Abstract
OBJECTIVE
Blood pressure (BP) control for renal protection is essential for patients with type 2 diabetes. Our objective in this analysis of Veterans Affairs Diabetes Trial (VADT) data was to learn whether on-study systolic BP (SBP), diastolic BP (DBP), and pulse pressure (PP) affected renal outcomes measured as albumin-to-creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR).
RESEARCH DESIGN AND METHODS
The VADT was a prospective, randomized study of 1,791 veterans with type 2 diabetes to determine whether intensive glucose control prevented major cardiovascular events. In this post hoc study, time-varying covariate survival analyses and hazard ratios (HR) were used to determine worsening of renal outcomes.
RESULTS
Compared with SBP 105–129 mmHg, the risk of ACR worsening increased significantly for SBP 130–139 mmHg (HR 1.88 [95% CI 1.28–2.77]; P = 0.001) and for SBP ≥140 mmHg (2.51 [1.66–3.78]; P < 0.0001). Compared with a PP range of 40–49 mmHg, PP <40 was associated with significantly lowered risk of worsening ACR (0.36 [0.15–0.87]; P = 0.022) and PP ≥60 with significantly increased risk (2.38 [1.58–3.59]; P < 0.0001). Analyses of BP ranges associated with eGFR worsening showed significantly increased risk with rising baseline SBP and an interaction effect between SBP ≥140 mmHg and on-study A1C. These patients were 15% more likely than those with SBP <140 mmHg to experience eGFR worsening (1.15 [1.00–1.32]; P = 0.045) for each 1% (10.9 mmol/mol) A1C increase.
CONCLUSIONS
SBP ≥130 mmHg and PP >60 mmHg were associated with worsening ACR. The results suggest that treatment of SBP to <130 mmHg may lessen ACR worsening. The interaction between SBP ≥140 mmHg and A1C suggests that the effect of glycemic control on reducing progression of renal disease may be greater in hypertensive patients.
Introduction
Control of blood pressure (BP) in patients with type 2 diabetes is an essential treatment goal to prevent the onset and progression of nephropathy and the associated morbidity and mortality (1–3). Nephropathy, a destructive microvascular complication of diabetes, encompasses persistent albuminuria, chronic kidney disease (CKD), arterial hypertension, and eventually end-stage renal failure (2,3). The optimal levels for systolic BP (SBP), diastolic BP (DBP), and pulse pressure (PP) for renal and cardiovascular protection are less certain (1,3–10). In the UK Prospective Diabetes Study (UKPDS), BP control was twice as effective as glucose control in preventing any diabetes end points including nephropathy (11,12). In the Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation (ADVANCE) trial, active BP treatment reduced the risk for renal events by 21% with SBP as low as 110 mmHg regardless of the baseline BP (13). A recent meta-analysis of trials treating BP in patients with type 2 diabetes noted that SBP levels <130 mmHg decreased risk of stroke but did not add benefit regarding renal event risk (14). The Action to Control Cardiovascular Risk in Diabetes-BP (ACCORD-BP) trial results analyzed for microvascular outcomes showed that intensive BP (mean SBP 119.3 mmHg) versus standard BP (mean 133.5 mmHg) lead to a 16% reduction in microalbuminuria but no reductions in either macroalbuminuria or renal failure (15). The Joint National Committee (JNC)-8 recommends treatment of BP in patients with diabetes to a target of <140/<90 mmHg, and the current American Diabetes Association (ADA) target is <140/<80 mmHg (9,16). There is uncertainty as to how far the SBP and DBP can be lowered safely for renal protection (3,7,9,15,16).
The Veterans Affairs Diabetes Trial (VADT) was a prospective, randomized study of 1,791 veterans with type 2 diabetes. The primary goal was to determine whether intensive glucose control prevented major cardiovascular disease events while BP and other risk factors were controlled equally in both glycemic treatment groups (17). An analysis of risk factors and renal outcomes in the VADT has previously been published (2). This report differs from the previous study in three aspects. First, the current study focuses on two renal risk factors, BP and glycemic control, while the previous work included a more global examination of risk factors. Second, the current study includes baseline as well as on-study (time-varying) parameters, while the risk factors examined in the previous publication were exclusively baseline. Finally, analyses of PP associations are included. The objectives of the current study were as follows: 1) to determine whether baseline and on-study SBP, DBP, and PP were associated with renal outcomes and 2) to ascertain whether there was an interaction between on-study BP variables and on-study A1C.
Research Design and Methods
The design of VADT and the results have previously been reported (17,18). In this trial, lipids, diet, and lifestyle were treated identically in both the intensive and standard glycemic treatment arms. The VADT was associated with attenuation of worsening albuminuria in the intensive glycemic treatment group of patients during the trial (17). The initial results and effects of BP on the cardiovascular outcomes and renal outcomes were published recently (1,2). Baseline characteristics of subjects available for the current analyses for worsening albumin-to-creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) are presented in Table 1. All who entered the trial with new or treated hypertension were given stepped treatment to maintain BP <130/80 mmHg, which was the ADA target at the time. After starting with ACE inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), the following agents were added as needed: diuretics, cardioselective β-blockers, calcium channel blockers, clonidine, hydralazine and minoxidil. The primary outcome was the time from randomization to the first occurrence of myocardial infarction, stroke, congestive heart failure, surgery for vascular disease, inoperable coronary disease, amputation for ischemic gangrene, or cardiovascular disease death. This study was approved by the institutional review boards of each participating site, and all subjects gave informed consent.
Table 1.
Baseline characteristics by standard and intensive glycemic control treatment groups for the subjects available for analyses of worsening ACR and eGFR
| Standard |
Intensive |
P |
|
|---|---|---|---|
| ACR subjects | |||
| A1C, % (mmol/mol) | 9.37 ± 1.48 (700) (79 ± 16.2) | 9.38 ± 1.44 (686) (79 ± 15.7) | <0.0001 |
| SBP, sitting, mmHg | 130.80 ± 16.41 (697) | 131.20 ± 15.64 (683) | <0.0001 |
| DBP, sitting, mmHg | 76.05 ± 10.11 (697) | 75.64 ± 10.05 (683) | <0.0001 |
| PP, mmHg | 54.77 ± 13.23 (697) | 54.46 ± 13.22 (683) | <0.0001 |
| No albuminuria (n) | 461 | 437 | |
| Microalbuminuria (n) | 239 | 249 | |
| ACEI or ARB (n/n) | 465/48 | 482/43 | |
| eGFR subjects | |||
| A1C, % (mmol/mol) | 9.42 ± 1.54 (842) (79 ± 16.8) | 9.38 ± 1.46 (826) (79 ± 16.0) | <0.0001 |
| SBP, sitting, mmHg | 131.70 ± 16.81 (837) | 131.20 ± 16.22 (824) | <0.0001 |
| DBP, sitting, mmHg | 76.09 ± 10.23 (837) | 75.81 ± 10.13 (824) | <0.0001 |
| PP, mmHg | 55.62 ± 13.81 (838) | 55.38 ± 13.83 (823) | <0.0001 |
| Stage 1 CKD (n) | 345 | 348 | |
| Stage 2 CKD (n) | 418 | 400 | |
| Stage 3 CKD (n) | 80 | 79 | |
| ACEI or ARB (n/n) | 577/51 | 547/59 |
Data are means ± SD (n) unless otherwise indicated.
Definition of BP Categories
The previous ADA and JNC-7 target BP defined the desirable BP measurements (8,19). For this analysis, we divided BP and PP into the following categories: SBP <105 mmHg, 105–129 mmHg as the standard comparison group, 130–139 mmHg, and ≥140 mmHg; DBP <70 mmHg, 70–79 mmHg as the standard comparison group, and ≥80 mmHg; and PP (calculated as SBP − DBP) <40 mmHg, 40–49 mmHg as the comparison group, 50–59 mmHg, and ≥60 mmHg (6).
Worsening of Renal Outcomes
The worsening of renal outcomes was determined using ACR and eGFR measurements. Baseline ACR measurements were categorized as follows: 0–29 mg/g, no albuminuria; 30–300 mg/g, microalbuminuria; and >300, macroalbuminuria. These values were compared with each subsequent on-study ACR measures that were monitored yearly. Worsening albuminuria was defined as an increase in the category of albuminuria at follow-up visits without reversion to an improved level (e.g., from no albuminuria to either micro- or macroalbuminuria or from microalbuminuria to macroalbuminuria). The time of worsening was taken when the patient started the worsening of ACR. Baseline eGFR measurements, calculated according to the MDRD equation (20), were used to categorize patients as follows: eGFR >90 mL/min/1.73 m2 = CKD stage 1, 60–89 mL/min/1.73 m2 = CKD stage 2, 30–59 mL/min/1.73 m2 = CKD stage 3, 15–29 mL/min/1.73 m2 = CKD stage 4, and <15 mL/min/1.73 m2 = CKD stage 5. The baseline eGFR value for each patient was compared with the subsequent quarterly (every 3 months) recordings of eGFR. If the measurement during the follow-up period was worse than the baseline eGFR by increasing at least one stage and persisted from that time forward, that patient was considered to have the outcome of persistent worsening of eGFR and/or progression of CKD. The first time at which the measurement was worse than the baseline value was used as the time to the first event for both ACR and eGFR.
Statistical Analysis
For baseline data, a two-tailed t test was used for comparison between the intensive and standard glycemic treatment groups. Cox proportional hazard models were performed to assess on-study BP and A1C interaction as a predictor of time to the first worsening of each ACR and eGFR renal outcome separately. Baseline-only measurements of A1C, SBP, DBP, and PP were used as covariates for all models. These models were useful in determining which level of baseline BP was predictive of the time to the first renal outcome. Both baseline variables and quarterly BP measures were used as on-study covariates in proportional hazard models, including the subgroup analyses of the risk associated with separate SBP, DBP, and PP. For the on-study analyses, any missing BP data were imputed with the last observation carried forward. The Cox proportional hazard models were used to test on-study SBP, DBP, and PP as continuous variables with ACEI and ARB use as on-study covariates to determine their potential contributions to time to the first worsening of each ACR and eGFR outcome separately. Two BP models were tested to determine whether there were any interaction effects between on-study covariates: 1) a two-way interaction effect between on-study A1C and on-study variable of SBP with three dummy variables for the four SBP categories and between on-study A1C and the on-study variable of DBP with two dummy variables for three DBP categories and 2) a two-way interaction effect between on-study A1C and on-study PP with three dummy variables for four PP categories, compared with the standard PP category, 40–49 mmHg. When no interaction was found between on-study A1C and any on-study BP or any of the on-study BP categories, we reduced the model with an interaction variable beginning from the highest P value until any interaction had a P value <0.05. However, all the main effects (on-study A1C and on-study BP) and the baseline covariates would still remain in the model selection procedure regardless of P values.
The derived effects of BP on the risk of an event were an estimate of the effect of the BP level at the time of a given worsening of renal event. Hazard ratios (HRs), 95% CIs, and P values are reported. HRs are interpreted as percent increased risk compared with the given reference category for categorical models. All analyses were done with a significance level of 0.05, using SAS, version 9.2, for Windows (SAS Institute, Cary, NC).
Results
At baseline, the mean ± SD age was 60.4 ± 8.7 years, BMI 31 ± 4 kg/m2, A1C 9.4 ± 1.5% (79 ± 16.4 mmol/mol), and diabetes duration 11.5 ± 7.5 years. Forty percent of patients had prior CVD events. Mean BP overall in the population analyzed for this study was 131/76 mmHg. The cohort entering VADT had near-optimal mean BP. This was further reduced within 1 year and was maintained below target for up to 7 years with added BP treatment (1,17). Though there were statistical differences between the two treatments in all baseline variables (SBP, DBP, A1C, and PP), the differences were clinically negligible (Table 1). However, these baseline variables were controlled for the on-study analyses.
At baseline, 65% (898) of patients had no albuminuria and 35% (488) had microalbuminuria for a total of 1,386 patients available for analysis of ACR worsening (Table 1). Patients with macroalbuminuria were not included because the ACR could not worsen based on our definition for the analyses. Of these patients, 68% (947) were taking ACEI, and 7% (91) were taking an ARB agent (Table 1). In the main VADT outcome results (17), 4.7% of the patients progressed from normal urine albumin to microalbuminuria (which would represent 36 patients in our analysis), and 0.4% progressed from microalbuminuria to macroalbuminuria (representing two patients in our group analyzed), with no significant differences between the intensive and standard glycemic control groups.
For the study of eGFR worsening, at baseline 41.5% (693) had stage 1 CKD, 49% had stage 2 (818), and 9.5% had stage 3 (159) for a total of 1,670 patients eligible for analysis. No patients had stages 4 or 5 CKD at baseline (17). Of these patients, 67% (1,124) were taking ACEI and 7% (110) were taking an ARB agent (Table 1). In our main VADT outcomes report, 8.8% of patients progressed to a doubling of serum creatinine, with 1.4% rising to >3.0 mg/dL and 1% progressing to stage 5 CKD. Again, there were no significant differences between the intensive and standard glycemic control groups for these items, but any increase in albuminuria was significantly lower (P = 0.05) in the intensive treatment group (17).
Relationship of On-Study SBP, DBP, and PP Categorical Variables With Renal Outcomes
To further characterize which BP and PP ranges might be associated with significant risks for worsening renal outcomes, we selected the ranges of separate SBP, DBP, and PP measurements to evaluate, as shown in Tables 2 and 3. ACR worsening with categorical SBP in a multivariate analysis was significant for SBP 130–139 (HR 1.88, P = 0.001) and ≥140 mmHg (HR 2.51, P < 0.0001). Categorical DBP levels were not associated with ACR worsening. There were no significant interaction effects with on-study A1C, but on-study A1C was significantly associated with worsening ACR (HR 1.19, P = 0.0002) (Table 2). Compared with a PP range of 40–49 mmHg, ACR worsening with categorical PP was significantly attenuated in those with PP <40 mmHg (HR 0.36, P = 0.022) and was accelerated significantly at PP ≥60 mmHg (HR 2.38, P < 0.0001). There were no significant interaction effects for worsening ACR with PP and A1C, but the time-varying A1C was significant (HR 1.22, P < 0.0001) (Table 3). There was no association with DBP.
Table 2.
HRs for worsening ACR and eGFR with SBP and DBP categories relative to each reference BP category and A1C used in the multivariate analyses
| ACR | eGFR | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| BP, mmHg | ||||||
| DBP 70–79 | Reference | Reference | ||||
| DBP <70 | 1.08 | 0.74–1.57 | 0.687 | 1.01 | 0.80–1.26 | 0.959 |
| DBP ≥80 | 1.03 | 0.69–1.55 | 0.887 | 0.96 | 0.73–1.26 | 0.763 |
| SBP 105–129 | Reference | Reference | ||||
| SBP <105 | 0.59 | 0.23–1.47 | 0.257 | 1.22 | 0.83–1.81 | 0.315 |
| SBP 130–139 | 1.88 | 1.28–2.77 | 0.001 | 1.08 | 0.84–1.38 | 0.566 |
| SBP ≥140 | 2.52 | 1.66–3.78 | <0.0001 | 0.42 | 0.13–1.31 | 0.135 |
| A1C | 1.19 | 1.09–1.31 | 0.0002 | 0.91 | 0.85–0.98 | 0.017 |
| A1C X† ≥140 mmHg | — | — | — | 1.15 | 1.00–1.32 | 0.045 |
| A1C baseline | 1.06 | 0.96–1.16 | 0.247 | 1.06 | 0.99–1.13 | 0.091 |
| SBP baseline | 1.00 | 0.99–1.01 | 0.792 | 1.01 | 1.00–1.02 | 0.008 |
| DBP baseline | 0.99 | 0.97–1.01 | 0.385 | 1.00 | 0.98–1.01 | 0.507 |
The total number of participants available for the final model of worsening ACR was 1,374, and for that of worsening eGFR, the number was 1,649. All variables are on study except baseline covariates.
†X represents the interaction between on-study A1C and the on-study SBP noted. Boldfaced text indicates data with statistically significant results.
Table 3.
HRs for worsening ACR and eGFR with PP categories relative to the reference category chosen from multivariate analyses that included baseline SBP, DBP, and A1C and on-study A1C among the covariates
| ACR |
eGFR |
|||||
|---|---|---|---|---|---|---|
| HR |
95% CI |
P |
HR |
95% CI |
P |
|
| PP, mmHg | ||||||
| 40–49 | Reference | Reference | ||||
| <40 | 0.36 | 0.15–0.87 | 0.022 | 1.02 | 0.71–1.45 | 0.934 |
| 50–59 | 1.11 | 0.71–1.72 | 0.651 | 1.00 | 0.76–1.30 | 0.986 |
| ≥60 | 2.38 | 1.58–3.59 | <0.0001 | 1.11 | 0.85–1.45 | 0.460 |
| A1C | 1.22 | 1.11–1.34 | <0.0001 | 0.95 | 0.89–1.01 | 0.105 |
| A1C baseline | 1.06 | 0.97–1.17 | 0.207 | 1.07 | 1.00–1.14 | 0.038 |
| SBP baseline, mmHg | 0.99 | 0.98–1.01 | 0.223 | 1.01 | 1.00–1.02 | 0.004 |
| DBP baseline, mmHg | 1.01 | 0.99–1.03 | 0.476 | 1.00 | 0.98–1.01 | 0.443 |
The total number of participants available for the final model of worsening ACR was 1,374, and for that of worsening eGFR, the number was 1,649. All variables are on study except baseline covariates. Boldfaced text indicates data with statistically significant results.
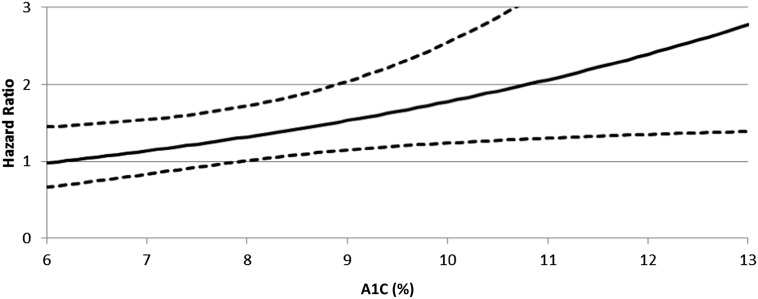
For worsening of eGFR, there was a significant interaction between SBP ≥140 mmHg and on-study A1C (HR 1.15, P = 0.045) (Fig. 1). On-study A1C also was significantly associated with worsening of eGFR (P = 0.017), as was baseline SBP (HR = 1.01, P = 0.008) in the multivariate analysis (Table 2). There were no significant associations or interaction effects with categorical DBP and eGFR worsening (Table 2). There also were not significant interactions or associations of categorical PP with eGFR worsening, although the baseline A1C (HR 1.07, P = 0.038) and baseline SBP (HR 1.01, P = 0.004) were significant in the on-study covariate survival analysis (Table 3).
Figure 1.
HR and 95% CIs for the interaction between on-study A1C (%) and on-study SBP ≥140 mmHg associated with worsening of the eGFR outcome. Baseline continuous values are fixed at mean values (DBP 75.95 mmHg, SBP 131.45 mmHg, A1C 9.4% [79 mmol/mol]) after controlling for all other on-study categorical BPs, which are held constant. When on-study A1C is ≥8% (64 mmol/mol), the 95% CIs are greater than an HR of 1, which indicates worsening of the eGFR outcome. Corresponding A1C, for each value starting with 6%, is as follows: 42, 53, 64, 75, 86, 97, 108, and 119 mmol/mol, respectively.
Relationship of On-Study SBP, DBP, and PP Continuous Variables With Renal Outcomes
We tested on-study SBP, DBP, and PP as continuous variables to evaluate their potential associations with ACR and eGFR worsening. We included on-study ACEI and ARB treatment to learn whether the use of these agents affected renal outcomes. SBP (HR 1.03, P < 0.0001) and PP (HR 1.03, P < 0.0001), but not DBP, showed significant risk for worsening of ACR. There was no significant increased risk of ACR worsening with on-study ACEI or ARB when evaluated with SBP, DBP, or PP as continuous variables. SBP (HR 1.01, P = 0.03) and PP (HR 1.01, P = 0.047), but not DBP, showed significant risk for worsening of eGFR. Again, there was no significant increased risk of eGFR worsening with on-study ACEI or ARB when evaluated with SBP, DBP, or PP as continuous variables. Of interest in this continuous variable analysis was that risk for worsening of eGFR was significant with baseline PP (HR 1.01, P = 0.03).
Conclusions
When the VADT BP data were analyzed with respect to the reference range BP (105–129/70–79 mmHg), SBP 130–139, ≥140 mmHg, and PP ≥60 mmHg were associated with increased risk for adverse renal outcomes for these patients with type 2 diabetes over time (on-study). The results underlined the need for treatment of type 2 diabetes patients with SBP ≥140 mmHg and into target range as recommended in ADA (<140/<80 mmHg) and JNC-8 (<140/<90 mmHg) BP guidelines (9,16). Current Kidney Disease: Improving Global Outcomes (KDIGO) guidelines also emphasize a lower target (≤130/≤80 mmHg) for patients with CKD and proteinuria (not on dialysis) and a higher target (≤140/≤90 mmHg) for patients with CKD without albuminuria (3). In our study, there was a step-wise increased risk of ACR worsening starting at SBP 130 mmHg and accelerating at ≥140 mmHg. There was a 19% increased risk for each 1% (10.9 mmol/mol) increase in on-study A1C, a result that suggests increasing A1C alone would contribute to worsening ACR. This increased risk occurred at a lower on-study SBP (130–139 mmHg) than the generally recommended target of <140 mmHg for people with diabetes (16). Thus, for example, a patient with SBP of 135 mmHg would be <140 mmHg but still at higher than optimal risk based on our analysis. This does not necessarily suggest that treating to SBP of <130 mmHg, and subsequent lowering of ACR, would decrease risk of advancing kidney failure. The recent Ongoing Telmisartan Alone and in Combination with Ramipril Global End point Trial (ONTARGET), the Aliskiren Trial in Type 2 Diabetes Using Cardiorenal End points (ALTITUDE), and the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial failed to demonstrate that intensified combination therapy of proteinuria with ACEI and ARB agents reduced renal risk beyond standard treatment with a single agent (21–23). Analogous to this, most of the VADT patients were taking an ACEI or an ARB, so it is not clear that further lowering of albuminuria with intensive glycemic control would provide additional renal benefit. In our study, there was no relationship of worsening of ACR to DBP (Table 2).
Worsening of ACR was attenuated in those with lower PP (<40 mmHg) and accelerated in those with higher PP (≥60 mmHg). The worsening of ACR with increasing PP likely represents effects from an increasing SBP, as there were no signals in the multivariate analyses associating categorical levels of DBP with significant risk for renal outcomes (Table 3). Given the age of our study population, the increased PP may represent arterial stiffening with increased SBP without elevation of the DBP as a major contributor to this finding. We identify a PP of ≥60 mmHg as a level of concern for increasing ACR in patients with type 2 diabetes.
Increased risk of worsening eGFR was related to on-study SBP when analyzed as a continuous variable and was associated with baseline SBP in the categorical analysis (Table 2). There was a 15% increased risk of worsening eGFR for each 1% (10.9 mmol/mol) increase in on-study A1C only in those with SBP ≥140 mmHg compared with SBP 105–129 mmHg (Table 2). This confirms data from the ACCORD-BP (15) and ADVANCE (24) trials in which there was no interaction between SBP and A1C in the setting of an SBP that was generally <140 mmHg. For example, in ACCORD-BP, the intensive glycemia arm patients who were in the intensive BP arm (mean SBP 119.3 mmHg) appeared to have a lower incidence of microalbuminuria and macroalbuminuria, but the interaction P value for these two interventions was not significant (15). However, in our study, if SBP was not well controlled to a level of ≤140 mmHg, on-study A1C appeared to be associated with greater renal impact. There was no relationship of worsening of eGFR to DBP, and there was no relationship between PP and eGFR as categorical variables, but the risk of eGFR worsening increased significantly when analyzed with on-study PP as a continuous variable (HR 1.01, P = 0.047).
Our results showed some similarities and substantial differences compared with the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) (7). This was a population-based study of 5,554 individuals of whom 5.8% (322) had type 2 diabetes. A number of these patients were followed up for ∼5 years after the baseline visit. Just as in this current VADT analysis, baseline SBP was associated with a decline in eGFR but was only significant when the DBP was <70 mmHg in the AusDiab trial (7). Similarly, there was no direct relationship between DBP and renal outcomes in AusDiab, as was the case in VADT. There were major differences, however. The AusDiab investigators reported that higher PP (defined as ≥61 mmHg) was a significant risk factor for eGFR decline over 5 years and that PP was not a significant risk factor for albuminuria (7), results that were the opposite of our VADT findings when analyzed as categorical variables. Several aspects of the studies may account for the discrepancies. First, the populations were different in that the AusDiab population consisted of approximately one-third women versus 2.9% of women in VADT. Second, the subgroup of patients with diabetes in AusDiab likely had less severe disease, since only 25–35% were taking antidiabetes medicines compared with 100% of patients on antidiabetes medicines in VADT. Finally, the AusDiab analysis used baseline PP, whereas the VADT analysis used on-study PP. Both studies did demonstrate an adverse effect of increased PP on a renal outcome, albeit baseline PP for worsening eGFR in AusDiab and on-study PP for worsening ACR in VADT. But, as mentioned earlier, the risk of eGFR worsening in our study increased significantly when analyzed with on-study SBP and PP as continuous variables. The differences in SBP and PP associations with worsening of eGFR in the continuous versus categorical analyses may be due to the slow progression of renal disease, especially in patients with relatively good baseline renal function. Decreased numbers of patients in the categorical groups may have contributed to the differences. Longer study lengths likely will be needed to detect expected increased risks for worsening eGFR in the categorical SBP and PP level groups.
Approximately 75% of our patients were taking either an ACEI (67–68%) or an ARB (7%). There were no significant increased risks of ACR or eGFR worsening with on-study ACEI or ARB when evaluated with SBP, DBP, or PP as continuous variables. It is reassuring that the extensive use of these agents did not appear to contribute to worsening of renal function that might occur when used in these patients. The potential attenuation of the progression of renal disease may be an added benefit with the use of ACEI or ARB agents in this group of patients.
Major strengths of our study include the well-characterized, large population with type 2 diabetes with frequent BP monitoring and adjustments for control, renal function monitoring, the longitudinal follow-up for up to 7 years on study, the inclusion of a larger percentage of minorities than most studies of this type, and the hypothesis-driven direction of the current analyses. A weakness was that this is a post hoc, retrospective analysis of patients who were not in a designed BP treatment trial. The results from this older, primarily male population may not be applicable to younger individuals and women.
Our results for categorical BP and PP values are in accord with our previous analysis in that baseline SBP, but not DBP, was related to a decline in eGFR (2). On-study SBP as a continuous variable showed significant risk for worsening of eGFR. Also, as in the previous report, baseline SBP and DBP were not related to worsening albuminuria. New findings were the association of a lower PP with attenuation of ACR worsening, and a PP >60 mmHg association with significant worsening of ACR, but not with worsening eGFR in a categorical PP analysis (Table 3). However, on-study and baseline PP studied in the continuous variable analyses were associated with significant worsening of eGFR. We cannot comment on potential beneficial lower limits of SBP based on our results. Our comparison SBP reference range was 105–129 mmHg, levels that were inclusive of the ACCORD-BP intensive treatment target of <120 mmHg (15). In ACCORD-BP, the achieved intensive mean SBP of 119.3 mmHg was associated with significant improvement in microalbuminuria (15). But the long-term renal benefit of treatment to such an SBP level is uncertain because the incident renal failure did not change compared to the standard BP group (mean SBP 133.5 mmHg) (15). Likewise, the simultaneous combination of intensive SBP control with intensive glycemic control failed to provide significant prevention of renal failure compared to standard BP control (15). Our results support strong consideration for treatment of higher levels of systolic hypertension for renal protection, with the level for increased risk for worsening ACR emerging at 130–139 mmHg, and a more critical SBP level of ≥140 mmHg for both ACR and eGFR worsening. The significant interaction of eGFR worsening with on-study A1C highlights the potential deleterious effects of an SBP level ≥140 mmHg in patients with poor glycemic control (Table 2).
Article Information
Acknowledgments. The authors thank the Veteran patients who volunteered for this study. Without their dedication, the VADT could not have been done. The authors acknowledge the data-management support of Derrick Kaufman, Ling Ge, Lizy Thottaparathu, Tamara Paine, and Barbara Christine, all at the Cooperative Studies Program Coordinating Center. The authors acknowledge the prolonged contributions of the co-chairmen of this cooperative study (Carlos Abraira, MD, and William C. Duckworth, MD).
Funding. This study was supported by the Veterans Affairs Cooperative Studies Program, Department of Veterans Affairs Office of Research and Development. Additional support was received from the American Diabetes Association and the National Eye Institute of the National Institutes of Health.
Duality of Interest. Pharmaceutical, other supplies, and financial assistance were provided by GlaxoSmithKline, Novo Nordisk, Roche Diagnostics, Sanofi, Amylin, and Kos Pharmaceuticals. N.V.E. discloses the receipt of honoraria from Merck.
The funding companies had no role in the design of the study, in the accrual or analysis of the data, or in the preparation of the manuscript.
Author Contributions. R.J.A. wrote the manuscript and researched data. G.D.B. researched and analyzed data, contributed to results, and reviewed and edited the manuscript. N.V.E. reviewed data and contributed to the manuscript writing and editing. J.B.M. researched data, contributed to the introduction, and edited the manuscript. W.C.D. contributed to analysis and reviewed the manuscript. R.J.A. and G.D.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
Footnotes
Clinical trial reg. no. NCT00032487, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0284/-/DC1.
A list of members of the VADT Study Group can be found in the Supplementary Data.
References
- 1.Anderson RJ, Bahn GD, Moritz TE, Kaufman D, Abraira C, Duckworth W, VADT Study Group Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care 2011;34:34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal L, Azad N, Emanuele NV, et al. Veterans Affairs Diabetes Trial (VADT) Study Group Observation on renal outcomes in the Veterans Affairs Diabetes Trial. Diabetes Care 2011;34:2090–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012;2:337–414 [Google Scholar]
- 4.Kovesdy CP, Bleyer AJ, Molnar MZ, et al. Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann Intern Med 2013;159:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rifkin DE, Sarnak MJ. How low can you go? Blood pressure and mortality in chronic kidney disease. Ann Intern Med 2013;159:302–303 [DOI] [PubMed] [Google Scholar]
- 6.Pastor-Barriuso R, Banegas JR, Damián J, Appel LJ, Guallar E. Systolic blood pressure, diastolic blood pressure, and pulse pressure: an evaluation of their joint effect on mortality. Ann Intern Med 2003;139:731–739 [DOI] [PubMed] [Google Scholar]
- 7.van den Hurk K, Magliano DJ, Alssema M, et al. Type 2 diabetes strengthens the association between pulse pressure and chronic kidney disease: the AusDiab study. J Hypertens 2011;29:953–960 [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 9.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–520 [DOI] [PubMed] [Google Scholar]
- 10.Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 12.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 13.de Galan BE, Perkovic V, Ninomiya T, et al. ADVANCE Collaborative Group Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 2009;20:883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation 2011;123:2799–2810, 9, 810 [DOI] [PubMed] [Google Scholar]
- 15.Ismail-Beigi F, Craven TE, O’Connor PJ, et al. ACCORD Study Group Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney Int 2012;81:586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 17.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 18.Abraira C, Duckworth W, McCarren M, et al. VA Cooperative Study of Glycemic Control and Complications in Diabetes Mellitus Type 2 Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications 2003;17:314–322 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Standards of medical care in diabetes—2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, et al. Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–254 [DOI] [PubMed] [Google Scholar]
- 21.Mann JFE, Schmieder RE, McQueen M, et al. ONTARGET investigators Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008;372:547–553 [DOI] [PubMed] [Google Scholar]
- 22.Parving H-H, Brenner BM, McMurray JJ, et al. ALTITUDE Investigators Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367:2204–2213 [DOI] [PubMed] [Google Scholar]
- 23.Fried LF, Emanuele N, Zhang JH, et al. VA NEPHRON-D Investigators Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–1903 [DOI] [PubMed] [Google Scholar]
- 24.Zoungas S, de Galan BE, Ninomiya T, et al. ADVANCE Collaborative Group Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: new results from the ADVANCE trial. Diabetes Care 2009;32:2068–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]