Abstract
Our previous studies showed that biomodification of demineralized dentin collagen with proanthocyanidin (PA) for a clinically practical duration improves the mechanical properties of the dentin matrix and the immediate resin–dentin bond strength. The present study sought to evaluate the ability of PA biomodification to reduce collagenase-induced biodegradation of demineralized dentin matrix and dentin/adhesive interfaces in a clinically relevant manner. The effects of collagenolytic and gelatinolytic activity on PA-biomodified demineralized dentin matrix were analysed by hydroxyproline assay and gelatin zymography. Then, resin-/dentin-bonded specimens were prepared and challenged with bacterial collagenases. Dentin treated with 2% chlorhexidine and untreated dentin were used as a positive and negative control, respectively. Collagen biodegradation, the microtensile bond strengths of bonded specimens and the micromorphologies of the fractured interfaces were assessed. The results revealed that both collagenolytic and gelatinolytic activity on demineralized dentin were notably inhibited in the PA-biomodified groups, irrespective of PA concentration and biomodification duration. When challenged with exogenous collagenases, PA-biomodified bonded specimens exhibited significantly less biodegradation and maintained higher bond strengths than the untreated control. These results suggest that PA biomodification was effective at inhibiting proteolytic activity on demineralized dentin matrix and at stabilizing the adhesive/dentin interface against enzymatic degradation, is a new concept that has the potential to improve bonding durability.
Keywords: bonding durability, collagenolysis, crosslinking agents, dentin, proanthocyanidins
Introduction
There is common consensus that resin–dentin bonds created with contemporary hydrophilic dentin bonding systems deteriorate over time.1,2 Collagen disorganisation in the incompletely resin-infiltrated demineralized dentin matrix is one of the main degradation patterns found in unsuccessful adhesive restorations.3 Continuing efforts have focused on preservation of the integrity of the collagen matrix of the hybrid layer in an attempt to achieve durable dentin bonding. Considering the key role of matrix metalloproteinases (MMPs) in interfacial ageing over time, inhibiting the activity of host-derived MMPs and reducing the susceptibility of collagen matrices to MMP-induced degradation via bonding procedures may thus be a rational and effective approach for the improvement of bonding durability.2
Many attempts have been made to apply MMP inhibitors to acid-etched dentin prior to the application of adhesives.4,5,6 The only such inhibitor that has proved to be effective for reducing the degradation of resin–dentin bonds after in vivo ageing is chlorhexidine (CHX), a classic nonspecific MMP inhibitor.7 However, its MMP-inhibitory effect lasts only 9–12 months, and then weakens over time as CHX slowly leaches from the resin/dentin interface.8 Proanthocyanidins (PAs), a group of naturally occurring polyphenolic compounds, have the potential to control MMP-mediated diseases, such as periodontitis, by inhibiting both MMP production and activity.9 Epasinghe et al.10 demonstrated that PA can inactivate more than 90% of soluble recombinant MMP-2, -8 and -9 and approximately 70%–80% of cysteine cathepsin B and K; these results are significantly better those derived from the use of CHX.
Apart from being a potential non-selective MMP inhibitor, PA has gained much attention over the past few years because of its crosslinking capabilities, which are beneficial for enhancing demineralized dentin matrix and improving resin-dentin bond strength. Castellan et al.11,12,13 reported that PA pre-treatment increased the immediate elastic modulus of dentin matrix and was effective even after bacterial collagenase challenge or 1-year of storage in artificial saliva. Additionally, it provided enhanced immediate adhesion and long-term stabilisation to demineralized dentin after 1-year of ageing in water. Hechler et al.14 evaluated the long-term performance of PA application both as an additive to the adhesive and as a primer in an extra bonding step. They found that after 52 weeks' exposure to collagenase digestion, the bond strength of the PA-primer group was significantly higher than that of the control, whereas no significant difference was found between the PA-adhesive group and the control. This observation strongly supports the use of PA as a primer. However, all of the above-mentioned studies had a common limitation: the PA priming time was over 10 min, which is not clinically practical. An extra bonding step in PA biomodification for a clinically relevant duration improved the mechanical properties of demineralized dentin collagen15 as well as the immediate resin-dentin bond strength to dentin,16 and was effective at reducing the interfacial ageing caused by thermal cycling.17
The combined crosslinking and potential anti-collagenolytic effects of PA would be beneficial in preventing degradation of dentin collagen within the hybrid layer. Using a gravimetric collagenase digestion assay, Liu et al.18 indirectly showed that PA biomodification for clinically relevant time periods (as short as 10 s and 1 min) can enhance dentinal collagen's resistance towards enzymatic challenge. Nevertheless, further studies are still needed to directly confirm the anti-proteolytic effect of PA applied to demineralized dentin matrix for clinically relevant time periods, and its role in improving the resistance of the bonding interface to enzymatic degradation. Therefore, the aims of the present study were to investigate the potential effect of PA on endogenous gelatinolytic activity on demineralized dentin matrices, as well as to determine the capability of PA biomodification to reduce collagen biodegradation in demineralized dentin matrices and adhesive/dentin interfaces on challenge with bacterial collagenases in a clinically relevant manner. The null hypothesis was that transient PA biomodification would have no effect on gelatinolytic activity on demineralized dentin and would not increase the resistance of the dentin matrix or the bonding interface to collagenolysis.
Materials and methods
Extracted intact human third molars were collected with patients' informed consent, under a protocol approved by the Ethics Committee Board of the Fourth Military Medical University. The teeth were cleaned of adhering soft tissue, stored in physiological saline at 4 °C and used within 2 weeks of extraction. PA biomodifiers were prepared by dissolving PA-rich (≥95%) grape seed extract (Vitis vinifera L; Acetar Bio-Tech, Xi'an, China) into ethanol at 15% and 10%. CHX (2%) (DaSheng Chemical Tech, Xi'an, China) was used for the pre-treatment of positive controls, and untreated teeth were used as negative controls. The experimental design and procedures are summarized in Figure 1.
Figure 1.
Schematic illustration of experimental design and procedures. CHX, chlorhexidine; PA, proanthocyanidin.
Collagenolytic/gelatinolytic activity on PA-biomodified demineralized dentin matrix
Collagenase-mediated biodegradation
The effect of PA biomodification on collagenolytic activity on demineralized dentin matrix was assessed by measuring the hydroxyproline (Hyp) released from demineralized dentin after enzymatic challenge. Collagenase solutions were prepared by dissolving collagenase type I and type II (from Clostridium histolyticum, ≥125 collagen digestion units (CDU) per mg solid; Sigma-Aldrich, St Louis, MO, USA) in 0.2 mmol⋅L−1 phosphate-buffered solution (pH=7.4) at a concentration of 0.2% (m/m). Completely demineralized dentin slabs of approximately 0.5 mm×1.7 mm×7.0 mm were prepared from the mid-coronal dentin of 40 mol.15 Forty slabs were randomly assigned to each group based on the treatment (pre-treated with 15% PA, 10% PA or 2% CHX for 60 s, 120 s or 0 s (without pre-treatment)). Then, the slabs in each group were equally subdivided into two subgroups and exposed to 1 mL of either collagenase type I or type II solution at 37 °C, until the untreated specimens had completely disappeared, as judged by the naked eye. The collagenase solutions were changed every 24 h during the course of exposure. A 50 µL aliquot of the supernatant was collected for each group and was subjected to Hyp content analysis (Hydroxyproline Assay Kit; Sigma-Aldrich, St Louis, MO, USA) at pre-arranged time points, following the manufacturer's instructions. The absorbance was measured at 560 nm using a spectrophotometer (Tecan Group, Männedorf, Switzerland). The Hyp content in each group was determined as the average of duplicate measurements. The amount of dissolved collagen from demineralized dentin slabs was expressed as micrograms of hydroxyproline per milligram of the dry mass of the demineralized dentin before incubation.19,20
Activity of host-derived gelatinases
Seven aliquots (1 g each) of demineralized dentin powder were obtained and subjected to the above-mentioned biomodification. Then, specimens in each group were incubated in 1 mL of Adper Single Bond 2 (3M ESPE, St. Paul, MN, USA) for 24 h at 4 °C in the dark, thoroughly rinsed with acetone and repeatedly centrifuged (4 000g for 10 min each) at 4 °C. Enzyme extraction and zymographic analyses were carried out as previously described4,21 to evaluate the effect of PA on the gelatinolytic activity of host-derived MMPs on dentin.
Briefly, the specimens were suspended in the extraction buffer (50 mmol⋅L−l Tris-HCl, pH 6.0) to ultrasonically extract the enzyme proteins. The supernatants were collected by centrifugation. Then, proteins were precipitated at 4 °C by adding powdered ammonium sulphate, redissolved and further dialysed through a 30-kDa membrane overnight. Total protein concentrations in demineralized dentin powder extracts were determined by Bradford assay (Beyotime Biology, Haimen, China). Dentin proteins were electrophoresed under non-reducing conditions on 7.5% sodium dodecyl sulfate (SDS)–polyacrylamide gels copolymerized with 2 g⋅L−1 gelatin (GMS30071.1; Gemend, Shanghai, China). Gels were stained in 0.2% Coomassie Brilliant Blue R-250 and destained. Wet gelatine zymograms were scanned using an EagleEye II imaging system (Stratagene, Santa Clara, CA, USA).
Resistance of resin-dentin bonds to collagenase-mediated degradation
Microtensile testing
Bond strength testing is the gold standard for the assessment of bonding durability. Changes in the microtensile bond strength (μTBS) after enzymatic challenge were used as an indirect measure of collagenase-induced biodegradation of resin-dentin bonds.
The occlusal surfaces of 105 teeth were ground flat to expose the middle coronal dentin and abraded to create a uniform smear layer under water cooling. Each tooth was further cut perpendicularly to the exposed dentin surface into four blocks. All blocks were randomly assigned to seven groups and pre-treated as mentioned above. The bonded specimens were prepared using Single bond 2 as previously described20 and sectioned perpendicularly to the bonding interface into beams with a rectangular dimension of 0.8 mm×0.8 mm×8.0 mm, using a slow-speed diamond wafering blade under constant water irrigation.
All of the beams were stored in distilled water at 37 °C for 24 h. Then, the beams in each group were equally subdivided into four subgroups and exposed to the aforementioned collagenase type I or type II solution for various durations (120 h or 24 h, 40 beams for each subgroup). The collagenase solution was changed every 24 h during the experiment. After exposure, the specimens were rinsed before the μTBS testing was performed in a universal testing machine (EZ-test; Shimadzu, Tokyo, Japan), at a crosshead speed of 0.5 mm⋅min−1. The means and standard deviations of μTBS were calculated as previously described.22
Fracture mode analyses
All of the dentin fragments that had debonded from the specimens after μTBS testing were collected to determine the fracture mode under a stereomicroscope (MLC-150; Motic, San Antonio, TX, USA) as previously described.16 These fragments were classified as resulting from adhesive failure, mixed failure or cohesive failure of the resin or the dentin. Two representative fractured beams from each group with a μTBS close to the mean bond strength of that group were fixed, dried and gold sputter-coated for micromorphological evaluation under a field emission scanning electron microscope (S-4800; Hitachi, Tokyo, Japan).
Amount of collagen biodegradation
The amount of dissolved collagen from the resin–dentin bonding interface following collagenase digestion was measured as another, indirect, index of collagenase-induced hydrolysis in dentin collagen matrices by using the Hyp assay. This assay was performed as previously described with minor modifications.20 After fracture mode analyses, dentin halves within 1 mm of the fractured surface were collected (specimens with cohesive failure in dentin were excluded because their dentin halves were not involved with the hybrid layer), pulverized in liquid nitrogen and then incubated in distilled water for 24 h (100 mg⋅mL−1) at 37 °C with gentle shaking. All mixtures were separated by centrifugation (×1 000g, 5 min) at 4 °C. The supernatants were subjected to amino-acid analyses to determine the amount of dissolved collagen from the resin/dentin bonding interface as mentioned above.
Statistical analysis
Because the normality and homoscedasticity assumptions of the data were valid, a General Linear Model SPSS 13.0 program (SPSS, Chicago, IL, USA) for analysis of variance (ANOVA) (biomodification duration vs. exposure time to collagenase solution) was conducted to test the significance of variations in (i) biodegradation of demineralized dentin and (ii) μTBS values and collagen biodegradation in bonding surfaces among groups challenged with collagenases. Multiple comparisons between groups were evaluated by Tukey's post hoc test. Differences were deemed significant at P<0.05.
Results
Collagenolytic/gelatinolytic activity on PA-biomodified demineralized dentin matrix
Inhibitory effect on collagenase-mediated biodegradation
As observed with the naked eye, the untreated demineralized dentin slabs completely disappeared in collagenase type I or type II solution after 72 h or 60 h of exposure, respectively. In contrast, PA-biomodified specimens remained mostly intact, and defects could be seen in CHX-pre-treated specimens. Significantly greater collagen biodegradation was observed in the untreated control than in the other groups (P<0.05) (Figure 2). All PA groups presented significantly lower collagen biodegradation than did the CHX groups at the same time point (P<0.05). The lowest biodegradation was in the 15% PA-120 s group.
Figure 2.
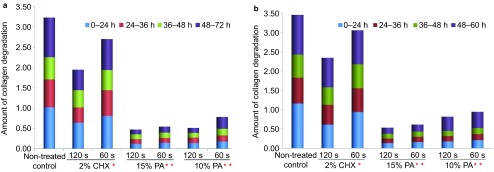
The amount of collagen biodegradation of demineralized dentin after challenge with collagenase. (a) Collagenase type I; (b) Collagenase type II. Amount of collagen degradation in ×10−4, m/m. *Significantly lower collagen biodegradation was observed in the 2% CHX group than the untreated group at the same time point (P<0.05). **PA preconditioning significantly reduced the collagen biodegradation compared with the other groups, regardless of PA concentration and treatment duration (P<0.01). CHX, chlorhexidine; PA, proanthocyanidin.
Inactivation of host-derived gelatinases
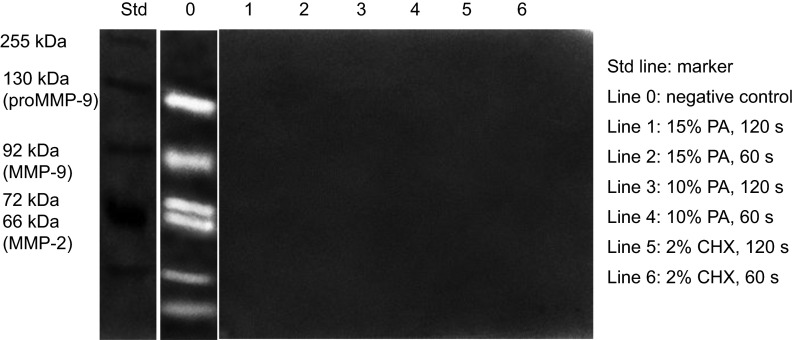
Multiple forms of gelatinases were detected in demineralized dentin powder after Single Bond 2 application without biomodification (Figure 3), including an intense band at 66 kDa identified as the active form of MMP-2, a 72 kDa band identified as proMMP-2 and 130 and 92 kDa bands identified as the latent and activated MMP-9 forms. In addition, other minor gelatinolytic bands with lower molecular weights were fainter but detectable. Complete inhibition of all forms of MMP-2 and MMP-9 in dentin matrices was exhibited in PA-biomodified groups.
Figure 3.
Gelatin zymography of MMPs from dentin extracts with or without PA pre-treatment. The relative molecular masses of MMP-2 and MMP-9, expressed in kDa, are presented in Std lane. After pre-treatment, gelatinolytic bands produced by MMP-2 and MMP-9 were visible only in the untreated group. CHX, chlorhexidine; MMP, matrix metalloproteinase; PA, proanthocyanidin.
Resistance of resin–dentin bonds to collagenase-mediated degradation
The μTBS values of the PA groups were not significantly reduced after challenge with collagenase type I for 24 h, irrespective of PA concentration and biomodification duration (P>0.05). By contrast, the μTBS values of the untreated control and CHX groups decreased significantly (P<0.05) (Table 1). The µTBS advantage of PA groups over the untreated control persisted even after 120 h exposure (P<0.05), although the values decreased to some extent. The highest μTBS value was exhibited by the 15% PA-120 s group. No significant differences were observed between the 10% PA (120 or 60 s) groups and the CHX-120 s group. Exposure to collagenase type II for 24 h significantly reduced the μTBS of all groups. Prolonging the exposure time to 120 h resulted in a notably decreased μTBS for the untreated control (P<0.05), whereas no significant changes occurred in the biomodified groups (P>0.05).
Table 1. μTBS of bonded specimens with or without PA pre-treatment after collagenase challenge.
| Non-treated controls | Pre-treatment groups | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure time | Collagenase | 2% CHX | 10% PA | 15% PA | ||||
| 120 s | 60 s | 120 s | 60 s | 120 s | 60 s | |||
| 0 h | 27.89a,A (6.42) | 33.42b,A (7.14) | 31.61 b,A (5.18) | 32.38 b,A (3.72) | 29.37a,A (5.00) | 33.56 b,A (6.64) | 31.65b,A (8.04) | |
| 24 h | Type I | 22.45a,B (5.68) | 25.71a,B (5.19) | 23.99a,B (5.58) | 29.04b,A (6.44) | 26.13a,A (4.81) | 32.01 b,A (6.10) | 29.67b,A (5.63) |
| Type II | 16.28a,C (5.16) | 22.78b,C (4.83) | 20.29b,C (4.91) | 23.48b,B (5.41) | 21.75b,B (5.36) | 26.50 c, B (5.93) | 23.96b,B (6.58) | |
| 120 h | Type I | 13.89a,C (3.05) | 20.17b,C (5.19) | 17.09a,C (4.85) | 21.63b,B (5.96) | 19.26b,B (4.51) | 26.52 c,B (4.61) | 24.06c,B (5.01) |
| Type II | 10.97a,D (3.12) | 20.28 b,C (5.54) | 18.26b,C (3.59) | 21.03b,B (5.79) | 19.87b,B (5.28) | 23.87 b,B (4.73) | 22.73b,B (3.41) | |
CHX, chlorhexidine; PA, proanthocyanidin; μTBS, microtensile bond strength.
Values are mean (±standard deviation), in MPa, n=40. Group identified by different upper and lower case letters are significantly different for columns and rows, respectively (P<0.05).
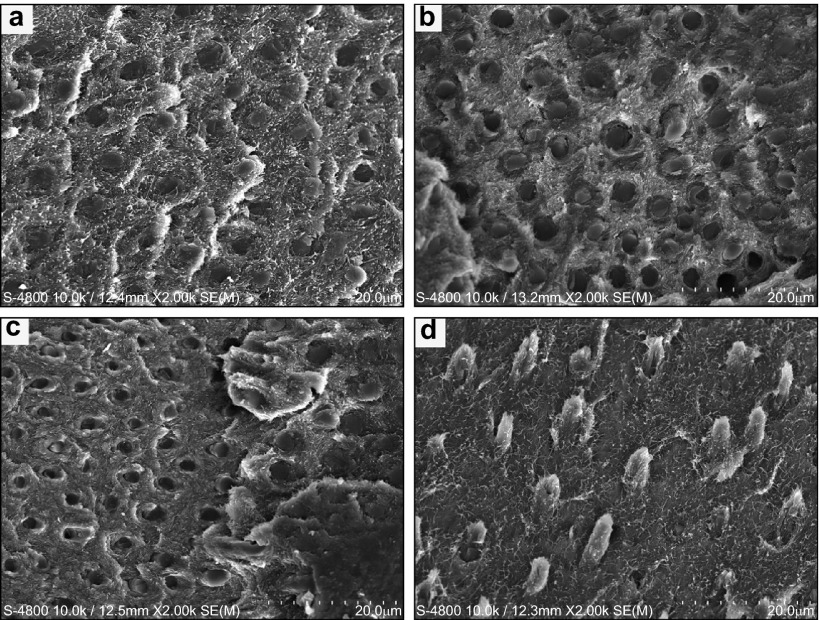
Fracture mode analyses revealed that biomodified specimens exhibited a high percentage of mixed failures (Figure 4). Fractures mostly happened at the top of the hybrid layer, with numerous resin tags tightly filling the dentinal tubules, and intertubular dentin was partially sealed by resin (Figure 5a–5c). Adhesive failures accounted for a large percentage of failures except for the mixed failures in the untreated control group, mostly at the bottom of the hybrid layer (Figure 5d).
Figure 4.
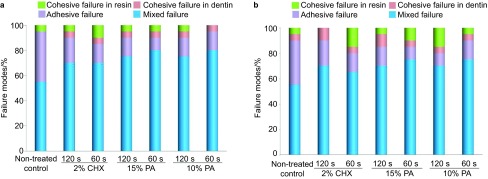
Comparison of the failure modes in different groups after exposed to collagenase type I or type II solution for 120 h. (a) Collagenase type I; (b) Collagenase type II.
Figure 5.
Representative FESEM micrographs of fractured dentin surfaces. (a) 15% PA-120 s group failed at the top of the hybrid layer; (b) Mixed failure happened at the top of hybrid layer was the most prevalent fracture mode in 10% PA-120 s group; (c) 2% CHX-120 s group presented adhesive failure; (d) Non-treated control group fractured at the bottom of the hybrid layer. FESEM, field emission scanning electron microscope; PA, proanthocyanidin; CHX, chlorhexidine.
Biomodified groups presented significantly less collagen biodegradation than the untreated control (P<0.05) (Table 2). No significant differences were found in collagen biodegradation among groups pre-treated for 120 s (P>0.05). Among those pre-treated for 60 s, the 15% PA group presented the least collagen degradation, followed by the 10% PA and 2% CHX groups, regardless of the type of collagenase and the exposure time (24 h or 120 h) (P<0.05).
Table 2. Collagen biodegradation of bonded specimens with or without PA pre-treatment after collagenase challenge.
| Non-treated controls | Pre-treatment groups | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure time | Collagenase | 2% CHX | 10% PA | 15% PA | ||||
| 120 s | 60 s | 120 s | 60 s | 120 s | 60 s | |||
| 24 h | Type I | 0.129a,A (0.008) | 0.022b,A (0.004) | 0.063d,A (0.001) | 0.019b,A (0.005) | 0.037c,A (0.009) | 0.008 b,A (0.001) | 0.023b,A (0.002) |
| Type II | 0.229a,C (0.010) | 0.096b,B (0.023) | 0.137b,C (0.002) | 0.091b,B (0.050) | 0.118b,C (0.016) | 0.082 b,B (0.005) | 0.083b,B (0.003) | |
| 120 h | Type I | 0.166a,B (0.012) | 0.096b,B (0.016) | 0.104c,B (0.008) | 0.074b,B (0.016) | 0.099b,B (0.013) | 0.069b,B (0.003) | 0.095b,B (0.001) |
| Type II | 0.256a,D (0.020) | 0.169 b,C (0.002) | 0.217c,D (0.016) | 0.154b,C (0.022) | 0.187b,D (0.016) | 0.145b,C (0.009) | 0.173b,C (0.009) | |
CHX, chlorhexidine; PA, proanthocyanidin.
Values are mean (±standard deviation), in ×10−4, m/m. Group identified by different upper and lower case letters are significantly different for columns and rows, respectively (P<0.05).
Discussion
Several MMPs, such as MMP-1, -2, -8 and -9, are secreted as proenzymes in the human dentin matrix and in saliva. These enzymes have been suggested to be activated in the low-pH environment of the mouth and to be responsible for the progressive breakdown of collagen matrices in resin-sparse regions at the bottom of bonding interfaces.21,23 Two mechanisms have been proposed to explain the degradation of collagen fibrils: namely, collagenolysis and gelatinolysis. Collagenases (e.g., MMP-1, -8) and gelatinase A (MMP-2) can cleave collagens into 1/4- and 3/4-fragments at the Gly-Leu/Ile peptide bond, where the collagen peptide structure determines both the specific cleavage and the binding sites for MMPs.24 The fragments then denature at body temperature and can be further degraded by gelatinases (e.g., MMP-2, -9) and other nonspecific tissue proteinases.25 As MMP-2 and MMP-9 are distributed widely in mature human coronal dentin,26 we directly evaluated the activity of these host-derived gelatinases on PA-biomodified dentin matrix by gelatin zymography. We indirectly assessed the collagenolytic activity of bacterial collagenases on PA-biomodified demineralized dentin matrix by measuring the release of Hyp into hydrolysates of media because only small amounts of endogenous collagenases (e.g., MMP-1, -8) are present in dentin matrix. The results revealed that transient PA biomodification was effective at inhibiting proteolytic activity and markedly improved the resistance of bonding interfaces to enzymatic degradation. The null hypothesis was consequently rejected.
CHX, a classic non-selective MMP inhibitor, was used for the pre-treatment of positive controls in this study because the application of 2% CHX to the demineralized dentin surface is the only procedure that has been proven to be clinically effective against bond degradation.7,27 Importantly, the µTBS of the PA groups was significantly higher than that of the CHX groups after collagenase exposure, yet the extent of collagen biodegradation was similar (Table 2). This finding may be ascribed to the considerable proportion of mineralized dentin involved in the specimens (dentin halves within 1 mm of the fractured surface). This may have reduced the differences between the collagenase-mediated biodegradation of PA groups and that of CHX groups, leading to no significant difference (Table 2). In light of this finding, the results of the collagenase-mediated biodegradation of completely demineralized dentin slabs exposed to a high concentration of collagenase solution (the section on ‘Collagenase-mediated biodegradation') may provide a more realistic assessment of the clinical situation and can be used as a reference for the long-term assessment of dentin biomodification on resistance to collagenase challenge. Figure 2 shows that PA exhibited significantly higher protease inactivation potential than CHX at the same exposure duration. This result may be ascribed to the use of a higher concentration of PA than of CHX because PA has better biocompatibility than CHX. Additionally, the distinct modes of action of PA and CHX in stabilising collagen could explain the superiority of PA. CHX is capable of competitively binding to metal ions, such as zinc and calcium, which are essential for proteases such as MMPs, thereby inhibiting the catalytic activity of these proteases.6 For PA, three other mechanisms of action have been proposed,28,29,30 in addition to its metal chelating activity similar to CHX, although PA–protease interactions have not yet been well elucidated. First, the protease resistance may be achieved via irreversible conformational changes of proteases within the catalytic domain or allosteric inhibition of other modular domains that coparticipate in collagen biodegradation.2,31 Second, PA may indirectly interfere with protease production and activation by modulating host immune responses.32 Third, the induction of exogenous crosslinks in dentin matrix by crosslinkers, such as PA, increases the density of the collagen network and reduces the swelling ratio of demineralized dentin, indicating a decrease in the collagenase absorption,33 thereby enhancing the matrix resistance against enzymatic degradation. The advantage of inactivating proteolytic enzymes in the dentin matrix by crosslinking is that the mechanism is nonspecific—that is, it crosslinks all types of MMPs and cysteine cathepsins.34 These crosslinks involve covalent bonds that are stable over time, unlike the reversible electrostatic binding of CHX.5 Accordingly, Macedo et al.35 proposed that collagenase inactivation by crosslinking agents should be long-lasting and may be even more effective than using inhibitors because collagenases do not turnover in dentin. Our results for the anti-proteolytic effect of PA, in comparison to CHX, also support this notion.
In accordance with previous reports,16,33 PA-biomodified groups challenged with collagenase showed a higher percentage of mixed failures, mainly at the top of the hybrid layer. Our results suggested that the poorly infiltrated demineralized dentin at the bottom of the hybrid layer was mechanically strengthened by PA biomodification and contributed to the stabilisation of the bonding interface.
Clinically, our PA biomodification protocol is less favourable than directively adding PA into adhesive system, because it adds an extra step to the bonding protocol, which is in contrast to the clinician's preference for simplification. However, this may result in a lower risk of interfering with the well-balanced monomer-solvent cocktails. Our previous investigation confirmed that the degree of conversion was not significantly affected by this additional PA biomodification step.16 A critical problem encountered with PA biomodification when attempting to achieve durable dentin bonding is that the resin-sparse collagen matrices are still retained within the hybrid layer. The present results further revealed that the μTBS values of PA-biomodified specimens were reduced than that before degradation and that Hyp was still detectable after enzymatic challenge. So the denuded collagen fibrils are structurally unstable and face enduring challenges as long as they lack protection from resin or apatite. Even if these demineralized collagen fibrils can be stiffened 50-fold with crosslinking agents, the resulting modulus of elasticity is still far inferior to that of resin-infiltrated dentin.2 Despite the limitations of this protocol, PA biomodification is helpful to limit the negative influence of host-derived proteases on the hybrid layer formed during dentin bonding, to maintain the stability of the bonding interface and to gain time for the slow process of mineral precipitation in resin-sparse regions in the hybrid layer to seal the denuded collagen fibrils (i.e., for remineralisation).36,37 In this regard, the application of PA in the bonding procedure could be a beneficial complementary approach to other strategies in the attempt to overcome the critical barriers currently encountered in dentin bonding.
Conclusions
Within the limits of this study, it may be concluded that transient PA biomodification of demineralized dentin decreased proteolytic activity on collagen matrices and enhanced the resistance of the bonding interface to enzymatic degradation. This new protocol has the potential to improve bonding durability and could be an adjunct approach to other strategies, such as remineralisation, in an attempt to achieve more durable dentin bonding.
Acknowledgments
The present study was supported by research funds from the Natural Science Foundation of China (No. 81130078 and No. 81000458) and Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13051).
References
- Breschi L, Mazzoni A, Ruggeri A, et al. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24 (1:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tjaderhane L, Breschi L, et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90 (8:953–968. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Ohno H, Kaga M, et al. In vivo degradation of resin–dentin bonds in humans over 1 to 3 years. J Dent Res. 2000;79 (6:1385–1391. doi: 10.1177/00220345000790060601. [DOI] [PubMed] [Google Scholar]
- Breschi L, Martin P, Mazzoni A, et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater. 2010;26 (6:571–578. doi: 10.1016/j.dental.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschi L, Mazzoni A, Nato F, et al. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dent Mater. 2010;26 (4:320–325. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Munck J, van den Steen PE, Mine A, et al. Inhibition of enzymatic degradation of adhesive–dentin interfaces. J Dent Res. 2009;88 (12:1101–1106. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- Ricci HA, Sanabe ME, de Souza Costa CA, et al. Chlorhexidine increases the longevity of in vivo resin–dentin bonds. Eur J Oral Sci. 2010;118 (4:411–416. doi: 10.1111/j.1600-0722.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- Sadek FT, Braga RR, Muench A, et al. Ethanol wet-bonding challenges current anti-degradation strategy. J Dent Res. 2010;89 (12:1499–1504. doi: 10.1177/0022034510385240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La VD, Howell AB, Grenier D. Cranberry proanthocyanidins inhibit MMP production and activity. J Dent Res. 2009;88 (7:627–632. doi: 10.1177/0022034509339487. [DOI] [PubMed] [Google Scholar]
- Epasinghe DJ, Yiu CK, Burrow MF, et al. The inhibitory effect of proanthocyanidin on soluble and collagen-bound proteases. J Dent. 2013;41 (9:832–839. doi: 10.1016/j.jdent.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Castellan CS, Pereira PN, Grande RH, et al. Mechnical characterization of proanthocyandin–dentin matrix interaction. Dent Mater. 2010;26 (10:968–973. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan CS, Bedran-Russo AK, Karol S, et al. Long-term stability of dentin matrix following treatment with various natural collagen cross-linkers. J Mech Behav Biomed Mater. 2011;4 (7:1343–1350. doi: 10.1016/j.jmbbm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan CS, Bedran-Russo AK, Antunes A, et al. Effect of dentin biomodification using naturally derived collagen cross-linkers: one-year bond strength study. Int J Dent. 2013;2013:918010. doi: 10.1155/2013/918010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechler B, Yao X, Wang Y. Proanthocyanidins alter adhesive/dentin bonding strengths when included in a bonding system. Am J Dent. 2012;25 (5:276–280. [PMC free article] [PubMed] [Google Scholar]
- Liu R, Fang M, Xiao Y, et al. The effect of transient proanthocyanidins preconditioning on the cross-linking and mechanical properties of demineralized dentin. J Mater Sci Mater Med. 2011;22 (11:2403–2411. doi: 10.1007/s10856-011-4430-4. [DOI] [PubMed] [Google Scholar]
- Fang M, Liu R, Xiao Y, et al. Biomodification to dentin by a natural crosslinker improved the resin–dentin bonds. J Dent. 2012;40 (6:458–466. doi: 10.1016/j.jdent.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Liu RR, Fang M, Zhao SJ, et al. [The potential effect of proanthocyanidins on the stability of resin-dentin bonds against thermal cycling.] Zhonghua Kou Qiang Yi Xue Za Zhi 201247(5268–272.Chinese. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen M, Yao X, et al. Enhancement in dentin collagen's biological stability after proanthocyanidins treatment in clinically relevant time periods. Dent Mater. 2013;29 (4:485–492. doi: 10.1016/j.dental.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Shiiba M. Lysine hydroxylation and cross-linking of collagen. Methods Mol Biol. 2008;446:95–108. doi: 10.1007/978-1-60327-084-7_7. [DOI] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Agee KA, Hoshika T, et al. The inhibitory effect of polyvinylphosphonic acid on functional matrix metalloproteinase activities in human demineralized dentin. Acta Biomater. 2010;6 (10:4136–4142. doi: 10.1016/j.actbio.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Mannello F, Tay FR, et al. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res. 2007;86 (5:436–440. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- Hosaka K, Nishitani Y, Tagami J, et al. Durability of resin–dentin bonds to water- vs. ethanol-saturated dentin. J Dent Res. 2009;88 (2:146–151. doi: 10.1177/0022034508328910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkala M, Tervahartiala T, Sorsa T, et al. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52 (2:121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Perumal S, Antipova O, Orgel JP. Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc Natl Acad Sci U S A. 2008;105 (8:2824–2829. doi: 10.1073/pnas.0710588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaderhane L, Nascimento FD, Breschi L, et al. Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater. 2013;29 (1:116–135. doi: 10.1016/j.dental.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu LN, Zhang L, Jiao K, et al. Localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in human coronal dentine. J Dent. 2011;39 (8:536–542. doi: 10.1016/j.jdent.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Carrilho MRO, Geraldeli S, Tay F, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86 (6:529–533. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- La VD, Bergeron C, Gafner S, et al. Grape seed extract suppresses lipopolysaccharide-induced matrix metalloproteinase (MMP) secretion by macrophages and inhibits human MMP-1 and -9 activities. J Periodontol. 2009;80 (11:1875–1882. doi: 10.1902/jop.2009.090251. [DOI] [PubMed] [Google Scholar]
- Ebrahimi A, Schluesener H. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res Rev. 2012;11 (2:329–345. doi: 10.1016/j.arr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Ku CS, Sathishkumar M, Mun SP. Binding affinity of proanthocyanidin from waste Pinus radiata bark onto proline-rich bovine achilles tendon collagen type I. Chemosphere. 2007;67 (8:1618–1627. doi: 10.1016/j.chemosphere.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Sela-Passwell N, Rosenblum G, Shoham T, et al. Structural and functional bases for allosteric control of MMP activities: can it pave the path for selective inhibition. Biochim Biophys Acta. 2010;1803 (1:29–38. doi: 10.1016/j.bbamcr.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Feghali K, Feldman M, La VD, et al. Cranberry proanthocyanidins: natural weapons against periodontal diseases. J Agric Food Chem. 2012;60 (23:5728–5735. doi: 10.1021/jf203304v. [DOI] [PubMed] [Google Scholar]
- Nam K, Kimura T, Kishida A. Physical and biological properties of collagen-phospholipid polymer hybrid gels. Biomaterials. 2007;28 (20:3153–3162. doi: 10.1016/j.biomaterials.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cova A, Breschi L, Nato F, et al. Effect of UVA-activated riboflavin on dentin bonding. J Dent Res. 2011;90 (12:1439–1445. doi: 10.1177/0022034511423397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo GV, Yamauchi M, Bedran-Russo AK. Effects of chemical cross-linkers on caries-affected dentin bonding. J Dent Res. 2009;88 (12:1096–1100. doi: 10.1177/0022034509351001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. Biomimetic remineralization of resin-bonded acid-etched dentin. J Dent Res. 2009;88 (8:719–724. doi: 10.1177/0022034509341826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008;29 (8:1127–1137. doi: 10.1016/j.biomaterials.2007.11.001. [DOI] [PubMed] [Google Scholar]