Abstract
The incidence and prevalence of diabetes mellitus have grown significantly throughout the world, due primarily to the increase in type 2 diabetes. This overall increase in the number of people with diabetes has had a major impact on development of diabetic kidney disease (DKD), one of the most frequent complications of both types of diabetes. DKD is the leading cause of end-stage renal disease (ESRD), accounting for approximately 50% of cases in the developed world. Although incidence rates for ESRD attributable to DKD have recently stabilized, these rates continue to rise in high-risk groups such as middle-aged African Americans, Native Americans, and Hispanics. The costs of care for people with DKD are extraordinarily high. In the Medicare population alone, DKD-related expenditures among this mostly older group were nearly $25 billion in 2011. Due to the high human and societal costs, the Consensus Conference on Chronic Kidney Disease and Diabetes was convened by the American Diabetes Association in collaboration with the American Society of Nephrology and the National Kidney Foundation to appraise issues regarding patient management, highlighting current practices and new directions. Major topic areas in DKD included 1) identification and monitoring, 2) cardiovascular disease and management of dyslipidemia, 3) hypertension and use of renin-angiotensin-aldosterone system blockade and mineralocorticoid receptor blockade, 4) glycemia measurement, hypoglycemia, and drug therapies, 5) nutrition and general care in advanced-stage chronic kidney disease, 6) children and adolescents, and 7) multidisciplinary approaches and medical home models for health care delivery. This current state summary and research recommendations are designed to guide advances in care and the generation of new knowledge that will meaningfully improve life for people with DKD.
Introduction
The incidence and prevalence of diabetes mellitus have grown significantly throughout the world, due primarily to the increase in type 2 diabetes. This increase in the number of people developing diabetes has had a major impact on the development of diabetic kidney disease (DKD) (1). Although kidney disease attributable to diabetes is referred to as DKD, diabetes and various kidney diseases are common chronic conditions. Thus, people with diabetes may have other etiologies of chronic kidney disease (CKD) in addition to diabetes. Notably, DKD remains one of the most frequent complications of both types of diabetes, and diabetes is the leading cause of end-stage renal disease (ESRD), accounting for approximately 50% of cases in the developed world. Although incidence rates for ESRD attributable to DKD have stabilized over the past few years (2), differences remain among high-risk subgroups. Middle-aged African Americans, Native Americans, and Hispanics continue to have higher rates of ESRD. These disparities in health care may be linked, in part, to the increasing rates of obesity and type 2 diabetes in youth, which disproportionately occur in these populations and allow for the development of diabetes complications earlier in life.
The overall costs of care for people with DKD are extraordinarily high, due in large part to the strong relationship of DKD with cardiovascular disease (CVD) and development of ESRD (3). For example, overall Medicare expenditures for diabetes and CKD in the mostly older (≥65 years of age) Medicare population were approximately $25 billion in 2011. At the transition to ESRD, the per person per year costs were $20,000 for those covered by Medicare and $40,000 in the younger (<65 years of age) group. Increased albuminuria and decreased glomerular filtration rate (GFR) are each independently and additively associated with an increase in all-cause and CVD mortality, and, in fact, most of the excess CVD of diabetes is accounted for by the population with DKD.
Due to both very high human and societal costs, the Consensus Conference on Chronic Kidney Disease and Diabetes was convened by the American Diabetes Association (ADA) in collaboration with the American Society of Nephrology (ASN) and the National Kidney Foundation (NKF). The objectives of convening the conference and publishing this consensus report were to address vital issues regarding patient care, highlighting current practices, gaps in knowledge, and new directions for improving outcomes in this high-risk population.
The major sponsoring organization (ADA) and conference leadership (K.R.T. and M.E.M.) chose major topic areas meeting these objectives based on recent publications, public health trends, and input from stakeholders representing professional, academic, clinical, industry, and patient groups. This report contains summaries of the topic areas based on the conference proceedings and feedback from participants. Major topic areas in DKD included 1) identification and monitoring, 2) CVD and management of dyslipidemia, 3) hypertension and use of renin-angiotensin-aldosterone system (RAAS) blockade and mineralocorticoid receptor (MR) blockade, 4) glycemia measurement, hypoglycemia, and drug therapies, 5) nutrition and general care in advanced-stage CKD, 6) children and adolescents, and 7) multidisciplinary approaches and medical home models for health care delivery.
This current state summary with research recommendations is designed to guide advances in patient care and the generation of new knowledge that will meaningfully improve life for people with DKD. This consensus conference and corresponding report are not all-inclusive of important considerations. For example, the topics of geriatrics, pregnancy, and kidney disease progression in DKD were not specifically addressed. However, these topics were comprehensively covered in the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines for diabetes and CKD and the evidence reviews and recommendations made therein remain germane (4).
Identification and Monitoring of DKD
Laboratory Assessment of DKD
Identifying and monitoring DKD relies upon assessments of kidney function, usually with an estimated GFR (eGFR) <60 mL/min/1.73 m2, and kidney damage, usually by estimation of albuminuria >30 mg/g creatinine. Widespread utilization of these simple laboratory measures has facilitated earlier recognition of DKD and has formed the basis for clinical staging. However, understanding the imprecision associated with these tests is critical to their appropriate utilization in clinical care.
Limitations of eGFR
Routine reporting of eGFR with serum creatinine concentration has been widely implemented. However, many clinicians and patients remain unaware of the uncertainty associated with GFR estimating equations. P30, the performance measure for estimating equations, is the likelihood that the eGFR is within ± 30% of the measured GFR. The P30 for the most commonly used estimating equations is generally between 80 and 90%. Thus, the eGFR has, at best, a 90% chance of being within 30% of the measured GFR. In addition, the characteristics of the existing estimating equations make them significantly less precise at higher GFRs. This is of particular concern early in the course of DKD, which may be associated with an elevated GFR (also called hyperfiltration) (5).
Hyperfiltration is thought to be a manifestation of increased intraglomerular capillary pressure and has been implicated in the development and progression of experimental nephropathy in diabetic rodents. Reduction in intraglomerular capillary pressure and single nephron GFR by RAAS blockade in these animal models formed the basis for subsequent clinical trials (6). However, the link between glomerular hyperfiltration and subsequent albuminuria or eGFR loss in humans has not been consistently confirmed. A meta-analysis suggested that there was a 2.7-fold increased risk for the development of “microalbuminuria” (30–300 mg/24 h, moderately increased) in those with prior hyperfiltration, but this increased risk was lost when the level of glycemia was taken into account (5). Studies using RAAS-blocking agents generally show an acute reduction in eGFR, which is thought to be due to a reduction in glomerular hyperfiltration (7). One post hoc analysis of a RAAS antagonist has shown a significant inverse relationship between reduction of eGFR at 6 months and subsequent rate of loss of eGFR (8). In other words, the greater the initial reduction in eGFR, the lower the rate of later eGFR loss. This finding needs confirmation in prospective studies.
Limitations of Albuminuria
Albuminuria is a marker for kidney/glomerular disease as well as for CVD risk and is often the first clinical indicator of the presence of DKD (9). It is a clinically useful tool for predicting prognosis and for monitoring response to therapy. Despite the strength of albuminuria as a risk biomarker for DKD and CVD outcomes, there are considerable limitations (Table 1). Importantly, not all people with DKD and reduced eGFR have increased albuminuria. In the UK Prospective Diabetes Study (UKPDS), 51% of those who developed an estimated creatinine clearance of <60 mL/min/1.73 m2 ever tested positive for albuminuria (10). Some, but not all, observational studies show that the rate of loss of GFR is slower in those type 2 diabetic patients with low or normal albuminuria (11,12).
Table 1.
Albuminuria: biomarker use and major limitations
| Biomarker use | Major limitations |
|---|---|
| DKD | Not sensitive |
| Higher albuminuria levels associate with faster eGFR decline | • Low eGFR present in half or more without increased albuminuria |
| Discordance between lowering albuminuria by treatment and clinical events | |
| CVD | Nonstandardized measurement and reporting |
| Independently predicts events and mortality | • Assays vary by ∼40% |
| • Variably reported as concentration, ratio to creatinine, or timed excretion | |
| Individual variability is large | |
| • Day-to-day variability ∼40% | |
| • Episodic increases with fever, urinary tract infection, exercise, congestive heart failure, hypertension, hyperglycemia, high-protein diet | |
| Categorical nomenclature does not reflect continuous nature of association with DKD and CVD risks | |
| • Moderately increased albuminuria (“microalbuminuria”) | |
| • Severely increased albuminuria (“macroalbuminuria”) |
The absence of albuminuria in persons with a reduced eGFR and diabetes raises the possibility of nondiabetic CKD. The NKF KDOQI Work Group for Diabetes and CKD concluded that the presence of retinopathy in patients with albuminuria >300 mg/g creatinine was strongly suggestive of DKD, and its absence in those with reduced eGFR and albuminuria <30–300 mg/g creatinine suggested nondiabetic CKD (4). These findings were confirmed in a recent meta-analysis (13). Recommendation 1.4 from the NKF KDOQI diabetes and CKD guidelines (Table 2) is particularly relevant for those with diabetes who have normal levels of albuminuria and an eGFR <60 mL/min/1.73 m2 (4).
Table 2.
Other cause(s) of CKD should be considered in the presence of any of the following circumstances
| • Absence of diabetic retinopathy; |
| • Low or rapidly decreasing GFR; |
| • Rapidly increasing proteinuria or nephrotic syndrome; |
| • Refractory hypertension; |
| • Presence of active urinary sediment; |
| • Signs or symptoms of other systemic disease; or |
| • >30% reduction in GFR within 2–3 months after initiation of an ACE inhibitor or ARB. |
Reproduced with permission from NKF (4).
Measurement of albuminuria is not standardized and demonstrates significant imprecision. The most common assays were compared with a recently developed isotope dilution mass spectrometry assay and varied by approximately 40% across albumin concentrations from 13 mg/L to 1,084 mg/L (14). Other barriers to the effective use of albuminuria in management of patients with diabetes include the nonstandardized reporting of results by clinical laboratories. Additionally, providers do not always understand how to interpret albuminuria results. Methods of assessment include the collection of urine specimens for albumin excretion rate over a specified time frame (typically 24 h) or the measurement of the urine albumin/creatinine ratio (ACR) in a spot collection, the latter being more commonly used because of patient convenience. Variation within individuals and studies may confound interpretation and risk assessment. There is considerable intraindividual daily variation in albuminuria. A coefficient of variation of 40% has traditionally been reported for those with type 1 diabetes and an ACR of 30–300 mg/g creatinine. Vagaries of study outcomes also cloud interpretation of albuminuria measurements. Examples include measurement of a single urine sample, collection at various times of the day, long periods between samplings, and measurement of only albumin concentration (10,15–18).
Albuminuria may also be increased by episodic hyperglycemia, high blood pressure (BP), high-protein diet, exercise, fever, urinary tract infection, and congestive heart failure. To the contrary, sustained regression of moderately increased albuminuria from the 30–300 mg/g creatinine range to the normal range was three times more likely in patients who had a hemoglobin A1c (A1C) <8.0%, systolic BP (SBP) <115 mmHg, and serum lipids in target (total cholesterol <198 mg/dL and triglycerides <145 mg/dL) than those who did not meet these targets (19). Overall, standardizing urine collection by correlating the patient’s clinical situation (glycemia, BP, lipids, etc.) with the number and timing of the samples is as important as the method of measurement and reporting of the albumin concentration. Recommendations from the ADA, NKF, and National Kidney Disease Education Program (NKDEP) support measuring albuminuria more than once and state that two of three samples should be elevated over a 3- to 6-month period for confirmation of a diagnosis of increased albuminuria (4,20–22).
Discordance between changes in albuminuria and kidney disease events has also been observed in a series of clinical trials. For example, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial in people with long-duration type 2 diabetes, intensive glycemic control resulted in significantly fewer individuals developing albuminuria at moderately increased levels (>30–300 mg/g creatinine) or severely increased levels (>300 mg/g creatinine) but increased the risk of doubling of serum creatinine (23). There was a reduction in both of these parameters in the intensive treatment arm of the UKPDS study in newly diagnosed patients, although the number of serum creatinine–doubling events was very few (10). Thus, it is possible that the timing of the intervention in terms of diabetes duration may be critical. Some complications such as DKD onset and progression may be more amenable to prevention in short- rather than long-duration diabetes. On the other hand, patients with type 1 diabetes in the intensive arm of Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC) had reductions in both albuminuria and their risk for developing CKD (defined as a sustained eGFR <60 mL/min/1.73 m2) (24).
The NKDEP Laboratory Working Group and the National Institute of Standards and Technology standardized the laboratory measurement of creatinine and are now collaborating with the International Federation of Clinical Chemistry and Laboratory Medicine to standardize the laboratory measurement and reporting of urine albumin. Reference methods and reference materials have been developed and are undergoing additional validation. However, even with standardization of serum creatinine and urine albumin measurements, residual imprecision of these biomarkers makes it likely that improved predictive tools will incorporate other biomarkers and patient characteristics. Until validated algorithms are available, clinicians are cautioned about predicting prognosis based on any single measurement of a particular biomarker, such as albuminuria. Serial monitoring of biomarkers is likely to reduce confounding “noise” and establish a temporal trend that may be more informative for prognosis. However, this approach has been challenged by the American College of Physicians, which recommended against monitoring albuminuria in patients with or without diabetes who are treated with RAAS antagonists (Grade: weak recommendation, low-quality evidence) (25).
It is clear that the relationship of albuminuria to ESRD and CVD risk is a continuum, starting from “normal” levels <30 mg/g creatinine. In this regard, there has been a trend to no longer refer to categorical nomenclature of “microalbuminuria” (30–300 mg/g creatinine) and “macroalbuminuria” (>300 mg/g creatinine). Instead, reporting the urine albumin level as a continuous variable (e.g., albumin excretion rate of XX mg/24 h or ACR of XX mg/g creatinine) may be preferred. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines have recently recommended a similar change for assessing CKD in general with albuminuria reported as normal to mildly increased (up to 30 mg/g creatinine), moderately increased (30–300 mg/g creatinine), or severely increased (>300 mg/g creatinine) and framed in the context of CKD stages 1 to 5 to determine risks (22).
Future Clinical Research
What are the reporting cutoffs for the definition of normal albuminuria and what is the proper nomenclature?
Should urine albumin results be reported as a continuous variable (i.e., eliminate “macro,” >300 mg/g creatinine, and “micro,” 30–300 mg/g creatinine, prefixes)?
Should there be sex-specific cutoffs that identify patients at increased risk of CVD as well as of progressive DKD?
Is there a practical strategy for screening patients that reduces intraindividual variability in ACR?
Can algorithms be developed to predict risk for progressive DKD, and which factors must be incorporated (e.g., eGFR, albuminuria, rate of change in eGFR or albuminuria, BP, new biomarkers)?
What is the role of albuminuria monitoring in guiding therapy?
Is there a strategy to target aggressive management on those patients at greatest risk of progressive DKD (e.g., patients on single-agent RAAS blockade and a rapidly declining eGFR of >5 mL/min/year)?
Can albuminuria be the primary end point in clinical trials to establish an evidence base for ongoing monitoring?
CVD and Management of Dyslipidemia
Cardiovascular Risks of DKD
Among patients with diabetes, those with kidney disease are consistently observed to have substantially elevated mortality rates (26). Much of this mortality is due to CVD, although noncardiovascular mortality is also increased. Albuminuria and eGFR are independently and additively associated with increased risks of CVD events, CVD mortality, and all-cause mortality (26). Both diabetes and CKD have been observed to have incidence rates of CVD events similar to patients with established coronary heart disease, leading to recommendations that patients with diabetes, CKD, or both should be treated for prevention of CVD as if they had already experienced such an event (27). In both type 1 and 2 diabetes, cohort studies suggest that increased risks of mortality and CVD are limited to patients who have evidence of DKD, and patients with normal levels of albuminuria and eGFR have risks similar to the general nondiabetic population (28–30). These observations suggest that treatment strategies focused on mitigating the high CVD risk of patients with DKD should be a high priority for improving diabetes outcomes.
While DKD may be in part a marker of systemic end-organ damage of diabetes, abundant evidence suggests that DKD may contribute to the pathogenesis of CVD. DKD may promote CVD through a number of pathways, including atherosclerosis, myocardial hypertrophy, cardiac fibrosis, and medial artery calcification, leading to myocardial infarction, stroke, congestive heart failure, sudden cardiac arrest, and peripheral vascular disease. The mechanisms through which DKD may promote CVD include augmentation of traditional cardiovascular risk factors (e.g., hyperglycemia, hypoglycemia, volume regulation and hypertension, lipoprotein metabolism, systemic inflammation, oxidative stress, and endothelial dysfunction) and initiation of mechanisms that are more specific to kidney disease (e.g., accumulation of small molecule toxins, anemia, and disordered mineral metabolism). Moreover, the presence of CKD may alter the risks and benefits of existing therapies targeting CVD in diabetes, including blood glucose control, BP control, lipid therapies, antiplatelet therapies, and coronary revascularization.
Dyslipidemia in DKD
DKD is accompanied by abnormalities in lipid metabolism related to decline in kidney function that varies depending on CKD stage. While LDL cholesterol is an established risk factor for CVD in the general population, its prognostic value appears to be less in those with CKD due to DKD or other causes (31). The magnitude of reduction in cholesterol levels in the CKD population (including those who are dialysis-treated) with statin therapy is similar to that in those with preserved kidney function (32). Clinical trials in nondialysis-dependent CKD suggest that CVD events and mortality are reduced with statins and statins/ezetimibe compared with placebo (32). The beneficial effects do not seem to be modified by the presence or absence of diabetes. While the CVD benefits of statins are well established, statins did not alter kidney disease progression in those with preexisting CKD (33). Thus, as recommended by the recently released KDIGO guidelines, statins are recommended for all diabetic patients with nondialysis-dependent CKD (34). It also recommends specific dosage for various statins in CKD population based on the dose used in the clinical trials (34). While dose titration is not recommended, follow-up measurements could, at a minimum, help assess adherence to statin therapy.
Clinical trials examining statins in the dialysis population consistently show no CVD or survival advantage, precluding recommendations for initiation of statins in dialysis patients. However, it is appropriate to consider continuing statin therapy in those who progress to treatment by chronic dialysis. Among kidney transplant recipients, an extension of the Assessment of Lescol in Renal Transplantation (ALERT) trial showed CVD benefit supporting statin use in this population (35). A meta-analysis examining data from over 50 trials (elevated creatine kinase levels, abnormal liver function tests, withdrawal from studies due to any adverse events) supports the safety of statins in CKD (32). Doses of statins used in clinical trials of CKD populations can be reasonably applied in practice. Despite the beneficial effects of statins, a significant proportion of the CKD population suffers from CVD events, providing an opportunity for study of other strategies to reduce risk. While post hoc analyses of clinical trials using fibrates in the general population showed benefits on CVD risk in the CKD (eGFR 30–59 mL/min/1.73 m2) population, further studies are warranted before widespread use of these agents in CKD is recommended (36). Another conundrum is that fibrates may elevate serum creatinine by effects independent of clearance by the kidneys, thus confounding eGFR estimates (37).
Future Clinical Research
How should we tailor existing common treatments for CVD risk reduction in the presence of DKD to increase safety, efficacy, or both?
Does follow-up measurement of plasma lipids after the initiation of a statin further reduce CVD risk in DKD by enhancing adherence, facilitating dose titration, or leading to the addition of other lipid-lowering agents?
Are there novel kidney-specific therapies that can be used to mitigate excess CVD risk in DKD?
Hypertension and Use of RAAS Blockade and MR Blockade
Hypertension
Based on the most recent Joint National Committee (JNC) 8 and KDIGO guidelines, BP levels in diabetes are recommended to be below 140/90 mmHg (38,39) in order to reduce CVD mortality and slow CKD progression. The support for these BP levels is derived from a limited number of randomized trials among patients with diabetes with a focus on CVD event outcomes. However, there are no randomized controlled trials of BP levels that examine CKD events. The data that support the BP level of <140/90 mmHg to slow CKD progression come exclusively from three randomized trials of non-DKD that include a participant mix of predominantly African Americans with hypertensive nephropathy, patients with IgA nephropathy, and patients with CKD without a specific diagnosis (40).
The relevance of this recommendation has been called into question based on data from 24-h BP monitoring studies that identified masked hypertension and failure of nocturnal dipping as confounders for the relationships between BP levels and CKD progression (41). There is a need for future studies to include a nested cohort, or subset of patients within a larger clinical trial, with an ambulatory BP monitoring evaluation. In this way, more complete information can be provided to interpret the effects of BP levels on clinical outcomes.
Another notable area that needs close consideration is monitoring of diastolic blood pressure (DBP) when treating SBP in those with DKD. While there are insufficient data to guide a lower limit for SBP in DKD, there is an adverse safety signal in clinical trials when DBP is treated to below 70 mmHg, and particularly below 60 mmHg, especially in older populations (42). Data from patients with stage 3 or later CKD demonstrate that DBP <60 mmHg is associated with higher incident rates of ESRD (43), while other studies in those without CKD found that <65 mmHg and/or 70 mmHg are associated with poor CVD outcomes (44). Data from the ongoing Systolic Blood Pressure Intervention Trial (SPRINT) will likely provide further information on low DBP in the context of treating to a target SBP of 120 mmHg in CKD, although it should be noted that the trial does not include people with diabetes (45).
RAAS Blockade
It is clear from a body of clinical trial data that interruption of the RAAS with either inhibition of the ACE or angiotensin receptor blocker (ARB) contributes to reductions in kidney disease events in those with stage 3 or later CKD who have severely increased albuminuria (previously termed “macroalbuminuria”), hypertension, and diabetes (46–48). Recently, there has been intense focus on whether combinations of these agents could further improve outcomes in DKD. To the contrary, data from studies testing this hypothesis found serious safety concerns, with two clinical trials being stopped prematurely due to risks of hyperkalemia and/or acute kidney injury as well as for futility, although the trials were underpowered for their primary outcomes (CKD and/or CVD events) at the time of termination (49,50). A considerable number of persons with DKD remain on RAAS combination therapy after many years of use in routine practice despite the clinical trial findings (49–51). If the use of agents for dual RAAS blockade is continued for those with DKD, caution and close monitoring for the status of serum potassium levels and kidney function are advised (52).
MR Blockade
The incorporation of MR blockade in combination with other RAAS inhibitors remains an area of great interest that has been explored in several short-term studies with a positive effect on albuminuria reduction in DKD (53). There was an increase in hyperkalemic episodes in those on dual therapy (49), and larger trials are needed, especially in light of the safety concerns with dual RAAS blockade employing other agents. Newer, nonsteroidal MR blockers are in phase 2 trials for CKD. In the meantime, there are clinical trial data in patients with systolic heart failure, with and without diabetes, showing a benefit of eplerenone on CVD outcomes with a low rate of hyperkalemia in the subset with stage 3 CKD (54).
CVD clinical trials of various RAAS inhibitors and/or their combinations have typically excluded patients with stages 3b and 4 CKD. As a result, efficacy and safety cannot be reliably assessed in the more advanced CKD subsets. The advent of new potassium binding agents such as patiromer (a polymer) and ZS-9 (an inorganic crystal) may allow further exploration of combined RAAS therapies that previously were limited by concerns about hyperkalemia. However, combination RAAS blockade therapies are also associated with an increased incidence of acute kidney injury and possibly other ischemic complications and, thus, cannot be recommended for CVD protection in people with CKD at present (49,50,52,55).
Emerging Antihypertensive Therapies
Phase 2 studies combining a selective endothelin receptor antagonist with RAAS therapy in DKD suggest that this may be a potential strategy for targeting further reductions in BP and albuminuria (56). However, the effect of this combination on CKD events remains to be determined and should be tested (57).
Device therapies for BP control have recently been the subject of intense interest. Renal denervation for BP reduction in patients with resistant hypertension has been intensively investigated (58,59). The latest clinical trial showed no benefit for BP control, and therefore, this approach to clinical management is not recommended in general or in CKD (59). Baroreflex activation therapy shows promise but is still experimental and under development (60).
Future Clinical Research
Are combination therapies for the control of BP safe and effective for reduction of kidney disease events in DKD?
Are MR blockade agents in the DKD population safe and effective for reducing kidney disease or CVD events?
Does combined RAAS inhibition with endothelin receptor antagonists reduce kidney disease events in DKD?
Glycemia Measurement, Hypoglycemia, and Drug Therapies
Glycemia Measurement
A1C has limitations in the general population and is even less precise in the setting of DKD (61). In the typical 120-day life cycle of a red blood cell, the A1C reflects time-averaged exposure to glucose. Accelerated red blood cell turnover is a major cause of imprecision of A1C. Erythrocyte survival times become shorter as eGFR falls, resulting in lower A1C. Glycation rate can also be influenced by temperature, acid–base balance, and hemoglobin concentration (62). Onset of anemia associated with advancing DKD is linked to deficiencies of iron, folate, and erythropoietin, each of which can influence A1C levels. Therapy with erythrocyte-stimulating agents lowers A1C further, perhaps due to rapid changes in hemoglobin concentrations (63,64).
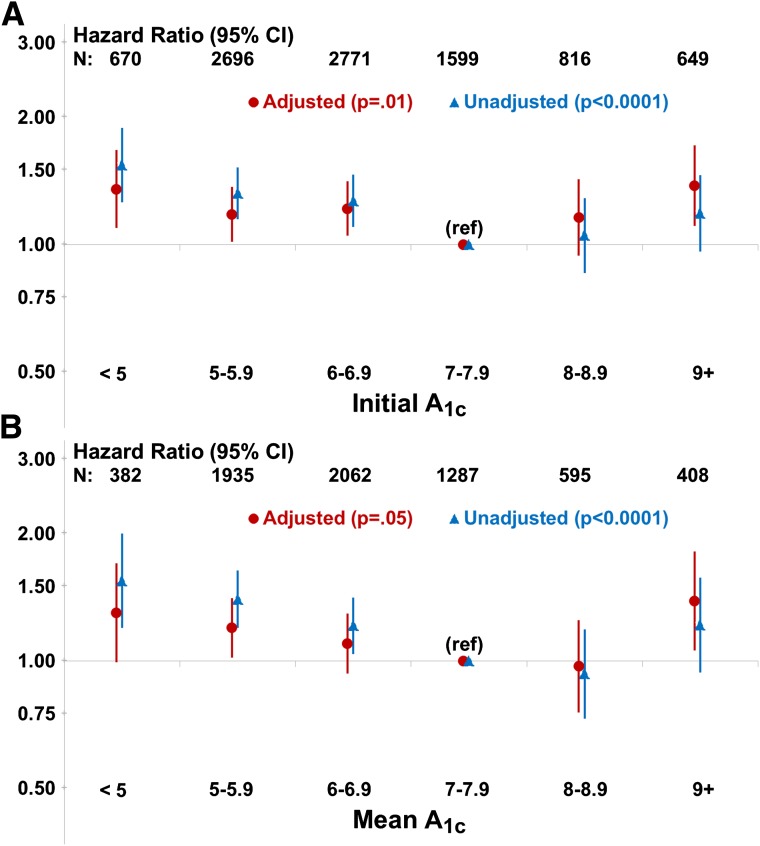
Patients with eGFR levels <60 mL/min/1.73 m2 are more prone to hypoglycemia. The reasons behind this association are multifactorial but include the prolonged action of hypoglycemic agents (particularly sulfonylureas and insulin), alcohol intake, chronic malnutrition, acute caloric deprivation, and the deficiency of gluconeogenic precursors as kidney function declines. In the ACCORD study, compared with patients with normal kidney function, those with baseline serum creatinine of 1.3–1.5 mg/dL had a 66% increased risk of severe hypoglycemia (defined as hypoglycemia requiring the assistance of another person) (65). There is a U-shaped relationship between A1C and mortality (Fig. 1), suggesting that hypoglycemia may be a reason for higher mortality in those with A1C levels <6.5% (66–68). However, there are other potential etiologies for higher mortality in this population with impaired kidney function. While A1C levels between 7–8% appear to be associated with the highest survival rates in retrospective analyses of DKD patients, the imprecision of A1C measurements makes specific targets for people with DKD difficult to define. However, measurement of A1C should still be performed, as the trending of the levels can assist in therapy decisions. Importantly, an A1C that is low or trending lower due to measurement imprecision and/or a reduction in kidney function may be taken as an indication of improved glycemic control when it is not. Rather, it may be an ominous sign of progressive DKD.
Figure 1.
Risk of mortality in patients with diabetes and ESRD. A: Risk of mortality by initial A1C, adjusted for age, sex, race, BMI, years of dialysis, albumin, creatinine, 10 comorbid conditions, insulin use, hemoglobin, HDL cholesterol, country, and study phase. B: Risk of mortality by mean A1C, adjusted for age, sex, race, BMI, years of dialysis, albumin, creatinine, 10 comorbid conditions, insulin use, hemoglobin, HDL cholesterol, country, and study phase. Reproduced with permission from ADA (68).
Serum fructosamine has been proposed as an alternate glycemic biomarker particularly in settings where A1C is less reliable. While fructosamine generally reflects the previous 2 to 3 weeks of glycemia, it also reflects total serum proteins that undergo glycation. Since the most abundant serum protein is albumin, hypoalbuminemia will result in low fructosamine levels. This is a major limitation in DKD patients. Similarly, serum levels of 1,5-anhydroglucitol, a sugar alcohol from the diet, are lost in the urine with glucose and are dependent on the kidney’s tubular threshold for glucose reclamation. Since this process is maximal at a blood glucose level of approximately 180 mg/dL, it will be lost in the urine in many diabetic patients. In particular, this test is not recommended for CKD stage 4 or 5 (69). Another emerging marker for glycemia is glycated albumin (GA), a ketoamine formed via nonenzymatic glycation of albumin reflecting average glycemia over 2 to 3 weeks. Unlike A1C and other glycemic biomarkers, GA is less affected by low eGFR, anemia, or other confounding conditions (70). Outcome studies are limited, but initial data suggest GA is associated with mortality and hospitalization (71). However, GA is not clinically available in the U.S. Notably, there are no clinical outcome studies assessing GA levels with microvascular or macrovascular complications in diabetes, and the relationship between GA and A1C is not linear. Therefore, GA levels cannot be extrapolated to corresponding A1C levels to assess risks of complications. Data are scant for continuous glucose monitoring (CGM) in people with either type 1 or 2 diabetes and low eGFR. However, given the high risk of hypoglycemia in this population, CGM is a potential tool for glycemic monitoring.
Given the limitations of the most frequently used glycemic biomarker, A1C, and the high risk of hypoglycemia, specific decisions on therapy should be based on self-monitoring of blood glucose (SMBG). Specific glycemic targets must consider overtreatment as well as undertreatment of blood glucose. Both preprandial and postprandial glycemic targets need to be individualized based on a patient’s knowledge and drug regimen, especially if it includes insulin. Blood glucose testing supplies need to be available in adequate quantities to allow sufficient monitoring to achieve therapeutic goals.
Drug Therapies
Hypoglycemia
Risk of hypoglycemia is increased in people with DKD when the eGFR is <60 mL/min/1.73 m2. This is partly due to decreased clearance of hypoglycemic agents and decreased gluconeogenesis by the kidney (72,73). Accordingly, dose adjustments are required for many hypoglycemic agents when used in people with DKD (Table 3). Insulin clearance decreases in parallel with a decline in eGFR (73–75). As is true with insulin use in general, frequent SMBG and appropriate patient-specific dose titration are critically important to achieve individual treatment goals and avoid hypoglycemia (73–75). Once patients are initiated on chronic dialysis treatment, exogenous insulin requirements often decline due to reduced insulin resistance on the one hand and emergence of malnutrition on the other (76). It should also be pointed out that older patients with DKD tend to progress to ESRD less commonly than younger patients (77), largely due to the competing risk of death from CVD (78). Older individuals also are at greater risk for hypoglycemia and for adverse consequences from hypoglycemia (79,80). Thus, greater care to avoid hypoglycemia is needed in the older patient with CKD and less stringent A1C targets of treatment are recommended (81).
Table 3.
Recommended dose adjustments for noninsulin antihyperglycemic agents in DKD
| Medication | In patients with impaired GFR | In dialysis patients |
|---|---|---|
| Biguanides Metformin | U.S. prescribing information states “do not use if serum creatinine ≥1.5 mg/dL in men, ≥1.4 mg/dL in women” | Contraindicated |
| British National Formulary and the Japanese Society of Nephrology recommend cessation if eGFR <30 mL/min/1.73 m2 | ||
| Second-generation sulfonylureas | ||
| Glipizide | No dose adjustment required | No dose adjustment required |
| Glimepiride | Initiate conservatively at 1 mg daily | Initiate conservatively at 1 mg daily |
| Glyburide | Avoid use | Avoid use |
| Meglitinides | ||
| Repaglinide | Initiate conservatively at 0.5 mg with meals if eGFR <30 mL/min/1.73 m2 | No clear guidelines exist |
| Nateglinide | Initiate conservatively at 60 mg with meals if eGFR <30 mL/min/1.73 m2 | No clear guidelines exist |
| TZDs | ||
| Pioglitazone | No dose adjustment required | 15–30 mg daily has been used (190) |
| α-Glucosidase inhibitors | ||
| Acarbose | Avoid if eGFR <30 mL/min/1.73 m2 | Avoid use |
| Miglitol | Avoid if eGFR <25 mL/min/1.73 m2 | Avoid use |
| GLP-1 receptor agonists | ||
| Exenatide | Not recommended with eGFR <30 mL/min/1.73 m2 | Avoid use |
| Liraglutide | Not recommended with eGFR <60 mL/min/1.73 m2 | Manufacturer does not recommend use (currently under study) |
| Albiglutide | No dose adjustment required | No clear guidelines exist—limited clinical experience in severe impairment of kidney function |
| DPP-4 inhibitors | ||
| Sitagliptin | 100 mg daily if eGFR >50 mL/min/1.73 m2 | 25 mg daily |
| 50 mg daily if eGFR 30–50 mL/min/1.73 m2 | ||
| 25 mg daily if eGFR <30 mL/min/1.73 m2 | ||
| Saxagliptin | 5 mg daily if eGFR >50 mL/min/1.73 m2 | 2.5 mg daily |
| 2.5 mg daily if eGFR ≤50 mL/min/1.73 m2 | ||
| Linagliptin | No dose adjustment required | No dose adjustment required |
| Alogliptin | 25 mg daily if eGFR >60 mL/min/1.73 m2 | 6.25 mg daily |
| 12.5 mg daily if eGFR 30–60 mL/min/1.73 m2 | ||
| 6.25 mg daily if eGFR <30 mL/min/1.73 m2 | ||
| Amylinomimetics | ||
| Pramlintide | No dose adjustment required with eGFR >30 mL/min/1.73 m2 | Avoid use |
| Not recommended with eGFR <30 mL/min/1.73 m2 | ||
| SGLT2 inhibitors | ||
| Canagliflozin | No dose adjustment required if eGFR ≥60 mL/min/1.73 m2 | Avoid use |
| 100 mg daily if eGFR 45–59 mL/min/1.73 m2 | ||
| Avoid use and discontinue in patients with eGFR <45 mL/min/1.73 m2 | ||
| Dapagliflozin | Avoid use if eGFR <60 mL/min/1.73 m2 | Avoid use |
Metformin
Metformin use is contraindicated, per current U.S. Food and Drug Administration (FDA) prescribing information, in men with a serum creatinine ≥1.5 mg/dL and in women with a serum creatinine ≥1.4 mg/dL. Metformin should also be used cautiously in patients with conditions that interfere with the metabolism and excretion of lactic acid, such as heart failure and liver disease, and during acute illness and/or instances of tissue hypoxia (82,83). Although lactic acidosis occurs in people with diabetes regardless of metformin use, the role of metformin per se in lactic acidosis is controversial at best. However, metformin may predispose to lactic acidosis in the event of serious intercurrent illness (84). Despite these concerns and published case reports (85), current data indicate the overall risk of metformin-associated lactic acidosis is low (86,87). It has been suggested that eGFR may be a more appropriate measure to assess continued metformin use considering that the serum creatinine level can translate into widely varying eGFR levels depending on race, age, and muscle mass (73). In turn, a recent review proposed metformin use should be reevaluated at an eGFR <45 mL/min/1.73 m2 with a reduction in maximum dose to 1,000 mg/day and discontinued when <30 mL/min/1.73 m2 (Table 4) (83). However, metformin should be discontinued in situations that are associated with a high risk of acute kidney injury, such as sepsis, hypotension, acute myocardial infarction, and use of radiographic contrast or other nephrotoxic agents.
Table 4.
Recommended dose adjustments for metformin based on eGFR
| eGFR (mL/min/1.73 m2) | Proposed action |
|---|---|
| ≥60 | No contraindication to metformin |
| Monitor kidney function annually | |
| <60 and ≥45 | Continue use |
| Increase monitoring of renal function (every 3–6 months) | |
| <45 and ≥30 | Prescribe metformin with caution |
| Use lower dose (e.g., 50%, or half-maximal dose) | |
| Closely monitor renal function (every 3 months) | |
| Do not start new patients on metformin | |
| <30 | Stop metformin |
Adapted with permission from ADA (83).
Sulfonylureas and Glinides
Sulfonylurea use in CKD requires careful attention to dosing to avoid hypoglycemia (73). Glyburide is extensively metabolized in the liver into several active metabolites that are excreted by the kidney and is not recommended for use in CKD (73,88). Glimepiride is associated with less hypoglycemia when compared with glyburide (89). Glipizide is metabolized by the liver into several inactive metabolites, and its clearance and elimination half-life are not affected by a reduction in eGFR (90), thus dose adjustments in patients with CKD are not necessary (91). Considering the inherent risk of hypoglycemia with sulfonylurea use, however, cautious use is warranted even with glipizide. Similar to the sulfonylureas, the main concern with repaglinide and nateglinide use in CKD is a potentially increased risk of hypoglycemia. Conservative initial doses of these agents are recommended since lower doses are typically needed in this population (73,92,93).
Thiazolidinediones
The thiazolidinediones (TZDs) are nearly completely metabolized by the liver (94–96). Despite the lack of a need for dosage adjustments in patients with CKD, TZD use is generally avoided in CKD due to side effects such as refractory fluid retention, hypertension, and increased fracture risk (73,97).
α-Glucosidase Inhibitors
The α-glucosidase inhibitors, acarbose and miglitol, are minimally absorbed from the gastrointestinal tract, yet plasma levels can increase in CKD (76). Therefore, caution is advised for use of these agents in diabetic patients with low eGFR (<30 mL/min/1.73 m2) (73).
Incretins
The prescribing information for exenatide recommends discontinuation with an eGFR <30 mL/min/1.73 m2. The kidneys are not a major pathway of elimination for liraglutide; however, its use is not recommended with an eGFR <60 mL/min/1.73 m2 due to a current lack of data in this population. GLP-1 receptor agonist use has been associated with postmarketing reports of decreased kidney function (98), yet such toxicity has not been observed in clinical trials or population-based observational studies to date (99–102). The majority of case reports of decreased kidney function with exenatide have involved at least one contributory factor such as congestive heart failure, pancreatitis, infection, and/or the use of concomitant medications such as diuretics, RAAS inhibitors, and nonsteroidal anti-inflammatory drugs (98).
The dipeptidyl peptidase-4 (DPP-4) inhibitors have potential advantages in people with CKD as they are associated with a low risk of hypoglycemia and are weight-neutral (103,104). All of the currently available DPP-4 inhibitors can be used in CKD, but sitagliptin, saxagliptin, and alogliptin require downward dose titration based on eGFR (105–108). Linagliptin, in contrast, does not require dose adjustment based on kidney function (109,110). A meta-analysis has shown that DPP-4 inhibitors appear to be especially effective in Asian people (111).
Sodium-Glucose Cotransporter 2 Inhibitors
There are currently two sodium-glucose cotransporter 2 (SGLT2) inhibitors available in the U.S.—canagliflozin and dapagliflozin. SGLT2 inhibitors improve glycemia by increasing disposal of glucose via the urine (112). Dapagliflozin is not recommended for use with an eGFR <60 mL/min/1.73 m2 as glycemic efficacy is negligible (113). Canagliflozin is recommended to be used at a reduced dose of 100 mg/day with an eGFR of 45–59 mL/min/1.73 m2 and is not recommended with an eGFR <45 mL/min/1.73 m2. SGLT2 inhibitors have been associated with an initial slight decrease in eGFR in clinical trials (114). This decrease in eGFR may be a hemodynamic effect to decrease glomerular hyperfiltration because eGFR trends back toward baseline with continued treatment (114,115). However, longer-term follow-up in larger groups of patients with diabetes and CKD is needed to confirm safety for kidney disease and other outcomes.
Future Clinical Research
What is the relationship between estimated average glucose and A1C and GA for individuals with advanced-stage CKD?
Can CGM improve our understanding of the frequency and impact of hypoglycemia in DKD?
What are ideal targets for glycemia based on biomarkers and direct glucose monitoring in DKD?
In a comparative effectiveness study, what is the effect of using different insulin and noninsulin regimens in patients with diabetes and CKD on glycemic control and hypoglycemic events?
Nutrition and General Care in Advanced-Stage CKD
Nutritional Therapy
For the goals of reducing DKD onset and progression, approaches to nutritional therapy are a subject of much debate. Extensive discussion of dietary management in diabetes and obesity is beyond the scope of this review. Instead, the focus is on extremes of macronutrient intake that have been associated with adverse outcomes, followed by assessment of concepts for healthful eating that is supported by clinical evidence relevant to DKD. It is well recognized that very low−protein diets can lead to protein malnutrition (116). Conversely, excessive protein intake is associated with increased albuminuria, more rapid kidney function loss, and CVD mortality (117–121). Likewise high-fat diets, defined as more than 30% of total calories, exacerbate hyperlipidemia and, therefore, can be inferred to increase CVD risk. An increasing body of evidence suggests that dietary pattern intake rather than a sole focus on individual nutrients may offer a more practical approach to dietary management of chronic diseases (122–124).
Dietary Protein and DKD
Both quantity and quality of protein and amino acids have been identified to be important for maintenance of adequate nutritional status in CKD, whether related to diabetes or other causes (125). Identification of optimal dietary protein intake is further complicated in DKD by the fact that kidney disease confers unique metabolic abnormalities that can include alterations in mineral metabolism, metabolic acidosis, anemia, vitamin D deficiency, loss of lean muscle mass, and susceptibility to malnutrition. The relationship of dietary protein to DKD prevention and progression has been widely debated for many years. Nutritional studies are inherently difficult to conduct and are subject to numerous limitations such as variable composition and adherence for study diets, multiple nutrients changed, different outcome measurements for kidney disease, small sample sizes, and short duration of studies.
Dietary protein reduction has produced variable findings across clinical trials (126–133). The effects of a low-protein (daily intake of 0.6 g protein/kg ideal body weight), low-phosphorus (500–1,000 mg/day) diet were compared with those of a control diet containing ≥1.0 g protein/kg ideal body weight per day and ≥1,000 mg phosphorus per day in 35 patients with type 1 diabetes and DKD. Study participants on the low-protein, low-phosphorus diet had a slower rate of decline in iothalamate GFR over the course of the study. Another study (134) evaluated a reduced-protein versus a usual-protein diet in 82 patients with type 1 diabetes and progressive DKD over 4 years. Actual protein intake during the follow-up period was 0.89 g/kg/day in the reduced-protein group and 1.02 g/kg/day in the usual-protein group. ESRD or death occurred in 10% of patients on the reduced-protein diet versus 27% of patients on the usual-protein diet (P = 0.042). The relative risk of death or ESRD after baseline adjustment for CVD and diabetes risk factors was 0.23 for patients on the reduced-protein diet (P = 0.01). A meta-analysis of nutrition studies evaluated 13 randomized controlled clinical trials and reported an overall effect of reduced-protein intake to slow GFR decline that was greater in diabetic than nondiabetic participants with evidence of a greater effect over time. To the contrary, similar benefits of a low-protein diet were not observed in 69 patients with either type 1 (n = 32) or type 2 (n = 37) diabetes and moderately to severely increased albuminuria on a low-protein (0.6 g/kg/day) diet or a “free” (nonstandardized) protein diet for 12 months (116). Other studies and meta-analyses have also reported negative results (127,135). However, there are many limitations of the previous studies, including combining type 1 and type 2 diabetic patients with varying stages of CKD, inconsistent concurrent management strategies (e.g., RAAS blockers), small sample sizes resulting in lack of statistical power, varying durations of intervention, lack of identification and uniformity of protein sources (e.g., plant versus animal) and other dietary components (fats, carbohydrates, phosphorus, and sodium), and incomplete assessment of dietary adherence.
Despite ongoing controversy, NKF KDOQI (4), KDIGO (22), and the ADA (20) provide clinical guidelines for dietary management of diabetes and CKD (4,20,22,136). The NKF KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease recommend a target protein intake of 0.8 g/kg body weight per day (the recommended daily allowance) for nondialysis-dependent DKD (Grade B evidence) (4). KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease also suggests a dietary protein intake of 0.8 g/kg/body weight per day in adults with diabetes and GFR <30 mL/min/1.73 m2 with appropriate nutritional education (Grade 2c evidence) (22). The ADA recommends “usual” (not high) dietary protein intake (Grade A evidence) (136). Both NKF KDOQI and KDIGO guidelines recommend avoidance of high levels of protein intake, defined as more than 20% of kcal from protein (4) or >1.3 g/kg/day of protein for individuals with CKD (22). Table 5 summarizes these recommendations along with those for other macronutrients for DKD.
Table 5.
Macronutrient recommendations in DKD
| Organization | Lower ranges of dietary protein intake | Higher ranges of dietary protein intake | Carbohydrate | Fatty acids | Sodium |
|---|---|---|---|---|---|
| KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease (22) | 0.8 g protein/kg/day in adults with diabetes and GFR <30 mL/min/1.73 m2 with appropriate education | Avoid protein intake >1.3 g/kg/day in adults with CKD at risk for progression; specific comment for DKD not provided | Specific recommendation not provided | Specific recommendation not provided | Lower salt intake to <2 g of sodium per day (5 g of sodium chloride), unless contraindicated |
| KDOQI 2007 Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease (4) | Recommended dietary allowance of 0.8 g/kg body weight per day for people with DKD and CKD stages 1–4 | Avoid high-protein diets defined as ≥20% of total daily calories | Specific recommendation not provided | Increase intake of omega-3 and omega-9 fatty acids | Reduction of intake 2.3 g/day as recommended by the DASH diet |
| ADA Standards of Medical Care in Diabetes—2014 (20) and Nutrition Therapy Recommendations for the Management of Adults With Diabetes (136) | Maintain usual level of dietary protein intake (136); approximated by reported studies surveying diet intake in people with diabetes to be approximately 16–18% of total calories (20) | Specific comment not provided | Specific recommendation for DKD not provided. For diabetes, include carbohydrates from vegetables, fruits, whole grains, legumes, and dairy products over intake from carbohydrates containing added sugar, fat, and sodium. Avoid beverages, products with high-fructose corn syrup, and sucrose | Total fat: individualized | Specific recommendation for DKD not provided |
| Omega-3: same recommendation as for general public. Cholesterol, saturated, trans fats: same as for general public | For individuals with diabetes, reduce sodium to <2,300 mg/day as recommended for the general public | ||||
| Mono- and polyunsaturated fats: integrated to comment regarding potential benefits of a Mediterranean diet pattern |
Carbohydrates and Fats
Whole-grain carbohydrates and fiber and fresh fruits and vegetables are recommended as part of a healthy diet for individuals with DKD (125,136). The number of portions and specific food selections from these food groups often need to be limited in advanced stages of CKD due to the potassium and phosphorus loads imposed by these foods (125). Carbohydrates are an important component of lower-protein calories. Whether a change in carbohydrate food selections will result in improvement in DKD outcomes is not known.
There is a growing body of literature suggesting beneficial effects of omega-3 fatty acids on albuminuria in DKD (137,138). However, definitive conclusions to support dietary recommendations are not yet available. The general recommendation for DKD is to include omega-3 and omega-9 fatty acids as part of total dietary fat intake while decreasing intake of saturated fats and food sources of trans fatty acids (4).
Sodium
Dietary sodium reduction in individuals with CKD has been shown to reduce BP irrespective of diabetes status. The recommended range of dietary sodium intake for individuals with kidney disease is 1,500–3,000 mg/day (Table 6). To accomplish this lower level of sodium intake, nutrition recommendations include increasing dietary intake of fresh cooked foods and reducing intake of fast foods and highly processed food products (125,136).
Table 6.
Approaches to incorporating diet patterns for diet management of DKD for type 1 and type 2 diabetes*
| Nutrient | Concept | How? | What? | Quantity |
|---|---|---|---|---|
| Protein | Explore/sample plant proteins | Incorporate vegan protein sources into meal plan, de-emphasize intake of fatty animal protein sources such as marbled red meats, poultry products with skin, shellfish | Dried beans and peas | Amount to maintain optimal glycemic control, as tolerated; maintain or obtain optimal nutritional status |
| Dairy products: emphasize nonfat and low-fat versions in diet, sample nondairy milk products | Legumes | |||
| Nuts and seeds | ||||
| Soy | ||||
| Quinoa | ||||
| Nonfat yogurts, milks, lower-fat cheese selections | ||||
| Include almond, rice, soy milk | ||||
| Carbohydrates: complex | Explore/sample | Include high-fiber, whole-grain products, de-emphasize refined white flour−based products | Whole/mixed-grain breads, pastas, cereals; wild, brown rice types | Within carbohydrate counting/diabetes management plan, as tolerated |
| Fruits and vegetables | High-fiber fruits/vegetables | Include as part of meals snacks and different formats such as smoothies | Fresh fruits and vegetables of choice, fresh cooked vegetables ideal, precooked choices available without seasonings | 6–8 servings per day as appropriate for meal plan and carbohydrate counting |
| Fat | Omega-9 and omega-3 fatty acids as a component of fat source | Enrich diet with olive oil, fish oil, and vegetarian sources of omega-3 fatty acids, de-emphasize saturated fat sources and generic vegetable oils that are enriched in omega-6 fatty acids | Include olive oil/canola oil−based margarines and fats, choose omega-3–enriched whole-grain breads and cereals when available | Within meal plan for calories and palatability |
| Sodium | Maximize approaches to lower sodium and salt intake | Reduce free salt use | Use sodium-free fresh and dried herbs, spices, and herbal blends, when available | 1,500–3,000 mg daily; transition toward lower range of intake |
| Use fresh cooked foods, purchase unseasoned options of foods, put sauces/flavorings on side | ||||
| Weight management | If overweight, work on weight reduction | Decrease calories, increase calorie utilization through a regular exercise program, avoid excessively high-protein diets (i.e., >20% kcal from protein) | Balanced proportions of protein, carbohydrate, and fat within individualized approach to maintain euglycemia | Based on individually determined ideal/healthy body weight, gradual weight loss toward goal to allow for altered eating pattern, ongoing modifications in diet as weight goal approached and glycemia management is modified |
Inclusion of vegan protein sources, complex carbohydrates, and increased intake of fruits and vegetables may increase serum levels of potassium and phosphorus in later stages of eGFR (i.e., GFR <30 mL/min/m2). Serum levels of these minerals will need to be monitored in those individuals.
Examining Dietary Patterns of Intake
Recent approaches to managing DKD apply dietary patterns that incorporate the above principles within whole diets. Both the Mediterranean (122) and Dietary Approaches to Stop Hypertension (DASH) diets (123) comprise an enhanced intake of whole-grain (complex, unrefined) carbohydrates, fruits, vegetables, and plant proteins, including nuts, seeds, and beans. Although fish is included in these diets, intake of other animal proteins and whole-fat dairy products is decreased compared with the Western diet (124). The Mediterranean diet also incorporates olive oil and red wine. Focusing on dietary patterns in conjunction with principles of healthy lifestyle management is a progressive approach to dietary management of DKD. Whether a healthy diet pattern will affect albuminuria, DKD progression, CVD outcomes, or weight management is unclear. However, the current Western dietary pattern, enriched in animal protein, fat (total and saturated), sodium, sugar, and calories, is strongly associated with many chronic diseases and exacerbation of risk factors (i.e., hypertension, obesity, CVD) (124). Patterns of eating that have been associated with improvement in BP, weight, other risk factors, and overall disease prevention can be incorporated into a diet for individuals with DKD (Table 6). It is important that individuals achieve and maintain adequate nutritional intakes of nutrients as well as a healthy BMI to enhance risk reduction and promote overall health.
General Care in Advanced-Stage CKD
Given the inherently progressive nature of CKD, people with DKD, if they survive through other complications of diabetic macro- and microvascular disease, often experience the advanced stages of CKD with their eGFR reaching values <30 mL/min/1.73 m2 (139). Kidney replacement therapy will be needed for these people to survive the ravages of uremia with a progressive worsening of kidney function. This section will briefly review certain, but not all, aspects of advanced DKD. How the choice of modality of treatment—hemodialysis, peritoneal dialysis, and transplantation—is made is beyond the scope of this discussion.
Type 2 diabetes is the leading cause of ESRD in the U.S. and many countries globally. Approximately half of the entire 450,000 dialysis patients in the U.S. have ESRD secondary to type 2 diabetes (140). These patients have a high prevalence of comorbid conditions, a high rate of hospitalization, a low health-related quality of life, as well as an excessively high mortality rate (15–20% per year), mostly because of CVD events (140). Observational studies in dialysis patients, including those with type 2 diabetes, have indicated the lack of a significant association between traditional CVD risk factors and mortality. The existence of a paradoxical or reverse association in which obesity, hypercholesterolemia, and hypertension appear to confer survival advantages has been described (141,142). The time discrepancy between the competing risk factors, i.e., overnutrition (long-term risks) versus undernutrition (short-term risks), may explain the overwhelming role of protein-energy wasting, inflammation, and cachexia in causing this so-called reverse epidemiology (143–146). Other comorbidities of advanced stages of CKD, such as secondary hyperparathyroidism, appear to have similar associations in diabetic and nondiabetic patients for complications, health care costs, and survival (147).
Glycemia and Mortality Risk in Dialysis Patients
The role of improved glycemic control in ameliorating the exceedingly high mortality risk of dialysis treatment and diabetes is unclear. The treatment of hyperglycemia in dialysis patients is challenging, given changes in glucose homeostasis, the questionable accuracy of glycemic control metrics, and the altered pharmacological properties of glucose-lowering drugs by kidney dysfunction, the uremic milieu, and the dialysis procedure, as previously discussed. Up to one-third of dialysis patients with type 2 diabetes experience falling glucose levels with A1C levels <6%. The causes and clinical implications of this observation have not been determined, although undernutrition and limited substrate availability are likely operative (139,148–150). Conventional methods of glycemic control assessment are confounded by the laboratory abnormalities and comorbidities associated with kidney failure. Similar to more recent approaches in the general population, there is concern that intensive glycemic control regimens aimed at glucose normalization may be harmful in diabetic dialysis patients. There is uncertainty surrounding the optimal glycemic target in this population, although recent epidemiologic data suggest that A1C ranges from 6–8% to 7–9% are associated with better survival rates (151). This association exists in both hemodialysis (152,153) and peritoneal dialysis patients with diabetes (154). Pretransplant glycemic control is also associated with posttransplant outcomes in kidney transplant recipients with diabetes (155).
New-Onset Diabetes After Transplantation
A clinically important and unique condition is the development of new-onset diabetes after transplantation (NODAT) (156). NODAT is defined as persistence of hyperglycemia (meeting criteria for diabetes) beyond initial hospitalization in transplanted patients without preexisting diabetes and occurs in 15–25% of patients who undergo organ transplantation (156,157). Immunosuppressive regimens including steroid and calcineurin inhibitors, in particular tacrolimus, have been implicated in the development of NODAT (156). Calcineurin inhibitors may lead to pancreatic cell apoptosis with resultant decline in insulin secretion, or they may also interfere with the calcineurin/nuclear factor of activated T-cell pathway, leading to distortion of the skeletal muscle glucose uptake (157). Posttransplant increases in appetite and weight gain may also play a role in the development of NODAT. NODAT independently increases the risk of cardiovascular events and infections and shortens kidney allograft longevity and patient survival (158). Judicious glycemic control and other preventative and management strategies have been suggested, including resting the pancreatic β-cells by insulin administration during the period immediately after transplant and intensive lifestyle modification upon kidney transplantation to lower the incidence of NODAT (158).
Future Clinical Research
What are the effects of a Mediterranean diet and/or a DASH diet pattern versus a conventional “diabetes diet” on eGFR, albuminuria, and nutritional status in individuals with DKD?
What is the comparative effect of animal and plant protein sources on eGFR, albuminuria, and lipid profiles in DKD?
What is the impact of fatty acid sources such as omega-3 versus omega-9 fats on eGFR, albuminuria, and CVD risk factors in DKD?
What changes risks of adverse outcomes in ESRD patients with diabetes versus patients with earlier-stage CKD and diabetes?
Why do many dialysis patients experience spontaneous resolution of hyperglycemia?
How can NODAT be prevented and managed without shortening allograft or patient longevity?
Children and Adolescents
Risks of Hypertension and DKD
Historically, it was assumed that diabetes complications primarily affected adults with long-standing and/or poorly controlled disease and spared children with recent-onset disease. A paucity of clinical research in the pediatric population perpetuated this assumption. However, recent studies contradict those tenets and paint a remarkably different picture. The multicenter Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study prospectively evaluated the incidence, prevalence, and risk factors for developing hypertension and increased albuminuria in youth with early type 2 diabetes (ages 10–17 years, <2 years diabetes duration, n = 699). In the relatively short follow-up period (average follow-up 3.9 years), 33.8% had hypertension (11.6% at baseline) and 16.6% had moderately increased albuminuria (30–300 mg/24 h) (6.3% at baseline) (159). Hypertension in youth with type 2 diabetes required multiple medications and was refractory to treatment. In less than 4 years, the prevalence of moderately to severely increased albuminuria tripled, with disease progression rates in the youth (2.6% annual rate) similar to that seen in the UKPDS population.
Cross-sectional studies in type 1 diabetes corroborate the TODAY findings. The multicenter Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT) (n = 3,353, age 10–16 years) used ACR to assign risk: those in the highest tertile were treated with ACE inhibitors and statins, while those in the middle and lower tertiles were observed. Vascular measurements, kidney disease markers, and cardiovascular markers demonstrated that youth in the highest tertile had more rapid decline in kidney and cardiovascular function despite treatment; however, those in the lower-risk groups also showed evidence of endothelial dysfunction, suggesting that ACR is a continuous risk factor for kidney and cardiovascular dysfunction (160). Another study in youth with type 1 diabetes (age >11 years, mean 14 years; mean A1C 8.3%; >2-year duration, mean 6.3 years) found that 16.1% already had moderately increased albuminuria, 30.3% had dyslipidemia, and 12.3% had hypertension (161).
Treatment of Hypertension and DKD
The KDIGO recommendations for children with CKD do not specifically address youth with DKD. However, since there are few data for treating youth with DKD, following the general KDIGO recommendations may be appropriate for this population. For youth, KDIGO recommends treating BP levels that are consistently above the 90th percentile to achieve systolic and diastolic readings ≤50th percentile for age, sex, and height, unless achieving these targets is limited by signs or symptoms of hypotension. An ARB or ACE inhibitor may be used in youth with CKD when BP-lowering agents are indicated, regardless of the proteinuria levels (22). Meticulous glycemic control, lifestyle modification, and smoking cessation are also means of preventing and treating albuminuria (20). The FDA-approved therapeutic options for treating youth with type 1 and type 2 diabetes are currently limited to metformin (age ≥10 years) and insulin. In youth with evidence of CKD, the options are even more limited, since metformin may be contraindicated.
The horizon for future therapies remains bleak due to drug development and regulatory challenges: the absolute number of youth with diabetes remains relatively small compared with the adult population (∼190,000 youth compared with 26 million adults) (162,163); study recruitment is difficult, especially in racial and ethnic minorities predominantly affected by type 2 diabetes; there are potential drug safety concerns; and regulatory hurdles are seemingly insurmountable. After adjusting for completeness of ascertainment, recent reports on the increasing incidence of type 1 (representing a 21.1% increase from 2001–2009; 95% CI 15.6–27.0) and type 2 (representing a 30.5% increase from 2001–2009; 95% CI 17.3–45.1) diabetes in youth indicate that future clinical research is not only needed but mandatory for this highly vulnerable population (164).
Future Clinical Research
In youth, is there a difference between type 1 and type 2 diabetes in the development of DKD?
Which laboratory measures are most appropriate to assess kidney function in youth with diabetes?
What are the best therapeutic options for treating DKD in youth?
What is the trajectory for youth with diabetes who subsequently develop DKD?
Multidisciplinary Approaches and Medical Home Models for Health Care Delivery
Multidisciplinary Approaches for Comprehensive Care
Optimal care for patients with DKD is complex and best managed using comprehensive multifactorial risk-reduction strategies (160,161). There is a growing consensus that patient outcomes are most improved with simultaneous control of BP, glucose, and lipids; use of antiplatelet agent therapy when indicated; and lifestyle modifications that include smoking cessation, a healthy diet, exercise, and weight reduction among those who are overweight or obese (4,20). Smoking is associated with progressive kidney disease (167,168) and is strongly associated with CVD events, supporting the inclusion of smoking cessation among risk-reduction strategies. Dietary approaches may facilitate control of BP and management of DKD (4,20,123). Physical activity is associated with a delay in the decline of kidney function (169) and improves other risk factors in patients with CKD (170). A high BMI is a strong, potentially modifiable, risk factor for both CKD and ESRD (171). However, caution is also warranted considering observations about so-called reverse epidemiology, associating higher weight within the overweight/obese range with higher survival in ESRD, as previously discussed. In earlier stages of CKD, weight loss is associated with reduced albuminuria and a slowing decline in kidney function (172). While addressing single risk factors or even a few together may be effective, targeting multiple risk factors concomitantly can result in dramatic reductions in microvascular and macrovascular complications (165,166,173–175). Although new therapies hold promise for improving outcomes among patients with DKD, simultaneous control of multiple conventional risk factors effectively reduces the high risks of ESRD, CVD events, and death (4,20).
Yet control of multiple risk factors often remains suboptimal (4,176) leading to poor outcomes. Nearly 95% of the complex tasks for self-management fall upon patients themselves (177), yet most patients encounter considerable difficulty in following recommended treatments and behaviors. Long-term nonadherence rates to physician recommendations range between 33–75% (178). Many patients also lack assistance with their self-management, a problem exacerbated by physicians who vary widely in their provision of recommendations despite the challenges that patients face. Emotional well-being is a critical component for optimal care and self-management; however, depression is often under-recognized despite affecting about one in four people with either diabetes (179) or CKD (180).
To overcome all of these challenges, a large number of programs have emerged to facilitate improved self-management, including disease management, case management, group clinics, or other organizational and practice changes (177,181). Several multifactorial interventions have been evaluated in dozens of studies with a variety of study designs, interventions, and intended targets (e.g., health system, providers, and patients) (177,181,182). Many have been effective in improving intermediate outcomes, including testing for complications and control of BP and metabolic abnormalities. Multiple risk factor intervention is essential to reducing risks of death and kidney disease progression (Table 7).
Table 7.
| Risk factor | General recommendations for diabetes | Modifications for DKD |
|---|---|---|
| Hyperlipidemia | Goal LDL <100 mg/dL or 30–40% reduction from baseline | No specific goal for LDL cholesterol, consider measuring lipids to assess adherence to medication regimen |
| Treatment consists of dietary modifications | Treatment consists of dietary modifications | |
| Statins are recommended in patients with overt CVD and those over the age of 40 years with another risk factor for CVD | Statin or statin-ezetimibe combination is recommended in patients with nondialysis-dependent CKD | |
| For high-CVD-risk patients, <70 mg/dL is an option | Reduced doses of statins are recommended for eGFR <60 mL/min/1.73 m2 | |
| Initiation of statin therapy has not been shown to be beneficial in patients undergoing chronic dialysis treatment | ||
| Statins may reduce CVD risk in kidney transplant recipients | ||
| Hypertension | Goal BP is <140/80 mmHg | Goal BP is <140/90 mmHg |
| Treatment consists of lifestyle modifications and oral medications that generally should include RAAS blockers | Goal BP is <130/80 mmHg if urine ACR >30 mg/g creatinine | |
| Goals for treatment are based primarily on studies of patients with nondiabetic CKD | ||
| Treatment consists of lifestyle modifications and oral medications that usually include RAAS blockers | ||
| Use of more than one RAAS blocker should generally be avoided | ||
| Hyperglycemia | Goal is A1C <7% | A1C <8% when GFR <60 mL/min/1.73 m2 due to increased risks of hypoglycemia |
| A goal of <6.5% may be appropriate in early-onset diabetes in younger patients | Imprecision of A1C with CKD strengthens reliance of SMBG in making treatment decisions | |
| Treatment consists of lifestyle modification, oral medications, and injectable medications, including insulin | Doses of insulin and other injectable and oral medications used to lower blood glucose often need to be reduced for eGFR <60 mL/min/1.73 m2 |
Despite the promise of simultaneously targeting multiple risk factors, translating approaches from research settings to routine clinical practice is challenging and broad implementation has been limited. Optimal management requires team-based approaches with multidisciplinary expertise and involvement from internists, diabetologists, nephrologists, nutritionists, behavioralists, nurses, educators, and pharmacists at various times during the clinical course. Such comprehensive care is often limited and challenging to implement in routine clinical practice. Although people routinely work together in health care, several barriers prevent explicit efforts to develop interprofessional, multidisciplinary team-based care (185). Establishing cohesive and high-functioning patients and teams requires dedicated effort and resources. If the potential benefits to team members are not outweighed by the investments in the effort required, then other incentives may be necessary. When considering various specialties, colocalization to minimize patient barriers to access may be difficult or infeasible. Finally, common barriers include other logistical barriers, lack of experience or expertise, deficient infrastructure, cultural silos, resistance to change, and inadequate or absent reimbursement (185).
Medical Home Models for Health Care Delivery
Although numerous challenges impede translation of multidisciplinary approaches to clinical practice, new efficient intervention paradigms are emerging that may better provide the comprehensive, multidisciplinary care teams that support the patient self-management necessary to manage the complex clinical entity of DKD. Team-based approaches include those delivered through integrated delivery systems, patient-centered medical homes (PCMH), and accountable care organizations. Such multidisciplinary approaches are able to deliver high-quality care by identifying high-risk individuals using registries, increasing patient engagement and self-management, managing complex patients with coordinated multispecialty input, and optimizing management with decision support at the point of care. Until recently, such integrated approaches have evolved primarily within integrated delivery systems because of an alignment of incentives that support the additional investments necessary to provide team-based comprehensive care (186–188). However, recent evolution of financing mechanisms for chronic disease care has increased the focus toward greater population-based accountability through capitated payment models.
Several demonstration projects of PCMHs for diabetes have been highly effective at improving outcomes while also being more efficient (189). In a detailed review of eight PCMH initiatives (Table 8) (189), prepost comparisons support the ability of PCMHs to improve the quality of care across a number of dimensions. Although each demonstration project varied according to how PCMHs were established, all included reimbursement enhancement and most utilized case management. Payment mechanisms included fee-for-service with per member, per month fees as well as bonus payments for achieving predefined performance metrics with or without shared savings. Practice coaches, learning collaboratives, or registry-based population management software facilitated practice changes. The National Committee for Quality Assurance certification for medical homes certifies practices across the dimensions that fulfill medical home criteria. In addition to managing the various barriers that often arise, good leadership and dedication to transformation are critical to the fruitful teamwork required to be successful. Online resources and specific strategies to implement practice change are available through the National Diabetes Education Program (www.betterdiabetescare.nih.gov).
Table 8.
PCMH demonstration projects in diabetes (189)
| PCMH demonstration projects | Outcomes |
|---|---|
| Community Care of North Carolina—1998 | Estimated annual savings of $161 million for diabetes care |
| Geisinger—2006 | 2.5-fold improvement in meeting nine quality indicators |
| Pennsylvania Chronic Care Initiative—2008 | Self-management goals increased from 20 to 70% |
| Group Health Cooperative—2007 | 11% reduction in hospitalizations |
| By 21 months, return on investment of $1.50 for each $1 spent | |
| Health Partners—2002 | 24% reduction in hospitalizations |
| Southeastern Pennsylvania Chronic Care Initiative—2007−2011 | 5% more A1C testing but more abnormal results |
| 11% greater monitoring for diabetic nephropathy | |
| Pioneer accountable care organizations | 25 of 32 had reduced risk-adjusted readmission rates compared with benchmark plans |
| 68% of people with diabetes reached BP targets compared with 55% in benchmark plans | |
| 57% with diabetes reached LDL cholesterol targets compared with 48% in benchmark plans |
The passage of the Affordable Care Act has provided support for a variety of innovative delivery systems in an effort to “bend the cost curve” of medical care. As accountable care organizations continue to develop, their impact on population management for complex patients with DKD will become clearer. Ongoing payment reform will be critical to stimulate broader dissemination and implementation of multiple risk factor and multidisciplinary approaches and to ensure long-term sustainability.
Future Clinical Research
What are the resources required to provide necessary care for people with DKD?
What incentives improve medical care and patient adherence in DKD?
What is the most efficient delivery system for complex cases of diabetes such as those with DKD?
When should patients with DKD be referred to other members of the treatment team?
What is the best way to utilize multidisciplinary health care providers (certified diabetes educators, pharmacists, nurse practitioners, physician assistants) for care of people with DKD?
What is the best way to continue optimal diabetes care in the context of treating ESRD by dialysis or kidney transplant?
How can other important clinical outcomes be optimized in ESRD patients (e.g., amputations, retinopathy, CVD)?
Conclusions
DKD has emerged as a major aftermath of the worldwide diabetes pandemic. Therefore, diabetes prevention must remain at the cornerstone of reducing DKD. Identification of DKD depends upon screening for increased albuminuria and low eGFR. Both measurements have considerable imprecision, highlighting the need for better identification methods, especially for people at high risk of DKD complications. Prevention of CVD, a major cause of death in DKD, centers upon management of LDL cholesterol and BP. More needs to be learned about risk factors unique to the DKD population to improve risk stratification as well as treatment strategies. Along with efficacy, heightened awareness surrounding safety of new therapeutic approaches is essential. Safety must also be carefully evaluated when approved drugs are used in combination or for new indications. For example, the recently halted clinical trials of dual RAAS blockade in DKD underscored this point by revealing increased risks of hyperkalemia and acute kidney injury.
Glycemic control is at the core of good diabetes care. However, effects of intensive glycemic control vary with severity of DKD. Those with low eGFR are at high risk for hypoglycemia, an immediate and serious adverse event. The number of oral agents that can be used to treat hyperglycemia in patients with DKD is quite limited due to decreased drug clearance and side effects. Insulin doses commonly require reduction, particularly due to the risk of hypoglycemia. Diabetes management is further complicated by challenges with glycemic monitoring due to a bias to the low of A1C related to heightened red blood cell turnover. As such, SMBG is critical to achieve glycemic goals and mitigate risk of hypoglycemia. In older adults with long-standing diabetes and CKD, greater care to avoid hypoglycemia is needed and less stringent A1C targets are recommended (81).
Nutritional recommendations are modified for advanced DKD (stages 4–5 CKD) to reduce risk of hyperkalemia, hyperphosphatemia, bone mineral metabolism disorders, hypertension, and kidney disease progression. With the adjustment of various macro- and micronutrients, dietary recommendations become extraordinarily complex. Therefore, current strategies emphasize whole diets (e.g., Mediterranean-style or DASH) with modification for kidney disease. Relationships between conventional risk factors for CKD and CVD (e.g., body weight, BP, lipids) are reversed or “U-shaped” with advanced DKD, suggesting that goals for the general population cannot be simply extrapolated to this group.
Care of patients with DKD is extraordinarily challenging due to multiple comorbidities, disparities, and complexities of health care delivery systems. Disparities in DKD appear to be linked to increasing rates of type 2 diabetes in youth, which disproportionately occurs in racial and ethnic minorities and allows for the development of diabetes complications earlier in life. These special populations, particularly high-risk youth, merit focused attention. Medical home models and integration of multiple risk factor management, keeping the patient-at-center, are of great potential for high-risk groups. Importantly, team care including health care professionals from various disciplines and effective communication are requirements for successful attainment of integrated, whole-person care. Novel therapies for DKD are urgently needed to improve clinically relevant outcomes, yet they must be accompanied by better methods for health care delivery and implementation in order to succeed. The key issues and accompanying research recommendations for DKD highlighted by this conference will help lead the way forward, filling gaps in knowledge that advance care and meaningfully improve life for people with DKD.
Article Information
Acknowledgments. ADA thanks K.R.T. and M.E.M. for serving as co-chairs for the Consensus Conference on Chronic Kidney Disease and Diabetes, held 20−22 March 2014. ADA also thanks ASN (Tod Ibrahim, Phillip Kokemueller, U.D.P.) and NKF (Emily Howell, Kerry Willis, G.L.B.) for participating in the conference as collaborators. The authors thank ADA staff members Erika Gebel Berg, PhD, for significant editorial contributions and Sonya Pendleton for administrative support.
Duality of Interest. All authors have completed the Unified Competing Interest form at www.icmje.org/conflicts-of-interest (available upon request from J.L.C.) and declare the following conflicts of interest. K.R.T. has received personal fees from Eli Lilly and Co. G.L.B. has received grants from Takeda and consultancy fees for his institution from AbbVie, Takeda, Medtronic, Relypsa, Daiichi Sankyo, Janssen, Novartis, and Bayer. R.W.B. has received personal fees from Roche Pharma, AbbVie, Mitsubishi, and Boehringer Ingelheim. I.H.d.B. has received grants from AbbVie. I.B.H. has received grants from Sanofi and Halozyme and personal fees from Abbott and Roche. K.K.-Z. has received honoraria from Abbott, AbbVie, Amgen, Fresnius, DaVita, Keryx, Otsuka, and Shire. S.D.N. received grants from the National Institutes of Health/NIDDK and Genzyme. J.J.N. has received grants from AstraZeneca, Johnson & Johnson, Merck, and Novo Nordisk. U.D.P. has received personal fees from Amgen and grants from Amgen, Daiichi Sankyo, Eli Lilly and Co., Luitpold Pharmaceuticals, Angion Biomedica Corp., CSL Limited, Ablative Solutions Inc., Kia Pharmaceuticals, Reata Pharmaceuticals, Keryx, and Gilead Sciences. M.E.M. has received grants from Sanofi, Bayer, Eli Lilly and Co., Novo Nordisk, and Novartis and consultant fees from AbbVie, Merck, Janssen, AstraZeneca, and Bristol-Myers Squibb. No other potential conflicts of interest relevant to this article were reported.
Footnotes
In collaboration with the American Society of Nephrology and the National Kidney Foundation
Organizational sponsors and participants: American Diabetes Association, National Kidney Foundation, and American Society of Nephrology
This article is being published concurrently in Diabetes Care and AJKD. The articles are identical except for stylistic changes in keeping with each journal’s style. Either of these versions may be used in citing this article.
© 2014 by the American Diabetes Association and the National Kidney Foundation. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
References
- 1.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013: 1.7.2; 1.8.2. [Google Scholar]
- 3.U.S. Renal Data System USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013:7.6.1; 7.9.1; 7.20.1. [Google Scholar]
- 4.National Kidney Foundation KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007;49(Suppl. 2)S12–S154 [DOI] [PubMed] [Google Scholar]
- 5.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 2009;52:691–697 [DOI] [PubMed] [Google Scholar]
- 6.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest 1986;77:1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans M, Bain SC, Hogan S, Bilous RW, Collaborative Study Group Participants Irbesartan delays progression of nephropathy as measured by estimated glomerular filtration rate: post hoc analysis of the Irbesartan Diabetic Nephropathy Trial. Nephrol Dial Transplant 2012;27:2255–2263 [DOI] [PubMed] [Google Scholar]
- 8.Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 2011;80:282–287 [DOI] [PubMed] [Google Scholar]
- 9.Bakris GL, Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care 2014;37:867–875 [DOI] [PubMed] [Google Scholar]
- 10.Bilous R. Microvascular disease: what does the UKPDS tell us about diabetic nephropathy? Diabet Med 2008;25(Suppl. 2):25–29 [DOI] [PubMed] [Google Scholar]
- 11.Nosadini R, Velussi M, Brocco E, et al. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes 2000;49:476–484 [DOI] [PubMed] [Google Scholar]
- 12.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004;27:195–200 [DOI] [PubMed] [Google Scholar]
- 13.He F, Xia X, Wu XF, Yu XQ, Huang FX. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia 2013;56:457–466 [DOI] [PubMed] [Google Scholar]
- 14.Bachmann LM, Nilsson G, Bruns DE, et al. State of the art for measurement of urine albumin: comparison of routine measurement procedures to isotope dilution tandem mass spectrometry. Clin Chem 2014;60:471–480 [DOI] [PubMed] [Google Scholar]
- 15.Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–259 [PubMed] [Google Scholar]
- 16.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 17.Bilous RW, Parving H-H, Orchard TJ, et al. Renin angiotensin system blockade is effective in preventing microalbuminuria in hypertensive but not normotensive people with type 2 diabetes; further analysis of the DIRECT Programme [Internet]. Stockholm, Sweden, EASD, 2010
- 18.Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013;59:675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003;348:2285–2293 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Disease Education Program NKDEP [Internet]. Bethesda, MD, National Institutes of Health. Available from http://nkdep.nih.gov/ Accessed 19 May 2014
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int 2013;3:S1–S150 [DOI] [PubMed] [Google Scholar]
- 23.Ismail-Beigi F, Craven T, Banerji MA, et al. ACCORD trial group Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossing P. Prediction, progression and prevention of diabetic nephropathy. The Minkowski Lecture 2005. Diabetologia 2006;49:11–19 [DOI] [PubMed] [Google Scholar]
- 25.Qaseem A, Hopkins RH, Jr, Sweet DE, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2013;159:835–847 [DOI] [PubMed] [Google Scholar]
- 26.Fox CS, Matsushita K, Woodward M, et al. Chronic Kidney Disease Prognosis Consortium Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonelli M, Muntner P, Lloyd A, et al. Alberta Kidney Disease Network Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012;380:807–814 [DOI] [PubMed] [Google Scholar]
- 28.Groop P-H, Thomas MC, Moran JL, et al. FinnDiane Study Group The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borch-Johnsen K, Andersen PK, Deckert T. The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1985;28:590–596 [DOI] [PubMed] [Google Scholar]
- 31.Tonelli M, Muntner P, Lloyd A, et al. Alberta Kidney Disease Network Association between LDL-C and risk of myocardial infarction in CKD. J Am Soc Nephrol 2013;24:979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GFM. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012;157:263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haynes R, Lewis D, Emberson J, et al. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol. 8 May 2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanner C, Tonelli M, Cass A, et al. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int2014;85:1303−1309 [DOI] [PubMed]
- 35.Holdaas H, Fellström B, Cole E, et al. Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant 2005;5:2929–2936 [DOI] [PubMed] [Google Scholar]
- 36.Jun M, Zhu B, Tonelli M, et al. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol 2012;60:2061–2071 [DOI] [PubMed] [Google Scholar]
- 37.Ansquer J-C, Dalton RN, Caussé E, Crimet D, Le Malicot K, Foucher C. Effect of fenofibrate on kidney function: a 6-week randomized crossover trial in healthy people. Am J Kidney Dis 2008;51:904–913 [DOI] [PubMed] [Google Scholar]
- 38.Wheeler DC, Becker GJ. Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney Int 2013;83:377–383 [DOI] [PubMed] [Google Scholar]
- 39.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–520 [DOI] [PubMed] [Google Scholar]
- 40.Taler SJ, Agarwal R, Bakris GL, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis 2013;62:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pogue V, Rahman M, Lipkowitz M, et al. African American Study of Kidney Disease and Hypertension Collaborative Research Group Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009;53:20–27 [DOI] [PubMed] [Google Scholar]
- 42.Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006;144:884–893 [DOI] [PubMed] [Google Scholar]
- 43.Peralta CA, Norris KC, Li S, et al. KEEP Investigators Blood pressure components and end-stage renal disease in persons with chronic kidney disease: the Kidney Early Evaluation Program (KEEP). Arch Intern Med 2012;172:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovesdy CP, Bleyer AJ, Molnar MZ, et al. Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann Intern Med 2013;159:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wake Forest Baptist Health Systolic Blood Pressure Intervention Trial. In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine, 2010. Available from http://clinicaltrials.gov/ct2/show/NCT01206062?term=nct01206062&rank=1 Accessed 19 May 2014
- 46.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 47.Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 48.Lewis EJ, Hunsicker LG, Clarke WR, et al. Collaborative Study Group Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 49.Fried LF, Emanuele N, Zhang JH, et al. VA NEPHRON-D Investigators Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–1903 [DOI] [PubMed] [Google Scholar]
- 50.Parving H-H, Brenner BM, McMurray JJV, et al. ALTITUDE Investigators Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367:2204–2213 [DOI] [PubMed] [Google Scholar]
- 51.Mann JFE, Schmieder RE, McQueen M, et al. ONTARGET investigators Renal Outcomes With Telmisartan, Ramipril, or Both, in People at High Vascular Risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008;372:547–553 [DOI] [PubMed] [Google Scholar]
- 52.European Medicines Agency. Renin-angiotensin-system (RAS)-acting agents [Internet]. Available from http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Renin-angiotensin_system_(RAS)-acting_agents/human_referral_prac_000026.jsp&mid=WC0b01ac05805c516f Accessed 19 May 2014
- 53.Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol 2009;20:2641–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pitt B, Bakris G, Ruilope LM, DiCarlo L, Mukherjee R, EPHESUS Investigators Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 2008;118:1643–1650 [DOI] [PubMed] [Google Scholar]
- 55.Toto RD. RAS blockade, hyperkalemia and AKI—look and you will find. Nat Rev Nephrol 2012;8:257–258 [DOI] [PubMed] [Google Scholar]
- 56.Kohan DE, Pritchett Y, Molitch M, et al. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol 2011;22:763–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.AbbVie. A Randomized, Multicountry, Multicenter, Double-Blind, Parallel, Placebo-Controlled Study of the Effects of Atrasentan on Renal Outcomes in Subjects With Type 2 Diabetes and Nephropathy SONAR: Study of Diabetic Nephropathy With Atrasentan. In: ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine, 2013. Available from http://clinicaltrials.gov/show/NCT01858532 Accessed 19 May 2014
- 58.Briasoulis A, Bakris G. The future of interventional management of hypertension: threats and opportunities. Curr Vasc Pharmacol 2014;12:69–76 [DOI] [PubMed] [Google Scholar]
- 59.Bhatt DL, Kandzari DE, O’Neill WW, et al. SYMPLICITY HTN-3 Investigators A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370:1393–1401 [DOI] [PubMed] [Google Scholar]
- 60.Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol 2011;58:765–773 [DOI] [PubMed] [Google Scholar]
- 61.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose Study Group Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ansari A, Thomas S, Goldsmith D. Assessing glycemic control in patients with diabetes and end-stage renal failure. Am J Kidney Dis 2003;41:523–531 [DOI] [PubMed] [Google Scholar]
- 63.Vos FE, Schollum JB, Coulter CV, Doyle TCA, Duffull SB, Walker RJ. Red blood cell survival in long-term dialysis patients. Am J Kidney Dis 2011;58:591–598 [DOI] [PubMed] [Google Scholar]
- 64.Nakao T, Matsumoto H, Okada T, et al. Influence of erythropoietin treatment on hemoglobin A1c levels in patients with chronic renal failure on hemodialysis. Intern Med 1998;37:826–830 [DOI] [PubMed] [Google Scholar]
- 65.Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shurraw S, Hemmelgarn B, Lin M, et al. Alberta Kidney Disease Network Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med 2011;171:1920–1927 [DOI] [PubMed] [Google Scholar]
- 67.Kalantar-Zadeh K. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: A1C remains the gold standard outcome predictor in diabetic dialysis patients. Diabetes Care 2012;35:1625–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramirez SPB, McCullough KP, Thumma JR, et al. Hemoglobin A(1c) levels and mortality in the diabetic hemodialysis population: findings from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Diabetes Care 2012;35:2527–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim WJ, Park C-Y, Lee K-B, et al. Serum 1,5-anhydroglucitol concentrations are a reliable index of glycemic control in type 2 diabetes with mild or moderate renal dysfunction. Diabetes Care 2012;35:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inaba M, Okuno S, Kumeda Y, et al. Osaka CKD Expert Research Group Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007;18:896–903 [DOI] [PubMed] [Google Scholar]
- 71.Freedman BI, Andries L, Shihabi ZK, et al. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin J Am Soc Nephrol 2011;6:1635–1643 [DOI] [PubMed] [Google Scholar]
- 72.Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 2001;24:382–391 [DOI] [PubMed] [Google Scholar]
- 73.National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012;60:850–886 [DOI] [PubMed] [Google Scholar]
- 74.Mühlhauser I, Toth G, Sawicki PT, Berger M. Severe hypoglycemia in type I diabetic patients with impaired kidney function. Diabetes Care 1991;14:344–346 [DOI] [PubMed] [Google Scholar]
- 75.Scheen AJ. Pharmacokinetic considerations for the treatment of diabetes in patients with chronic kidney disease. Expert Opin Drug Metab Toxicol 2013;9:529–550 [DOI] [PubMed] [Google Scholar]
- 76.Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab 2000;26(Suppl. 4):73–85 [PubMed] [Google Scholar]
- 77.Burrows NR, Li Y, Geiss LS. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care 2010;33:73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS Group Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63:225–232 [DOI] [PubMed] [Google Scholar]
- 79.Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care 2005;28:2948–2961 [DOI] [PubMed] [Google Scholar]
- 80.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011;154:554–559 [DOI] [PubMed] [Google Scholar]
- 82.Roberts F, Ryan GJ. The safety of metformin in heart failure. Ann Pharmacother 2007;41:642–646 [DOI] [PubMed] [Google Scholar]
- 83.Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care 2011;34:1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Metformin ok in CKD? Drug Ther Bull 2014;52:37. [DOI] [PubMed] [Google Scholar]
- 85.Kalantar-Zadeh K, Uppot RN, Lewandrowski KB. Case records of the Massachusetts General Hospital. Case 23-2013. A 54-year-old woman with abdominal pain, vomiting, and confusion. N Engl J Med 2013;369:374–382 [DOI] [PubMed] [Google Scholar]
- 86.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2010;4:CD002967. [DOI] [PubMed] [Google Scholar]
- 87.Rachmani R, Slavachevski I, Levi Z, Zadok B, Kedar Y, Ravid M. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. Eur J Intern Med 2002;13:428. [DOI] [PubMed] [Google Scholar]
- 88.Rydberg T, Jönsson A, Røder M, Melander A. Hypoglycemic activity of glyburide (glibenclamide) metabolites in humans. Diabetes Care 1994;17:1026–1030 [DOI] [PubMed] [Google Scholar]
- 89.Holstein A, Plaschke A, Egberts EH. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev 2001;17:467–473 [DOI] [PubMed] [Google Scholar]
- 90.Harrower AD. Pharmacokinetics of oral antihyperglycaemic agents in patients with renal insufficiency. Clin Pharmacokinet 1996;31:111–119 [DOI] [PubMed] [Google Scholar]
- 91.Balant L, Zahnd G, Gorgia A, Schwarz R, Fabre J. Pharmacokinetics of glipizide in man: influence of renal insufficiency. Diabetologia 1973;9(Suppl. 1):331–338 [DOI] [PubMed] [Google Scholar]
- 92.Nagai T, Imamura M, Iizuka K, Mori M. Hypoglycemia due to nateglinide administration in diabetic patient with chronic renal failure. Diabetes Res Clin Pract 2003;59:191–194 [DOI] [PubMed] [Google Scholar]
- 93.Hasslacher C, Multinational Repaglinide Renal Study Group Safety and efficacy of repaglinide in type 2 diabetic patients with and without impaired renal function. Diabetes Care 2003;26:886–891 [DOI] [PubMed] [Google Scholar]
- 94.Budde K, Neumayer H-H, Fritsche L, Sulowicz W, Stompôr T, Eckland D. The pharmacokinetics of pioglitazone in patients with impaired renal function. Br J Clin Pharmacol 2003;55:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chapelsky MC, Thompson-Culkin K, Miller AK, Sack M, Blum R, Freed MI. Pharmacokinetics of rosiglitazone in patients with varying degrees of renal insufficiency. J Clin Pharmacol 2003;43:252–259 [DOI] [PubMed] [Google Scholar]
- 96.Thompson-Culkin K, Zussman B, Miller AK, Freed MI. Pharmacokinetics of rosiglitazone in patients with end-stage renal disease. J Int Med Res 2002;30:391–399 [DOI] [PubMed] [Google Scholar]
- 97.Grey AB. Skeletal toxicity of thiazolidinediones. Ann Intern Med 2008;148:563. [DOI] [PubMed] [Google Scholar]
- 98.U.S. Food and Drug Administration. Drug safety information for healthcare professionals—information for healthcare professionals: reports of altered kidney function in patients using exenatide (marketed as Byetta) [Internet]. Washington, DC, U.S. Food and Drug Administration, 2009. Available from http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm188656.htm Accessed 19 May 2014
- 99.Tuttle KR, Heilmann C, Hoogwerf BJ, Brown C, Anderson PW. Effects of exenatide on kidney function, adverse events, and clinical end points of kidney disease in type 2 diabetes. Am J Kidney Dis 2013;62:396–398 [DOI] [PubMed] [Google Scholar]
- 100.Pawaskar M, Tuttle KR, Li Q, Best JH, Anderson PW. Observational study of kidney function and albuminuria in patients with type 2 diabetes treated with exenatide BID versus insulin glargine. Ann Pharmacother 2014;48:571–576 [DOI] [PubMed] [Google Scholar]
- 101.Davidson JA, Brett J, Falahati A, Scott D. Mild renal impairment and the efficacy and safety of liraglutide. Endocr Pract 2011;17:345–355 [DOI] [PubMed] [Google Scholar]
- 102.Pendergrass M, Fenton C, Haffner SM, Chen W. Exenatide and sitagliptin are not associated with increased risk of acute renal failure: a retrospective claims analysis. Diabetes Obes Metab 2012;14:596–600 [DOI] [PubMed] [Google Scholar]
- 103.Deacon CF, Mannucci E, Ahrén B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Diabetes Obes Metab 2012;14:762–767 [DOI] [PubMed] [Google Scholar]
- 104.Aroda VR, Henry RR, Han J, et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther 2012;34:1247–1258.e22 [DOI] [PubMed] [Google Scholar]
- 105.Giorda CB, Nada E, Tartaglino B. Pharmacokinetics, safety, and efficacy of DPP-4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. A systematic review of the literature [published correction appears in Endocrine 2014;46:420−22]. Endocrine 2014;46:406−419 [DOI] [PubMed] [Google Scholar]
- 106.Januvia (sitagliptin) [package insert] Whitehouse Station, NJ, Merck & Co. Inc., February 2014 [Google Scholar]
- 107.Onglyza (saxagliptin) [package insert] Princeton, NJ, Bristol-Myers Squibb Co, May 2013 [Google Scholar]
- 108.Nesina (alogliptin) [package insert] Deerfield, IL, Takeda Pharmaceuticals American, Inc, June 2013 [Google Scholar]
- 109.Tradjenta (linagliptin) [package insert] Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, May 2014 [Google Scholar]
- 110.Friedrich C, Emser A, Woerle H-J, Graefe-Mody U. Renal impairment has no clinically relevant effect on the long-term exposure of linagliptin in patients with type 2 diabetes. Am J Ther 2013;20:618–621 [DOI] [PubMed] [Google Scholar]
- 111.Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia 2013;56:696–708 [DOI] [PubMed] [Google Scholar]
- 112.Neumiller JJ, White JR, Jr, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs 2010;70:377–385 [DOI] [PubMed] [Google Scholar]
- 113.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014;85:962–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yale J-F, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013;15:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cherney DZI, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–597 [DOI] [PubMed] [Google Scholar]
- 116.Meloni C, Morosetti M, Suraci C, et al. Severe dietary protein restriction in overt diabetic nephropathy: benefits or risks? J Ren Nutr 2002;12:96–101 [DOI] [PubMed] [Google Scholar]
- 117.Wrone EM, Carnethon MR, Palaniappan L, Fortmann SP, Third National Health and Nutrition Examination Survey Association of dietary protein intake and microalbuminuria in healthy adults: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41:580–587 [DOI] [PubMed] [Google Scholar]
- 118.Hoogeveen EK, Kostense PJ, Jager A, et al. Serum homocysteine level and protein intake are related to risk of microalbuminuria: the Hoorn Study. Kidney Int 1998;54:203–209 [DOI] [PubMed] [Google Scholar]
- 119.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med 2003;138:460–467 [DOI] [PubMed] [Google Scholar]
- 120.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cirillo M, Lombardi C, Chiricone D, De Santo NG, Zanchetti A, Bilancio G. Protein intake and kidney function in the middle-age population: contrast between cross-sectional and longitudinal data. Nephrol Dial Transplant. 21 March 2014 [Epub ahead of print] [DOI] [PubMed]
- 122.Estruch R, Ros E, Salas-Salvadó J, et al. PREDIMED Study Investigators Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–129023432189 [Google Scholar]
- 123.Sacks FM, Obarzanek E, Windhauser MM, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol 1995;5:108–118 [DOI] [PubMed] [Google Scholar]
- 124.Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol 2011;301:F919–F931 [DOI] [PubMed] [Google Scholar]
- 125.Goldstein-Fuchs DJ, LaPierra AF. Nutrition and Kidney Disease. National Kidney Foundation. National Kidney Foundation’s primer on kidney diseases. 6th ed. Gilbert SJ, Weiner DE, Gipson DS, Perazella MA, Tonelli M, Eds. Philadelphia, PA, Elsevier/Saunders, 2014, p. 467–475 [Google Scholar]
- 126.Pijls LTJ, de Vries H, van Eijk JTM, Donker AJM. Protein restriction, glomerular filtration rate and albuminuria in patients with type 2 diabetes mellitus: a randomized trial. Eur J Clin Nutr 2002;56:1200–1207 [DOI] [PubMed] [Google Scholar]
- 127.Dussol B, Iovanna C, Raccah D, et al. A randomized trial of low-protein diet in type 1 and in type 2 diabetes mellitus patients with incipient and overt nephropathy. J Ren Nutr 2005;15:398–406 [DOI] [PubMed] [Google Scholar]
- 128.Pedrini MT, Levey AS, Lau J, Chalmers TC, Wang PH. The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: a meta-analysis. Ann Intern Med 1996;124:627–632 [DOI] [PubMed] [Google Scholar]
- 129.Robertson L, Waugh N, Robertson A. Protein restriction for diabetic renal disease. Cochrane Database Syst Rev 2007;4:CD002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hansen HP, Christensen PK, Tauber-Lassen E, Klausen A, Jensen BR, Parving HH. Low-protein diet and kidney function in insulin-dependent diabetic patients with diabetic nephropathy. Kidney Int 1999;55:621–628 [DOI] [PubMed] [Google Scholar]
- 131.Kasiske BL, Lakatua JD, Ma JZ, Louis TA. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am J Kidney Dis 1998;31:954–961 [DOI] [PubMed] [Google Scholar]
- 132.Raal FJ, Kalk WJ, Lawson M, et al. Effect of moderate dietary protein restriction on the progression of overt diabetic nephropathy: a 6-mo prospective study. Am J Clin Nutr 1994;60:579–585 [DOI] [PubMed] [Google Scholar]
- 133.Dullaart RP, Beusekamp BJ, Meijer S, van Doormaal JJ, Sluiter WJ. Long-term effects of protein-restricted diet on albuminuria and renal function in IDDM patients without clinical nephropathy and hypertension. Diabetes Care 1993;16:483–492 [DOI] [PubMed] [Google Scholar]
- 134.Hansen HP, Tauber-Lassen E, Jensen BR, Parving H-H. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int 2002;62:220–228 [DOI] [PubMed] [Google Scholar]
- 135.Pan Y, Guo LL, Jin HM. Low-protein diet for diabetic nephropathy: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2008;88:660–666 [DOI] [PubMed] [Google Scholar]
- 136.Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2014;37(Suppl. 1):S120–S143 [DOI] [PubMed] [Google Scholar]
- 137.Shapiro H, Theilla M, Attal-Singer J, Singer P. Effects of polyunsaturated fatty acid consumption in diabetic nephropathy. Nat Rev Nephrol 2011;7:110–121 [DOI] [PubMed] [Google Scholar]
- 138.Lee CC, Sharp SJ, Wexler DJ, Adler AI. Dietary intake of eicosapentaenoic and docosahexaenoic acid and diabetic nephropathy: cohort analysis of the diabetes control and complications trial. Diabetes Care 2010;33:1454–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kovesdy CP, Sharma K, Kalantar-Zadeh K. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis 2008;52:766–777 [DOI] [PubMed] [Google Scholar]
- 140.Kalantar-Zadeh K, Abbott KC, Kronenberg F, Anker SD, Horwich TB, Fonarow GC. Epidemiology of dialysis patients and heart failure patients. Semin Nephrol 2006;26:118–133 [DOI] [PubMed] [Google Scholar]
- 141.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 2003;63:793–808 [DOI] [PubMed] [Google Scholar]
- 142.Kalantar-Zadeh K. What is so bad about reverse epidemiology anyway? Semin Dial 2007;20:593–601 [DOI] [PubMed] [Google Scholar]
- 143.Kalantar-Zadeh K. Causes and consequences of the reverse epidemiology of body mass index in dialysis patients. J Ren Nutr 2005;15:142–147 [DOI] [PubMed] [Google Scholar]
- 144.Kalantar-Zadeh K, Anker SD, Coats AJS, Horwich TB, Fonarow GC. Obesity paradox as a component of reverse epidemiology in heart failure. Arch Intern Med 2005;165:1797; author reply 1797–1798 [DOI] [PubMed] [Google Scholar]
- 145.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, Wu DY. Reverse epidemiology: a spurious hypothesis or a hardcore reality? Blood Purif 2005;23:57–63 [DOI] [PubMed] [Google Scholar]
- 146.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension 2005;45:811–817 [DOI] [PubMed] [Google Scholar]
- 147.Schumock GT, Andress DL, Marx SE, Sterz R, Joyce AT, Kalantar-Zadeh K. Association of secondary hyperparathyroidism with CKD progression, health care costs and survival in diabetic predialysis CKD patients. Nephron Clin Pract 2009;113:c54–c61 [DOI] [PubMed] [Google Scholar]
- 148.Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial 2014;27:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park J, Lertdumrongluk P, Molnar MZ, Kovesdy CP, Kalantar-Zadeh K. Glycemic control in diabetic dialysis patients and the burnt-out diabetes phenomenon. Curr Diab Rep 2012;12:432–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr 2009;19:33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ricks J, Molnar MZ, Kovesdy CP, et al. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes 2012;61:708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care 2007;30:1049–1055 [DOI] [PubMed] [Google Scholar]
- 153.Hoshino J, Mehrotra R, Rhee CM, et al. Using hemoglobin A1c to derive mean blood glucose in peritoneal dialysis patients. Am J Nephrol 2013;37:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duong U, Mehrotra R, Molnar MZ, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol 2011;6:1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Molnar MZ, Kovesdy CP, Bunnapradist S, et al. Associations of pretransplant serum albumin with post-transplant outcomes in kidney transplant recipients. Am J Transplant 2011;11:1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sarno G, Mehta RJ, Guardado-Mendoza R, Jimenez-Ceja LM, De Rosa P, Muscogiuri G. New-onset diabetes mellitus: predictive factors and impact on the outcome of patients undergoing liver transplantation. Curr Diabetes Rev 2013;9:78–85 [PubMed] [Google Scholar]
- 157.Chakkera HA, Mandarino LJ. Calcineurin inhibition and new-onset diabetes mellitus after transplantation. Transplantation 2013;95:647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chakkera HA, Weil EJ, Pham P-T, Pomeroy J, Knowler WC. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care 2013;36:1406–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.TODAY Study Group Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 2013;36:1735–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marcovecchio ML, Woodside J, Jones T, et al. AdDIT Investigators Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT): urinary screening and baseline biochemical and cardiovascular assessments. Diabetes Care 2014;37:805–813 [DOI] [PubMed] [Google Scholar]
- 161.Demirel F, Tepe D, Kara O, Esen I. Microvascular complications in adolescents with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol 2013;5:145–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pettitt DJ, Talton J, Dabelea D, et al. SEARCH for Diabetes in Youth Study Group Prevalence of diabetes in U.S. youth in 2009: the SEARCH for Diabetes in Youth Study. Diabetes Care 2014;37:402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dabelea D, Mayer-Davis EJ, Saydah S, et al. SEARCH for Diabetes in Youth Study Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gaede P, Lund-Andersen H, Parving H-H, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 166.Gaede P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 167.Shankar A, Klein R, Klein BEK. The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol 2006;164:263–271 [DOI] [PubMed] [Google Scholar]
- 168.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PWF, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA 2004;291:844–850 [DOI] [PubMed] [Google Scholar]
- 169.Robinson-Cohen C, Katz R, Mozaffarian D, et al. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med 2009;169:2116–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen P-Y, Huang Y-C, Kao Y-H, Chen J-Y. Effects of an exercise program on blood biochemical values and exercise stage of chronic kidney disease patients. J Nurs Res 2010;18:98–107 [DOI] [PubMed] [Google Scholar]
- 171.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 2008;73:19–33 [DOI] [PubMed] [Google Scholar]
- 172.Morales E, Valero MA, León M, Hernández E, Praga M. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis 2003;41:319–327 [DOI] [PubMed] [Google Scholar]
- 173.Chan JC, So W-Y, Yeung C-Y, et al. SURE Study Group Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study. Diabetes Care 2009;32:977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leung WYS, So W-Y, Tong PCY, Chan NN, Chan JCN. Effects of structured care by a pharmacist-diabetes specialist team in patients with type 2 diabetic nephropathy. Am J Med 2005;118:1414. [DOI] [PubMed] [Google Scholar]
- 175.Olivarius NF, Beck-Nielsen H, Andreasen AH, Hørder M, Pedersen PA. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. BMJ 2001;323:970–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rossi MCE, Nicolucci A, Arcangeli A, et al. Associazione Medici Diabetologi Annals Study Group Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care 2008;31:2166–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Agency for Healthcare Research and Quality. Patient Self-Management Support Programs An Evaluation [Internet], 2007. Available from http://www.ahrq.gov/research/findings/final-reports/ptmgmt/index.html Accessed 19 May 2014
- 178.World Health Organization Adherence to Long-Term Therapies: Evidence for Action. Sabaté E, Ed. Geneva, World Health Organization, 2003, p. 198 [Google Scholar]
- 179.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2006;23:1165–1173 [DOI] [PubMed] [Google Scholar]
- 180.Palmer S, Vecchio M, Craig JC, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int 2013;84:179–191 [DOI] [PubMed] [Google Scholar]
- 181.Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care 2001;24:1821–1833 [DOI] [PubMed] [Google Scholar]
- 182.Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA 2006;296:427–440 [DOI] [PubMed] [Google Scholar]
- 183.Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int 2013;3 (Suppl.):259–305 [Google Scholar]
- 184.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2012;2(Suppl.):337–414 [Google Scholar]
- 185.Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care [Internet], 2012. Washington, DC, Institute of Medicine. Available from http://www.iom.edu/Global/Perspectives/2012/TeamBasedCare.aspx Accessed 19 May 2014
- 186.Lee BJ, Forbes K. The role of specialists in managing the health of populations with chronic illness: the example of chronic kidney disease. BMJ 2009;339:b2395. [DOI] [PubMed] [Google Scholar]
- 187.Lee B, Turley M, Meng D, et al. Effects of proactive population-based nephrologist oversight on progression of chronic kidney disease: a retrospective control analysis. BMC Health Serv Res 2012;12:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bayliss EA, Bhardwaja B, Ross C, Beck A, Lanese DM. Multidisciplinary team care may slow the rate of decline in renal function. Clin J Am Soc Nephrol 2011;6:704–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bojadzievski T, Gabbay RA. Patient-centered medical home and diabetes. Diabetes Care 2011;34:1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Abe M, Okada K, Maruyama T, Maruyama N, Soma M, Matsumoto K. Clinical effectiveness and safety evaluation of long-term pioglitazone treatment for erythropoietin responsiveness and insulin resistance in type 2 diabetic patients on hemodialysis. Expert Opin Pharmacother 2010;11:1611–1620 [DOI] [PubMed] [Google Scholar]