SUMMARY
Recalcitrant microbial infections demand new therapeutic options. Here we present an approach that exploits two prongs of the host immune cell antimicrobial response: the oxidative burst and the compartmentalization of copper (Cu) within phagolysosomes. The prochelator QBP is a nontoxic protected form of 8-hydroxyquinoline (8HQ) in which a pinanediol boronic ester blocks metal ion coordination by 8HQ. QBP is deprotected via reactive oxygen species produced by activated macrophages, creating 8HQ and eliciting Cu-dependent killing of the fungal pathogen Cryptococcus neoformans in vitro and in mouse pulmonary infection. 8HQ ionophoric activity increases intracellular Cu, overwhelming the Cu-resistance mechanisms of C. neoformans to elicit fungal killing. The Cu-dependent antimicrobial activity of 8HQ against a spectrum of microbial pathogens suggests that this strategy may have broad utility. The conditional activation of Cu ionophores by innate immune cells intensifies the hostile antimicrobial environment and represents a promising approach to combat infectious disease.
INTRODUCTION
The need for new therapeutics to treat infectious disease is widely recognized, but the scarcity of candidates in the drug pipeline is alarming (Butts and Krysan, 2012). Opportunistic fungal infections in particular are difficult to treat, as fungi are ubiquitously found in the environment, are biochemically similar to mammalian cells, and have limited classes of drugs available (Day et al., 2013). New strategies and molecules with innovative mechanisms of action to increase efficacy against their pathogenic targets must be developed.
The transition metal copper (Cu) is essential for most forms of life but can also be toxic, a duality that provides a promising tactic for antimicrobial therapy development. Metallic Cu, Cu salts, and Cu compounds have long been used to control bacterial, fungal, and algal growth in agricultural and health care settings (Borkow and Gabbay, 2005; Grass et al., 2011). However, pathogenic microbes ultimately infect their host, and it is becoming apparent that mammalian hosts use Cu to fight infections (Hodgkinson and Petris, 2012; Samanovic et al., 2012). At the macroscopic level, serum Cu levels increase in response to infection, and Cu-deficient individuals are highly susceptible to infections (Milanino and Buchner, 2006; Percival, 1998). At the cellular level, an emerging model of Cu mobilization within macrophages during infection is now coming into focus (Achard et al., 2012; Hodgkinson and Petris, 2012; Wagner et al., 2005; White et al., 2009).
One of the many roles of macrophages is to ingest and destroy pathogens in specialized phagosomal compartments. Upon macrophage activation, these compartments present a hostile environment that includes an oxidative burst of hydrogen peroxide and nitric oxide, along with lytic enzymes and acidic pH (Flannagan et al., 2009). In addition, macrophages activated with lipopolysaccharide (LPS) or interferon-gamma (IFN-γ) increase expression of the cell-surface Cu importer Ctr1 while concurrently increasing levels of the Cu exporter ATP7A, which partially localizes to phagosomes (White et al., 2009). The concentration of Cu in Mycobacteria-containing phagolysosomes can reach hundreds of micromolar (Wagner et al., 2005). This paradigm of compartmentalizing phagosomal Cu in response to infection directly contrasts the concurrent movement of biologically important metals, iron (Fe) and zinc (Zn), which are actively expelled to starve pathogens of these essential metals (Hood and Skaar, 2012).
Increasingly, the importance of Cu resistance pathways in pathogenic bacteria and fungi is being recognized (Achard et al., 2012; Ding et al., 2013; González-Guerrero et al., 2010). Although the mechanism by which macrophage-associated Cu elicits microbial toxicity are unclear, bacterial pathogens counter Cu stress by inducing the expression of genes encoding multi-Cu oxidases and Cu export machinery (Achard et al., 2010; Fu et al., 2013; Solioz et al., 2010; Ward et al., 2010; White et al., 2009). Fungi also adapt to elevated Cu during infection; for example, Cryptococcus neoformans induces the expression of genes encoding metallothioneins (MTs), cysteine-rich metal binding proteins, to handle elevated host Cu in the lung (Ding et al., 2013). These advances in Cu biology expose unexplored opportunities to develop antimicrobial agents that accentuates host Cu.
We hypothesized that small molecules can be designed as novel antimicrobial agents that operate by manipulating Cu at the host-pathogen axis. Prerequisites for such molecules are that they selectively mobilize endogenous Cu during infection, avoid disrupting host metal status, exhibit Cu-based pathogen killing, and evade the Cu-resistance mechanisms of the pathogen. Here, we report a compound based on 8-hydroxyquinoline (8HQ), a metal-binding scaffold with known antimicrobial activity (Anderson and Swaby, 1951). Although the mechanism of action of 8HQ is multifaceted, its ability to form lipophilic, neutral complexes with Zn(II) and Cu(II) that translocate these metal ions across cell membranes independent of metal pumps and transporters has been well documented (Li et al., 2010; Tardito et al., 2011; Zhai et al., 2010). In addition to antimicrobial activity, compounds in this family have also shown metal-dependent activity against cancer and neurodegenerative diseases (Adlard et al., 2008; Tardiff et al., 2012; Tardito et al., 2011; Zhai et al., 2010). However, the metal-dependent toxicity extends to healthy mammalian cells as well. Intraperitoneal administration of 8HQ and its derivatives as Cu chelates results in toxicity that is generally far worse than the compounds alone (Bernstein et al., 1963; Leanderson and Tagesson, 1996; Oliveri et al., 2012; Tardito et al., 2012). Surface administration of 8HQ has been deemed safe at low concentrations, but the data are insufficient to support safety at higher concentrations or for systemic administration (Andersen, 2006).
To mitigate off-target effects, we take advantage of QBP, a prochelator form of 8HQ that is inactive and unable to bind Cu unless its boronic ester masking group is removed by reacting with hydrogen peroxide to release 8HQ (Figure 1A) (Dickens and Franz, 2010). The oxidative burst generated by activated macrophages is sufficient to mediate conversion of nontoxic QBP to 8HQ, which subsequently elicits Cu-dependent cytotoxicity. We use the opportunistic fungal pathogen C. neoformans to show that 8HQ exerts its fungicidal effects by increasing cell-associated Cu to overwhelm the Cu detoxification capacity of C. neoformans. This work establishes prochelators as a class of antimicrobial compounds that synergize with the host’s response to infection and thereby disrupt the efficacy of microbial Cu detoxification.
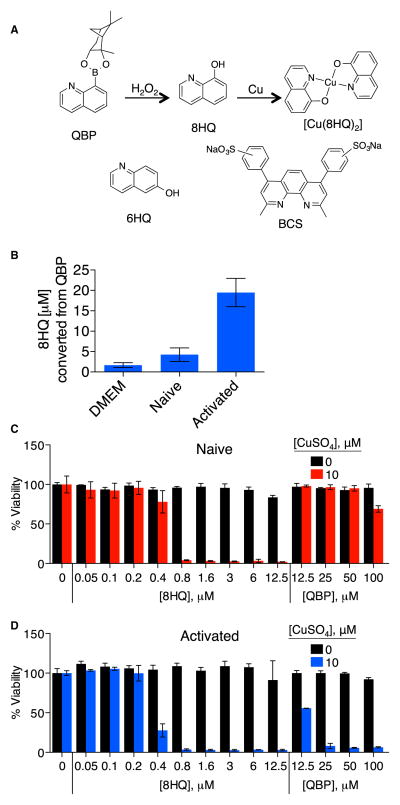
Figure 1. QBP Is Converted to 8HQ in RAW 246.7 Macrophages.
(A) Schematic of the reaction of prochelator QBP, which contains a pinanediol boronic ester masking group that blocks metal binding, with hydrogen peroxide (or peroxynitrite) giving the metal-binding agent 8HQ (nontoxic boric acid and pinanediol side products not shown). Other compounds used in this study include 6HQ, in which the position of the OH eliminates the O,N chelating motif that makes 8HQ an effective agent for binding a range of di- and trivalent metal ions, and BCS, a Cu(I) chelator.
(B) Plot showing the concentration of 8HQ detected by LC-MS analysis of RAW 246.7 cells treated with 200 μM QBP overnight. Whereas 8HQ was detected at appreciable levels in activated RAW 264.7 cells, 8HQ was not observed above the limit of detection in naive cells or cell-free medium (DMEM). Cells were activated with 30 μg/ml of LPS and 5 ng/ml of IFN-γ. Error bars represent SD.
(C and D) Viability plots of RAW 264.7 macrophage-like cells. Cell viability of naive (C) and activated (D) RAW cells in the presence or absence of 10 μM supplemental CuSO4 and increasing concentrations of 8HQ or QBP. Error bars represent SD.
RESULTS
QBP Converts to 8HQ in the Presence of Activated RAW Macrophages
We hypothesized that the oxidative conditions present upon macrophage activation would facilitate the conversion of QBP, which does not bind Cu, to the Cu-chelating ionophore 8HQ (Figure 1A). QBP is stable in aqueous solutions between pH 5 and 8, although some hydrolysis of the pinanediol occurs at lower and higher pH values, leaving quinoline boronic acid (QBA), a molecule that remains ill equipped to bind metals (Dickens and Franz, 2010). We previously showed that boronate-based prochelators such as QBP and QBA require interaction with specific oxidative species of either H2O2 or peroxynitrite (OONO−) to convert to their metal-binding phenol derivative (Kielar et al., 2012; Sikora et al., 2011). QBP, originally conceived in the context of Alzheimer’s disease, converts to the [Cu(8HQ)2] complex when reacted in vitro with a peroxide-generating combination of amyloid-beta peptide and Cu (Dickens and Franz, 2010).
Here we activate murine RAW 264.7 macrophage-like cells (RAW cells) with LPS and IFN-γ treatment. Both naive and activated RAW cells were incubated with 200 μM QBP overnight, and the amount of converted 8HQ was quantitated by liquid chromatography and mass spectrometry (LC-MS) (Figure 1B; Figures S1 and S2 available online). Samples from naive RAW cells provided LC-MS chromatograms showing the presence of QBP but only trace quantities of 8HQ, which were not significantly different from the 4 μM detection limit of the cell-free control (Figure 1B; Figure S2). However, samples from activated RAW cells provided chromatograms with a mass signal consistent with the presence of about 20 μM 8HQ, indicative of approximately 10% QBP conversion and indicating that the niche of activated macrophage-like cells is suitable to convert QBP to 8HQ (Figure 1B).
QBP and 8HQ Display Differential Toxicity to RAW Cells
The Cu-dependent cytotoxic effects of 8HQ have been documented in a number of cell lines (Darby and Nathan, 2010; Tardito et al., 2012). Because the metal-binding capacity of 8HQ is masked in QBP, we reasoned that QBP has diminished toxicity compared with 8HQ. In culture media without supplemental Cu, naive RAW cells were not viable in 8HQ concentrations above 12.5 μM but tolerated QBP at concentrations as high as 100 μM, the highest dose tested. Upon coaddition with 10 μM CuSO4, viability was lost at concentrations of 0.8 μM 8HQ and higher. In contrast, naive RAW cells remained viable at all combinations of Cu and QBP, with a slight decrease at the highest 100 μM dose of QBP (Figure 1C).
Activated RAW cells cultured with Cu also showed 8HQ-dependent cytotoxicity but were even more sensitive, with a cytotoxic threshold occurring at 0.4 μM 8HQ (Figure 1D). As expected, activated RAW cells cultured with both Cu and QBP showed a dramatic decrease in viability (Figure 1D), supporting previous data that activated macrophages stimulate conversion of QBP to 8HQ to elicit Cu-dependent killing.
8HQ and Peroxide-Activated QBP Are Antifungal in the Presence of Cu
Consistent with previous reports (Ding et al., 2013), we found that C. neoformans grows normally in culture supplemented with 1 mM Cu (Figure 2A), which is much higher than what many cell types withstand. Without supplemental Cu, the minimal inhibitory concentration (MIC) of 8HQ that prevents C. neoformans growth is 400 μM (Table 1). However, the combination of 5 μM 8HQ with either high (1 mM) or low (10 μM) concentrations of Cu inhibits growth (Figure 2A; Figure S4). With supplementation of 1, 10, and 100 μM Cu, the MIC values for 8HQ decrease to 50, 6, and 6 μM, respectively (Table 1). In contrast to 8HQ, neither 6-hydroxyquinoline (6HQ), an isomer of 8HQ that does not bind Cu, nor QBP inhibits growth at concentrations as high as 400 μM in the presence of 1 mM Cu (Figure 2A; Figure S3).
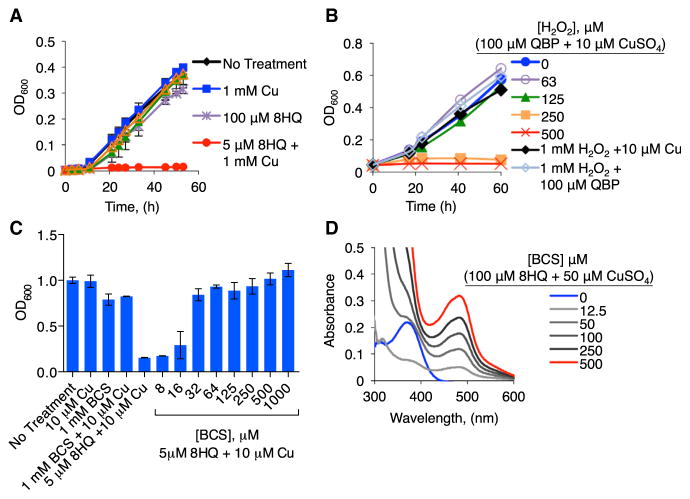
Figure 2. Effects of Cu, 8HQ, QBP, and H2O2 on C. neoformans Growth.
(A and B) Growth, as monitored by optical density after 60 hr, of WT C. neoformans H99 strain grown in SC medium at 30°C with (A) 8HQ, QBP with or without 1 mM CuSO4 (B) 100 μM QBP and 10 μM CuSO4 with indicated concentrations of hydrogen peroxide. 8HQ plus Cu inhibits growth, while QBP requires stimulatory H2O2 to have an effect, consistent with prochelator-to-chelator conversion under these conditions.
(C) BCS at various concentrations and in combination with 5 μM 8HQ and 10 μM CuSO4. BCS reverses the growth inhibition of 8HQplus Cu.
(D) UV-visible spectra of 100 μM 8HQ and 50 μM CuSO4 in SC media with increasing concentrations of BCS from 0 to 500 μM. Error bars represent SD.
Table 1.
MICs of 8HQ and QBP in the Presence of Varying Concentrations of Cu
| Organism | 8HQ
|
QBP
|
|||
|---|---|---|---|---|---|
| 0 mM Cu | 1 mM Cu | 10 mM Cu | 100 mM Cu | 100 mM Cu | |
| C. neoformans | 400 | 50 | 6 | 6 | >400 |
|
| |||||
| C. glabrata | 500 | 50 | 12 | 6 | >400 |
|
| |||||
| S. aureus | 100 | 25 | 12 | 6 | >400 |
|
| |||||
| E. coli | 100 | 100 | 200 | a | >400b |
|
| |||||
| S. typhimurium | 400 | 400 | 200 | 100 | >400 |
MIC represented in micromolar compound.
E. coli was unable to grow in 100 μM Cu in M9 minimal media.
In M9 minimal media, E. coli was unable to grow in 100 μM Cu. The value presented here is in the presence of 10 μM Cu.
The ineffectiveness of 6HQ and QBP to inhibit growth emphasizes the importance of Cu binding as a critical property of 8HQ’s antifungal activity. To ascertain if QBP can be conditionally converted to 8HQ and bind Cu to inhibit C. neoformans growth in vitro, 100 μM QBP was coincubated with 10 μM Cu and increasing concentrations of H2O2. In the presence of 250 μM H2O2 and 10 μM Cu, otherwise inert concentrations of QBP became antifungal (Figure 2B). H2O2 at these concentrations, alone or with Cu, did not inhibit growth of C. neoformans (Figure 2B); therefore, this activity cannot be attributed simply to H2O2 and Cu reactivity. Although the concentration of H2O2 resulting from this bolus dose appears higher than would be expected in a macrophage, its effective concentration likely diminishes rapidly as a result of enzymatic consumption and does not mimic the persistent peroxide generation that occurs during macrophage activation (Antunes and Cadenas, 2000). Regardless of these considerations, our observation that QBP converts to 8HQ upon macrophage activation argues that the reactive oxygen species (ROS) produced by these cells are sufficient for activating QBP (Figure 1B). Combined, these data support the hypothesis that 8HQ, converted from peroxide-activated QBP, interacts with Cu in a manner that inhibits growth of C. neoformans.
Metal specificity of 8HQ-dependent antifungal activity was determined by growth in the presence of sublethal (10 μM) concentrations of Cu(II), Zn(II), Fe(III), and Ag(I) (Figure S4). C. neoformans growth was not inhibited by Fe(III) at any of the 8HQ concentrations tested. The addition of Zn(II) or Ag(I) with 100 μM 8HQ prolonged the transition from lag-phase to exponential growth. None of these metals demonstrated a synergistic antifungal effect with 8HQ as observed with Cu.
As further validation of the Cu requirement on 8HQ toxicity, the cell impermeable Cu(I) chelator bathocuproine disulfonic acid (BCS) showed a dose-dependent ability to restore growth of C. neoformans in the presence of inhibitory concentrations of Cu and 8HQ (Figure 2C). To bolster the assumption that this result was due to BCS competing with 8HQ for binding Cu, a solution of 100 μM 8HQ and 50 μM CuSO4 was prepared in synthetic complete (SC) growth medium, and an absorption band centered at 375 nm was observed spectrophotometrically as a characteristic feature of Cu(II) complexation by 8HQ (Figure 2D). Addition of BCS resulted in the dose-dependent appearance of a new absorption band centered at 483 nm, a characteristic feature of the [Cu(BCS)2]3− complex. These data reveal that BCS competes with 8HQ for binding Cu, which is readily reduced from Cu(II) to Cu(I) upon BCS binding.
8HQ-Cu Complexes Are Fungicidal
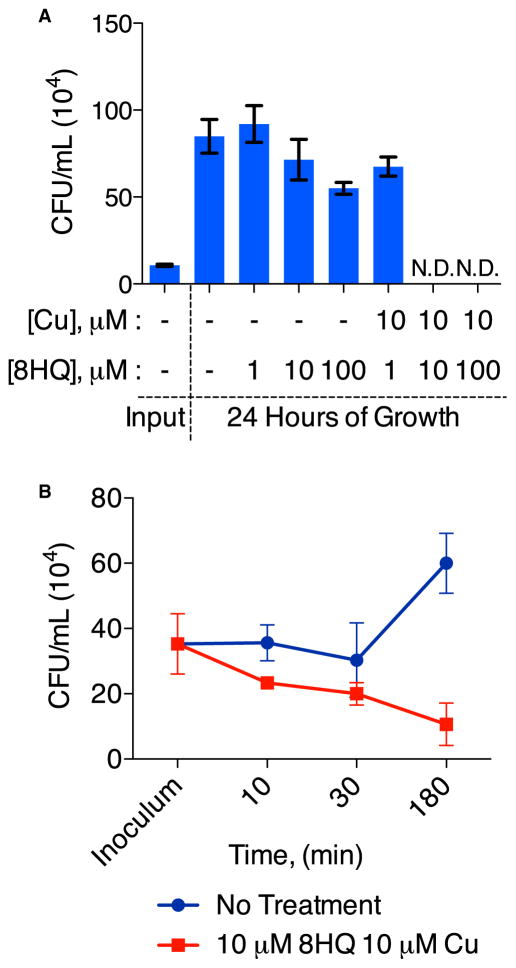
To determine whether 8HQ-Cu is fungicidal or fungistatic, C. neoformans was incubated with 8HQ or 8HQ plus Cu, and colony-forming units (cfu) were quantitated for each condition after 24 hr. Fungi grown in medium containing up to 100 μM 8HQ without Cu were able to replicate. With coadministration of 10 μM Cu, replication was observed only for concentrations of 8HQ lower than the MIC value (Figure 3A). No viable fungi were detected for higher concentrations of 8HQ in combination with 10 μM Cu (Figure 3A). Moreover, after 3 hr, we observed a reduction in the number of colonies recovered from cultures containing 10 μM 8HQ and 10 μM Cu, whereas untreated C. neoformans cells were able to propagate (Figure 3B), suggesting that the 8HQ-Cu combination is a fast-acting fungicide.
Figure 3. 8HQ-Cu Combination Is Fungicidal.
(A) The cfu obtained from samples of C. neoformans initially grown in liquid SC medium containing indicated concentrations of 8HQ with or without supplemental CuSO4 for 24 hr.
(B) Time course of cell replication as measured by the number of C. neoformans colonies formed after incubation in SC medium without treatment or with the addition of 10 μM 8HQ and 10 μM CuSO4. The fungicidal effect of 10 μM 8HQ plus 10 μM CuSO4 on fungal growth occurs within the first 3 hr of treatment, as shown by a steady decrease in the recovery of viable cfu. Error bars represent ±SEM in (A) and SD in (B).
8HQ Increases Cellular Cu Accumulation and Bioavailability
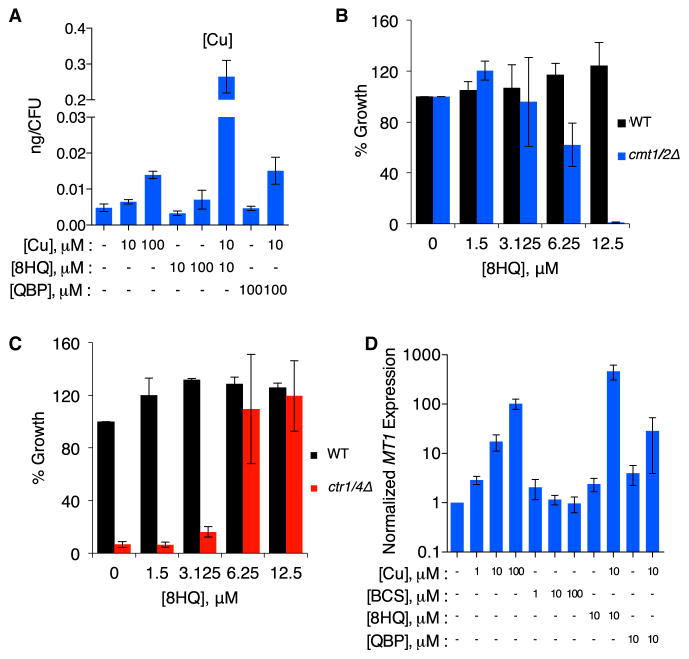
To evaluate the effect of 8HQ on metal allocation, total metal content of C. neoformans cells was measured by inductively coupled plasma mass spectrometry (ICP-MS). Figure 4A shows that treatment of C. neoformans with 8HQ and Cu together results in ~40 times more cell-associated Cu than treatment with equivalent amounts of Cu alone. Incubation with QBP under the same conditions did not increase C. neoformans-associated Cu, consistent with the absence of Cu-binding activity by QBP. In addition, cell-associated Zn and Fe levels were unchanged in conditions tested, providing further evidence for Cu specific action of 8HQ (Figure S5).
Figure 4. 8HQ Transports Cu into C. neoformans.
(A) Plot of the amount of Cu detected by ICP-MS analysis of WT C. neoformans treated with or without various combinations of CuSO4 and/or compound, as indicated. The combination of 10 μM 8HQ and 10 μM CuSO4 increases cell-associated Cu significantly more than 100 μM CuSO4 alone (notice the break in the y axis).
(B) Comparison of the growth of WT C. neoformans with the double MT knockout strain cmt1Δcmt2Δ as a function of 8HQ concentration in SC media. The growth defect of the mutant strain at concentrations of 8HQ that are well tolerated by the WT suggests that MTs are required to tolerate the increased Cu stress promoted by 8HQ.
(C) Growth comparison of WT C. neoformans versus the ctr1Δctr4Δ strain as a function of 8HQ concentration in YPEG medium without Cu supplementation. Without a cell-surface Cu transporter to import Cu, ctr1Δctr4Δ cells are unable to grow in these conditions. 8HQ overcomes this growth defect, suggesting that 8HQ transports trace Cu from the medium.
(D) The relative expression level, as quantified by quantitative PCR, of the MT gene CMT1 as a function of the indicated concentrations of CuSO4, BCS, 8HQ, or QBP. The strength of CMT1 induction increases with concentration of Cu. As a control, the extracellular Cu(I) chelator BCS does not lead to a change in CMT1 expression. 8HQ and QBP (10 μM) alone do not cause robust induction of CMT1, while 8HQplus Cu (10 μM each) leads to CMT1 levels greater than what is observed with 100 μM CuSO4 alone. ICP-MS and expression data are represented by SEM; error bars in growth assays represent SD.
The ICP-MS data, combined with the differential effects on fungal growth to the extracellular chelator BCS versus the cell permeable ionophore 8HQ, endorse a mechanism of action whereby 8HQ increases intracellular Cu. Consistent with this hypothesis, a C. neoformans strain lacking the Cu-detoxifying MT proteins (CMT1 and CMT2) was predicted to be hypersensitive to treatment with 8HQ (Ding et al., 2013). In SC medium with trace Cu, the cmt1Δcmt2Δ mutant grows equivalently to the isogenic wild-type (WT) parental strain. However, addition of 8HQ partially inhibits growth of the cmt1Δcmt2Δ mutant at 6.25 μM 8HQ and completely inhibits at 12.5 μM 8HQ (Figure 4B).
The reciprocal experiment was performed using a C. neoformans mutant lacking the functionally redundant high-affinity Cu(I) importers Ctr1 and Ctr4 (Ding et al., 2011). Growth of C. neoformans in YPEG medium containing ethanol and glycerol as sole carbon sources requires Cu for cytochrome oxidase-dependent respiration. Although WT cells grow in YPEG, the ctr1Δctr4Δ mutant does not (Ding et al., 2011). However, growth of this mutant was restored to near WT levels by addition of 6.25 μM 8HQ (Figure 4C). These results suggest that 8HQ shuttles Cu present in the growth medium into cells, bypassing the Ctr1- and Ctr4-dependent Cu(I) import mechanisms and rendering Cu bioavailable for cytochrome oxidase.
On the basis of this Cu shuttling activity, we predicted that 8HQ would activate the Cu-sensing transcriptional activator Cuf1, leading to increased expression of CMT1. Consistent with published reports (Ding et al., 2011), CMT1 expression increased with increasing Cu and was unchanged in response to Cu limitation by BCS, as determined by quantitative real-time PCR (Figure 4D). Compared with modest CMT1 gene activation observed upon treatment of cells with 10 μM Cu alone (~17-fold), treatment with 10 μM 8HQ in addition to Cu resulted in a strongly synergistic ~460-fold CMT1 induction. The addition of 100 μM QBP along with 10 μM Cu, on the other hand, resulted in only a ~29-fold induction, similar to Cu alone. These results suggest that C. neoformans responds to and mounts a high-Cu response in the presence of 8HQ by increasing expression of CMT1.
8HQ-Cu Has Broad-Spectrum Antimicrobial Activity
To assess the possible broad-spectrum applicability of the QBP strategy, we tested the effects of 8HQ against other pathogens in vitro. The fungal commensal and opportunistic pathogen of mucosal surfaces, Candida glabrata, was treated with increasing concentrations of 8HQ or QBP in the presence of increasing Cu and monitored for growth to determine the MIC. Similar to the results observed with C. neoformans, QBP did not inhibit growth, whereas 8HQ inhibited growth upon increasing the concentration of Cu (Table 1). In the absence of supplemental Cu, the concentration required for 8HQ alone to prevent growth of C. glabrata was 400 μM, but the MIC decreased to 50, 12.5, and ~6.25 μM in the presence of 1, 10, and 100 μM CuSO4, respectively, revealing that 8HQ is similarly effective against C. glabrata compared with C. neoformans.
We chose Staphylococcus aureus Newman strain as an example of Gram-positive bacteria (Table 1). As was the case with the two fungi, S. aureus failed to grow in the presence of 8HQ and increasing concentrations of supplemental Cu. For this bacterium, the MIC shifted to lower concentrations of 8HQ as the concentration of Cu increased, with the lowest MIC being the combination of 6.25 μM 8HQ with 100 μM CuSO4, similarly susceptible to the 8HQ-Cu combination when compared with the two fungi.
Two Gram-negative bacteria, Escherichia coli and Salmonella typhimurium, were tested against 8HQ and QBP (Table 1); these organisms responded to treatment with 8HQ differently than the other pathogens. The MIC of 8HQ for these organisms was ≥100 μM 8HQ at all concentrations of supplemental Cu, suggesting either a different antimicrobial mechanism of 8HQ or an enhanced resistance mechanism of these bacteria. These results demonstrate that 8HQ has broad-spectrum antimicrobial activity, with the level of in vitro activity varying by organism.
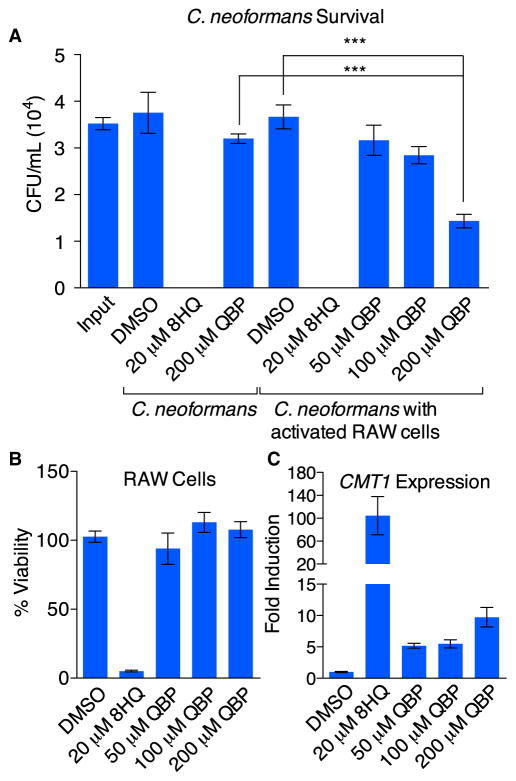
QBP Enhances the Fungicidal Activity of Activated RAW Cells
Given that 8HQ and Cu are fungicidal to C. neoformans, and that activated macrophages convert QBP to 8HQ, the impact of QBP on C. neoformans viability in the presence of activated RAW cells was evaluated. We observed a dose-dependent reduction in C. neoformans cfu when activated RAW cells were treated with QBP. C. neoformans showed 41% survival after 6 hr of coincubation with activated macrophages and 200 μM QBP with 2 μM supplemental Cu (Figure 5A). However, 91% of C. neoformans survived when treated with QBP in the absence of macrophages, confirming that killing of C. neoformans by QBP was mediated by the action of RAW cells. As expected, treatment with 20 μM 8HQ was fungicidal but concurrently reduced RAW cell viability as well (Figure 5B). Importantly, the reduction in C. neoformans proliferation in the presence of activated RAW cells and QBP did not diminish the viability of RAW cells (Figure 5B). In these coculture assays, expression of CMT1 increased as a function of QBP concentration, with ~10-fold induction of the MT at the highest concentration of QBP tested (200 μM) (Figure 5C). Treatment with 20 μM 8HQ resulted in a 100-fold induction of CMT1. Although Figure 5A indicates that there were no viable cells recovered after coculture for 6 hr upon 8HQ treatment in a 96-well format, we were still able to recover RNA when the assay was adapted to a 6-well format for RNA isolation. These data further support the evidence that QBP conversion to 8HQ by macrophages leads to an increased Cu response by C. neoformans, highlighting a role of Cu overload in its antifungal activity.
Figure 5. QBP Aids Killing of C. neoformans by Macrophages.
(A) Comparison of the survival of C. neoformans in the absence or presence of activated RAW 264.7 cells (MOI = 1:10). C. neoformans viability was quantified 6 hr after incubation at 37°C by enumeration of cfu produced for each condition. No C. neoformans cells were recovered upon treatment with 8HQ. A statistically significant reduction in the survival of C. neoformans was detected when cocultured with macrophages and 200 μM QBP compared with QBP in cell-free medium, suggesting that the macrophages convert QBP to 8HQ. Data are represented as mean ± SEM (***p < 0.001).
(B) Cell viability of the infected and treated macrophages described in (A). Whereas 8HQ was toxic, QBP was not toxic at any concentration tested, even at 200 μM, which was shown in (A) to reduce C. neoformans survival.
(C) Expression of CMT1 in C. neoformans cocultured with macrophages in the same conditions as in (A) and (B) increases as the concentration of QBP rises and correlates with the observed antifungal activity in (A). All error bars represented are SEM.
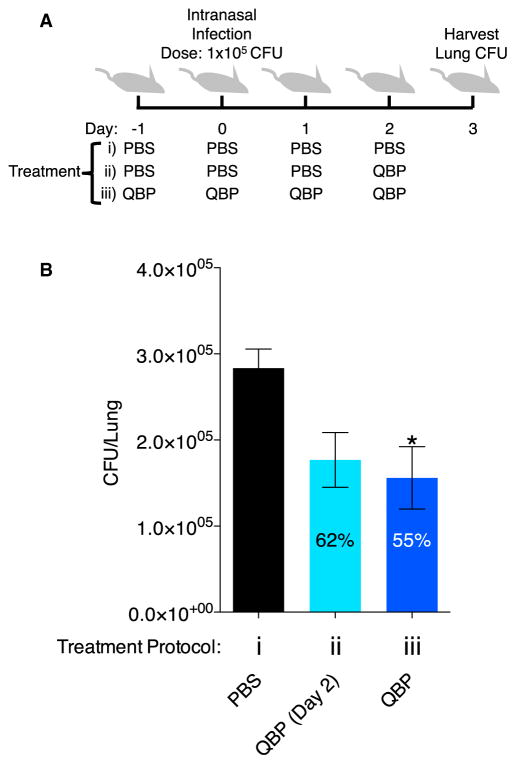
QBP Reduces Fungal Burden during Mouse Lung Infection
To test the effect of QBP on the burden of C. neoformans in a mouse model of lung infection, we intranasally infected mice. Mice were divided into three groups: a vehicle-only control group and two treatment groups that received different dosing regimens of QBP. The control group was given four doses of PBS/1% DMSO vehicle: one the day before infection, one the day of infection, and two doses after infection (Figure 6Ai). One treatment group received only one dose total of QBP on day 2 (5 mg/kg QBP, 50 μl intranasal dose), administered 2 days after infection (Figure 6Aii), while the second treatment group received four QBP doses (5 mg/kg QBP, 50 μl intranasal dose) the day before infection, the day of infection, and doses 1 and 2 days after infection (Figure 6Aiii). At 3 days after infection, the lungs were harvested to quantify the remaining cfu of C. neoformans. Compared with PBS control, mice receiving four doses of QBP had a significantly reduced fungal burden in their lungs (Figure 6B). The animals that received only one dose of QBP the day prior to lung harvest did show a decrease in fungal burden, but this was not significant (Figure 6B). QBP was active in the mouse lung, resulting in a reduction in the number of viable C. neoformans cells, suggesting that it is converted to the anti-fungal compound 8HQ in the context of an infected lung.
Figure 6. Mice Treated with QBP Have a Decreased Fungal Burden.
(A) Schematic of the treatment regimen for three groups of animals (each with n = 7) on indicated days. Treatment group 1 received DMSO vehicle control (1% DMSO, PBS) from day −1 to day 2, treatment group 2 received DMSO vehicle control from day −1 to day 1 and then one final dose of 5 mg/kg QBP on day 2, and treatment group 3 received 5 mg/kg QBP from day −1 to day 2. Data are presented as mean ± SEM (*p < 0.05).
(B) On day 3, lungs were harvested from mice, and the cfu per lung were enumerated by homogenizing the entire organ and plating a dilution series on SC medium.
On the basis of reports that the immune response and immune cell counts can be perturbed by either high or low levels of serum Cu (Kelley et al., 1995; Pocino et al., 1991), we sought to determine if administration of QBP or 8HQ had any observable effects on immune cell types in the serum. We dosed mice intraperitoneally daily with QBP, 8HQ, or a DMSO control over the course of 2 weeks, after which complete blood counts revealed that there was no significant change in various cell types (lymphocytes, monocytes, eosinophils, and basophils) (Figure S6). Furthermore, no appreciable changes in weight were observed for mice treated with QBP or 8HQ. Combined, these results provide preliminary evidence that these doses of QBP have no adverse effects in mice in the conditions tested.
DISCUSSION
In the face of escalating antibiotic resistance and lethal fungal infections, there is a clear need for new strategies to identify antimicrobial therapeutics with minimal toxicity to host cells. Cu mobilization is used by the immune system to kill pathogens, but these pathogens have developed mechanisms to cope with elevated Cu. The results presented here provide a strategy for the development of compounds that exploit the activated immune response and override Cu detoxification machinery in fungal and bacterial pathogens, thereby boosting antimicrobial activity by the host. The prodrug QBP takes advantage of two prongs of the immune response, the first being the increase of ROS in phagolysosomes and surrounding tissues that convert QBP to 8HQ. We show by analytical methods that QBP is nontoxic and remains intact in naive RAW macrophages but converts to 8HQ in activated cells. The second element of the immune system responsible for activating QBP is the mobilization of Cu during infection to facilitate formation of antimicrobial 8HQ-Cu complexes. It is unclear if the antimicrobial Cu used by macrophages derives from intracellular stores or extracellular recruitment. Although no direct role in Cu donation has been reported, the major Cu-containing protein in the plasma, ceruloplasmin, is elevated during infection and inflammation, potentially providing a source of Cu to immune cells (Bakhautdin et al., 2013; Conforti et al., 1982). In principle, these two requirements for efficacy of QBP as a prodrug endow it with a unique conditional antimicrobial activity that focuses the toxicity of 8HQ to sites of infection. Although we cannot conclude that the activity of QBP in the mouse is Cu dependent, data from our in vitro experiments strongly support this hypothesis.
C. neoformans, a fungal pathogen that causes lethal meningitis in humans, expresses several Cu-dependent enzymes, including laccase for melanin production, Cu/Zn superoxide dismutase for superoxide disproportionation and multi-Cu oxidases for Fe uptake. Although the presence of these proteins implies a requirement for Cu acquisition, conditions of acute Cu deprivation created by BCS in culture media do not limit growth. C. neoformans grows equally well in high or low Cu conditions, and it is striking that low concentrations of Cu combined with 8HQ kill C. neoformans. We present evidence that this fungicidal effect is due to 8HQ’s ionophoric properties, which increase cell-associated and bioavailable Cu and strongly induce Cu-responsive transcription.
Although both BCS and 8HQ bind Cu by forming five-membered chelate rings, significant differences in the chemistry of these Cu-chelate complexes directly relate to their observed biological activity. BCS enforces anionic tetrahedral Cu(I) complexes, of formula [Cu(BCS)2]3−, that resist aerial oxidation and remain extracellular. Its much greater affinity for Cu(I) than Cu(II) (log β2 = 19.8 versus 11.8, respectively) makes BCS an excellent reagent for limiting Cu bioavailability via sequestration of Cu(I) (Xiao et al., 2011). On the other hand, 8HQ forms neutral, flat, square planar complexes with Cu(II), of formula [Cu(8HQ)2], that can permeate cell membranes. Although its overall affinity for Cu(II) predicts strong binding (log β2 = 26.2) (Johnston and Freiser, 1952), 8HQ has negligible affinity for Cu(I), suggesting that Cu can be transferred via reductive transmetalation from 8HQ to a Cu(I) binding agent, as we observed in the competition experiment shown in Figure 2D. These properties categorize 8HQ as an ionophore that operates by increasing intracellular bioavailable Cu. Corroborating the data presented here, the hydroxyquinoline derivative clioquinol increases the amount of cell associated Cu in Saccharomyces cerevisiae, providing bioavailable Cu to the Sod1 chaperone Ccs1 (Li et al., 2010). Hyperinduction of CMT1 in the presence of 8HQ and Cu and recovery of growth of the ctr1Δctr4Δ mutant by 8HQ suggest that 8HQ similarly supplies Cu in a bioavailable form to C. neoformans.
Our results show that 8HQ-derived Cu evades the normally robust Cu tolerance and detoxification mechanisms used by C. neoformans to survive in Cu-rich environments in vitro. We recently reported that pulmonary infection of mice with C. neoformans resulted in induction of the CMT genes, which are essential for virulence (Ding et al., 2013). These in vivo results in combination with the data presented here suggest that pharmacologically targeting the mechanisms of C. neoformans for Cu tolerance may be a promising antifungal strategy.
The mechanistic details of how Cu, supplied by 8HQ, evades the C. neoformans Cu maintenance machinery to induce toxicity remain to be elucidated. Perhaps increased oxidative stress driven by Fenton-type reactions, or displacement of metals from physiological protein coordination sites, in particular exposed Fe-sulfur clusters, plays a role in 8HQ-Cu toxicity (Macomber et al., 2007; Valko et al., 2005). Both possibilities suggest that Cu is released from 8HQ in cells, a probable scenario given that the reducing intracellular environment favors reduction of Cu(II) to the more labile Cu(I), which would dissociate from 8HQ. An alternative, or perhaps synergistic, activity could result from the liberation of free 8HQ inside the cell, which could then bind other metals or inhibit metalloenzymes, as has been observed in some cases (Baum et al., 2007; Fraser and Creanor, 1975). Yet another possibility is that intact [Cu(8HQ)2] complexes are a source of toxicity. Molecules in the hydroxyquinoline family have been suggested to inhibit the proteasome as intact, neutral complexes that induce cell death (Cater and Haupt, 2011; Daniel et al., 2005; Tardito et al., 2012; Zhai et al., 2010). Although such pathways have not been well characterized in C. neoformans, we cannot rule them out for the fungicidal activity of 8HQ and Cu. However, the strong transcriptional response of CMT1 gives weight to a role for Cu toxicity being driven mostly by released Cu, not intact [Cu(8HQ)2] complexes.
Treatment of a panel of bacterial and fungal pathogens showed that 8HQ has potential to be broadly antimicrobial. The differing sensitivity of organisms to 8HQ-Cu cotreatment remains an interesting subject for further investigation, as the antimicrobial mechanisms against prokaryotes and eukaryotes may be different. The fungi and Gram-positive organisms tested here appear to be sensitive to the 8HQ and Cu combination at similar levels. S. aureus appears to be more sensitive to Cu alone compared with C. neoformans (growth inhibition in culture at 300 μM versus 2.5 mM, respectively) (Baker et al., 2010; Ding et al., 2013). The two Gram-negative bacteria were less sensitive to 8HQ than the other organisms tested in our study, and this toxicity was independent of exogenous Cu (Table 1). The acid-fast bacterium Mycobacterium tuberculosis has also been shown to be sensitive to the effects of 8HQ, but a role for Cu was not elucidated in that study (Darby and Nathan, 2010). The antimicrobial properties of Cu-8HQ may be due to a combination of effects that are specific to each organism, such as altered metabolism, different effects of bioavailable Cu, species-specific targets of 8HQ, or differences in their Cu homeostasis machinery.
Significance
In this work, we have laid the foundation for the utilization of molecules that are conditionally activated in the niche of infection to bind Cu and override the Cu detoxification machinery of microbial pathogens. In doing so, these molecules utilize and bolster key aspects of the immune system to kill fungi yet minimize cytotoxicity to the host. Discovery of new antifungal drugs is notoriously difficult because of the biochemical similarities of typical drug targets between eukaryotic pathogens and eukaryotic host cells. QBP exploits the unique chemical milieu created by the host in an antifungal targeting strategy. Furthermore, this work represents an important advance in developing broad-spectrum antimicrobial agents on the basis of Cu biology. Because Cu homeostasis is a universal aspect of biology, microbes have devised a number of ways to survive when faced with increased Cu during infection. Small molecules that sneak Cu through these biochemical checkpoints therefore provide a powerful paradigm for targeting a broad spectrum of pathogens. Furthermore, both pathogenic and commensal microorganisms have evolved to deal with metal availability in general that is controlled by the host. Prochelators that are optimized for different metals or under different activating conditions may therefore enable selective redistribution of metals more broadly, and not just at sites of infection but in other disease states as well.
EXPERIMENTAL PROCEDURES
Detailed outline of experimental procedures for LC-MS detection of QBP and 8HQ, fungicidal assay, UV-visible analysis, ICP-MS analysis, quantitative real-time PCR, and intraperitoneal mice experiments are outlined in Supplemental Experimental Procedures.
Strains and Media
C. neoformans H99 and C. glabrata strains were streaked onto solid SC medium (MP Biomedicals) from frozen stocks stored at −80°C. For experiments, one colony was picked and grown in an overnight culture of SC medium. Overnight cultures were diluted, washed, or aliquoted as appropriate for the experimental protocols described below. E. coli and S. typhimurium ser. typhimurium strains were maintained on Luria broth solid medium and grown in liquid M9 minimal medium. S. aureus Newman strain was grown on tryptic soy broth (BD Biosciences) for the duration of experiments. MIC experiments were performed in triplicate in 96-well plate format in the media stated above.
Growth Curves
The broth microdilution method described by the Clinical and Laboratory Standards Institute, with minor modifications for Cryptococcus, was used to determine the susceptibility of strains in this study. Briefly, C. neoformans cultures in liquid SC medium were inoculated from a single colony grown on solid SC medium and incubated overnight at 30°C with shaking at 200 rpm. Cell suspensions were diluted to an optical density at 600 nm (OD600) of 0.002 with fresh SC medium and aliquoted into 96-well plates. Test compounds were added from DMSO stock solutions to final concentrations ranging from 400 to 0.4 μM, with <0.5% DMSO. For each test run, a compound-free positive growth control and a cell-free negative control were included. For experiments using added metal ions, fresh stock solutions of CuSO4, ZnCl2, ferric ammonium citrate, and AgNO3 were prepared in deionized water and added to appropriate wells at final concentrations as indicated in figure legends. Plates were incubated at 30°C and read every 4 hr for 48 hr. Growth curves were generated by plotting OD600 readings versus time. All tests were performed in triplicate for each condition in a single experiment, and two separate experiments were carried out. MIC was defined as the lowest concentration of compound at which growth was not detected at 24 hr.
Cell Culture
The mouse RAW 264.7 macrophage-like cell line obtained from the Duke Cell Culture Facility was maintained, and experiments were carried out, unless otherwise noted, in supplemented Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 g/L D-glucose (Gibco, Invitrogen) and 10% fetal bovine serum (FBS), 100 U penicillin-streptomycin, at 37°C and 5% CO2. Experiments were carried out on cells passaged between three and ten times. For experiments requiring activated macrophages, IFN-γ and LPS (Sigma-Aldrich) were added simultaneously with the compound treatments at final concentrations of 5 ng/ml and 30 μg/ml, respectively. Concentrations used were determined empirically by treating RAW cells with increasing amounts of these activators and assessing the fluorescence response of the resulting oxidation of 2′,7′-dichlor-odihydrofluorescein diacetate oxidation.
Cytotoxicity Assay
Macrophages were seeded in 100 μl of supplemented DMEM at 2.5 × 105 cells/ml in a 96-well plate. Aliquots of 8HQ, QBP, and CuSO4 were added to final concentrations as indicated in figure legends. The concentration of DMSO as treatment vehicle was adjusted to 0.1% in all wells, including the no-treatment control. Plates were incubated at 37°C, and cell viability was assessed after 24 hr by using the Cell Titer Glo assay (Promega), per the manufacturer’s protocol. All tests were performed in triplicate for each condition in a single experiment, and two separate experiments were carried out.
Macrophage Coculture Assay
Macrophages were seeded in 100 μl of supplemented DMEM at 2.5 × 105 cells/ml in a 96-well plate and incubated at 37°C. After 24 hr, the growth medium was removed, cells were washed 2 times with PBS buffer (pH 7.4), and cells were activated by the addition of IFN-γ (5 ng/ml) and LPS (30 μg/ml) in 100 μl of nonsupplemented DMEM with 4.5 g/LD-glucose. Cells were simultaneously treated with 2 μM CuSO4 and indicated concentrations of 8HQ or QBP in DMEM without FBS supplementation. Cells were infected immediately with C. neoformans (H99) at a multiplicity of infection (MOI) of 1:10 (macrophage/yeast). After a 6 hr incubation at 37°C, the cell medium of infected macrophages was removed and saved. Macrophages were lysed with 0.1% Triton X-100 in deionized water, and lysates were combined with spent medium previously collected. Dilutions of this preparation were plated on solid SC, and cfu were enumerated after incubation at 30°C for 2 days. Coculture experiments in which RNA was isolated for quantitative real-time PCR analysis were designed in the same manner, with the exception of being performed in 6-well plates, as detailed in Supplemental Experimental Procedures.
Animal Infection and Treatment with QBP
Female A/J mice were purchased from the National Cancer Institute at age 4 to 6 weeks. The mice were given a week to acclimate at Duke facilities. Infections were carried out according to published protocols (Crabtree et al., 2012). Doses (50 μl/day) of PBS control (1% DMSO dissolved in PBS) or QBP (5 mg/kg equivalent dissolved in PBS) were administered intranasally to anesthetized mice (250 mg/kg tribromoethanol; Avertin). The dose of QBP administered to mice was determined as the maximal amount soluble in a 50 μl preparation.
Statistical Analysis
The error bars in all growth curves and cytotoxicity assays represent the SD of an experiment done in triplicate. The conversion of QBP to 8HQ in macrophages error bars represents the SD of at least six samples per treatment over two different passages of macrophages. In the fungicidal assays, ICP-MS assay, and macrophage coculture assays, the error bars represent the SEM from three experiments. In the animal infection experiment, the error bars represent the SEM of seven mice per group. One-way ANOVA was performed to determine significance for macrophage coculture assay and for animal infection experiments.
Ethics Statement
All experiments involving animals were approved by the Institutional Animal Care and Use Program (protocol number A013-13-01) at Duke University.
Supplementary Material
Acknowledgments
We gratefully acknowledge Kim Hutchison for running ICP-MS samples at North Carolina State University, Raleigh. We thank Dr. George Dubay for thoughtful insight with the LC-MS experiments, Chen Ding for helpful suggestions, and Andrew Franks for help with ROS fluorescence assays. This work was supported by NIH grants GM100678-02 (R.A.F.), GM007105-40 (M.E.H.), GM041840 and AI106013 (D.J.T.), and GM084176 (K.J.F.).
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and six figures and can be found with this article online at http://dx.doi.org/10.1016/j.chembiol.2014.06.009.
AUTHOR CONTRIBUTIONS
R.A.F. and M.E.H. designed and performed experimental assays. K.J.F. and D.J.T. designed and supervised this study. All authors reviewed and interpreted data and provided input in writing and reviewing the manuscript.
References
- Achard MES, Tree JJ, Holden JA, Simpfendorfer KR, Wijburg OLC, Strugnell RA, Schembri MA, Sweet MJ, Jennings MP, McEwan AG. The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect Immun. 2010;78:2312–2319. doi: 10.1128/IAI.01208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard MES, Stafford SL, Bokil NJ, Chartres J, Bernhardt PV, Schembri MA, Sweet MJ, McEwan AG. Copper redistribution in murine macrophages in response to Salmonella infection. Biochem J. 2012;444:51–57. doi: 10.1042/BJ20112180. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, et al. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Andersen A. Final amended report on the safety assessment of oxyquinoline and oxyquinoline sulfate as used in cosmetics. Int J Toxicol. 2006;25(Suppl 1):1–9. doi: 10.1080/10915810600716570. [DOI] [PubMed] [Google Scholar]
- Anderson BI, Swaby RJ. Factors influencing the fungistatic action of 8-hydroxyquinoline (oxine) and its metal complexes. Aust J Sci Res, B. 1951;4:275–282. doi: 10.1071/bi9510275. [DOI] [PubMed] [Google Scholar]
- Antunes F, Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000;475:121–126. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- Baker J, Sitthisak S, Sengupta M, Johnson M, Jayaswal RK, Morrissey JA. Copper stress induces a global stress response in Staphylococcus aureus and represses sae and agr expression and biofilm formation. Appl Environ Microbiol. 2010;76:150–160. doi: 10.1128/AEM.02268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhautdin B, Febbraio M, Goksoy E, de la Motte CA, Gulen MF, Childers EP, Hazen SL, Li X, Fox PL. Protective role of macrophage-derived ceruloplasmin in inflammatory bowel disease. Gut. 2013;62:209–219. doi: 10.1136/gutjnl-2011-300694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum EZ, Crespo-Carbone SM, Klinger A, Foleno BD, Turchi I, Macielag M, Bush K. A MurF inhibitor that disrupts cell wall biosynthesis in Escherichia coli. Antimicrob Agents Chemother. 2007;51:4420–4426. doi: 10.1128/AAC.00845-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein EH, Pienta PW, Gershon H. Acute toxicity studies on 8-quinolinol and some derivatives. Toxicol Appl Pharmacol. 1963;5:599–604. doi: 10.1016/0041-008x(63)90005-x. [DOI] [PubMed] [Google Scholar]
- Borkow G, Gabbay J. Copper as a biocidal tool. Curr Med Chem. 2005;12:2163–2175. doi: 10.2174/0929867054637617. [DOI] [PubMed] [Google Scholar]
- Butts A, Krysan DJ. Antifungal drug discovery: something old and something new. PLoS Pathog. 2012;8:e1002870. doi: 10.1371/journal.ppat.1002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cater MA, Haupt Y. Clioquinol induces cytoplasmic clearance of the X-linked inhibitor of apoptosis protein (XIAP): therapeutic indication for prostate cancer. Biochem J. 2011;436:481–491. doi: 10.1042/BJ20110123. [DOI] [PubMed] [Google Scholar]
- Conforti A, Franco L, Milanino R, Velo GP. Copper and ceruloplasmin (Cp) concentrations during the acute inflammatory process in the rat. Agents Actions. 1982;12:303–307. doi: 10.1007/BF01965394. [DOI] [PubMed] [Google Scholar]
- Crabtree JN, Okagaki LH, Wiesner DL, Strain AK, Nielsen JN, Nielsen K. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect Immun. 2012;80:3776–3785. doi: 10.1128/IAI.00507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel KG, Chen D, Orlu S, Cui QC, Miller FR, Dou QP. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005;7:R897–R908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby CM, Nathan CF. Killing of non-replicating Mycobacterium tuberculosis by 8-hydroxyquinoline. J Antimicrob Chemother. 2010;65:1424–1427. doi: 10.1093/jac/dkq145. [DOI] [PubMed] [Google Scholar]
- Day JN, Chau TTH, Wolbers M, Mai PP, Dung NT, Mai NH, Phu NH, Nghia HD, Phong ND, Thai CQ, et al. Combination anti-fungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368:1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MG, Franz KJ. A prochelator activated by hydrogen peroxide prevents metal-induced amyloid β aggregation. ChemBioChem. 2010;11:59–62. doi: 10.1002/cbic.200900597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Yin J, Tovar EMM, Fitzpatrick DA, Higgins DG, Thiele DJ. The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol Microbiol. 2011;81:1560–1576. doi: 10.1111/j.1365-2958.2011.07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Festa RA, Chen YL, Espart A, Palacios Ò, Espín J, Capdevila M, Atrian S, Heitman J, Thiele DJ. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe. 2013;13:265–276. doi: 10.1016/j.chom.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Cosío G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- Fraser RSS, Creanor J. The mechanism of inhibition of ribonucleic acid synthesis by 8-hydroxyquinoline and the antibiotic lomofungin. Biochem J. 1975;147:401–410. doi: 10.1042/bj1470401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Tsui HCT, Bruce KE, Sham LT, Higgins KA, Lisher JP, Kazmierczak KM, Maroney MJ, Dann CE, 3rd, Winkler ME, Giedroc DP. A new structural paradigm in copper resistance in Streptococcus pneumoniae. Nat Chem Biol. 2013;9:177–183. doi: 10.1038/nchembio.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guerrero M, Raimunda D, Cheng X, Argüello JM. Distinct functional roles of homologous Cu+ efflux ATPases in Pseudomonas aeruginosa. Mol Microbiol. 2010;78:1246–1258. doi: 10.1111/j.1365-2958.2010.07402.x. [DOI] [PubMed] [Google Scholar]
- Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson V, Petris MJ. Copper homeostasis at the host-pathogen interface. J Biol Chem. 2012;287:13549–13555. doi: 10.1074/jbc.R111.316406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston WD, Freiser H. Structure and behavior of organic analytical reagents. III Stability of chelates of 8-hydroxyquinoline and analogous reagents. J Am Chem Soc. 1952;74:5239–5242. [Google Scholar]
- Kelley DS, Daudu PA, Taylor PC, Mackey BE, Turnlund JR. Effects of low-copper diets on human immune response. Am J Clin Nutr. 1995;62:412–416. doi: 10.1093/ajcn/62.2.412. [DOI] [PubMed] [Google Scholar]
- Kielar F, Helsel ME, Wang Q, Franz KJ. Prochelator BHAPI protects cells against paraquat-induced damage by ROS-triggered iron chelation. Metallomics. 2012;4:899–909. doi: 10.1039/c2mt20069d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanderson P, Tagesson C. Iron bound to the lipophilic iron chelator, 8-hydroxyquinoline, causes DNA strand breakage in cultured lung cells. Carcinogenesis. 1996;17:545–550. doi: 10.1093/carcin/17.3.545. [DOI] [PubMed] [Google Scholar]
- Li C, Wang J, Zhou B. The metal chelating and chaperoning effects of clioquinol: insights from yeast studies. J Alzheimers Dis. 2010;21:1249–1262. doi: 10.3233/jad-2010-100024. [DOI] [PubMed] [Google Scholar]
- Macomber L, Rensing C, Imlay JA. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol. 2007;189:1616–1626. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanino R, Buchner V. Copper: role of the ‘endogenous’ and ‘exogenous’ metal on the development and control of inflammatory processes. Rev Environ Health. 2006;21:153–215. doi: 10.1515/reveh.2006.21.3.153. [DOI] [PubMed] [Google Scholar]
- Oliveri V, Giuffrida ML, Vecchio G, Aiello C, Viale M. Gluconjugates of 8-hydroxyquinolines as potential anti-cancer prodrugs. Dalton Trans. 2012;41:4530–4535. doi: 10.1039/c2dt12371a. [DOI] [PubMed] [Google Scholar]
- Percival SS. Copper and immunity. Am J Clin Nutr. 1998;67(Suppl):1064S–1068S. doi: 10.1093/ajcn/67.5.1064S. [DOI] [PubMed] [Google Scholar]
- Pocino M, Baute L, Malavé I. Influence of the oral administration of excess copper on the immune response. Fundam Appl Toxicol. 1991;16:249–256. doi: 10.1016/0272-0590(91)90109-h. [DOI] [PubMed] [Google Scholar]
- Samanovic MI, Ding C, Thiele DJ, Darwin KH. Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe. 2012;11:106–115. doi: 10.1016/j.chom.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora A, Zielonka J, Lopez M, Dybala-Defratyka A, Joseph J, Marcinek A, Kalyanaraman B. Reaction between peroxynitrite and boronates: EPR spin-trapping, HPLC Analyses, and quantum mechanical study of the free radical pathway. Chem Res Toxicol. 2011;24:687–697. doi: 10.1021/tx100439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solioz M, Abicht HK, Mermod M, Mancini S. Response of gram-positive bacteria to copper stress. J Biol Inorg Chem. 2010;15:3–14. doi: 10.1007/s00775-009-0588-3. [DOI] [PubMed] [Google Scholar]
- Tardiff DF, Tucci ML, Caldwell KA, Caldwell GA, Lindquist S. Different 8-hydroxyquinolines protect models of TDP-43 protein, α-synuclein, and polyglutamine proteotoxicity through distinct mechanisms. J Biol Chem. 2012;287:4107–4120. doi: 10.1074/jbc.M111.308668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardito S, Bassanetti I, Bignardi C, Elviri L, Tegoni M, Mucchino C, Bussolati O, Franchi-Gazzola R, Marchiò L. Copper binding agents acting as copper ionophores lead to caspase inhibition and paraptotic cell death in human cancer cells. J Am Chem Soc. 2011;133:6235–6242. doi: 10.1021/ja109413c. [DOI] [PubMed] [Google Scholar]
- Tardito S, Barilli A, Bassanetti I, Tegoni M, Bussolati O, Franchi-Gazzola R, Mucchino C, Marchiò L. Copper-dependent cytotoxicity of 8-hydroxyquinoline derivatives correlates with their hydrophobicity and does not require caspase activation. J Med Chem. 2012;55:10448–10459. doi: 10.1021/jm301053a. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Wagner D, Maser J, Lai B, Cai Z, Barry CE, 3rd, Höner Zu Bentrup K, Russell DG, Bermudez LE. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol. 2005;174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM. CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol Microbiol. 2010;77:1096–1110. doi: 10.1111/j.1365-2958.2010.07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Brose J, Schimo S, Ackland SM, La Fontaine S, Wedd AG. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J Biol Chem. 2011;286:11047–11055. doi: 10.1074/jbc.M110.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S, Yang L, Cui QC, Sun Y, Dou QP, Yan B. Tumor cellular proteasome inhibition and growth suppression by 8-hydroxyquinoline and clioquinol requires their capabilities to bind copper and transport copper into cells. J Biol Inorg Chem. 2010;15:259–269. doi: 10.1007/s00775-009-0594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.