Abstract
OBJECTIVE
The presence of large subcutaneous adipocytes in obesity has been proposed to be linked with insulin resistance and type 2 diabetes through the “adipose tissue expandability” hypothesis, which holds that large adipocytes have a limited capacity for expansion, forcing lipids to be stored in nonadipose ectopic depots (skeletal muscle, liver), where they interfere with insulin signaling. This hypothesis has, however, been largely formulated by cross-sectional findings and to date has not been prospectively demonstrated in the development of insulin resistance in humans.
RESEARCH DESIGN AND METHODS
Twenty-nine men (26.8 ± 5.4 years old; BMI 25.5 ± 2.3 kg/m2) were fed 40% more than their baseline requirement for 8 weeks. Before and after overfeeding, insulin sensitivity was determined using a two-step hyperinsulinemic-euglycemic clamp. Intrahepatic lipid (IHL) and intramyocellular lipid (IMCL) were measured by 1H-MRS and abdominal fat by MRI. Subcutaneous abdominal adipose and skeletal muscle tissues were collected to measure adipocyte size and markers of tissue inflammation.
RESULTS
Subjects gained 7.6 ± 2.1 kg (55% fat) and insulin sensitivity decreased 18% (P < 0.001) after overfeeding. IHL increased 46% from 1.5% to 2.2% (P = 0.002); however, IMCL did not change. There was no association between adipocyte size and ectopic lipid accumulation. Despite similar weight gain, subjects with smaller fat cells at baseline had a greater decrease in insulin sensitivity, which was linked with upregulated skeletal muscle tissue inflammation.
CONCLUSIONS
In experimental substantial weight gain, the presence of larger adipocytes did not promote ectopic lipid accumulation. In contrast, smaller fat cells were associated with a worsened metabolic response to overfeeding.
Introduction
Obesity prevalence (BMI ≥30 kg/m2) has increased dramatically from the 1980s to 2010 (1) and is being mirrored by an increase in type 2 diabetes (T2DM) (2). Based on the “experiment of nature” that has happened in the United States, with the average adult gaining >10 kg over the past 30 years, Unger and Scherer (3) proposed that the metabolic syndrome (insulin resistance, hyperlipidemia, elevated abdominal fat, and hypertension [4]) preceding T2DM onset occurs specifically through lipotoxicity, whereas the state of obesity per se is of lesser importance. However, only a well-controlled study of experimental weight gain mimicking the route to obesity can provide insight into the determinants of insulin resistance and metabolic syndrome.
The underlying mechanism linking lipotoxic obesity with insulin resistance is widely believed to be impaired adipogenesis, which is manifested as the presence of enlarged subcutaneous adipocytes (5). Larger adipocytes are thought to be close to a hypothesized critical volume where further expansion is no longer possible; therefore, excess lipid is shunted instead to nonadipose tissues (skeletal muscle, liver, heart, and pancreas), where it interferes with insulin signaling and causes tissue insulin resistance (reviewed by Samuel and Shulman [6]). Enlarged adipocytes may also secrete chemoattractants or have localized hypoxia, which trigger macrophage infiltration and activate the inflammatory process in adipose tissue, thus worsening insulin resistance (7,8). Adipose tissue containing primarily large adipocytes is more insulin resistant, as illustrated by reduced suppression of free fatty acid production, resulting in elevated free fatty acids (FFAs), which can directly activate inflammation via Toll-like receptors (9).
While evidence in support of the adipose expansion theory of insulin resistance is well documented in the animal literature (reviewed by Virtue and Vidal-Puig [5]), less is known about the pathway in humans. Obese people who are “metabolically healthy” have greater adipogenesis (smaller subcutaneous fat cells) along with less visceral adiposity and hepatic lipid accumulation, decreased inflammation, and preserved insulin sensitivity compared with metabolically unhealthy obese (10–12). Clinically, one of the mechanisms by which thiazolidinedione treatment works to improve insulin sensitivity in T2DM is by stimulating adipogenesis and subcutaneous fat accumulation, reducing circulating and hepatic lipids, and improving insulin sensitivity despite weight gain (13).
The question remains: Does the presence of relatively smaller subcutaneous fat cells protect against the accumulation of lipid in ectopic depots and development of insulin resistance during the period of weight gain en route to obesity? To address this question, we overfed men by 40% of their energy requirement for 8 weeks to determine the influence of baseline adipocyte size on insulin sensitivity, lipid accumulation in liver and muscle, tissue inflammation, and other facets of the metabolic syndrome in response to excess energy. According to the prevailing theory, we hypothesized that individuals with larger fat cells would have less capacity to expand their subcutaneous adipose tissue (SAT) and would deposit more lipid into ectopic depots, worsening the metabolic response.
Research Design and Methods
Participants
Volunteers aged 20–40 years with a BMI between 22.0 and 32.0 kg/m2 were eligible to participate. Subjects underwent screening tests before enrollment, including a physical exam, blood and urine analyses, and detailed medical history. Those reporting a history of chronic disease (diabetes, heart or liver disease, high blood pressure, gastrointestinal disorder), eating disorder, recent weight loss or gain (>2.5 kg over the past 6 months), or abnormal blood or urine values were excluded. Subjects must not have had a BMI >32 kg/m2 at any point in their lifetime. The study was approved by the Pennington Biomedical Research Center (PBRC) institutional review board, and all volunteers provided written informed consent before participation.
Diets
Before overfeeding, participants completed a 2-week measure of free-living energy expenditure using doubly labeled water (DLW) (14). During the second week of DLW, participants were fed to energy balance using a published equation (15). The baseline energy requirement was calculated as the average of the measured 2-week energy expenditure by DLW and the 1-week calorie level provided during feeding at weight maintenance, which was multiplied by 1.4 to determine the overfeeding prescription. This calorie level was initiated on the first day of overfeeding and was maintained until the last day (day 56). All meals were prepared by the PBRC Metabolic Kitchen using a validated 5-day rotating menu (16), and the diet was composed of 41% carbohydrate, 44% fat, and 15% protein. The fat content comprised a mixture of saturated (40%), monounsaturated (37%), and polyunsaturated (23%) fatty acids. Participants consumed all meals (3 per day, 7 days per week) in the PBRC Inpatient Unit under the direct supervision of nursing staff but were free-living the remainder of the time. On day 56, the number of calories needed to maintain energy balance at the new body weight was calculated using the run-in equation, and subjects were fed a weight-maintaining diet for 3 days before metabolic testing.
Measurements at Baseline and After Overfeeding
Overview
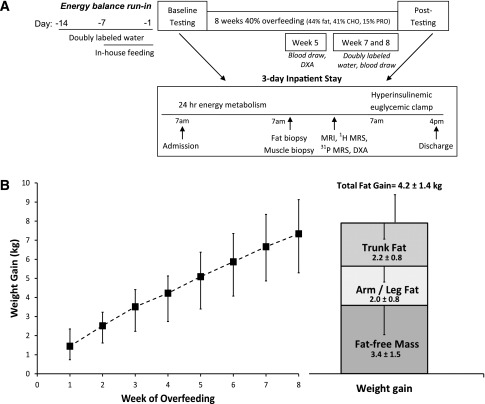
The study protocol is illustrated in Fig. 1A. Before and after overfeeding, subjects were admitted to the Inpatient Unit for 3 days to complete metabolic testing. On day 2, they underwent adipose and skeletal muscle (vastus lateralis) biopsies under fasting conditions, followed by measurement of intrahepatic lipid (IHL) and intramyocellular lipid (IMCL) by 1H-MRS, abdominal fat by MRI, and whole-body composition by DXA. Insulin sensitivity was measured on day 3 using a 2-step hyperinsulinemic-euglycemic clamp.
Figure 1.
A: Schematic of the study protocol. B: Average weekly weight gain from daily fasting weights (days 1–56) (left panel). Composition of the final weight gain as determined by DXA and fasting weight at metabolic testing (right panel).
Fat Depots
Abdominal SAT and visceral adipose tissue (VAT) volumes were measured using a 3.0T scanner (Excite HD System; General Electric, Milwaukee, WI). Images (240–340) were obtained from the highest point of the liver through the pubic symphysis and were analyzed by a single trained technician using Analyze software (AnalyzeDirect, Overland Park, KS). The mean coefficient of variation for three readings of the same scan was 9.9% for VAT and 1.8% for SAT. Estimates of MRI volumes were converted to mass using an assumed density of 0.92 kg/L (17). Because of an inability of patients to lie in the scanner or technical issues, data are reported for 26 of the 29 subjects.
Soleus and anterior tibialis IMCL and IHL were measured by 1H-MRS using the point resolved spectroscopy box technique (18). Three water-suppressed point-resolved spectroscopy voxels were collected and peak areas of interest were determined by time domain fitting using jMRUI (The MRUI Project) (19) and a set of information from Rico-Sanz et al. (20). Water-suppressed signals were deconvoluted with the unsuppressed water signal acquired from the same voxel locations and the resulting metabolite signals were analyzed with AMARES, a nonlinear least square fitting algorithm operating in the time domain (21). Data were normalized to an external oil phantom (peanut oil), as described by Perseghin et al. (22), and in our hands the coefficient of variation is 8.3% for IMCL and 9.9% for IHL.
Percentage fat of the whole body was measured using DXA (QDR 4500A; Hologics, Bedford, MA), and total fat mass (FM) and fat-free mass (FFM) were calculated from the percentage body fat and measured metabolic body weight.
Insulin Sensitivity
Insulin sensitivity was measured using a two-step hyperinsulinemic-euglycemic clamp. Insulin was infused for 180 min at 10 mU/min · m2 and for 150 min at 50 mU/min · m2, and glucose (20%) was infused at a variable rate to maintain plasma glucose concentration at 90 mg/dL. The average amount of glucose required during the final 30 min of each insulin infusion step (glucose infusion rate [GIR]) was considered the measure of insulin sensitivity (23) and was expressed per kilogram of estimated metabolic body size (FFM + 17.7 kg) (24). In a subset of subjects (n = 19), endogenous glucose production (EGP) was determined in the basal state (0.22 µmol 6,6-2H2 · kg−1 · min−1 for 4 h) and during insulin-stimulated conditions (1% 6,6-2H2 enrichment of the 20% glucose solution).
Circulating Biochemicals
Insulin and hs-CRP were measured by immunoassay with chemiluminescent detection (Siemens), and FFA concentrations were measured by enzymatic reaction with colorimetric detection (Wako). Adiponectin and leptin were measured by radioimmunoassay, and high–molecular weight adiponectin was determined using ELISA (Millipore). The enrichment of 6,6-2H2 glucose in plasma was analyzed using gas chromatography–mass spectrometry (Agilent Technologies).
Adipose and Skeletal Muscle Tissue
Abdominal SAT and skeletal muscle tissue were collected using the technique described by Bergstrom (25), and liposuction was used to collect additional adipose tissue. We chose to focus on abdominal fat because of its closer association with metabolic risk than other adipose depots (26). Adipocyte size was measured using an osmium tetroxide method adapted from Hirsch and Gallian (27) and Pasarica et al. (28). Cells were counted using the Multisizer 3 Coulter Counter (Beckman Coulter, Miami, FL) using a 400-μm aperture (linear range, 12–380 µm; only cells larger than 22 μm were included in this analysis), and the average of two runs for approximately 1,000–3,000 cells each was collected to calculate mean adipocyte size. Total RNA was extracted using the miRNeasy kit (Qiagen), and real-time PCR was performed using the ABI PRISM 7900 (Applied Biosystems) and normalized to the expression of ribosomal protein P0.
Statistical Analysis
Statistical analyses were performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC). Data are presented as mean ± SD, with the α level set at 0.05; statistical tests were two-tailed. Changes in continuous variables between baseline and after overfeeding were analyzed by paired t test or Wilcoxon signed rank test, depending on the normality of the data. Associations between subcutaneous adipocyte size at baseline, with and without adjusting for baseline FM, and overfeeding-induced changes in metabolic parameters (fat depots, glucose infusion, circulating and tissue markers) were analyzed using linear regression models. Subjects also were grouped by tertile according to their baseline FM–adjusted fat cell size (FCS), and differences between those in the lowest tertile (smallest fat cells) and highest tertile (largest fat cells) were analyzed by one-way ANOVA.
Results
Participant Characteristics
Thirty-three men began overfeeding and four withdrew in the first week (two because of too much food and two because of interference with job activities); therefore, 29 men (19 Caucasian, 10 African American) aged 26.8 ± 5.4 years completed the overfeeding protocol. Baseline energy requirements were 3,054 ± 396 kcal/day, and the overfeeding prescription was 4,235 ± 470 kcal/day. Subjects consumed an excess of 66,156 ± 9,960 kcal above baseline energy requirements over the 8-week period (range 42,476–80,052 kcal). Average weight gain was 7.6 ± 2.1 kg and comprised 4.2 ± 1.4 kg FM (55%) and 3.4 ± 1.5 kg FFM (45%); BMI rose more than two units (Table 1 and Fig. 1B).
Table 1.
Clinical changes with overfeeding (n = 29)
| Parameter | Before overfeeding | After overfeeding |
|---|---|---|
| Anthropometry | ||
| Weight (kg) | 81.9 ± 10.3 | 89.5 ± 9.4* |
| BMI (kg/m2) | 25.5 ± 2.3 | 27.8 ± 2.5* |
| FM (kg) | 16.0 ± 4.8 | 20.2 ± 5.6* |
| Body fat (%) | 19.4 ± 4.9 | 22.3 ± 5.2* |
| FFM (kg) | 65.9 ± 7.3 | 69.4 ± 7.3* |
| Waist (cm) | 84.6 ± 6.6 | 92.0 ± 7.2* |
| Hip (cm) | 98.0 ± 5.4 | 104.1 ± 7.0* |
| Waist-to-hip ratio | 0.87 ± 0.04 | 0.89 ± 0.05† |
| Fat depots | ||
| Abdominal SAT (kg) (n = 26) | 4.1 ± 1.5 | 5.4 ± 1.8* |
| VAT (kg) (n = 26) | 0.58 ± 0.49 | 0.94 ± 0.58* |
| IHL (% [mean ± SEM]) | 1.50 ± 0.6 | 2.19 ± 1.0† |
| IMCL, soleus (%) | 0.45 ± 0.24 | 0.49 ± 0.24 |
| Blood chemistry | ||
| Triglycerides (mg/dL) | 87 ± 42 | 96 ± 68 |
| Total cholesterol (mg/dL) | 171 ± 25 | 196 ± 31* |
| HDL (mg/dL) | 55 ± 12 | 57 ± 11 |
| LDL (mg/dL) | 99 ± 23 | 120 ± 28* |
| HDL-to-LDL ratio | 0.59 ± 0.20 | 0.50 ± 0.16* |
| Leptin (ng/mL) | 6.4 ± 4.9 | 11.1 ± 6.4* |
| Adiponectin (total, µg/mL) | 4.70 ± 2.80 | 4.60 ± 2.48 |
| hs-CRP (mg/L) | 0.87 ± 0.87 | 1.10 ± 1.18‡ |
| Alanine transaminase (IU/L) | 27.4 ± 12.4 | 38.3 ± 18.9* |
| Fasting insulin (µU/mL) | 5.4 ± 4.0 | 8.3 ± 7.8‡ |
| Fasting glucose (mg/dL) | 91.0 ± 6.7 | 92.7 ± 6.9 |
| Insulin sensitivity | ||
| Glucose infusion** (mU/min · m2 insulin) | ||
| 10 | 2.87 ± 0.94 | 2.35 ± 0.7 * |
| 50 | 11.51 ± 2.54 | 10.91 ± 2.46§ |
| Basal EGP (mg/min/kg) | 1.69 ± 0.18 | 1.59 ± 0.17† |
| EGP during low-dose insulin infusion (mg/min/kg) | 0.46 ± 0.34 | 0.56 ± 0.27‡ |
| EGP suppression (%) | 74 ± 18 | 66 ± 15† |
| EGP during higher-dose insulin infusion (mg/min/kg) | 0.06 ± 0.16 | 0.28 ± 0.30† |
| EGP suppression (%) | 96 ± 10 | 82 ± 20† |
| FFAs (nmol/L) | ||
| Basal | 0.26 ± 0.08 | 0.30 ± 0.11§ |
| Insulin infusion (mU/[min · m2]) | ||
| 10 | 0.04 ± 0.03 | 0.05 ± 0.04† |
| 50 | 0.03 ± 0.04 | 0.02 ± 0.02 |
Data are mean ± SD unless otherwise indicated. *P < 0.001,
†P < 0.01,
‡P < 0.05,
§P < 0.10.
**GIR (mg/min · [FFM+17.7]).
Clinical Response to Overfeeding
Mean changes with overfeeding are shown in Table 1. From DXA analysis, subjects gained 1.9 ± 0.8 kg fat (45% of total fat gain) in peripheral depots (arms and legs) and 2.1 ± 0.8 kg fat (50% of total) in the trunk. Within the abdomen, SAT and VAT increased by 31% and 62%, respectively (seen on MRI). IHL increased by 0.7 ± 2.8% (1.5–2.2%; P = 0.002); however, there was no change in IMCL in any muscle group studied (MRS). Waist and hip circumferences increased, as did the waist-to-hip ratio, indicating that a greater amount of weight was deposited in the abdomen relative to the hips. Blood pressure (systolic, 113 ± 8 to 115 ± 11 mmHg [P = 0.26]; diastolic, 73 ± 7 mmHg before and after overfeeding [P = 0.95]) and heart rate (66 ± 10 to 67 ± 7 bpm; P = 0.30) remained stable.
Total and LDL cholesterol (P < 0.001), alanine transaminase (P < 0.001), hs-CRP (P = 0.04), and FFAs (P = 0.08) increased with overfeeding, demonstrating dysregulated lipid metabolism, hepatic stress, and systemic inflammation, but plasma triglyceride concentrations did not change. The diet’s relatively high fat content may have contributed to the altered lipid profile, particularly the increase in LDL cholesterol and systemic inflammation. Concurrent with the increase in FM, leptin levels nearly doubled (P < 0.001) and the ratio of leptin per kilogram FM increased 45% after overfeeding (0.36 ± 0.18 to 0.52 ± 0.20; P < 0.001), showing that leptin concentrations increased significantly more than could be accounted for by increased FM. Ghrelin decreased from 675 ± 240 to 617 ± 200 pg/mL (P > 0.001). There was no change in either total or high–molecular weight adiponectin. Although fasting glucose remained unchanged, fasting insulin levels increased by 54% (P = 0.01) (Table 1). African American and Caucasian men responded similarly to overfeeding, with no significant effect of race on clinical or metabolic outcomes (data not shown).
The amount of glucose required to maintain euglycemia at 90 mg/dL decreased by 18% (P < 0.001) during low-dose insulin infusion and by 5% (P = 0.052) during higher-dose insulin infusion (Table 1). Decreases in GIR occurred despite similar levels of insulin during steady-state (SS) periods at baseline and after overfeeding; insulin during the low-dose infusion (10 mU/min · m2) SS was 13.4 ± 4.5 µU/mL at baseline and 13.0 ± 5.2 µU/mL after overfeeding (P = 0.57) and during the higher-dose insulin infusion (50 mU/min · m2) SS was 59.0 ± 11.3 and 60.8 ± 14.0 µU/mL (P = 0.30), respectively. The decrease in GIR after overfeeding despite similar SS plasma insulin demonstrates the presence of insulin resistance. Conversely, the ability of insulin to suppress FFA release into the circulation (presumably coming mostly from adipose tissue) was largely unaffected by overfeeding. At baseline, FFAs during the clamp were reduced from basal levels by 85 ± 11% during low-dose insulin infusion and by 90 ± 15% during higher-dose infusion, and the percent suppression was largely unchanged after overfeeding (low-dose insulin, 83 ± 11% [P = 0.19]; higher-dose insulin, 93 ± 8% [P = 0.28]). However, basal FFA levels were slightly higher after overfeeding (P = 0.08), possibly reflecting mild adipose or hepatic resistance during the non-insulin–stimulated state.
To determine whether the insulin resistance was hepatic-driven or systemic, we examined results of the 19 participants with complete tracer data. At baseline, basal EGP was 1.69 ± 0.18 mg/min/kg and decreased to 1.59 ± 0.17 mg/min/kg after overfeeding (P < 0.01), likely because of higher fasting plasma insulin concentrations. During low-dose insulin infusion, EGP was suppressed by 74 ± 18% at baseline but was reduced to 66 ± 15% after overfeeding (P = 0.01). During higher-dose insulin infusion, EGP was suppressed by 96 ± 10% at baseline but decreased to 82 ± 20% after overfeeding (P = 0.002), demonstrating hepatic insulin resistance (Table 1). Adjusting the GIR for EGP increased the absolute amount of glucose disposed but did not fully account for the reduction in glucose disposal rate observed after overfeeding (3.57 ± 0.94 to 3.03 ± 0.64 mg/min × [FFM + 17.7] [P = 0.001] for low-dose insulin infusion). This implies that, in addition to the liver, other tissues (namely, skeletal muscle) also became more insulin resistant.
Associations Between Adipocyte Size and Clinical/Metabolic Response to Overfeeding
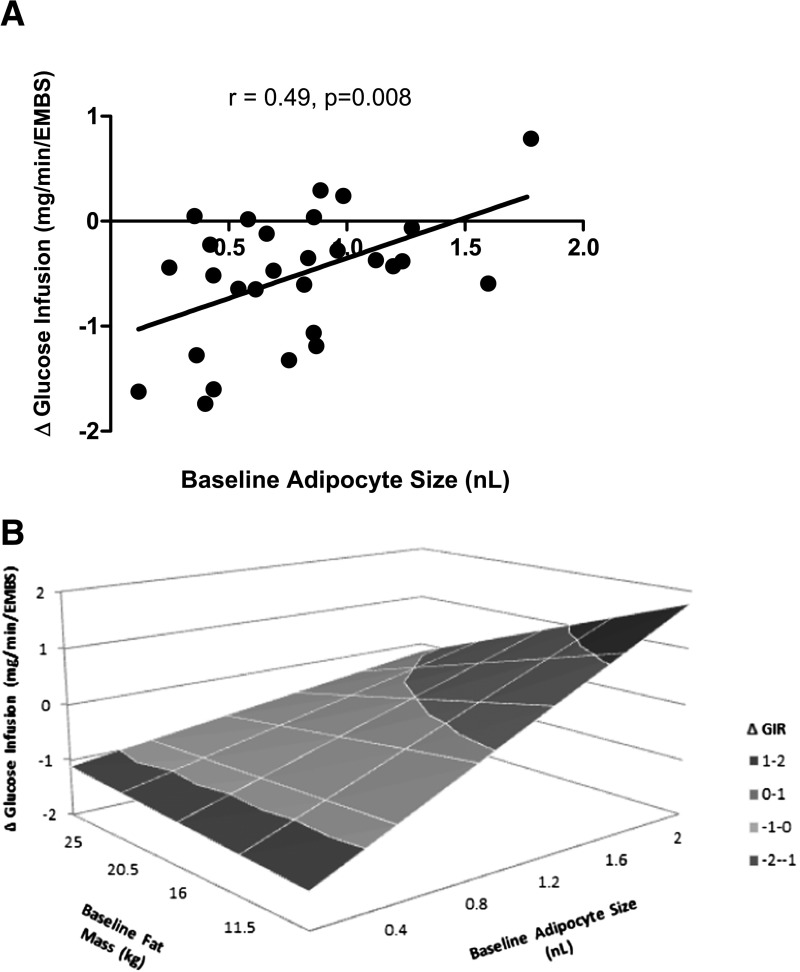
Despite similar absolute and percent weight gain, subjects with larger adipocytes at baseline had a smaller percent increase in abdominal SAT (r = −0.52; P = 0.007) and VAT (r = −0.43; P = 0.03) in response to overfeeding and overall tended to have a smaller increase in percent body fat (r = −0.35; P = 0.06). They did not, however, accumulate more lipid in the liver (r = 0.15; P = 0.43) or skeletal muscle (r = 0.08; P = 0.69). Moreover, subjects with smaller, not larger, adipocytes had a greater decrease in insulin sensitivity (ΔGIR at low-dose insulin: r = 0.49, P = 0.008) (Fig. 2A and B).
Figure 2.
A: Linear association between adipocyte size at baseline and the change in GIR with overfeeding with low-dose insulin infusion (10 mU/min/m2), expressed as milligrams per minute · (estimated metabolic body size [EMBS]) (EMBS = FFM + 17.7). The association remained significant after using Pearson correlation (r = 0.42; P = 0.03) but was attenuated after removing the subject with the largest adipocytes (r = 0.36; P = 0.06). B: The three-dimensional relationship between baseline adipocyte size and change in GIR across baseline levels of FM illustrates that smaller adipocytes are associated with larger decreases in GIR for any given value of FM.
Subjects who had larger fat cells also had a higher BMI (r = 0.46; P = 0.01), FM (r = 0.56; P = 0.002), and percent fat (r = 0.45; P = 0.01) to begin with compared with those with smaller cells; thus, the lesser relative expansion of subcutaneous fat could simply reflect higher FM at baseline. Therefore, we repeated the analysis after adjusting FCS for FM. Whereas the association between adipocyte size and Δ percent VAT lost significance, all other associations were unchanged. In addition, after adjusting FM, larger adipocytes tended to be related to less IHL accumulation after overfeeding (r = −0.35; P = 0.06). Of the weight gain, a larger percent was deposited as FFM (r = 0.39; P = 0.04) in subjects with larger fat cells compared with more as FM in those with smaller cells (r = −0.39; P = 0.04).
Associations Between Adipocyte Size and Tissue Molecular Markers With Overfeeding
We recently demonstrated that markers of tissue inflammation were increased in skeletal muscle but not in abdominal adipose tissue after overfeeding; however, both tissues showed significant upregulation of extracellular matrix gene expression (29). Here, we questioned whether adipocyte size influenced the change in these inflammatory or extracellular matrix markers in either tissue. At baseline, expression of adipogenic markers peroxisome proliferator–activated receptor (PPARγ) (r = 0.47; P = 0.03) and SREBP-1 (r = 0.55; P = 0.01) was higher in adipose tissue of subjects with larger fat cells, but there were no relationships in skeletal muscle tissue. After overfeeding, adipocyte size was not associated with changes in the expression of markers of adipogenesis, inflammation, or extracellular matrix remodeling in adipose tissue (Table 2). Conversely, in skeletal muscle the expression of markers of inflammation (CD40, CD11c, CD68 [trend], RelA) and extracellular matrix remodeling (collagens I, III, V, and VI; SPARC) were upregulated in subjects who had smaller adipocytes. Tertile analysis showed that FCS increased by 0.50 ± 0.38 nL (109% increase) in the lower tertile (i.e., the smallest fat cells) with overfeeding but did not change in the upper tertile (−0.01 ± 0.40 nL; 1% decrease). Compared with the upper tertile, the lower FCS tertile had a greater increase in FM (4.7 ± 1.3 vs. 3.6 ± 1.6 kg; P = 0.09) and decreased glucose disposal by 32% and 13% (low- and higher-dose insulin, respectively), whereas the upper tertile decreased glucose disposal by only 9% and <1%, respectively. Logistic regression analysis revealed that those in the lower (vs. upper) tertile had an 11.6-fold greater risk of developing a 20% or larger reduction in glucose disposal (P = 0.03, low-dose insulin). There were no differences between the groups in the change in VAT (P = 0.70), IHL (P = 0.14), IMCL (soleus; P = 0.35), or total weight gain (P = 0.75) in response to overfeeding. The larger increase in FM despite similar weight gain indicated that the lower FCS tertile group gained a larger percentage of the weight as fat (67 ± 14% vs. 50 ± 15% in the upper tertile; P = 0.02) (Table 3).
Table 2.
Linear associations between adipocyte size (adjusted for FM) and changes in gene expression after overfeeding (n = 29 unless otherwise indicated)
| Correlation coefficient | P value | |
|---|---|---|
| Adipose tissue | ||
| Inflammatory markers | ||
| Interleukin-6 (n = 20) | 0.09 | 0.73 |
| p65/RelA subunit of NF-κB (n = 21) | −0.03 | 0.89 |
| CCL2 (n = 17) | −0.17 | 0.54 |
| CD68 (n = 24) | 0.17 | 0.36 |
| Extracellular matrix remodeling | ||
| Collagen I (n = 23) | 0.07 | 0.74 |
| Collagen III (n = 24) | 0.09 | 0.66 |
| Collagen VI (n = 23) | −0.09 | 0.67 |
| SPARC (n = 24) | 0.04 | 0.86 |
| Adipogenesis | ||
| SREBP-1 (n = 19) | −0.13 | 0.60 |
| PPARγ (n = 21) | −0.18 | 0.43 |
| Skeletal muscle | ||
| Inflammatory markers | ||
| CD40 | −0.56 | 0.002 |
| CD11c | −0.37 | 0.05 |
| CD68 | −0.35 | 0.07 |
| p65/RelA subunit of NF-κB | −0.48 | 0.01 |
| Extracellular matrix remodeling | ||
| Collagen I | −0.43 | 0.02 |
| Collagen III | −0.42 | 0.03 |
| Collagen VI | −0.44 | 0.02 |
| SPARC | −0.44 | 0.02 |
CCL2, macrophage chemoattractant protein-1; mTOR, mammalian target of rapamycin; NF, nuclear factor; SPARC, secreted protein acidic and rich in cysteine.
Table 3.
Changes in fat stores and glucose disposal with overfeeding among participants in the lower and upper tertiles of baseline adipocyte size
| FCS |
P value | ||
|---|---|---|---|
| Upper tertile (n = 10) | Lower tertile (n = 10) | ||
| Excess kilocalorie intake (8 weeks) | 67,944 ± 9,491 | 66,952 ± 11,246 | 0.78 |
| Weight gain (kg) | 7.3 ± 2.5 | 7.6 ± 2.1 | 0.75 |
| Weight gained as fat (%) | 50 ± 51 | 67 ± 14 | 0.02 |
| ΔFFM (kg) | 3.7 ± 1.5 | 2.9 ± 1.6 | 0.26 |
| ΔTotal FM (kg) | 3.6 ± 1.6 | 4.7 ± 1.3 | 0.09 |
| ΔAbdominal SAT (kg) | 1.1 ± 0.5 | 1.4 ± 0.7 | 0.27 |
| ΔVAT (kg) | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.70 |
| ΔIHL (%) | −0.4 ± 2.2 | 0.9 ± 1.3 | 0.14 |
| ΔIMCL, soleus (%) | 0.12 ± 0.22 | 0.00 ± 0.31 | 0.35 |
| ΔGlucose disposal (mg/min · [FFM + 17.7]) | |||
| Low insulin | −0.26 ± 0.53 | −0.96 ± 0.53 | <0.01 |
| High insulin | −0.04 ± 1.59 | −1.73 ± 1.35 | 0.02 |
Conclusions
Eight weeks of 40% overfeeding in a group of young, healthy men caused a 7.6-kg weight gain and induced visceral and hepatic lipid deposition and mild hepatic and skeletal muscle insulin resistance and triggered systemic and skeletal muscle inflammation. The purpose of the study was to determine the influence of baseline adipocyte size on depot-specific fat expansion and the development of insulin resistance among subjects. In line with the adipose expandability theory, we found that subjects who had larger adipocytes expanded their subcutaneous fat depot significantly less; however, despite similar total weight gain, they did not accumulate more lipid in the liver or skeletal muscle. Moreover, in contrast to our hypothesis and contrary to the expandability theory, subjects who had smaller adipocytes—even after adjusting for baseline FM—had a larger decline in insulin sensitivity after overfeeding.
These results from a well-controlled prospectively designed study are in contrast to previous observational (30) and cross-sectional reports (31). Larger mean subcutaneous adipocytes were shown to change very little in size in response to weight gain but were associated with the onset of insulin resistance and, independent of body fatness, predicted future development of T2DM (30). In offspring of patients with T2DM, the size of the large adipocyte fraction was associated with lower insulin sensitivity independent of BMI (31), suggesting that larger fat cells impair insulin sensitivity, even in the absence of obesity. Conversely, in obese adolescents, a higher ratio of visceral to subcutaneous fat was associated with insulin resistance, impaired adipogenesis/lipogenesis, and a smaller proportion of large adipose cells (32). Gene expression of markers of de novo lipogenesis and lipid uptake was found to be lower in subjects with a greater proportion of small adipocytes; in this sense, small fat cells were considered detrimental because of the diminished capacity for fat storage and subsequently higher circulating plasma FFA and deposition of lipid in ectopic (liver) depots. These results are more in line with our longitudinal findings and suggest that a greater number of small adipocytes (not larger) and hypertrophy of adipocytes represent impaired adipogenesis and lead to insulin resistance. Notably, such discrepancy between data from cross-sectional versus longitudinal studies is reminiscent of early studies of energy metabolism, where cross-sectional analysis showed obese subjects had higher energy expenditure than lean adults; however, when examined longitudinally, adults who became obese had lower energy expenditure than those who remained lean (33). This popular misconception (that obese subjects had higher than normal energy expenditure) led initially to erroneous conclusions about causes of obesity.
Mechanistically, what might be driving the link between small adipocytes at baseline and adverse outcomes to overfeeding? First, smaller adipocytes were associated with a larger percent increase in circulating FFAs after overfeeding (r = −0.38; P = 0.04). Adipose tissue–derived nonesterified fatty acids have been shown to induce muscle insulin resistance (34,35) possibly by promoting the formation of lipid intermediates in skeletal muscle, including diacyglycerols, ceramides, and long-chain acyl-CoA, which activate kinases (protein kinase C, c-Jun-NH2-terminal kinase) that interfere with insulin signaling (36). Although we did not observe an increase in skeletal muscle triglyceride with overfeeding, we cannot rule out increases in other lipid species. There is also evidence that FFAs may act as a ligand for the Toll-like receptor 4 complex, thereby activating the classical inflammatory response, including macrophage accumulation (9,37). Indeed, we observed a greater increase in the expression of inflammatory factors (p65/RelA subunit of nuclear factor-κB) and macrophage markers (CD40, CD11c) in the skeletal muscle of subjects with smaller fat cells at baseline.
Second, whereas mean adipocyte size increased from 0.76 ± 0.40 to 1.06 ± 0.33 nL after overfeeding (P < 0.001), smaller fat cells increased most in size in response to excess energy (r = −0.56; P < 0.001). The large increase in adipocyte size suggests that the smaller adipocytes had a greater capacity to expand during overfeeding, whereas the larger cells may have been closer to a hypothesized “critical volume” and instead relied on adipogenesis to accommodate excess lipid (38), preventing storage of lipid in liver or muscle. Supporting this hypothesis, the higher “adipogenic potential” at baseline in subjects with larger fat cells (as represented by greater expression of PPARγ and SREBP-1) may have primed the adipose tissue for differentiation/proliferation upon exposure to excess energy, whereas those with smaller existing fat cells were able to accommodate the excess lipid by cellular hypertrophy. Indeed, an approximation of fat cell number using adipocyte size (nanoliters) and total subcutaneous adipose volume (liters) by MRI suggested that participants with larger adipocytes had a greater increase in fat cell number with overfeeding (r = 0.46; P = 0.02). A recent study of obese adolescents showed that a higher ratio of visceral to subcutaneous fat was associated with insulin resistance, impaired adipogenesis/lipogenesis, and a smaller proportion of large adipose cells (32). Gene expression of markers of de novo lipogenesis and lipid uptake was lower in subjects with a greater proportion of small adipocytes, and in this sense, small fat cells were considered detrimental because of a diminished capacity for fat storage and subsequently higher circulating plasma FFAs and deposition of lipid in ectopic (liver) depots. These results are more in line with our longitudinal findings and suggest that a greater number of small adipocytes (not larger) and hypertrophy of adipocytes represent impaired adipogenesis and lead to insulin resistance.
Although the expansion of subcutaneous adipocytes to accommodate lipid oversupply is considered to be a “safe” way to store fat, the rapid enlargement of fat cells may trigger macrophage infiltration, hypoxia, and cellular fibrosis (39). Supporting such pathological expansion, we found that smaller adipocytes were associated with upregulated expression of markers of inflammation and matrix remodeling, possibly indicating fibrotic changes, but, interestingly, this was limited to skeletal muscle and not detected in adipose tissue. We speculate that adipokines released from the rapidly expanding fat cell may have interfered with insulin sensitivity in skeletal muscle through tissue cross talk.
In conclusion, our data suggest that among young healthy men with no preexisting metabolic disorder, having larger subcutaneous adipocytes did not promote ectopic or visceral lipid accumulation during overfeeding-induced weight gain and did not induce insulin resistance. Rather surprisingly, participants with smaller fat cells had a larger reduction in insulin sensitivity despite a greater expansion of the subcutaneous adipose depot. We propose that during weight gain toward the development of obesity, smaller fat cells are not protective and respond to excess energy by rapidly increasing in size. This rapid expansion may induce insulin resistance through the release of adipokines that promote inflammation/extracellular matrix remodeling even in nonadipose tissues.
Article Information
Acknowledgments. The authors thank the dedicated staff of the Pennington Biomedical Research Center inpatient and outpatient units and imaging center for their contributions.
Funding. This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases grants K01-DK-089005 (to D.L.J.) and R01-DK-060412 and 2P30-DK-072476 (to E.R.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.L.J., Y.T., C.S.T., and J.D.C. performed the experiments and wrote the manuscript. W.X. assisted with statistical analysis. J.-M.S. performed the experiments and contributed to intellectual discussion. S.B. contributed to intellectual discussion. E.R. designed the study and wrote the manuscript. E.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. A preliminary form of this work was presented as an abstract at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, PA, 8–12 June 2012.
Footnotes
Clinical trial reg. no. NCT01672632, clinicaltrials.gov.
A slide set summarizing this article is available online.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–497 [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 2012;8:228–236 [DOI] [PubMed] [Google Scholar]
- 3.Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab 2010;21:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 5.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome—an allostatic perspective. Biochim Biophys Acta 2010;1801:338–349 [DOI] [PubMed] [Google Scholar]
- 6.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012;148:852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 2007;92:1023–1033 [DOI] [PubMed] [Google Scholar]
- 8.Lê KA, Mahurkar S, Alderete TL, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-κB stress pathway. Diabetes 2011;60:2802–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klöting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 2010;299:E506–E515 [DOI] [PubMed] [Google Scholar]
- 11.Karelis AD, Faraj M, Bastard JP, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 2005;90:4145–4150 [DOI] [PubMed] [Google Scholar]
- 12.O’Connell J, Lynch L, Hogan A, Cawood TJ, O’Shea D. Preadipocyte factor-1 is associated with metabolic profile in severe obesity. J Clin Endocrinol Metab 2011;96:E680–E684 [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki Y, Mahankali A, Matsuda M, et al. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care 2001;24:710–719 [DOI] [PubMed] [Google Scholar]
- 14.Redman LM, Heilbronn LK, Martin CK, et al. Pennington CALERIE Team Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE 2009;4:e4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heilbronn LK, de Jonge L, Frisard MI, et al. Pennington CALERIE Team Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 2006;295:1539–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bray GA, Smith SR, de Jonge L, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA 2012;307:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder WSCM, Nasset ES, Karhausen LR, Howells GP, Tipton IH. Report of the Task Group on Reference Man International Commission on Radiological Protection publication no. 23. Oxford, United Kingdom, Elsevier, 1975 [Google Scholar]
- 18.Larson-Meyer DE, Newcomer BR, Hunter GR. Influence of endurance running and recovery diet on intramyocellular lipid content in women: a 1H NMR study. Am J Physiol Endocrinol Metab 2002;282:E95–E106 [DOI] [PubMed] [Google Scholar]
- 19.Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA 2001;12:141–152 [DOI] [PubMed] [Google Scholar]
- 20.Rico-Sanz J, Thomas EL, Jenkinson G, Mierisová S, Iles R, Bell JD. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by (1)H-MRS. J Appl Physiol (1985) 1999;87:2068–2072 [DOI] [PubMed] [Google Scholar]
- 21.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 1997;129:35–43 [DOI] [PubMed] [Google Scholar]
- 22.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 1999;48:1600–1606 [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 24.Lillioja S, Bogardus C. Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev 1988;4:517–540 [DOI] [PubMed] [Google Scholar]
- 25.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 1975;35:609–616 [PubMed] [Google Scholar]
- 26.Kissebah AH, Vydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982;54:254–260 [DOI] [PubMed] [Google Scholar]
- 27.Hirsch J, Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res 1968;9:110–119 [PubMed] [Google Scholar]
- 28.Pasarica M, Xie H, Hymel D, et al. Lower total adipocyte number but no evidence for small adipocyte depletion in patients with type 2 diabetes. Diabetes Care 2009;32:900–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam CS, Covington JD, Bajpeyi S, et al. Weight gain reveals dramatic increases in skeletal muscle extracellular matrix remodeling. J Clin Endocrinol Metab 2014;99:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000;43:1498–1506 [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Eliasson B, Smith U, Cushman SW, Sherman AS. The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetic patients. Obesity (Silver Spring) 2012;20:932–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kursawe R, Eszlinger M, Narayan D, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes 2010;59:2288–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravussin E, Swinburn BA. Metabolic predictors of obesity: cross-sectional versus longitudinal data. Int J Obes Relat Metab Disord 1993;17(Suppl. 3):S28–S31; discussion S41–S42 [PubMed] [Google Scholar]
- 34.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002;32(Suppl. 3):14–23 [DOI] [PubMed] [Google Scholar]
- 35.Kraegen EW, Cooney GJ, Turner N. Muscle insulin resistance: a case of fat overconsumption, not mitochondrial dysfunction. Proc Natl Acad Sci U S A 2008;105:7627–7628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 2002;277:50230–50236 [DOI] [PubMed] [Google Scholar]
- 37.Varma V, Yao-Borengasser A, Rasouli N, et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab 2009;296:E1300–E1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marques BG, Hausman DB, Martin RJ. Association of fat cell size and paracrine growth factors in development of hyperplastic obesity. Am J Physiol 1998;275:R1898–R1908 [DOI] [PubMed] [Google Scholar]
- 39.Sun K, Scherer PE. Adipose Tissue Dysfunction: A Multistep Process. Berlin, Germany, Springer-Verlag Berlin Heidelberg, 2010 [Google Scholar]