants included in these analyses (i.e., those who had baseline and at least one follow-up DXA scan), 19 reported having bariatric surgery by the time of the Y4 visit (6 in ILI, 13 in DSE). Only 2 of these 19 participants (1 in each arm) had bariatric surgery by the time of their Y1 visit. There are no significant differences at Y1 in weight loss and bone loss of those with and without bariatric surgery by Y4. By Y4, those in both the ILI and DSE arms with the bariatric surgery had significantly more weight loss and bone loss than those without the surgery. The GLM models representing the data in Fig. 1 were rerun with the 19 bariatric surgery participants removed, showing only slight changes in the estimate values and no change in significance. There was no significant difference in hip and spine BMD for insulin users versus nonusers stratified by randomization arm and visit.
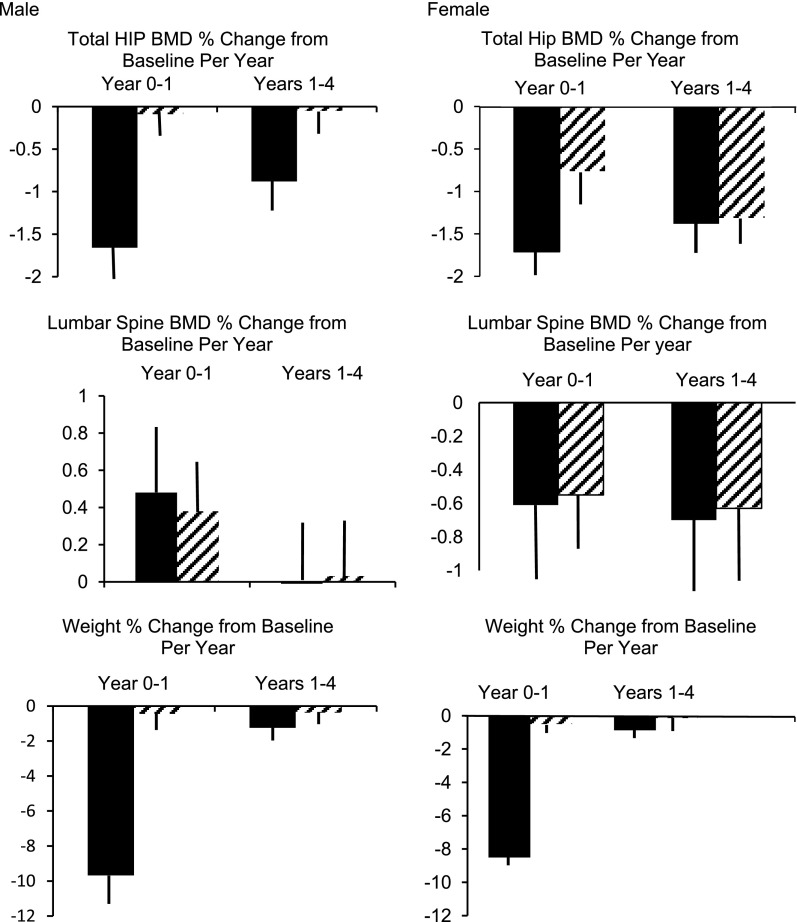
Figure 1.
Mean percent change in BMD per year (± SEM) for the intervals of 0–1 year and 1–4 years of follow-up derived from repeated-measures (mixed) modeling of the relationship between time and change in BMD. The solid bars represent the ILI group, and the striped bars represent the DSE group. Adjusted for age, ethnicity, study center, insulin use, TZD use, menstrual status (for female models only), duration of diagnosed diabetes, BMI, proteinuria, and smoking status (all baseline values).
Effect of Interventions on the Rate of Interval Bone Loss Between Years 0 and 1 and 1 and 4
The results of a fully adjusted repeated-measures GLM are summarized in Fig. 1. The purpose of this model was to determine whether the effect of the intervention on the annual rate of bone loss varied over time. A visual inspection of Fig. 1 shows marked differences in the pattern of bone loss and weight with time in men and women. There is a striking parallel between the changes in weight and hip BMD in men and a significant (P < 0.001) correlation between percent change in lean mass and percent change in hip BMD at both Y1 (r = 0.294) and Y4 (r = 0.339). There was also a significant (P < 0.001) correlation between percent change in total body fat and percent change in hip BMD at both Y1 (r = 0.341) and Y4 (r = 0.290). A significant (P ≤ 0.0001) time × arm interaction for hip BMD was found in men as well as in women. As a percentage of the initial BMD, these estimated hip losses for men and women, respectively, were −1.38% and −0.88% per year for ILI over the 1- to 4-year follow-up intervals. This model showed a greater rate of bone loss at Y1 compared with Y1–Y4 at the hip for both men and women. There was no significant effect on the lumbar spine (P value for time × arm interaction = 0.591 and 0.935 in men and women, respectively).
Conclusions
After 1-year follow-up, we found the intensive weight loss intervention of Look AHEAD to be associated with more rapid bone loss at the hip but not at the spine in both men and women (9). In men, a pattern of increased hip bone loss with ILI continued through Y4. An accelerated rate of hip bone loss in women at Y1 decreased with ILI by 4 years of follow-up but persisted in the DSE group such that the rate of hip bone loss was equivalent between groups by Y4. ILI was effective in producing significant weight loss and increased fitness throughout 4 years of follow-up but did not result in increased physical activity among women in the DXA subset.
Hip bone loss in men was only modestly increased with ILI, a difference of ∼0.4% per year or 1.6% over 4 years in BMD. The hip bone loss with ILI in men may reflect the greater weight loss in this group and correlated with loss of both lean mass and fat mass. Men in the ILI arm experienced an average weight loss of 9.8% in the first year, whereas DSE participants had stable weight. In the subsequent 3 years, there was a small regain of weight in the ILI arm (mean weight increase 4.1 kg in men, 1.5% per year weight regain). Of note, despite this weight regain, more rapid bone loss in the ILI group persisted after Y1, although the rate was reduced compared with the first year of the trial. Average weight remained lower in the ILI arm than in the DSE arm, which may account for the lower, yet persistent rate of hip bone loss. Neither increased fitness nor increased physical activity appeared to lessen the accelerated rate of hip bone loss observed in the ILI group in men. Explanations for the increased spine BMD observed in the ILI group must remain purely speculative because no mechanistic studies were included in the design of the trial. The change, however, could reflect artifact (22–26) due to spine degenerative changes, a common observation in older adults (10).
For women, although the ILI group continued to maintain lower weight than the DSE group, there was no longer a difference in the rate of hip bone loss across intervention groups after 4 years of follow-up, suggesting that more rapid bone loss in the ILI group occurred during the rapid weight loss phase. When this phase was over and weight regain began (+3.6 kg from Y1 to Y4), the rate of bone loss from Y1 to Y4 decreased in the ILI group but continued unabated in the DSE group, which continued to lose weight (−1.3 kg). By the end of 4 years, the amount of bone loss in the ILI and DSE groups thus converged.
The rate and magnitude of bone loss at the hip attributable to ILI, adjusting for all covariates, are small. The effect size is modest (13). The hip and lumbar spine BMD values with ILI observed at 4 years of follow-up in the current study were comparable to values previously reported in type 2 diabetic and age- and sex-matched control subjects who were not subject to sustained weight loss (27). Sensitivity analyses suggest that several potentially confounding factors did not affect the results, including the use of steroids, TZDs, or bisphosphonates; age; or the disproportionate number of individuals receiving bariatric surgery for weight loss in the different arms of the study.
The impact of voluntary weight loss on BMD in other studies of nondiabetic subjects has been variable and reported mostly in obese women (28–30). In two of these studies, bone loss was found with weight loss (28,29), but the other study focused on premenopausal women (30). In one study of overweight men, bone loss was found with weight loss and was comparable between diet-induced and exercise-induced weight loss (31). Other studies of nondiabetic subjects have shown a variable impact of exercise, a component of the current lifestyle modification intervention, on BMD. Exercise increased BMD in premenopausal compared with postmenopausal women in one study (32), but other studies have described both increased (33–38) and decreased (28) BMD in postmenopausal women. Exercise appears to increase BMD in men (38,39), but the effect is small and not seen in all studies (40).
The data from the current study suggest that a comprehensive lifestyle modification program does not completely compensate for the bone loss associated with weight loss. The results differ from those observed in nondiabetic elderly obese subjects where weight loss induced by much more intensive physical activity increased hip BMD (32–36). This may reflect different participant selection, a much more intensive physical activity intervention in the latter study, limitations of using a physical activity questionnaire for assessment of physical activity in the current study, or some aspect of type 2 diabetes rendering such individuals refractory to increasing BMD from lifestyle intervention.
The current findings suggest that the improved glycemic control associated with weight loss was also associated with greater bone loss. Our observations agree with epidemiological evidence (27) that individuals with worse glycemic control (higher A1C levels) have greater BMD either directly or indirectly through its association with increased BMI and elevated insulin levels, which may protect bone. In contrast, after 1 year of follow-up in Look AHEAD, improved glycemic control, reflected in a lower A1C, was associated with reduced total bone loss at the hip in men but not in women (9).
Strengths of the current study include it being the largest clinical trial to date of lifestyle modification with long-term follow-up and a randomized prospective design that successfully produced weight loss and focused specifically on individuals with type 2 diabetes. This study also is the first to show a positive time × intervention arm effect on hip BMD of ILI in men and women.
There are a number of potential limitations to the current study, including lack of data on calcium/vitamin D supplement use, the prevalence of bisphosphonate use in the postmenopausal women, and the possible underlying secondary hyperparathyroidism from deteriorating kidney function. However, there is no a priori reason that any of these factors should have been disproportionally represented in either arm of the study. No direct measures of vitamin D were included in the current study. The change in physical activity by the questionnaire was modest and limited to a subset of the DXA group, with limited power due to sample size. The questionnaire captured leisure-time physical activity, but this questionnaire has never been validated for assessing the risk of bone loss or fracture, does not distinguish between weight bearing and non-weight–bearing aerobic activity, and remains a major weakness of the study.
In conclusion, we have found that in diabetic patients who lose a substantial amount of weight over 1 year through ILI and then maintain their weight for the subsequent 3 years, there is no overall effect on hip bone loss in women compared with DSE. For men, hip bone loss is more rapid through ILI compared with DSE, but the amount of additional bone loss is modest. These results do not suggest a clinically important effect of weight loss on the skeleton; however, fracture results are needed to be confident of this conclusion.
Article Information
Funding. This study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK-57136, DK-57149, DK-56990, DK-57177, DK-57171, DK-57151, DK-57182, DK-57131, DK-57002, DK-57078, DK-57154, DK-57178, DK-57219, DK-57008, DK-57135, and DK-56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (IHS) provided personnel, medical oversight, and use of facilities. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the IHS or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (GCRC) (M01-RR-02719), the Massachusetts General Hospital Mallinckrodt GCRC and the Massachusetts Institute of Technology GCRC (M01-RR-01066), the University of Colorado Health Sciences Center GCRC (M01-RR-00051) and Clinical Nutrition Research Unit (P30-DK-48520), the University of Tennessee at Memphis GCRC (M01-RR-0021140), the University of Pittsburgh GCRC (M01-RR-000056), the Clinical Translational Research Center funded by a Clinical and Translational Science Award (UL1-RR-024153) and a National Institutes of Health grant (DK-046204), the VA Puget Sound Health Care System Medical Research Service, the Department of Veterans Affairs, and the Frederic C. Bartter GCRC (M01-RR-01346).
Duality of Interest. The following organizations have committed to making major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. E.W.L. contributed to the research design, interpretation of statistics, and writing of the paper. A.V.S., K.C.J., and G.A.B. contributed to the study design and review and revision of the manuscript. A.M.A. and C.D. provided database management, statistical evaluation and interpretation, and model development. E.W.G., R.B., A.L.P., C.L., and S.E.K. contributed to the review and revision of the manuscript. A.H. contributed as the project manager. E.W.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00017953, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0762/-/DC1.
A complete list of the Look AHEAD Research Group may be found in the Supplementary Data.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.National Diabetes Statistics. Bethesda, MD, National Diabetes Information Clearinghouse, 2007 [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res 2011;26:2851–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armamento-Villareal R, Sadler C, Napoli N, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res 2012;27:1215–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res 2007;22:1317–1328 [DOI] [PubMed] [Google Scholar]
- 7.Räkel A, Sheehy O, Rahme E, LeLorier J. Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes Metab 2008;34:193–205 [DOI] [PubMed] [Google Scholar]
- 8.Daly RM, Dunstan DW, Owen N, Jolley D, Shaw JE, Zimmet PZ. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos Int 2005;16:1703–1712 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz AV, Johnson KC, Kahn SE, et al. Look AHEAD Research Group Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res 2012;27:619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu G, Peacock M, Eilam O, Dorulla G, Braunstein E, Johnston CC. Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos Int 1997;7:564–569 [DOI] [PubMed] [Google Scholar]
- 11.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD Research Group Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628 [DOI] [PubMed] [Google Scholar]
- 12.Jakicic JM, Jaramillo SA, Balasubramanyam A, et al. Look AHEAD Study Group Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes (Lond) 2009;33:305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol 1978;108:161–175 [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Block G, McHenry K, Baron RB. Evaluation of two food frequency methods of measuring dietary calcium intake. Am J Epidemiol 1987;126:796–802 [DOI] [PubMed] [Google Scholar]
- 15.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988, p. 8–27 [Google Scholar]
- 16.Maricic M. Update on glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am 2011;37:415–431 [DOI] [PubMed] [Google Scholar]
- 17.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 18.Kahn SE, Zinman B, Lachin JM, et al. Diabetes Outcome Progression Trial (ADOPT) Study Group Rosiglitazone-associated fractures in type 2 diabetes: an analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care 2008;31:845–851 [DOI] [PubMed] [Google Scholar]
- 19.Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008;168:820–825 [DOI] [PubMed] [Google Scholar]
- 20.Bilezikian JP, Josse RG, Eastell R, et al. Rosiglitazone decreases bone mineral density and increases bone turnover in postmenopausal women with type 2 diabetes mellitus. J Clin Endocrinol Metab 2013;98:1519–1528 [DOI] [PubMed] [Google Scholar]
- 21.Harsløf T, Wamberg L, Møller L, et al. Rosiglitazone decreases bone mass and bone marrow fat. J Clin Endocrinol Metab 2011;96:1541–1548 [DOI] [PubMed] [Google Scholar]
- 22.Svendsen OL, Haarbo J, Hassager C, Christiansen C. Accuracy of measurements of body composition by dual-energy x-ray absorptiometry in vivo. Am J Clin Nutr 1993;57:605–608 [DOI] [PubMed] [Google Scholar]
- 23.Tothill P, Hannan WJ, Cowen S, Freeman CP. Anomalies in the measurement of changes in total-body bone mineral by dual-energy X-ray absorptiometry during weight change. J Bone Miner Res 1997;12:1908–1921 [DOI] [PubMed] [Google Scholar]
- 24.Tothill P, Avenell A. Errors in dual-energy X-ray absorptiometry of the lumbar spine owing to fat distribution and soft tissue thickness during weight change. Br J Radiol 1994;67:71–75 [DOI] [PubMed] [Google Scholar]
- 25.Van Loan MD, Johnson HL, Barbieri TF. Effect of weight loss on bone mineral content and bone mineral density in obese women. Am J Clin Nutr 1998;67:734–738 [DOI] [PubMed] [Google Scholar]
- 26.Evans EM, Mojtahedi MC, Kessinger RB, Misic MM. Simulated change in body fatness affects Hologic QDR 4500A whole body and central DXA bone measures. J Clin Densitom 2006;9:315–322 [DOI] [PubMed] [Google Scholar]
- 27.Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol 2012;27:319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen LB, Quaade F, Sørensen OH. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res 1994;9:459–463 [DOI] [PubMed] [Google Scholar]
- 29.Avenell A, Richmond PR, Lean MEJ, Reid DM. Bone loss associated with a high fibre weight reduction diet in postmenopausal women. Eur J Clin Nutr 1994;48:561–566 [PubMed] [Google Scholar]
- 30.Shapses SA, Von Thun NL, Heymsfield SB, et al. Bone turnover and density in obese premenopausal women during moderate weight loss and calcium supplementation. J Bone Miner Res 2001;16:1329–1336 [DOI] [PubMed] [Google Scholar]
- 31.Pritchard JE, Nowson CA, Wark JD. Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes Relat Metab Disord 1996;20:513–520 [PubMed] [Google Scholar]
- 32.Martyn-St. James M, Carroll S. Effects of different impact exercise modalities on bone mineral density in premenopausal women: a meta-analysis. J Bone Miner Metab 2010;28:251–267 [DOI] [PubMed] [Google Scholar]
- 33.Gleeson PB, Protas EJ, LeBlanc AD, Schneider VS, Evans HJ. Effects of weight lifting on bone mineral density in premenopausal women. J Bone Miner Res 1990;5:153–158 [DOI] [PubMed] [Google Scholar]
- 34.Rockwell JC, Sorensen AM, Baker S, et al. Weight training decreases vertebral bone density in premenopausal women: a prospective study. J Clin Endocrinol Metab 1990;71:988–993 [DOI] [PubMed] [Google Scholar]
- 35.Pruitt LA, Jackson RD, Bartels RL, Lehnhard HJ. Weight-training effects on bone mineral density in early postmenopausal women. J Bone Miner Res 1992;7:179–185 [DOI] [PubMed] [Google Scholar]
- 36.Snow-Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res 1992;7:761–769 [DOI] [PubMed] [Google Scholar]
- 37.Wolff I, van Croonenborg JJ, Kemper HCG, Kostense PJ, Twisk JWR. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 1999;9:1–12 [DOI] [PubMed] [Google Scholar]
- 38.Krall EA, Dawson-Hughes B. Walking is related to bone density and rates of bone loss. Am J Med 1994;96:20–26 [DOI] [PubMed] [Google Scholar]
- 39.Snow-Harter C, Whalen R, Myburgh K, Arnaud S, Marcus R. Bone mineral density, muscle strength, and recreational exercise in men. J Bone Miner Res 1992;7:1291–1296 [DOI] [PubMed] [Google Scholar]
- 40.Kelley GA, Kelley KS, Tran ZV. Exercise and bone mineral density in men: a meta-analysis. J Appl Physiol (1985) 2000;88:1730–1736 [DOI] [PubMed] [Google Scholar]