Abstract
OBJECTIVE
This study examined specific measures of weight loss in relation to incident diabetes and improvement in cardiometabolic risk factors.
RESEARCH DESIGN AND METHODS
This prospective, observational study analyzed nine weight measures, characterizing baseline weight, short- versus long-term weight loss, short- versus long-term weight regain, and weight cycling, within the Diabetes Prevention Program (DPP) lifestyle intervention arm (n = 1,000) for predictors of incident diabetes and improvement in cardiometabolic risk factors over 2 years.
RESULTS
Although weight loss in the first 6 months was protective of diabetes (hazard ratio [HR] 0.94 per kg, 95% CI 0.90, 0.98; P < 0.01) and cardiometabolic risk factors (P < 0.01), weight loss from 0 to 2 years was the strongest predictor of reduced diabetes incidence (HR 0.90 per kg, 95% CI 0.87, 0.93; P < 0.01) and cardiometabolic risk factor improvement (e.g., fasting glucose: β = −0.57 mg/dL per kg, 95% CI −0.66, −0.48; P < 0.01). Weight cycling (defined as number of 5-lb [2.25-kg] weight cycles) ranged 0–6 times per participant and was positively associated with incident diabetes (HR 1.33, 95% CI 1.12, 1.58; P < 0.01), fasting glucose (β = 0.91 mg/dL per cycle; P = 0.02), HOMA-IR (β = 0.25 units per cycle; P = 0.04), and systolic blood pressure (β = 0.94 mmHg per cycle; P = 0.01). After adjustment for baseline weight, the effect of weight cycling remained statistically significant for diabetes risk (HR 1.22, 95% CI 1.02, 1.47; P = 0.03) but not for cardiometabolic traits.
CONCLUSIONS
Two-year weight loss was the strongest predictor of reduced diabetes risk and improvements in cardiometabolic traits.
Introduction
Obesity is an established risk factor for a range of complex diseases, including type 2 diabetes, cardiovascular disease, several cancers, osteoarthritis, and periodontitis. Intentional weight loss, on the other hand, reduces the risk of most of these diseases. A person’s body weight can vary considerably in both the short- and long-term. Factors associated with fluctuations in body weight include many biopsychosocial processes, such as genetic predisposition, thoughts, and emotions, and barriers in a person’s social or cultural environment (1–3). Regaining weight after intentional weight loss is common (4,5). Although the extent and rate of weight regain varies greatly from one person to the next, few studies have considered the dynamic nature of changes in body weight in relation to diabetes and cardiometabolic risk (6,7). Studies that seek to determine the dynamic relationships between variations in body weight and susceptibility to disease may yield novel insights into the role of obesity in chronic disease and thus give rise to tailored interventions that seek to mitigate disease risk.
The purpose of this analysis was to compare the effects of weight loss variables on diabetes incidence and cardiometabolic risk factor levels in participants who received the Diabetes Prevention Program (DPP) weight loss intervention. We also sought to establish an optimized set of weight loss variables that are most strongly related to the risk of type 2 diabetes.
Research Design and Methods
Participants
The design, methods, and main outcomes of the DPP have been published previously (4). In the DPP, 3,234 participants aged 25 years or older with elevated fasting glucose (5.3–6.9 mmol/L, or ≤6.9 mmol/L for American Indians), impaired glucose tolerance (glucose 7.8–11.0 mmol/L 2 h after a 75-g oral glucose load), and elevated BMI (≥22 kg/m2 for Asian Americans, ≥24 kg/m2 for others) were randomly assigned to one of three treatments: intensive lifestyle intervention, metformin, or placebo control (4). This analysis was limited to participants randomized to the intensive lifestyle intervention. Participants included those who had completed at least 2 years of the lifestyle intervention to allow sufficient time to examine fluctuations in body weight after the initial 6 months of weight loss with the core curriculum. Of the 1,079 DPP participants originally randomized to intensive lifestyle, 63 were excluded due to less than 2 years of total participation and another 16 were excluded due to missing measurements, leaving 1,000 participants in the current analysis (93% of the total lifestyle cohort). Measurements for fasting glucose, HOMA-insulin resistance (IR), triglycerides, and weight at baseline were not systematically different between the 79 excluded subjects (Supplementary Table 1) and the 1,000 participants included in the analysis (Table 1).
Table 1.
Weight variables and cardiometabolic risk factors of the study cohort
| Mean | SD | Minimum | 25th percentile | Median | 75th percentile | Maximum | |
|---|---|---|---|---|---|---|---|
| Baseline | |||||||
| Fasting glucose (mg/dL) | 106.27 | 8.15 | 76.00 | 100.00 | 105.00 | 112.00 | 139.00 |
| HOMA-IR | 6.99 | 4.33 | 0.56 | 4.15 | 6.05 | 8.89 | 54.46 |
| Triglyceride (mg/dL) | 163.42 | 97.38 | 31.00 | 98.00 | 139.00 | 198.00 | 796.00 |
| SBP (mmHg) | 123.42 | 14.63 | 84.00 | 112.00 | 122.00 | 132.00 | 175.00 |
| At year 2 | |||||||
| Fasting glucose (mg/dL) | 104.92 | 14.78 | 74.00 | 97.00 | 103.00 | 110.00 | 331.00 |
| HOMA-IR | 5.97 | 4.67 | 0.46 | 3.20 | 4.87 | 7.31 | 60.03 |
| Triglyceride (mg/dL) | 140.95 | 84.00 | 31.00 | 86.00 | 118.00 | 169.00 | 672.00 |
| SBP (mmHg) | 120.12 | 14.72 | 84.00 | 110.00 | 119.00 | 129.00 | 209.00 |
| Baseline weight (kg) | 93.70 | 20.29 | 48.60 | 79.00 | 90.90 | 104.58 | 192.20 |
| Weight loss from baseline to (kg) | |||||||
| 1 month | 1.38 | 2.66 | −17.17 | −0.18 | 1.27 | 2.83 | 15.85 |
| 3 months | 4.40 | 3.87 | −9.62 | 1.79 | 4.13 | 6.51 | 25.27 |
| 6 months | 6.84 | 5.62 | −13.25 | 3.20 | 6.45 | 9.60 | 35.55 |
| 2 years | 5.39 | 7.56 | −19.15 | 1.05 | 4.50 | 8.50 | 77.50 |
| Weight loss from (kg) | |||||||
| 18 months to 2 years | −1.38 | 5.73 | −27.60 | −4.35 | −1.20 | 1.13 | 49.20 |
| 6 months to 2 years | −0.61 | 3.18 | −17.95 | −2.25 | −0.60 | 0.90 | 33.60 |
| 5-lb weight cycles (n) | 1.45 | 0.99 | 0 | 1 | 1 | 2 | 6 |
| Average weight (kg) | 83.03 | 19.87 | 44.93 | 74.02 | 84.57 | 98.66 | 188.68 |
All body composition variables are raw weight loss values in the units of kilograms. The study sample consisted of 1,000 intensive lifestyle participants with at least 2 years of follow-up and excluded participants who developed diabetes during those 2 years (n = 952).
Use of thiazide diuretics or β-adrenergic antagonists was an exclusion criterion, but other classes of antihypertensive agents were permitted. New medical therapies initiated by nonstudy physicians after enrollment were not restricted. Study procedures and documents were approved by institutional review boards at all participating sites. All participants provided written informed consent for their participation. The study was performed in accordance with the Declaration of Helsinki.
Intervention
The DPP lifestyle intervention has been previously described in detail (8). In brief, the lifestyle intervention had two specific goals: to facilitate ≥7% of body weight loss and to achieve ≥150 min/week of moderate intensity physical activity. Participants were given calorie goals to promote weight loss determined by initial body weight and fat gram goals that were based on 25% of calories from fat. Lifestyle coaches met with participants individually over the first 24 weeks to review a 16-session core curriculum that focused on diet, physical activity, and strategies for behavioral modification. After the first 6 months, lifestyle coaches offered tailored individual sessions at least once every 2 months and group classes/campaigns three times per year to help improve physical activity levels and sustain weight loss.
Outcomes Measures
Subjects were weighed in light clothing without shoes at each intervention and outcome visit. Diabetes incidence was determined by fasting plasma glucose levels every 6 months and 2-h glucose levels from 75-g oral glucose tolerance tests performed annually. Diabetes events through the 1.5-year checkup (before the 2-year follow-up) are excluded from our analysis for the diabetes outcome (n = 48), and only those between year 2 and the end of DPP (31 July 2001) are considered (n = 952). Therefore, the risk of diabetes conditional on the fact that a participant was diabetes-free before the 2-year follow-up is modeled. Cardiometabolic risk factor assessments were done for fasting glucose (mg/dL), insulin resistance (HOMA-IR) (9,10), fasting triglycerides (mg/dL), and systolic blood pressure (SBP; mmHg). Cardiometabolic outcome measures were made at the 2-year follow-up assessment.
Weight Loss Variables
To determine which aspects of weight loss were most strongly related to the incidence of diabetes and levels of cardiometabolic risk factors, nine separate variables for weight or weight loss over specific time intervals were examined. These included:
Baseline weight (kg)
-
Weight loss (kg) from baseline (immediate loss) to:
1 month
3 months
6 months
2 years
-
Weight loss (kg) up to 24 months (delayed loss):
From 6 to 24 months
From 18 to 24 months
Weight cycling: A complete weight cycle is defined as a 5-lb (2.25-kg) or more weight loss from the highest weight since last cycle and then a 5-lb (2.25-kg) or more weight regain from the lowest weight since the last cycle. Weight cycling for a given participant is the number of such cycles in the 2-year period starting from the DPP baseline.
Average weight in the first 2 years: The weighted average of the available weight measures from baseline to year 2, where each weight observation is weighted by the time duration between this measure and its previous measure.
Statistical Methods
The relationships of the weight variables with diabetes incidence and cardiometabolic risk factor levels were estimated using Cox proportional hazards and linear regression models, respectively. Covariates included age at randomization (years), race, sex, and the corresponding baseline measure of glucose, HOMA-IR, SBP, and triglyceride level. The Pearson correlation coefficient between the baseline weight and weight loss from 1, 3, and 6 months and 2 years to baseline, weight cycles, and average weight in the first 2 years were significantly different from zero (P < 0.01; Table 2). Therefore, baseline weight was not controlled for due to the moderate-to-high degree of correlations between baseline weight and some of the other measures of weight.
Table 2.
Pearson partial correlation coefficients between the nine weight metrics conditional on age at enrollment
| BL weight | BL to 1 month | BL to 3 months | BL to 6 months | 18 months to 2 years | BL to 2 years | 6 months to 2 years | 5-lb cycles (n) | Average weight | |
|---|---|---|---|---|---|---|---|---|---|
| BL weight | 1 | 0.10 | 0.26 | 0.33 | 0.01 | 0.20 | −0.06 | 0.35 | 0.96 |
| 0.00 | <0.01 | <0.01 | 0.78 | <0.01 | 0.10 | <0.01 | <0.01 | ||
| BL to 1 month | 1 | 0.80 | 0.60 | −0.07 | 0.38 | −0.10 | 0.05 | −0.06 | |
| <0.01 | <0.01 | 0.03 | <0.01 | 0.00 | 0.13 | 0.08 | |||
| BL to 3 months | 1 | 0.86 | −0.08 | 0.56 | −0.12 | 0.06 | 0.04 | ||
| <0.01 | 0.02 | <0.01 | 0.00 | 0.09 | 0.24 | ||||
| BL to 6 months | 1 | −0.10 | 0.67 | −0.12 | 0.05 | 0.08 | |||
| <0.01 | <0.01 | 0.00 | 0.14 | 0.02 | |||||
| 18 months to 2 years | 1 | 0.26 | 0.45 | −0.11 | 0.03 | ||||
| <0.01 | <0.01 | 0.00 | 0.39 | ||||||
| BL to 2 years | 1 | 0.66 | −0.10 | −0.06 | |||||
| <0.01 | 0.00 | 0.09 | |||||||
| 6 months to 2 years | 1 | −0.19 | −0.16 | ||||||
| <0.01 | <0.01 | ||||||||
| 5-lb cycles (n) | 1 | 0.38 | |||||||
| <0.01 |
BL, baseline.
To assess the effects of weight cycling beyond baseline weight, 2-year weight loss, and average weight, we performed secondary analyses where baseline weight, 2-year weight loss, and average weight were adjusted in addition to number of 5-lb weight cycles. Assessments of weight loss from 18 to 24 months were replaced in the Cox models by a variable quantifying weight loss in the most recent 6 months, which was treated as a time-varying coefficient. Outliers in the cardiometabolic outcomes (one in fasting glucose, one in HOMA-IR, and one in SBP) were deleted before linear regression analyses were performed for the corresponding outcomes.
One model was fitted for each combination of outcome and weight measure. For the same outcome, we compared the size of the coefficients corresponding to different standardized weight measures. For example, we fitted nine models for the same fasting glucose outcome and obtained nine coefficient estimates for the nine standardized weight measures. Weight measures were standardized by the corresponding ethnic- and sex-specific SDs, which yield directly comparable regression coefficient estimates. Therefore, the regression coefficient of each measure of weight is the difference in the outcome corresponding to 1 SD change in the given weight measure. Pairwise comparisons among the nine coefficients were made. We then tested whether the difference between a pair of coefficients varied from zero.
The most predictive single weight variable was selected, whose model was called the core model. To determine whether a combination of the weight variables could be defined that improved the predictive accuracy for incident diabetes of the core model, expanded models that included additional weight variables besides the covariates in the core model were selected, and the predictive ability of different models was assessed considering both statistical significance and clinical importance. Statistical selection of candidate weight measures was done by stepwise model selections, with a significance level for variable entry of P = 0.25 and a significance level for variable removal of P = 0.15. The three strongest predictors of diabetes identified through the stepwise model selection ranked by effect sizes are 1) weight loss from baseline to year 2, 2) average weight in the first 2 years, and 3) weight loss in the first month after randomization. None of these variables was collinear (variance inflation factor <2).
We also estimated the predictive accuracy of the logistic model including the top three weight measures (11). Furthermore, three sets of sensitivity analyses were tested to determine the prognostic power of weight loss from baseline to year 2 on diabetes risk by adding dietary and physical activity measurements to the model. In the first sensitivity analysis, the average daily caloric intake calculated from food frequency questionnaires at baseline and year 1 was added to the Cox model of 2-year weight loss and demographic variables. In the second sensitivity analysis, average physical activity measured in metabolic hours per week at baseline and year 1 was added in addition to the same core model. In the third sensitivity analysis, average daily caloric intake and weekly physical activity in metabolic hours per week were both added. All calculations were done using SAS 9.2 software (SAS Institute, Cary, NC).
Results
At baseline, this subgroup of lifestyle participants was 51 (SD 11) years old on average, 68% were women, and 54% were white, 18% African American, 16% Hispanic American, 5% Native American, and 6% Asian American by self-reported ethnicity. Participants’ mean BMI was 33.77 (SD 6.56) kg/m2. Other weight measures and cardiometabolic risk factors are outlined in Table 1. The cohort achieved a mean weight loss of 6.84 (SD 5.62) kg at 6 months and sustained a mean weight loss of 5.39 (SD 7.56) kg at 2 years. After completion of the core curriculum at 6 months, there was a modest mean weight regain of 0.61 (SD 3.18) kg over the ensuing 18 months, with more rapid weight gain (mean 1.38 [SD 5.73] kg) between 18 months and 24 months. From baseline to 2 years, there were small changes in fasting glucose and systolic blood pressure, with greater changes in HOMA-IR and triglyceride levels. The average number of 5-lb (2.25-kg) weight cycles was 1.45, with a range of 0–6 weight cycles over 2 years. Weight cycling was more frequent in men than in women (1.59 vs. 1.39 cycles/year, P < 0.01), younger participants (1.60 cycles/year for age <45 years vs. 1.40 for 45–59 years, and 1.35 for ≥60 years, P = 0.01), and in African Americans than other ethnic groups (1.68 vs. 1.00–1.44 cycles/year, P < 0.01).
Table 2 summarizes the pairwise partial correlation coefficients between weight variables conditional on age at enrollment. Baseline body weight and several weight loss variables were significantly correlated with one another. Higher baseline weight correlated with a larger number of weight cycles (r = 0.35, P < 0.01), weight loss (6 months: r = 0.33, P < 0.01; 2 years: r = 0.20, P < 0.01), and a larger average weight in the first 2 years (r = 0.96, P < 0.01), but not weight regain between 6 and 24 months (P = 0.10) or from 18 to 24 months (P = 0.78). More initial weight loss (e.g., baseline to 1-month weight loss) was associated with greater subsequent weight loss from baseline to 24 months (r = 0.38, P < 0.01) and less weight loss/greater weight regain between 6 and 24 months (r = −0.10, P < 0.01) but not with more weight cycling (number of 5-lb (2.25-kg) weight cycles; P = 0.13). Weight cycling was correlated with average weight in the first 2 years (r = 0.38, P < 0.001), less weight loss/greater weight regain between 6 and 24 months (r = 0.19, P < 0.01) and between 18 and 24 months (r = −0.11, P < 0.01), and less long-term weight loss at 2 years (r = −0.10, P < 0.01).
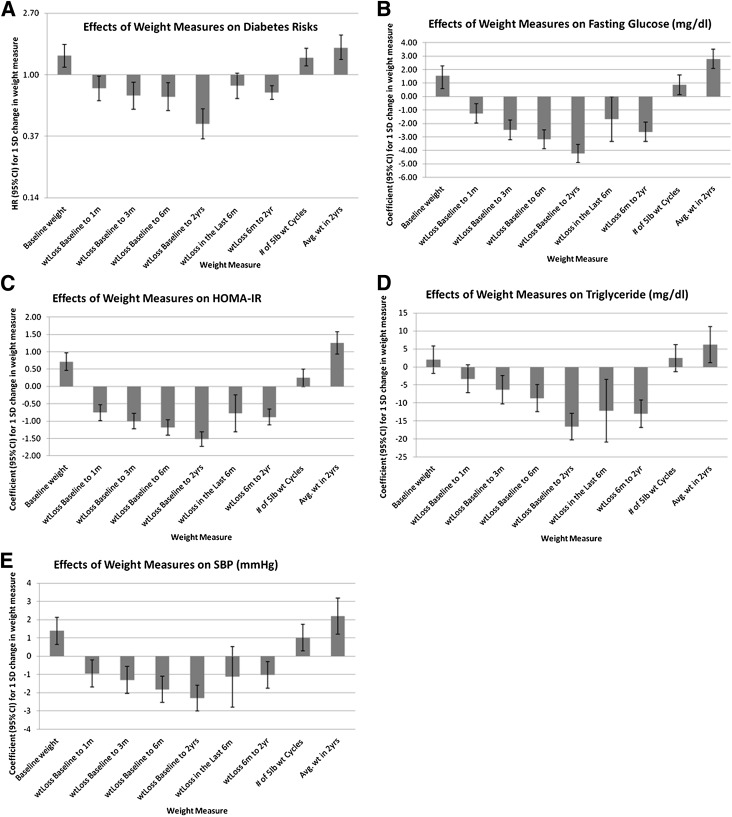
There were 99 incident diabetes events ascertained during a median follow-up of 3 years (range 2–4.5 years). Table 3 reports the estimated effects of a 1-unit (kg or weight cycle) change in the weight loss variable on diabetes incidence and glucose concentrations, HOMA-IR, SBP, and triglyceride concentrations at 2 years. Figure 1 shows the comparative effects of 1-SD change specific to ethnic/sex of the various body weight variables on diabetes incidence and cardiometabolic outcomes at 2 years. Greater weight loss in the first 6 months and from 6 months to 2 years were both predictive of reduced diabetes incidence; however, overall weight loss from baseline to 2 years was the strongest single predictor of lower diabetes incidence. The effects of 2-year weight loss on diabetes risk were not affected by further adjusting for covariates measuring caloric intake and physical activity (data not shown).
Table 3.
Estimated effects (HR or coefficient) of the body weight dynamics on diabetes incidence and cardiometabolic outcomes
| Diabetes incidence |
Fasting glucose (mg/dL) |
HOMA-IR |
Triglycerides (mg/dL) |
SBP (mmHg) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| BL weight | 1.02 | 1.01, 1.03 | <0.01 | 0.08 | 0.04, 0.12 | 0.01 | 0.04 | 0.02, 0.05 | <0.01 | 0.10 | −0.11, 0.30 | 0.36 | 0.09 | 0.05, 0.12 | <0.01 |
| BL to 1 month | 0.93 | 0.86, 1.00 | 0.05 | −0.47 | −0.75, 0.20 | <0.01 | −0.28 | −0.37, 0.19 | <0.01 | −1.34 | −2.83, 0.14 | 0.08 | −0.35 | −0.64, 0.07 | 0.01 |
| BL to 3 months | 0.91 | 0.86, 0.97 | <0.01 | −0.67 | −0.86, −0.47 | <0.01 | −0.27 | −0.33, −0.21 | <0.01 | −1.77 | −2.81, −0.73 | <0.01 | −0.37 | −0.56, −0.17 | <0.01 |
| BL to 6 months | 0.94 | 0.90, 0.98 | <0.01 | −0.59 | −0.72, −0.46 | <0.01 | −0.22 | −0.26, −0.18 | <0.01 | −1.68 | −2.38, −0.99 | <0.01 | −0.35 | −0.48, −0.21 | <0.01 |
| BL to 2 years | 0.90 | 0.87, 0.93 | <0.01 | −0.57 | −0.66, −0.48 | <0.01 | −0.20 | −0.23, −0.17 | <0.01 | −2.27 | −2.76, −1.78 | <0.01 | −0.30 | −0.40, −0.21 | <0.01 |
| Last 6 months | 0.95 | 0.89, 1.01 | 0.12 | −0.22 | −0.45, 0.01 | 0.06 | −0.11 | −0.18, −0.04 | <0.01 | −1.85 | −3.07, −0.64 | <0.01 | −0.15 | −0.39, 0.08 | 0.19 |
| 6 months to 2 years | 0.91 | 0.88, 0.95 | <0.01 | −0.46 | −0.58, −0.33 | <0.01 | −0.15 | −0.19, −0.11 | <0.01 | −2.38 | −3.03, −1.72 | <0.01 | −0.20 | −0.33, −0.07 | <0.01 |
| 5-lb cycles (n) | 1.33 | 1.12, 1.58 | <0.01 | 0.91 | 0.17, 1.65 | 0.02 | 0.25 | 0.01, 0.49 | 0.04 | 2.57 | −1.35, 6.50 | 0.20 | 0.94 | 0.19, 1.68 | 0.01 |
| Average weight in 2 years | 1.05 | 1.03, 1.07 | <0.01 | 0.30 | 0.22, 0.38 | <0.01 | 0.14 | 0.10, 0.16 | <0.01 | 0.68 | 0.26, 1.10 | <0.01 | 0.26 | 0.18, 0.34 | <0.01 |
BL, baseline. The P values are output from individual regressions for the specific outcome and weight metric combination without adjustments for multiple tests. All weight measures are in kilograms except for number of 5-lb weight cycles.
Figure 1.
Estimated effects (HR or coefficient) and the corresponding 95% CIs of body weight dynamics on diabetes risks (A), fasting glucose (B), HOMA-IR (C), triglyceride levels (D), and SBP (E).
Weight cycling (defined as number of 5-lb weight cycles) was predictive of an increased incidence of diabetes. Moreover, the effect of weight cycling on diabetes risk remained statistically significant in models adjusted for baseline weight (hazard ratio [HR] 1.22, 95% CI 1.02, 1.48; P = 0.03) or 2-year weight loss (HR 1.22, 95% CI 1.02, 1.47; P = 0.03). The effect of the number of 5-lb weight cycles was no longer significant when baseline weight and 2-year weight loss were both included in the same model (HR 1.11, 95% CI 0.91, 1.35; P = 0.29) or when average weight over 2 years was included in the model (HR 1.15, 95% CI 0.95, 1.39; P = 0.15).
The expanded model including demographic variables, 2-year weight loss, 1-month weight loss, and average weight adds significant predictive power to the core model (P < 0.01 compared with the core model). The areas under the curve (AUCs) of the core model (demographics and 2-year weight loss) and the expanded models described above were 0.72 and 0.76, respectively. The AUC in the model including the top three weight measures was 0.76, which was essentially the same as the logistic model including all candidate weight measures (AUC 0.76; P = 0.90 for difference between AUCs). Weight cycling raised the risk of diabetes (HR 1.33, 95% CI 1.12, 1.58; P = 0.001) but did not significantly add to the predictive accuracy of the core model (P = 0.28).
Cardiometabolic Outcomes
Weight loss was predictive of improved cardiometabolic outcomes in both the first 6 months and from 6 months to 2 years, but again, was not as predictive as total weight loss from baseline to 2 years. Weight loss from 18 months to 2 years was a significant predictor of improved triglyceride levels and insulin sensitivity at 2 years but was not a significant predictor of the other cardiometabolic traits or diabetes incidence. Weight cycling was associated with worsening of most cardiometabolic risk factor levels except for fasting triglycerides: fasting glucose (β = 0.91, 95% CI 0.17, 1.65 mg/dL per kg; P = 0.02), HOMA-IR (β = 0.25, 95% CI 0.01, 0.49 per kg; P = 0.04), and SBP (β = 0.94, 95% CI 0.19, 1.68 mmHg per kg; P = 0.01); however, these effects were no longer statistically significant when adjusted for baseline weight or 2-year weight loss.
Conclusions
This study expands on previous research by examining the relative importance of several measures of body weight as predictors of diabetes and changes in cardiometabolic risk factors and identifying an optimized set of weight loss variables associated with diabetes risk. We compared the strength and magnitude of the associations for various weight loss variables with the change in cardiometabolic levels and diabetes incidence to quantify the short- and long-term time-dependent effects of weight loss, recent weight loss, degree of exposure to excess weight, and weight cycling in the first 2 years of the DPP trial. Although weight loss during the first 6 months of the lifestyle intervention was a major predictor of reduced incidence of diabetes, the strongest predictor of reduced diabetes risk and improvements in cardiometabolic traits was the overall 2-year weight loss: every kilogram of weight loss in the first 6 months of the trial corresponded with a 6% reduction in risk of diabetes, whereas every kilogram of weight loss from baseline to 2 years corresponded with a 10% decrease in the risk of diabetes. Our results differ from those previously reported by Hamman et al. (12) because we examined the development of diabetes from year 2 until study end and excluded those who had less than 2 years of follow-up and those who developed diabetes within the first 1.5 years of the intervention.
For all cardiometabolic traits, the overall weight loss at 2 years was a better predictor than average weight in the first 2 years. Interestingly, the initial 6-month weight loss appears to be a better predictor for the reduction in fasting blood glucose, SBP, and HOMA-IR than the most recent 6-month weight loss, whereas the most recent 6-month weight loss was a predictor of both lower triglyceride levels and improved insulin sensitivity.
We also identified an optimized set of weight loss variables for predicting incident diabetes in the DPP, which includes 1-month weight loss, 2-year weight loss, and either initial body weight or exposure to excess body weight over 2 years. These results suggest that there are added benefits to early rapid weight loss in the first month in the context of successful 2-year weight loss results, particularly for those who have higher initial body weights.
Our results are consistent with previous reports that have shown that weight losses that are not maintained are not as effective in reducing the risk of diabetes as those that are maintained (12). Our findings regarding the importance of the pattern of weight loss are also consistent with findings from the Look AHEAD (Action for Health in Diabetes) trial, another long-term intentional weight loss study in people with diabetes. In Look AHEAD, greater overall weight losses had the strongest relationship with improved cardiovascular disease risk factors at 4 years; however, a pattern of larger month-to-month weight loss during the first year was also predictive of greater improvements in glycated hemoglobin, HDL cholesterol, and SBP at 4 years, independent of total weight loss (6). These studies and our data emphasize that early larger weight losses that are sustained seem to confer added benefits for reducing the risk of diabetes and improving cardiometabolic outcomes in the medium-term.
Weight cycling and the average weight in the first 2 years were both among the top three predictors of increased incidence of diabetes, implying that continued efforts to reduce exposure to excess body weight are important in reducing the risk of diabetes. The effect of weight cycling on diabetes risk was still significant even after adjusting for baseline weight or 2-year weight change, but not after adjusting for average weight in the first 2 years. Importantly, however, in a translational setting, one would not know the long-term weight change before or during the intervention period, and so focusing on reducing weight cycling and preventing weight gain are logical and complimentary objectives for diabetes prevention. Moreover, a patient’s baseline weight is clearly not the focus of an intervention. Thus, in the context of translation, the correlations among baseline weight, average weight, and weight cycling are important for a clinician to consider because heavier patients are more likely to weight cycle and develop diabetes. Nevertheless, this does not mean diabetes risk is not affected by weight cycling in these patients or that one should not focus on reducing weight cycling for diabetes prevention. By contrast, we conclude for the cardiometabolic traits that weight cycling does not convey effects that are independent of baseline weight or 2-year weight change.
Our findings generally agree with those reported elsewhere that indicate that weight cycling is associated with weight regain, increased risk of hypertension and diabetes, and elevations in other cardiometabolic traits (2,13–17). In contrast to previous studies that have categorized the magnitude and frequency of weight cycling (13,15,17) (e.g., 5–9, 10–19, 20–49, and ≥50 lb) (13), we measured the effects of the number of weight cycles (gain and loss) of at least 5 lb because most DPP participants did not have 10-lb weight cycles over 2 years of follow-up. Although our findings show that overall weight status is an important determinant of diabetes and cardiometabolic risk and that overall weight loss lowers risk, avoiding weight regain of more than 5 lb also appears to be clinically important.
A strength of our study is that we examined and compared the relative importance of a variety of weight loss variables in a well-characterized, ethnically diverse cohort of people with impaired glucose tolerance that was pursuing intentional weight loss. We also used weight change data from actual clinic visits rather than from self-reported weights to assess weight cycling.
However, these findings must also be interpreted with several limitations in mind. Many estimated effects are statistically significant but may not be clinically relevant due to the large sample size. The results from analyses focused on estimating the HRs of each weight variable on diabetes and cardiometabolic outcomes are not adjusted for multiple testing. This is because extensive existing evidence supports an effect of body weight change on diabetes risk; thus, the prior probability of association is likely to be greater than the null, which is not considered in conventional multiple test correction procedures. By clearly defining the number of tests performed and presenting nominal P values, we provide the necessary information for readers to determine for themselves the possibility that these associations represent true positive effects. Potential confounding factors include changes in diet or activity that vary within the intervention and could affect development or worsening of cardiometabolic risk factors. Although we measured changes in body weight, weight cycling, and magnitude of exposure to excess body weight, we did not examine body fat distribution by waist circumference or visceral fat, and weight as a proxy for change in adiposity may lack precision. Because the DPP is a multiethnic cohort of people at high risk for diabetes, these results may not be generalizable to individuals at a lower risk of diabetes.
These findings have several important clinical implications. First, current programs that focus on translating the first months of the DPP lifestyle intervention need to consider offering maintenance programs to maximize sustainability of weight loss, improvements in cardiometabolic risk factors, and the potential to prevent or delay diabetes. Early rapid weight loss that is sustained may confer additional benefits for reducing the risk of diabetes. Although lapses in eating behavior may lead to weight regain and some weight cycling, the ability to refocus on weight loss behaviors and achieve weight loss overall appears to be most important for diabetes prevention and improved cardiometabolic outcomes. Nevertheless, minimizing weight cycling is also important for reducing diabetes risk and for psychological well-being and perceived self-efficacy related to weight loss. Efforts to promote weight loss should seek to achieve this in a manner that is steady and consistent over time.
In conclusion, the overall 2-year weight loss among the DPP lifestyle intervention participants was the strongest predictor of reduced risk for diabetes and improvements in cardiometabolic traits compared with eight other body weight variables. Early rapid weight loss in the context of successful 2-year weight loss also provided added benefit in diabetes risk reduction. In the context of the DPP’s intentional weight loss program, weight cycling was associated with overall weight regain, increased risk of diabetes, and elevations in SBP, insulin resistance, and fasting glucose. Weight cycling conferred an increased risk for diabetes independent of baseline weight or 2-year weight change but not when both were considered simultaneously or when average weight over 2 years was considered. The effects of weight cycling on the cardiometabolic traits were not statistically significant when baseline weight or 2-year weight were conditioned upon. Defining the weight loss variables that are most strongly associated with the reduced incidence of diabetes and improvements in cardiometabolic traits may have implications for the choice of approaches in studies that examine genetic influences on weight loss and in the translation of findings from studies such as the DPP to clinical practice.
Article Information
Acknowledgments. The DPP investigators gratefully acknowledge the commitment and dedication of the participants of the DPP.
Funding. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) provided funding to the clinical centers and the coordinating center for the design and conduct of the study and the collection, management, analysis, and interpretation of the data (U01-DK-048489). The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding for data collection and participant support was also provided by the Office of Minority Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and Prevention, the Office of Research on Women’s Health, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also partly supported by the intramural research program of the NIDDK. LifeScan Inc., Health o meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slimfast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the coordinating center. A complete list of centers, investigators, and staff can be found in the Supplementary Data online. K.A.J. (the DPP Genetics Program) has a research grant from NIH (R01-DK072014). J.C.F. has received NIH grants (R01-DK072041). P.W.F. is supported by the Department of Clinical Sciences, Lund University and the Department of Nutrition, Harvard University. P.W.F. has received speaking honoraria from academic and nonprofit organizations.
Duality of Interest. L.M.D. has a financial interest in Omada Health, a company that develops online behavior-change programs, with a focus on diabetes. These interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. J.C.F. has received consulting honoraria from Novartis, Lilly, and Pfizer. P.W.F. has received speaking honoraria from Novo Nordisk and has research grants from Novo Nordisk.
Author Contributions. L.M.D., Q.P., and P.W.F. researched the data and wrote the manuscript. K.A.J., V.R.A., K.E.W., G.A.B., S.E.K., and J.C.F., and L.P. reviewed and edited the manuscript. P.W.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00004992, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0018/-/DC1.
A list of Diabetes Prevention Program Research Group investigators is provided in the Supplementary Data online.
The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies.
References
- 1.Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC. The built environment and obesity. Epidemiol Rev 2007;29:129–143 [DOI] [PubMed] [Google Scholar]
- 2.Teixeira PJ, Going SB, Sardinha LB, Lohman TG. A review of psychosocial pre-treatment predictors of weight control. Obes Rev 2005;6:43–65 [DOI] [PubMed] [Google Scholar]
- 3.Hill JO, Galloway JM, Goley A, et al. Scientific statement: sociological determinants of prediabetes and type 2 diabetes. Diabetes Care 2013;36:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wing RR, Look AHEAD Research Group Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neiberg RH, Wing RR, Bray GA, et al. Look AHEAD Research Group Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD Study. Obesity (Silver Spring) 2012;20:2048–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maruthur NM, Ma Y, Delahanty LM, et al. ; for the Diabetes Prevention Research Group. Early response to preventive strategies in the Diabetes Prevention Program. J Gen Intern Med 2013;28:1629–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetelogia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 10.Haffner S, Temprosa M, Crandall J, et al. Diabetes Prevention Program Research Group Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 2005;54:1566–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845 [PubMed] [Google Scholar]
- 12.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field AE, Byers T, Hunter DJ, et al. Weight cycling, weight gain, and risk of hypertension in women. Am J Epidemiol 1999;150:573–579 [DOI] [PubMed] [Google Scholar]
- 14.Guagnano MT, Pace-Palitti V, Carrabs C, Merlitti D, Sensi S. Weight fluctuations could increase blood pressure in android obese women. Clin Sci (Lond) 1999;96:677–680 [DOI] [PubMed] [Google Scholar]
- 15.Guagnano MT, Ballone E, Pace-Palitti V, et al. Risk factors for hypertension in obese women. The role of weight cycling. Eur J Clin Nutr 2000;54:356–360 [DOI] [PubMed] [Google Scholar]
- 16.Waring ME, Eaton CB, Lasater TM, Lapane KL. Incident diabetes in relation to weight patterns during middle age. Am J Epidemiol 2010;171:550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French SA, Folsom AR, Jeffery RW, Zheng W, Mink PJ, Baxter JE. Weight variability and incident disease in older women: the Iowa Women’s Health Study. Int J Obes Relat Metab Disord 1997;21:217–223 [DOI] [PubMed] [Google Scholar]