Abstract
As diabetes develops, we currently waste the first ∼10 years of the natural history. If we found prediabetes and early diabetes when they first presented and treated them more effectively, we could prevent or delay the progression of hyperglycemia and the development of complications. Evidence for this comes from trials where lifestyle change and/or glucose-lowering medications decreased progression from prediabetes to diabetes. After withdrawal of these interventions, there was no “catch-up”—cumulative development of diabetes in the previously treated groups remained less than in control subjects. Moreover, achieving normal glucose levels even transiently during the trials was associated with a substantial reduction in subsequent development of diabetes. These findings indicate that we can change the natural history through routine screening to find prediabetes and early diabetes, combined with management aimed to keep glucose levels as close to normal as possible, without hypoglycemia. We should also test the hypothesis with a randomized controlled trial.
Introduction
Diabetes is the major cause of kidney failure, blindness, and nontraumatic leg amputations in U.S. adults and a leading cause of stroke and heart disease (1). Diabetes cost the U.S. $176 billion in direct costs in 2012, amounting to 11% of U.S. health care dollars (2), and is a critical public health problem in other countries as well (3). Moreover, National Health and Nutrition Examination Surveys (NHANES) from 2003–2006 and 2007–2010 show that while there were slight improvements in the percentage of U.S. adults with diagnosed diabetes who had A1C levels >9% (75 mmol/mol) (decreasing from 13.0 to 12.6%) and those who had A1C levels <8% (64 mmol/mol) (increasing from 78.0 to 79.1%), there was no improvement in those with A1C <7.0% (53 mmol/mol) (decreasing from 56.8 to 52.2%) (4). We believe it does not have to be like this.
The ongoing “epidemic” of diabetes, which currently affects 11% of U.S. adults and 27% of those over 65 (1), is mostly type 2 diabetes and largely reflects the “success of society.” We are living longer, we eat too much, and we are inactive. Being older, overweight, and sedentary makes us resistant to insulin, and if our bodies cannot make enough insulin to compensate, glucose levels rise (5).
The earliest stage of type 2 diabetes is prediabetes (impaired glucose tolerance [IGT] and/or impaired fasting glucose [IFG]), where glucose levels are higher than normal but not in the diabetes range (1). Prediabetes tends to progress to diabetes, and over time, persistent hyperglycemia leads to the complications that are the major source of morbidity, mortality, and cost (Fig. 1A). This natural history reflects underlying loss of β-cell function (6,7), due in part to factors such as elevated glucose and lipid levels, inflammation, amyloid, and oxidative and endoplasmic reticulum stress.
Figure 1.

Diagram illustrating the natural history of diabetes (progression from prediabetes to diabetes and development of diabetes complications over time) without interventions (A); with interventions such as lifestyle change or a glucose-lowering medication that are successful in decreasing progression from prediabetes to diabetes, but then are stopped (B); and with interventions that are titrated to keep glucose and A1C levels in the normal range and are not stopped (C).
Unfortunately, we waste the first ∼10 years (8) of the natural history—when the disorder is easiest to treat. There are 79 million Americans with prediabetes and 7 million with early type 2 diabetes (1) who are largely unrecognized, because we do not screen to find these states of dysglycemia. Moreover, when we do make the diagnosis, we do not treat in a way that lowers glucose levels to normal. American Diabetes Association (ADA) guidelines call for adjusting therapy when A1C reaches 7.0% (53 mmol/mol) (9), which corresponds to glucose levels that average 154 mg/dL (10). And as adjustments in therapy are frequently delayed (11,12), many patients have glucose levels that are well above normal. For example, Nichols et al. (13) reported in 2007 that over half of Kaiser Permanente Northwest (KPNW) patients who initiated metformin-sulfonylurea combination therapy attained but failed to maintain A1C levels below 8.0% (64 mmol/mol), and continued combination therapy for an average of almost 3 years before insulin was added, with glucose exposure equivalent to 32 months of A1C levels of 9% (75 mmol/mol). Similarly, Khunti et al. (14) reported in 2013 that in the U.K. Clinical Practice Research Datalink (CPRD) database—representative of the U.K. general population—patients who were using two oral glucose-lowering drugs and had A1C levels of at least 8.0% (64 mmol/mol) had a median time of over 6.9 years before further intensification of therapy, with a mean A1C level of 9.1% (76 mmol/mol) when therapy was finally intensified.
As a consequence, the disease tends to progress, and patients need more and more medications. Many patients eventually come to need mealtime insulin and other drugs that can cause hypoglycemia—itself a potential cause of acute cardiovascular events. Johnston et al. (15) found ICD-9-CM coding for outpatient hypoglycemic events in patients with type 2 diabetes to be independently associated with acute cardiovascular events in a retrospective analysis of a large health care claims database. Glucose levels of 41 to 70 mg/dL (2.3 to 3.9 mmol/L) were associated with increased mortality in the prospective Normoglycemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study (16). As NICE-SUGAR involved intensive care unit inpatients, the findings may not be generalizable to outpatients. However, outpatients with type 2 diabetes who are treated with insulin and/or sulfonylureas exhibit an increased frequency of asymptomatic mild and severe hypoglycemia, and severe hypoglycemia is associated with increased ventricular ectopy (17,18). Severe hypoglycemia is also independently associated with QTc interval prolongation in both type 2 and type 1 diabetes (17,19), and there are plausible biological mechanisms through which hypoglycemia could trigger acute cardiovascular events (20,21).
But this natural history is not inevitable. If we found prediabetes and early diabetes when they first presented and treated them more effectively, we could change the natural history—preventing or delaying the need for the use of mealtime medications and other drugs that can cause hypoglycemia, as well as the development of complications. What is the evidence that we could do this?
Patients with diabetes who are given glucose-lowering medications earlier in their natural histories—when glucose levels are lower and/or lower glucose nadirs can be achieved—can go longer periods of time before another medication is needed. In another KPNW study (22), half of the patients initiated on metformin monotherapy who achieved an A1C nadir of 7–8% (53–64 mmol/mol) were given another drug after 3 additional years, but half of those reaching a nadir of 6–7% (42–53 mmol/mol) were given another drug after 6.5 years, and half of those achieving an A1C nadir of less than 6% (42 mmol/mol) were given another drug after 7.5 years. In the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study (23,24), diabetic youth were given metformin and the end point was preserving A1C levels below 8% (64 mmol/mol). Those who failed or succeeded with treatment were comparable in age, sex, and BMI. However, those who failed were begun on treatment when their A1C averaged 6.5% (48 mmol/mol), while those who succeeded were begun when their A1C averaged 5.7% (39 mmol/mol) and they had correspondingly higher measures of β-cell function. Such retrospective analyses might be subject to length time bias, but suggest the potential benefit of starting treatment earlier.
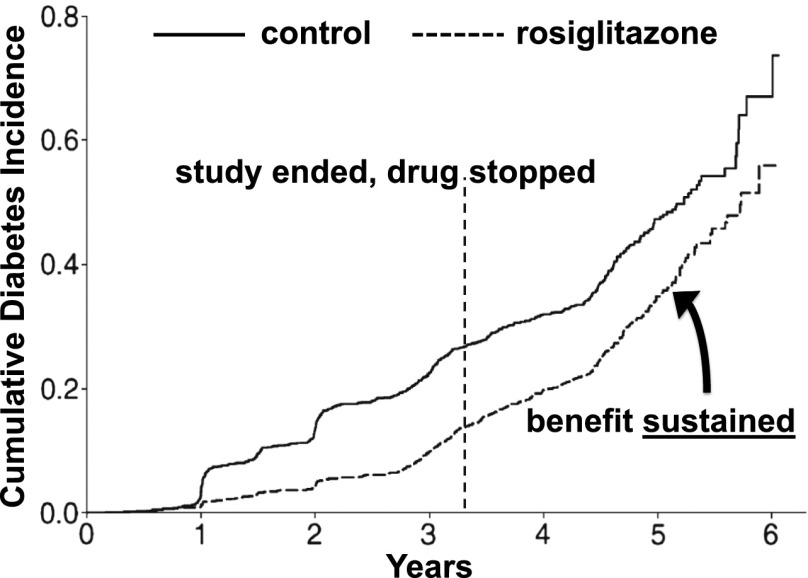
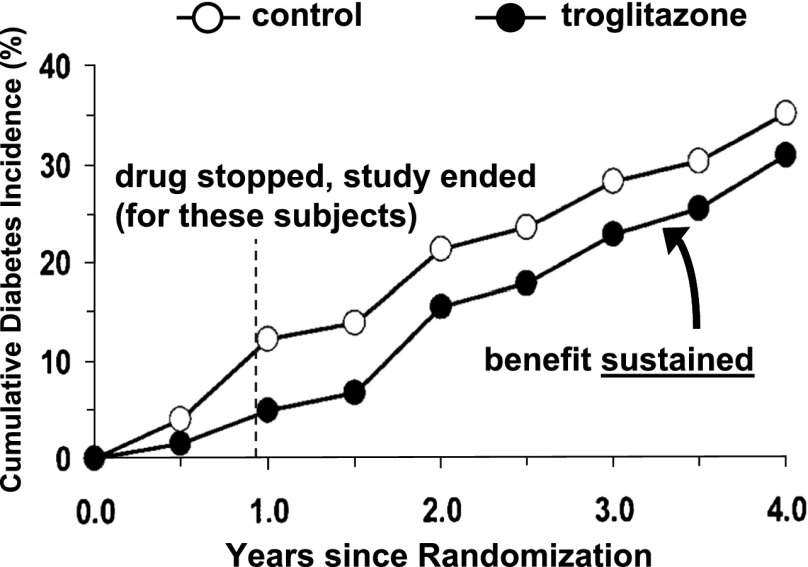
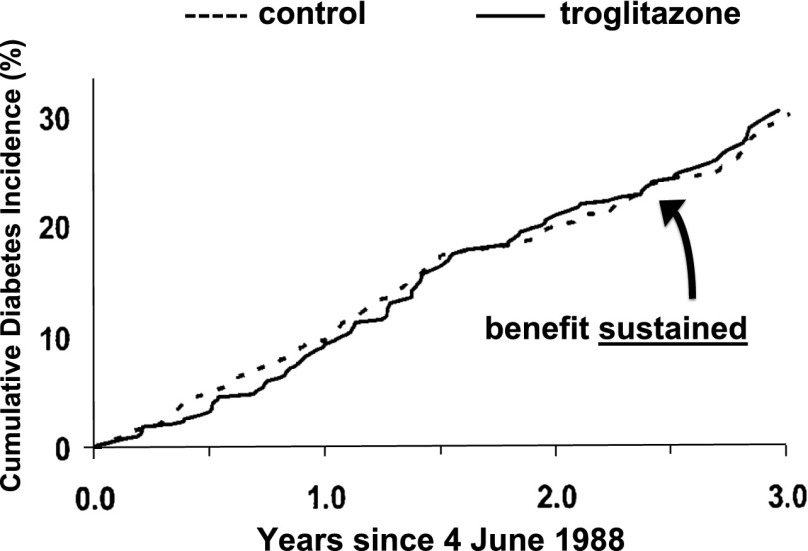
Stronger evidence for early intervention comes from randomized controlled trials: the Da Qing IGT and Diabetes Study in China (25), the Finnish Diabetes Prevention Study (26), the U.S. Diabetes Prevention Program (DPP) (27), and the multinational Diabetes Reduction Assessment With Ramipril and Rosiglitazone Medication (DREAM) study (28). These studies all showed that treatment with lifestyle change or glucose-lowering medications could reduce progression from prediabetes to diabetes. On follow-up after the studies ended and the interventions were stopped or reduced, additional study participants developed diabetes. However, there was little “catch-up”—the cumulative development of diabetes in the previously treated groups remained less than that in the untreated control groups (29–32). The follow-up findings of the DREAM study are shown in Fig. 2, and the pattern is particularly clear with the DPP subjects who had been randomized to the troglitazone arm (which was stopped after a little less than a year), as shown in Fig. 3 (33). During 0.9 years of treatment with troglitazone, the diabetes incidence rate was 3.0 cases/100 person-years, considerably lower than the rate of 12.0 cases/100 person-years with placebo. However, during the 3 years after troglitazone was stopped, the diabetes incidence was virtually identical to that of the placebo group—without evidence of “catch-up” (Figs. 3 and 4).
Figure 2.
Cumulative diabetes incidence in the DREAM study, where subjects with prediabetes were given rosiglitazone or placebo, including time points before and after the primary study was stopped (vertical dashed line). Adapted with permission from Gerstein et al. (32).
Figure 3.
Cumulative diabetes incidence in the U.S. DPP study, showing subjects with prediabetes who were given troglitazone or placebo, including time points before and after the primary study was stopped (vertical dashed line). Adapted with permission from Knowler et al. (33).
Figure 4.
Cumulative diabetes incidence in the U.S. DPP study, showing subjects with prediabetes who were given troglitazone or placebo, including only time points after the primary study was stopped on 4 June 1988. Adapted with permission from Knowler et al. (33).
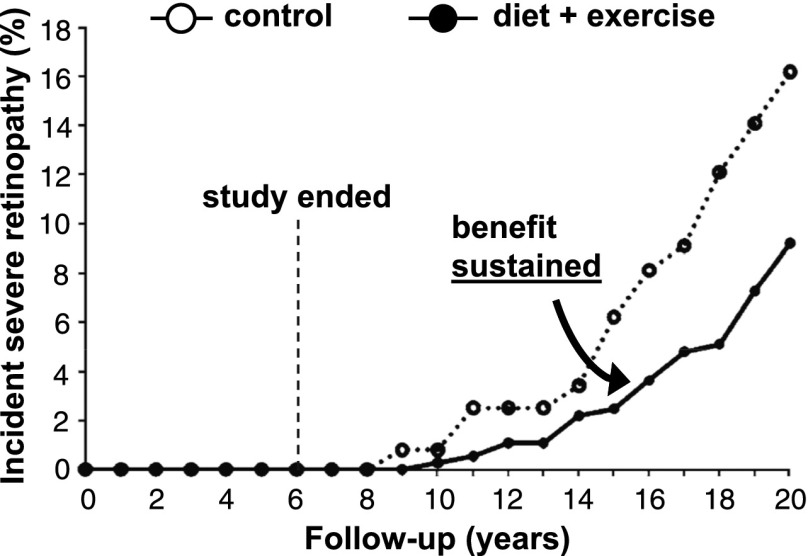
These patterns are consistent with a change in the natural histories of the subjects in the treated groups (Fig. 1B). Moreover, in the Da Qing study, follow-up 14 years after the trial ended showed that the lifestyle change group had a significant decrease in incident severe diabetic retinopathy compared with the control group (Fig. 5) (34); after an additional 3 years of follow-up, the lifestyle change group had decreases in cardiovascular and all-cause mortality (35). These are clinical benefits accompanying the glucose level benefit.
Figure 5.
Cumulative incidence of severe diabetic retinopathy in the Da Qing study, showing subjects with prediabetes who were randomized to receive instruction in diet + exercise or to be control subjects, including time points before and after the primary study was stopped (vertical dashed line). Adapted with permission from Gong et al. (34).
In the DPP subject population, achieving normal glucose levels appeared to be particularly beneficial. Among subjects who had not developed diabetes at the end of the primary study, those who achieved normal fasting and 2-h oral glucose tolerance test (OGTT) glucose levels at least once during the average 3.2 years of the primary study had a 56% decrease in development of diabetes during follow-up after the primary study ended (36). Moreover, it did not matter how normal glucose was achieved, as the reduction in the tendency to develop diabetes was comparable among subjects in the lifestyle change, metformin, and control groups who achieved normal glucose levels at least once. In addition, the risk of subsequent development of diabetes was decreased progressively according to the number of times normal glucose levels were achieved; the risk reduction was 47, 61, and 67% when normal glucose levels were achieved once, twice, or three times, respectively. Attaining normal glucose levels was predicted somewhat more strongly by better β-cell function than by better insulin sensitivity, although both were statistically significant.
Hypothesis and Implications
Thus, evidence supports a logical hypothesis: In patients who are early in their natural histories and already have prediabetes, 1) identifying the problem at such an early stage, and 2) keeping glucose levels normal or near-normal, will change the natural history of the disease—preventing or delaying progression from prediabetes to diabetes, and reducing the associated development of diabetes complications.
What are the implications of this hypothesis? Among DPP subjects, who at baseline had a fasting glucose level 95–125 mg/dL and a 2-h OGTT glucose level 140–199 mg/dL, 50% of subjects in the placebo arm had developed diabetes after 8 years (and over 30% of the lifestyle change group) (31). The hypothesis implies that instead of half of untreated DPP-type patients with prediabetes developing diabetes over 8 years and developing complications over another 10–20 years (depending on the level of diabetes control), identifying those at high risk early and keeping glucose levels normal or near-normal will extend the natural history. Patients with prediabetes should remain in a prediabetic state for a longer period of time—and subsequent development of complications should be reduced (Fig. 1C). As prediabetes and early diabetes are a continuum, there might be similar benefits from identification and intensive management of early diabetes.
As the natural history of diabetes—the tendency for the disease to get worse and for complications to develop—reflects underlying loss of β-cell function, which is due in part to glucotoxicity, treatment that normalizes glucose levels should help preserve β-cell function. Evidence for this is provided by several studies of patients with newly diagnosed diabetes showing that several weeks of intensive insulin treatment (titrated to normalize fasting and postprandial glucose levels) improved β-cell function and was associated with a high likelihood of being in remission 1 year later (37–39). In the study by Ryan et al. (38), patients were given 2–3 weeks of regular insulin before meals and NPH at bedtime, with target glucose levels <6 mmol/L before breakfast and <7 mmol/L 2 h after meals. Of the 16 subjects in the study, 7 who had mean baseline A1C levels of 12.3% (111 mmol/mol) remained in remission without pharmacologic therapy 1 year later. In the study by Weng et al. (39), patients given continuous subcutaneous insulin infusions or multiple daily insulin injections, titrated to achieve fasting glucose <6.1 mmol/L and 2-h postprandial glucose <8.0 mmol/L and then maintained for the following 2 weeks, exhibited improved acute insulin responses to glucose, decreased ratios of proinsulin to insulin levels, and remission rates of approximately 50% after 1 year. In these and other studies, the initial benefit was less well sustained when glucose normalization was achieved with the aid of sulfonylureas; such a lack of persistent benefits may reflect the tendency of sulfonylureas to be associated with “secondary failure” (40,41), possibly by contributing to β-cell apoptosis (42).
Indirect evidence of preservation of β-cell function is also provided by the Outcome Reduction With Initial Glargine Intervention (ORIGIN) study, in which long-acting insulin was titrated to keep fasting glucose levels in the normal range (at 95 mg/dL or less) (43). In patients with prediabetes, treatment with glargine insulin decreased progression to diabetes by about 30%. Moreover, in the combined treatment group (diabetes + prediabetes), the amount of insulin needed increased by only a small amount over 4 years—from a median dosage of 0.31 units/kg at 1 year to 0.40 units/kg at 5 years, equivalent to a rise in dosage from 28 to 36 units for a subject weighing 200 pounds. Such titration resulted in very little deterioration in A1C, with median levels of 6.4% at baseline, 5.9% at 1 year, and 6.2% after 7 years (46, 41, and 42 mmol/mol, respectively). Such relative stability of A1C levels was qualitatively different from the rise in A1C seen in the UK Prospective Diabetes Study (UKPDS) and in A Diabetes Outcome Progression Trial (ADOPT) (where glucose-lowering medications were administered in a fixed dosage, without titration) (41,44). In ADOPT, mean A1C levels in the glyburide-treated group were approximately 7.3% at baseline, 6.4% at 3 months, and 7.6% at 5 years (56, 46, and 60 mmol/mol, respectively), although A1C levels rose more slowly in the metformin and rosiglitazone groups (approximately 7.3% and 7.1% [56 and 54 mmol/mol, respectively]); only the rosiglitazone group was clearly still below baseline at 5 years.
Evidence of the potential benefit from earlier identification of disease and initiation of treatment comes from several clinical trials. In the UKPDS, subjects presenting with initial fasting glucose 140–179 and ≥180 mg/dL appeared to be recognized 3 and 5 years later in their natural histories, respectively, than those with fasting glucose <140 mg/dL (45). Earlier recognition was associated with slightly lower blood pressure, but the groups were comparable in age and lipid levels, and they were all treated similarly. However, subsequent development of microvascular disease was less in both earlier recognition groups, and the group recognized when initial fasting glucose was <140 mg/dL also had reduced mortality.
Similar findings were obtained in subgroups in more recent trials. Intensive treatment tended to reduce incident cardiovascular disease (CVD) in subjects in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study who had baseline A1C levels ≤8.0% (64 mmol/mol) or who had not yet had a CVD event (46). Intensive treatment also tended to reduce incident CVD in the Veterans Affairs Diabetes Trial (VADT) in subjects who had little coronary artery calcification at baseline (47) and in those with shorter duration of diagnosed diabetes, while it tended to increase incident CVD in those with longer duration (48)—generally consistent with the findings in the ACCORD study. In the ORIGIN study, subjects who had a baseline A1C ≥6.4% (46 mmol/mol) and were assigned to glargine had a median postrandomization change in A1C of –0.65% (compared with –0.33% in the standard care group) and a significant decrease in a microvascular composite outcome of kidney and eye disease (49)—evidence of the potential clinical benefit from keeping glucose levels as close to normal as possible.
How Medical Practice Should Change
If the hypothesis is correct, then medical practice should change in two ways (Table 1).
Table 1.
Changing the natural history of diabetes—how medical practice should change
| 1. Screening to identify early diabetes and prediabetes should become routine. |
| Oral glucose tolerance tests—most sensitive, least convenient |
| Fasting plasma glucose—lowest cost, intermediate sensitivity and convenience |
| A1C—most convenient, least sensitive |
| 2. Patients who are at high risk and have health prospects justifying improved glucose control should have management aimed to keep glucose levels as close to normal as possible without causing hypoglycemia. |
| Begin with lifestyle change |
| Include medications if appropriate |
| Begin with metformin for patients with diabetes |
| Consider metformin for patients with prediabetes if there is: |
| • IFG and |
| • IGT and |
| • At least one risk factor for progression to diabetes (age <60 years, BMI ≥35 kg/m2, a family history of diabetes, elevated triglycerides, reduced HDL cholesterol, hypertension, or A1C >6.0% [42 mmol/mol]) |
| Other medications that may be appropriate (DPP-4 inhibitors, SGLT-2 inhibitors, GLP-1 analogs, α-glucosidase inhibitors, and possibly basal insulin and thiazolidinediones) |
First, screening to identify early diabetes and prediabetes should become routine. The ADA guidelines currently recommend screening with OGTTs or measurement of fasting plasma glucose or hemoglobin A1C (9). OGTTs are the least convenient but most sensitive (50), while measurement of A1C is most convenient but least sensitive (51). As demonstrated in the Screening for Impaired Glucose Tolerance (SIGT) study, it may also be possible to use two-step screening, similar to screening for gestational diabetes mellitus—glucose measurement 1 h after a 50-g oral glucose challenge (at any time of day, without a prior fast), followed, if abnormal, by a 75-g OGTT (52). Having every patient fill out a brief diabetes risk questionnaire to identify individuals who are at particularly high risk might reduce the need for every patient to have screening by measurement of glucose levels. However, it may be more practical to use near-universal glucose screening, similar to what many countries do to identify gestational diabetes mellitus.
Second, patients who are at high risk (53) and have health prospects justifying improved glucose control (54,55) should have management aimed to keep glucose levels as close to normal as possible without causing hypoglycemia. Management should begin with lifestyle change and include medications if appropriate. The ADA recommends use of metformin for patients with diabetes and consideration of metformin for patients with prediabetes who have both 1) IFG and 2) IGT and 3) at least one risk factor for progression to diabetes (age <60 years, BMI ≥35 kg/m2, a family history of diabetes, elevated triglycerides, reduced HDL cholesterol, hypertension, or A1C >6.0%) (56). The great majority of patients who have both IFG and IGT have such risk factors, and a substantial percentage of patients with minimal IFG (fasting plasma glucose 100–109 mg/dL) also have IGT (57). Other medications that might be appropriate (in our opinion—not necessarily supported in each instance by clinical trial data) include dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT-2) inhibitors, GLP-1 analogs, and possibly basal insulin and thiazolidinediones. Many patients are likely to need such medications, as over 40% of the subjects in the DPP lifestyle change group developed diabetes after 10 years of follow-up (31). High-risk patients should also have appropriate management of other CVD risk factors.
Our recommendations may involve a qualitative change in the approach to management. Many practitioners may currently use a “stepped care” strategy (Table 2). With stepped care, patients with prediabetes and early diabetes would have management of cardiovascular risk factors, use of aspirin (if appropriate), screening for eye and renal complications, and education in medical nutrition management. If lifestyle change is insufficient to achieve glycemic goals, pharmacologic therapy would be initiated, usually with use of metformin as recommended by the ADA and the European Association for the Study of Diabetes (EASD) guidelines (55,58). If metformin is insufficient, another agent would then be added. Many practitioners would use a sulfonylurea as a second drug. Although such agents carry a risk of hypoglycemia, they are available in inexpensive generic forms, there is wide experience with their use, and they may be more cost-effective than other, newer drugs (59,60). Alternatives with less of a risk of hypoglycemia include GLP-1 analogs, DPP-4 inhibitors, thiazolidinediones, SGLT-2 inhibitors, α-glucosidase inhibitors, colesevelam, bromocriptine, and glinides. If goals cannot be attained with two or three such agents, insulin would then be added—either as basal insulin alone, basal plus mealtime insulin, or a premixed insulin preparation. With the stepped care strategy, insulin is typically added relatively late in the natural history, and in practice, often when patients have had elevated A1C levels for a substantial period of time (13,14). In addition, with this strategy, use of home glucose monitoring to guide management varies widely and may not be initiated until patients are treated with insulin—again, relatively late in the natural history.
Table 2.
Stepped care strategy
| • Insulin is typically added relatively late in the natural history. |
| • Use of home glucose monitoring to guide management varies and often is not initiated until patients are treated with insulin—relatively late in the natural history. |
| 1. All patients with prediabetes and early diabetes should have management of cardiovascular risk factors, use of aspirin (if appropriate), screening for eye and renal complications, and education in medical nutrition management. |
| 2. If lifestyle change is insufficient to achieve glycemic goals, initiate pharmacologic therapy; usually begin with use of metformin as recommended by ADA guidelines. |
| 3. If metformin is insufficient, add another pharmacologic agent. |
| • Sulfonylureas—inexpensive and cost-effective, but carry a risk of hypoglycemia |
| • Alternatives with less risk of hypoglycemia (GLP-1 analogs, DPP-4 inhibitors, thiazolidinediones, SGLT-2 inhibitors, α-glucosidase inhibitors, colesevelam, bromocriptine, and glinides) |
| 4. If glycemic goals cannot be attained with two or three such agents, add insulin—either as basal insulin alone, basal plus mealtime insulin, or a premixed insulin preparation. |
In contrast, bringing glucose levels as close to normal as possible while minimizing the risk of hypoglycemia in patients with prediabetes and early diabetes is likely to require a qualitatively different approach—“pattern care” (Table 3). Pattern care management differs from stepped care management in two major ways: both glucose monitoring and basal insulin may be needed relatively early in the natural history.
Table 3.
Pattern care strategy
| • Earlier use of home blood glucose monitoring to guide management | |
| • Use of basal insulin earlier in the natural history | |
| 1. Home glucose monitoring and A1C measurements: | |
| • Keeping A1C <5.7% or <5.5% (39 or 37 mmol/mol) may be sufficient if these goals can be met by management with lifestyle change + metformin. | |
| • In patients requiring additional medication treatment, some home glucose monitoring will likely be needed to determine whether therapy in individual patients needs to be directed toward fasting vs. postprandial glucose levels. | |
| • Target glucose levels: fasting <100 mg/dL, 2-h postprandial <140 mg/dL | |
| • Early in the natural history, some monitoring of fasting glucose levels along with measurement of A1C levels is likely to be sufficient. | |
| 2. Management according to glucose patterns: | |
| Fasting glucose <100 mg/dL | Postprandial glucose <140 mg/dL |
| Metformin | DPP-4 inhibitors |
| Basal insulin given in the evening | GLP-1 analogs |
| • Glargine—before supper | Pioglitazone |
| • Detemir—at bedtime | SGLT-2 inhibitors |
| Glipizide (1.25–5.0 mg) at bedtime | α-Glucosidase inhibitors |
| Glinides before meals | |
| Short-acting insulin analogs before meals | |
| 3. It is difficult to achieve near-normal glucose levels with the use of either mealtime insulin, long-acting sulfonylureas, or premixed insulin, due to problems with hypoglycemia. | |
A1C levels are strong predictors of the development of both micro- and macrovascular disease (61,62), and it may be possible in some patients to guide management with lifestyle change, metformin, and addition of a second drug largely on the basis of A1C levels. Keeping A1C levels <5.7% (39 mmol/mol) (based on ADA guidelines [58]) would provide assurance that in most patients, glucose levels were unlikely to be in the range of diabetes or prediabetes. Across the NHANES and SIGT data sets, 60–70% of subjects had normal glucose tolerance when A1C levels were <5.7% (39 mmol/mol) (50); keeping A1C levels <5.5% (37 mmol/mol) (based on the Norfolk prospective study, where higher levels were associated with increased CVD, cancer, and all-cause mortality [63]) would provide greater assurance that glucose levels would be normal.
Guiding Management by Glucose Monitoring
To the extent that the goal of management is both to attain A1C goals and to maintain glucose levels similar to those of the DPP patients who had normal glucose levels when OGTTs were performed (36), some home glucose monitoring will likely be needed to determine whether therapy in individual patients needs to be directed toward fasting versus postprandial glucose levels. Target glucose levels might be similar to those that are normal by ADA guidelines (58)—fasting glucose <100 mg/dL and 2-h postprandial glucose <140 mg/dL. The normal range of postprandial glucose levels is not well established, but random plasma glucose was measured 1.5–2.5 h after a meal in 705 subjects with normal OGTTs in the SIGT study (52); only 3.7% had values >130 mg/dL and only 2.1% had values >140 mg/dL, suggesting that a 2-h postprandial glucose target of <140 mg/dL is reasonable. However, for most patients who are early in their natural histories, some monitoring of fasting glucose levels along with measurement of A1C levels is likely to be sufficient.
Management According to Glucose Patterns
With pattern management, fasting glucose is best targeted with metformin and, if needed, basal insulin given in the evening. The use of insulin carries a risk of hypoglycemia, but the ORIGIN study showed that glargine insulin can be used with little risk of hypoglycemia in patients who are early in their natural histories (43); there tends to be less hypoglycemia with use of either glargine or detemir than with NPH, and the risk of nocturnal hypoglycemia can be minimized by giving detemir at bedtime and glargine before supper. Although in the ORIGIN study there was more hypoglycemia with the use of glargine compared with standard care (mostly metformin and sulfonylureas), it is not clear whether glargine was given before supper or at bedtime, and A1C levels were also 0.2–0.3% higher in the standard care group compared with the glargine group. Detemir insulin has a different time course of action (64) and reduced variability (65) compared with glargine insulin, which can sometimes allow it to be used successfully in place of glargine if problems with hypoglycemia are encountered with glargine, although it has not been used in a trial comparable to ORIGIN. The new insulin degludec also has less variability compared with glargine insulin (66). As an alternative to basal insulin, some patients may be able to achieve target fasting glucose levels by taking a small dose of glipizide (1.25–5.0 mg) at bedtime, but the use of this agent must be cautious because of the risk of nocturnal hypoglycemia.
While most glucose-lowering drugs reduce both fasting and postprandial hyperglycemia, some tend to produce greater absolute falls in postprandial glucose levels. These include DPP-4 inhibitors, GLP-1 analogs, pioglitazone (for which there has been extensive description of use early in the natural history), α-glucosidase inhibitors, and SGLT-2 inhibitors, and as they act by different mechanisms, all of these can be used in combination except DPP-4 inhibitors and GLP-1 analogs. If such drugs are insufficient, many patients can attain near-normal postprandial glucose levels by further addition of a glinide before meals. Only if such approaches fail would use of short-acting insulin analogs before meals be recommended, due to the increased risk of hypoglycemia with such agents (67). In our opinion, it is difficult to achieve near-normal glucose levels with the use of either mealtime insulin, long-acting sulfonylureas, or premixed insulin, due to problems with hypoglycemia.
Because of the complex underlying pathophysiology responsible for the metabolic abnormalities of type 2 diabetes, combination therapy may be required very early. Fortunately, even in the context of diabetes prevention, low-dose combinations can provide good efficacy with low rates of adverse effects, as shown with rosiglitazone and metformin (68). It is possible that pathophysiologic phenotyping aimed at restoring and preserving β-cell function and identifying disease evolution in individuals will be useful beyond pattern management (69,70). Individuals with higher A1C levels may have them because of longer duration of disease and/or more severe underlying pathophysiology. In both the TODAY study and the DPP, starting treatment when A1C levels were lower (in TODAY) and/or β-cell function was better (in both TODAY and DPP) was associated with improved outcomes (23,24,36). Alternatively, DeFronzo et al. (71) have recommended beginning management with the combination of metformin, pioglitazone, and exenatide to provide “pathogenic” targeting, but some patients may require less and others more than this combination to obtain normal glucose levels. Further, it is possible that other combinations may be as or possibly even more effective.
There are potential concerns with both the screening and the management that we recommend. With respect to population screening, the U.S. Preventive Services Task Force (USPSTF) currently recommends screening only for diabetes and only in patients with sustained blood pressure (treated or untreated) greater than 135/80 mmHg (72,73). While the USPSTF found “convincing evidence that intensive glycemic control in persons with clinically detected (as opposed to screening-detected) diabetes can reduce progression of microvascular disease,” it found “inadequate evidence that early diabetes control as a result of screening provides an incremental benefit for microvascular clinical outcomes compared with initiating treatment after clinical diagnosis” (73). However, the evidence that prompts our recommendations is more recent than the evidence upon which the USPSTF recommendations were based (72), and the clinical trial we outline below should provide evidence that addresses the USPSTF’s concern. The studies of long-term outcomes in the Da Qing study (34) and both short- and long-term outcomes in the UKPDS (44,74) also provide some reassurance that outcomes would be positive in the study we propose, and the ADA currently recommends more extensive screening (58).
Although population screening could in theory lead to adverse effects such as “false worry” about having abnormal test results and “false reassurance” in those with normal results (potentially leading to less healthful behavior), the USPSTF found little evidence that screening causes short-term harms. Screening also involves costs, but over 10 years, treatment with lifestyle change or metformin in the DPP was found to be cost-effective or cost-saving, respectively (75). In addition, Chatterjee et al. (51) have also projected screening plus treatment to be cost-effective or cost-saving, particularly in high-risk groups (76). Additional cost-effectiveness analyses will be needed to help determine the extent to which the expense of testing and monitoring, office visits, and glucose-lowering medications, used as we recommend, might be justified by potential savings from any reductions in the micro- and macrovascular complications that account for the major portion of diabetes-related health care system expenditures (2).
Implementation of our management recommendations by individual practitioners and health care systems also should be cautious in view of the findings in the ACCORD study, which was stopped early because intensification of management in subjects with type 2 diabetes led to increased mortality (46). However, the ACCORD subjects were overall relatively late in their natural histories (median duration of diabetes was 10 years) and many ACCORD subjects already had CVD. In the ACCORD study, the increase in mortality appeared to be limited to those who were assigned to intensive treatment but unable to achieve A1C levels below 7.0% (53 mmol/mol) (77), and increased mortality was not found in subgroups who were earlier in their natural histories (46). Moreover, increased mortality was not found in the ORIGIN study, where average duration of diabetes was 5.5 years (43).
The hypothesis that such management can change the natural history also should be tested in a randomized controlled trial. The trial could involve high-risk subjects with baseline A1C 6.0–6.6% (42–49 mmol/mol) or higher, who are all given support for change in lifestyle. The control subjects might not be given medications unless their A1C reached 7.0–7.5% (53–58 mmol/mol), (similar to the ongoing Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study [GRADE] [78]), whereas the treatment subjects would be managed with medications titrated to maintain glucose or A1C levels in the normal range (e.g., A1C <5.5% [37 mmol/mol], based on the Norfolk study [63]), without hypoglycemia. The feasibility of our recommended approaches to glucose lowering will be partly addressed by the ongoing Restoring Insulin Secretion (RISE) study (79) and A Study to Compare Combination Regimen With Vildagliptin and Metformin Versus Metformin in Treatment-naïve Patients With Type 2 Diabetes Mellitus (VERIFY) (80), and such management has been used in the clinical practice of one of the authors (L.S.P.) for the past several years. A1C differences between the groups would likely be sufficient to permit evaluation of clinically significant end points such as retinopathy and microalbuminuria, as well as β-cell function. Inclusion of CVD as an end point would require a larger, longer study. Such a trial should be a high priority, as positive findings would speed a change in medical practice.
Conclusions
We believe we are at a time when we can change the natural history of type 2 diabetes. Doing so should benefit the health of millions of patients and might also benefit health care systems, by reducing resource use and costs (51,76).
Article Information
Funding. This work was supported in part by National Institutes of Health awards DK066204 and UL1 RR025008, VA award HSR&D IIR 07-138, and a Cystic Fibrosis Foundation award PHILLI12A0 (L.S.P.); the National Center for Advancing Translational Sciences (UL1TR000083) (J.B.B.); P30 DK017047 (S.E.K.); the U.S. Department of Veterans Affairs (L.S.P. and S.E.K.); and the ADA (R.E.R.).
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Duality of Interest. Within the past several years, L.S.P. served on scientific advisory boards for Boehringer Ingelheim and Janssen, and has or had research support from Merck, Amylin, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, and the Cystic Fibrosis Foundation. In the past, he was a speaker for Novartis and Merck, but not for the last several years. J.B.B. is an investigator and/or consultant without any direct financial benefit under contracts between his employer and the following companies: Abbott, Amylin, Andromeda, AstraZeneca, BD Research Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Catabasis, Cebix, Diartis, Elcelyx, Eli Lilly, Exsulin, Genentech, GI Dynamics, GlaxoSmithKline, Halozyme, Hoffman-La Roche, Johnson & Johnson, LipoScience, Medtronic, Merck, Metabolic Solutions Development Co., Metabolon, Novan, Novartis, Novella Clinical, Novo Nordisk, Orexigen, Osiris, Pfizer, Rhythm, Sanofi, Spherix, Takeda, Tolerx, TransPharma, Veritas, and Verva. S.E.K. has received honoraria for advisory work and lectures from Boehringer Ingelheim, Bristol-Myers Squibb, Elcelyx, Eli Lilly, Genentech (Roche), GlaxoSmithKline, Intarcia, Janssen, Merck, Novo Nordisk, Receptos, and Takeda. The above activities involve diabetes, but have nothing to do with the manuscript. No other potential conflicts of interest relevant to this article were reported.
Footnotes
This work is not intended to reflect the official opinion of the Department of Veterans Affairs or the U.S. government.
References
- 1.Centers for Disease Control and Prevention 2011 National Diabetes Fact Sheet [article online], 2011. Available from http://www.cdc.gov/diabetes/pubs/factsheet11.htm Accessed 30 May 2011
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Y, Wang L, He J, et al. 2010 China Noncommunicable Disease Surveillance Group Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–959 [DOI] [PubMed] [Google Scholar]
- 4.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 6.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 8.Bertram MY, Vos T. Quantifying the duration of pre-diabetes. Aust N Z J Public Health 2010;34:311–314 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose Study Group Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips LS, Branch WT, Jr, Cook CB, et al. Clinical inertia. Ann Intern Med 2001;135:825–834 [DOI] [PubMed] [Google Scholar]
- 12.Phillips LS, Twombly JG. It’s time to overcome clinical inertia. Ann Intern Med 2008;148:783–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols GA, Koo YH, Shah SN. Delay of insulin addition to oral combination therapy despite inadequate glycemic control: delay of insulin therapy. J Gen Intern Med 2007;22:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013;36:3411–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care 2011;34:1164–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finfer S, Liu B, Chittock DR, et al. NICE-SUGAR Study Investigators Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012;367:1108–1118 [DOI] [PubMed] [Google Scholar]
- 17.Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014;63:1738–1747 [DOI] [PubMed] [Google Scholar]
- 18.Stahn A, Pistrosch F, Ganz X, et al. Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: silent hypoglycemias and silent arrhythmias. Diabetes Care 2014;37:516–520 [DOI] [PubMed] [Google Scholar]
- 19.Gruden G, Giunti S, Barutta F, et al. QTc interval prolongation is independently associated with severe hypoglycemic attacks in type 1 diabetes from the EURODIAB IDDM complications study. Diabetes Care 2012;35:125–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark AL, Best CJ, Fisher SJ. Even silent hypoglycemia induces cardiac arrhythmias. Diabetes 2014;63:1457–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright RJ, Newby DE, Stirling D, Ludlam CA, Macdonald IA, Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care 2010;33:1591–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols GA, Alexander CM, Girman CJ, Kamal-Bahl SJ, Brown JB. Treatment escalation and rise in HbA1c following successful initial metformin therapy. Diabetes Care 2006;29:504–509 [DOI] [PubMed] [Google Scholar]
- 23.Zeitler P, Hirst K, Pyle L, et al. TODAY Study Group A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.TODAY Study Group Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan X-R, Li G-W, Hu Y-H, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 26.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 27.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerstein HC, Yusuf S, Bosch J, et al. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 29.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 30.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Finnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 31.Knowler WC, Fowler SE, Hamman RF, et al. Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerstein HC, Mohan V, Avezum A, et al. DREAM On (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication Ongoing Follow-up) Investigators Long-term effect of rosiglitazone and/or ramipril on the incidence of diabetes. Diabetologia 2011;54:487–49521116607 [Google Scholar]
- 33.Knowler WC, Hamman RF, Edelstein SL, et al. Diabetes Prevention Program Research Group Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005;54:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300–307 [DOI] [PubMed] [Google Scholar]
- 35.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol; 2014;2:474−480 [DOI] [PubMed] [Google Scholar]
- 36.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, Diabetes Prevention Program Research Group Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ilkova H, Glaser B, Tunçkale A, Bagriaçik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care 1997;20:1353–1356 [DOI] [PubMed] [Google Scholar]
- 38.Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care 2004;27:1028–1032 [DOI] [PubMed] [Google Scholar]
- 39.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
- 40.Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care 2003;26:2231–2237 [DOI] [PubMed] [Google Scholar]
- 41.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 42.Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab 2005;90:501–506 [DOI] [PubMed] [Google Scholar]
- 43.Gerstein HC, Bosch J, Dagenais GR, et al. ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 44.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 45.Colagiuri S, Cull CA, Holman RR, UKPDS Group Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes? U.K. Prospective Diabetes Study 61. Diabetes Care 2002;25:1410–1417 [DOI] [PubMed] [Google Scholar]
- 46.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reaven PD, Moritz TE, Schwenke DC, et al. Veterans Affairs Diabetes Trial Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes 2009;58:2642–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duckworth WC, Abraira C, Moritz TE, et al. Investigators of the VADT The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA Diabetes Trial. J Diabetes Complications 2011;25:355–361 [DOI] [PubMed] [Google Scholar]
- 49.Gilbert RE, Mann JF, Hanefeld M, et al. ; ORIGIN Trial Investigators. Basal insulin glargine and microvascular outcomes in dysglycaemic individuals: results of the Outcome Reduction With an Initial Glargine Intervention (ORIGIN) trial. Diabetologia; 2014;57:1325−1331 [DOI] [PubMed] [Google Scholar]
- 50.Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 2010;33:2184–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatterjee R, Narayan KM, Lipscomb J, Phillips LS. Screening adults for pre-diabetes and diabetes may be cost-saving. Diabetes Care 2010;33:1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips LS, Ziemer DC, Kolm P, et al. Glucose challenge test screening for prediabetes and undiagnosed diabetes. Diabetologia 2009;52:1798–1807 [DOI] [PubMed] [Google Scholar]
- 53.Gregg EW, Geiss L, Zhang P, Zhuo X, Williamson DF, Albright AL. Implications of risk stratification for diabetes prevention: the case of hemoglobin A1c. Am J Prev Med 2013;44(Suppl. 4):S375–S380 [DOI] [PubMed] [Google Scholar]
- 54.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011;154:554–559 [DOI] [PubMed] [Google Scholar]
- 55.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nathan DM, Davidson MB, DeFronzo RA, et al. American Diabetes Association Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 57.Rhee MK, Herrick K, Ziemer DC, et al. Many Americans have pre-diabetes and should be considered for metformin therapy. Diabetes Care 2010;33:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klarenbach S, Cameron C, Singh S, Ur E. Cost-effectiveness of second-line antihyperglycemic therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin. CMAJ 2011;183:E1213−E1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhoads GG, Dain MP, Zhang Q, Kennedy L. Two-year glycaemic control and healthcare expenditures following initiation of insulin glargine versus neutral protamine Hagedorn insulin in type 2 diabetes. Diabetes Obes Metab 2011;13:711–717 [DOI] [PubMed] [Google Scholar]
- 61.Selvin E, Ning Y, Steffes MW, et al. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes 2011;60:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfister R, Sharp SJ, Luben R, Khaw KT, Wareham NJ. No evidence of an increased mortality risk associated with low levels of glycated haemoglobin in a non-diabetic UK population. Diabetologia 2011;54:2025–2032 [DOI] [PubMed] [Google Scholar]
- 64.Porcellati F, Rossetti P, Busciantella NR, et al. Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in type 1 diabetes: a double-blind, randomized, crossover study. Diabetes Care 2007;30:2447–2452 [DOI] [PubMed] [Google Scholar]
- 65.Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004;53:1614–1620 [DOI] [PubMed] [Google Scholar]
- 66.Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab 2012;14:859–864 [DOI] [PubMed] [Google Scholar]
- 67.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084 [DOI] [PubMed] [Google Scholar]
- 68.Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 2010;376:103–111 [DOI] [PubMed] [Google Scholar]
- 69.Kramer CK, Choi H, Zinman B, Retnakaran R. Determinants of reversibility of β-cell dysfunction in response to short-term intensive insulin therapy in patients with early type 2 diabetes. Am J Physiol Endocrinol Metab 2013;305:E1398–E1407 [DOI] [PubMed] [Google Scholar]
- 70.Ferrannini E, Natali A, Muscelli E, et al. RISC Investigators Natural history and physiological determinants of changes in glucose tolerance in a non-diabetic population: the RISC Study. Diabetologia 2011;54:1507–1516 [DOI] [PubMed] [Google Scholar]
- 71.DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 2013;36(Suppl. 2):S127–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Norris SL, Kansagara D, Bougatsos C, Fu R, U.S. Preventive Services Task Force Screening adults for type 2 diabetes: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2008;148:855–868 [DOI] [PubMed] [Google Scholar]
- 73.U.S. Preventive Services Task Force Screening for type 2 diabetes mellitus in adults [article online], 2013. Available from http://www.uspreventiveservicestaskforce.org/uspstf08/type2/type2rs.htm Accessed 12 December 2013
- 74.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 75.Herman WH, Edelstein SL, Ratner RE, et al. Diabetes Prevention Program Research Group Effectiveness and cost-effectiveness of diabetes prevention among adherent participants. Am J Manag Care 2013;19:194–202 [PMC free article] [PubMed] [Google Scholar]
- 76.Chatterjee R, Narayan KM, Lipscomb J, et al. Screening for diabetes and prediabetes should be cost-saving in patients at high risk. Diabetes Care 2013;36:1981–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riddle MC, Ambrosius WT, Brillon DJ, et al. Action to Control Cardiovascular Risk in Diabetes Investigators Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010;33:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nathan DM, Buse JB, Kahn SE, et al. GRADE Study Research Group Rationale and design of the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care 2013;36:2254–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.RISE Consortium Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Novartis Pharmaceuticals. VERIFY: A Study to Compare Combination Regimen With Vildagliptin and Metformin Versus Metformin in Treatment-naïve Patients With Type 2 Diabetes Mellitus. In: ClinicalTrials.gov [Internet]. Available from http://clinicaltrials.gov/show/NCT01528254. Accessed 18 July 2014.