Abstract
Leishmania (Viannia) parasites are etiological agents of cutaneous leishmaniasis in the New World. Infection is characterized by a mixed Th1/Th2 inflammatory response, which contributes to disease pathology. However, the role of T regulatory cells (Treg) in Leishmania (Viannia) disease pathogenesis is unclear. Using the mouse model of chronic L. (V.) panamensis infection, we have examined the hypothesis that Treg functionality contributes to control of pathogenesis. Upon infection, T regulatory cells (CD4+Foxp3+) presented with a dysregulated phenotype, in that they produced IFN-γ, expressed Tbet and had a reduced ability to suppress T cell proliferation in vitro. Targeted ablation of Tregs resulted in enlarged lesions, increased parasite load and enhanced production of IL-17 and IFN-γ with no change in IL-10 and IL-13 levels. Thus, indicating that an increased inflammatory response was commensurate with disease exacerbation and that the remaining impaired Treg cells were important in regulation of disease pathology. Conversely, adoptive transfer of Tregs from naïve mice halted disease progression, lowered parasite burden and reduced cytokine production (IL-10, IL-13, IL-17, IFN-γ). As Tregs appeared important for controlling infection, we hypothesized the expansion of Tregs could be used as an immunotherapeutic treatment approach. As a proof of principle, chronically infected mice were treated with rIL-2-anti-IL-2 antibody complex to expand Tregs. Treatment transitorily increased numbers and percentage of Treg (draining lymph node, spleen), that resulted in reduced cytokine responses, ameliorated lesions and reduced parasite load (105-fold). Thus, immunotherapy targeting Tregs could provide an alternate treatment strategy for leishmaniasis caused by L. (Viannia) parasites.
Keywords: Parasitic-Protozoan, inflammation, skin
INTRODUCTION
The members of the genus Leishmania, comprised of species of protozoan parasites (1), are the causative agents of leishmaniasis, which manifests as a wide spectrum of clinical diseases including cutaneous, mucocutaneous and visceral forms. The outcome of disease depends on the infecting parasite species, host, and environmental factors (2). Leishmania are taxonomically divided into two subgenera, Leishmania and Viannia, with the Viannia subgenus being predominant in Southern and Central Americas. In Colombia, L. (V.) panamensis is the agent responsible for the majority of leishmaniasis cases (3). Infection typically manifests as cutaneous disease, which can be chronic or recurrent; further, mucocutaneous forms have also been reported (4).
The immune response to Leishmania directly impacts the outcome of infection. The classic Th1/Th2 paradigm developed for L. major, however, does not consistently apply to the broad spectrum of species implicated in human disease (5–7). The control of disease can be subverted immunologically through multiple mechanisms, which include the Th2 cytokines but also Th1-IL-10+ CD4+ T cells, and the inhibition of macrophage activation through the production of TGF-β, IL-10 and other mediators (8). In the case of Leishmania (Viannia) panamensis, both the human disease and the mouse model present with a mixed cytokine response (9). Despite abundant IFN-γ production, parasite persistence and pathology still remain. Alongside IFN-γ, there is concomitant production of IL-10, IL-13, IL-17 and TNF-α. This imbalanced/mixed immune response may be partially responsible for disease pathology and indeed, in the mouse model, genetic depletion of IL-13 or IL-10 can result in disease resolution (9). However, excessive inflammation is known to play an important role in human pathology and infection with Leishmania (Viannia) organisms (10–12). An exaggerated immune response (high production of Th1 cytokines with reduced levels of IL-10) is associated with enhanced disease severity in infected patients (13–15). Additionally, there is a correlation with lesion size and the frequency of antigen specific cytokine producing cells (16); further, reductions in IFN-γ and TNF-α are found following disease resolution (17). From these findings, it follows that factors that control inflammation may improve the outcome of infection with Leishmania species.
Regulatory T cells (Tregs), characterized by the transcription factor Foxp3, are responsible for controlling aberrant immune responses through cell (CTLA-4, CD39, CD73) and cytokine mediated (IL-10, TGF-β) mechanisms (18, 19). Although Treg cells have been demonstrated to contribute to pathology and parasite persistence in leishmaniasis, these cells do not appear to play identical roles across Leishmania species. During L. major infection, Tregs prevent immune mediated parasite clearance leading to parasite persistence and potentially reactivation of disease (20). In the case of L. donovani, evidence indicates that the induction of T regulatory cells leads to disease exacerbation (21, 22). In contrast, in the L. amazonensis mouse model, it was found that Tregs have the opposite effect; these cells are beneficial to relieving a hyper-inflammatory state and aid in disease remediation (23).
Despite the increasing knowledge of immunopathological mechanisms that contribute to disease progression, the role of T regulatory cells during Leishmania (Viannia) infection has not been directly evaluated (24–27). Recently, it was found that L. (V.) panamensis infected patients had improved Treg suppressive capacity following successful treatment (28). To determine whether Tregs play a beneficial role during infection with L. (V.) panamensis, we have utilized the mouse model which closely mimics the human mixed cytokine response (9), to investigate the impact of these cells on infection. We found that during infection, T regulatory cells in the draining lymph node display characteristics of dysregulation (express Tbet and IFN-γ) and have a reduced suppressive capacity in comparison to Tregs from naïve uninfected mice. Consequently, we sought to determine the impact of T regulatory cells on infection. These studies have revealed that these cells significantly modulate both the immune response (IL-10, IL-13, IL-17, IFN-γ) and parasite levels. The further experimental depletion of Tregs during infection leads to disease exacerbation, while temporal enhancement of T regulatory cells (through cell transfer or rIL-2/anti-IL-2 antibody therapy) leads to disease amelioration. Taken together, these results indicate that Tregs can be beneficial in resolving infection with L. (V.) panamensis.
MATERIALS AND METHODS
Animals
Female BALB/c mice were obtained from the National Cancer Institute (Frederic, MD, USA) and housed at the Yale University School of Medicine facilities, approved by the American Association for Accreditation of Laboratory Animal Care facilities. All animal procedures were performed in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and have been reviewed and approved by the Yale University Committee for the Use and Care of Animals. DEREG (Foxp3-eGFP/DTR) mice employed were as previously described (29) and had been backcrossed for 10 generations onto the BALB/c background.
Parasite culture, infection, and parasite burden analyses
L. (V.) panamensis (strain MHOM/CO/1995/1989) were grown in Schneider’s Drosophila medium supplemented with 20% heat-inactivated FCS and 17.5 μg/ml gentamycin. The infection protocol has been described previously (9). Briefly, infective parasites were isolated from late stationary phase promastigotes from the 45/60% percoll gradient interface. Parasites (5×104) were injected intradermally into the top of a hind foot. Lesion development was monitored by measuring the foot thickness using a dial gauge caliper (Starrett Thickness Gauge) and calculating the ratio between the infected and the contralateral non-infected foot. At the termination of the experiment, parasites were quantified in infected tissue by limiting dilution assay, as previously described (6).
Indoleamine 2,3-dioxygenase (IDO) inhibition and in vivo depletion of T regulatory T cells
1-methyl-D-tryptophan (1-MT; Sigma-Aldrich) was formulated and administered to mice as previously described (30). Briefly, mice were treated with 2mg/ml 1-MT in their drinking water; starting 2 days post infection and continued for the duration of the experiment.
Depletion of Foxp3+ cells in DEREG mice was performed as previously described (31). Briefly three weeks post infection, mice were administered 0.5μg diphtheria toxin (DT; Enzo Life Sciences), intraperitoneally on 2 consecutive days per week for 2 weeks. PBMCs were isolated from mice one day following the last DT injection; flow cytometry was employed to confirm T regulatory cell depletion.
Isolation of lymphocytes, cellular transfer and suppression assays
CD4+ and CD4+CD25+ cells were isolated from the spleen or draining lymph node of mice using the CD4+CD25+ regulatory T cell isolation kit (MACS Miltenyi Biotec) according to the manufacturer’s protocol. CD4+CD25+ or CD4+CD25− cells (3×105) were injected once intralesionally in chronically infected mice (three to five weeks post infection) and infections monitored as indicated above.
For suppression assays, 5×104 isolated naïve CD4+CD25− cells (Teff) were labeled with 5uM CFSE (eBisoscience) and co-cultured with CD4+CD25+ cells (Treg) at varying ratios using 2×105 T cell depleted irradiated splenocytes as APCs. Cells were stimulated with 0.5μg/ml αCD3 clone 145-2C11 (16-0031, eBioscience). Treg suppressive capacity was assessed by examining CFSE dilution using flow cytometry. The percentage suppression was calculated as (% proliferation Teff alone−% proliferation Treg+Teff)/% proliferation Teff. The isolated CD4+ Tregs from both naïve and infected mice were found to have comparable levels of CD25 and Foxp3 expression (CD4+CD25+ purity was >90.0%).
Flow cytometry and cytokine analyses
Single cell suspensions were made from the draining lymph nodes and brought up to 5×106 cells/ml in RPMI supplemented with 10% FCS. Cells were cultured with PMA/Ionomycin (BD Pharmingen) for 4 hours, Fc receptors were blocked (αCD16/CD32, BD Pharmingen), and surface markers were stained with αCD3 (145-2C11, BD Pharmingen), αCD4 (RM4-5, BD Pharmingen), αCD8α (53-6.7, BD Pharmingen), αCD11b (M1/70, BD Pharmingen); αCD11c (N418, eBioscience); αCD19 (1D3, eBioscience); αLy6G (RB6-8C5, eBioscience); αCD25 (PC61.5, eBioscience), αCD39 (24DSM1, eBioscience), αCD73 (TY/23, BD Pharmingen), and/or αCTLA-4 (UC10-4F10-11, BD Pharmingen) with corresponding isotype controls (eBioscience or BD Pharmingen). Cells were fixed and permeabalized (Cytofix/Cytoperm, BD Pharmingen) for intracellular staining with the following antibodies: αFoxp3 (FJK-16s, eBioscience); αIFN-γ (XMG1.2, eBiosciences); αTbet (4B10, eBioscience) and the corresponding isotype controls IgG2aκ, IgG1κ (eBioscience). Alternatively, cells were cocultured for 72 hours with soluble leishmania antigen (SLA, L. (V.) panamensis freeze-thaw lysate). Supernatants were harvested and IFN-γ, IL-10, IL-13, IL-17, TNF-α and TGFβ were analyzed by sandwich ELISA following the manufacturer’s protocol (eBioscience).
Indoleamine 2,3-dioxygenase analyses
For the determination of indoleamine 2,3-dioxygenase (IDO), RNA was isolated from infected and naïve lymph nodes using Trizol (Invitrogen) and cDNA was synthesized using Superscript III Reverse Transcriptase (Invitrogen) following the manufacturer’s protocols. Real-time PCR was performed on the Mx3005P QPCR System (Stratagene) using iQ SYBR Green Supermix (Biorad) according to the manufacturer’s instructions. IDO mRNA was normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the comparative CT method (32). The following primers were used: IDO fwd, cggactgagaggacacaggttac; IDO rev, acacatacgccatggtgatgtac; GAPDH fwd, tgcaccaccaactgcttag; GAPDH rev, gatgcagggatgatgttc.
rIL-2/anti-IL-2 antibody complex treatment for Treg expansion
Anti-IL-2 antibody-rIL-2 complexes were formulated as previously described (33), using a 1:1 molar ratio. Briefly, mice with established lesions were given intraperitoneal injections of recombinant mouse IL-2 (PeproTech, 1ug) in complex with anti-IL-2 mAb (JES6-1, 5ug), every 3 days for a total of 4 doses. Due to the fact that rapamycin is a drug known to affect Leishmania (34) as TOR1 and TOR 2 are essential genes, rapamycin together with the rIL-2/anti-IL-2 antibody was not employed. Two days and 8 days following the final treatment and at the end of the experiment, the percentages of CD4+, CD8+ and CD4+Foxp3+ cells were analyzed in the draining lymph node and spleen. Cytokine analysis was conducted at the same time point and at the termination of the experiment. Lesion size was monitored as described above.
Statistical analyses
Statistical analyses were conducted using the Students t-test or the Wilcoxon signed-rank test. P values ≤0.05 were considered significant.
RESULTS
Regulatory T cells are phenotypically altered in response to L. (V.) panamensis infection
Inflammation plays an important role in leishmaniasis caused by organisms of the L. (Viannia) subgenus (10–12). Understanding the contribution of T regulatory cells (Tregs) in modulating inflammation and thus disease outcome potentially could highlight areas for immunotherapeutic interventions. Tregs have been found to play variable roles in studies of the diverse species of Leishmania; these range across a wide spectrum, from preventing strong anti-parasitic Th1 responses, and limiting responses necessary for development of memory, thus aiding parasite persistence to the control of pathology and infection (23, 35–37). Consequently, we sought to directly examine the effects of T regulatory cells on a member of the Viannia subgenus, L. (V.) panamensis, using a mouse model of chronic disease. Previous experiments (9) in the L. V. panamensis mouse model indicated that in response to infection, as expected, there was an early expansion of T cells (CD4+, CD8+) and B cells in both susceptible BALB/c and resistance IL-13 deficient mice. Notably, the expansion of Treg cells was higher (2.4-fold) in the resistant IL-13 deficient mice than the overall T cell expansion (1.6-fold), while the expansion of T cells and Treg populations were comparable in the susceptible mice. These data suggested the immunological balance toward regulation might also contribute to disease control.
To further evaluate the Treg population present during infection, we initially examined whether the phenotypic markers associated with regulatory capacity might have been altered due to infection. Functional Tregs are known to act through the high affinity IL-2 receptor CD25, the co-stimulatory molecule CTLA-4 and/or the ectonucleases CD39 and CD73. However, the expression of the regulatory surface markers CTLA-4, CD25, CD39, and CD73 remained unchanged in the CD4+Foxp3+ population in infected mice in comparison to naïve mice (Figure 1A). These results, suggested that these Treg functional mechanisms are unaltered and that these cells may remain capable of immunosuppression.
Figure 1. Characterization of T regulatory cells in the draining lymph node during L. (V.) panamensis infection.
BALB/c mice were infected with 5×104 parasites in the top of the rear foot. At various times post-infection, LN cell populations were removed and T regulatory cell markers were evaluated; LN cells from naïve mice were used as controls. (A) Cells from naïve and chronically infected mice (>12 weeks post-infection) were gated on CD4+Foxp3+ cells and analyzed for expression of CTLA-4, CD25, CD39, and CD73 (solid line, naïve; dashed, chronic infection; shaded, isotype control). (B) SLA-stimulated draining lymph node cells were gated on CD4+ cells and examined at the indicated times post-infection. Tbet and FoxP3 expression are shown. (C) Lymph node cells from chronically infected mice were directly cultured ex vivo with PMA/ionomycin and examined for Foxp3 and IFN-γ. Cells were gated on CD4+Foxp3+ cells. Experiments were independently repeated at least 2 times.*, p≤0.05.
Recent findings have shown a functional plasticity of Treg populations in inflammatory environments (38–42). Although lineage specific transcription factors are more generally expressed independently, subpopulations expressing transcription factors which are markers of multiple CD4+ T cell lineages have been observed in distinct inflammatory states (43, 44). Following ex vivo stimulation with leishmanial antigen, a population of CD4+ cells expressing Foxp3 and Tbet was present and remained detectable throughout the course of infection (Figure 1B). Further, after PMA/ionomycin stimulation, 20.8 ±4.5% of Foxp3+ cells from the draining lymph nodes of L. (V.) pananmensis infected mice expressed IFN-γ (Figure 1C). IFN-γ producing Tregs have been found in both human and mouse models (45) of autoimmune (38, 45) and infectious diseases (46) and have been associated with Tregs taking on proinflammatory capabilities. Taken together, these data suggested functional dysregulation of Tregs during L. (V.) panamensis infection.
Regulatory T cells from L. (V.) panamensis infected mice are functionally impaired
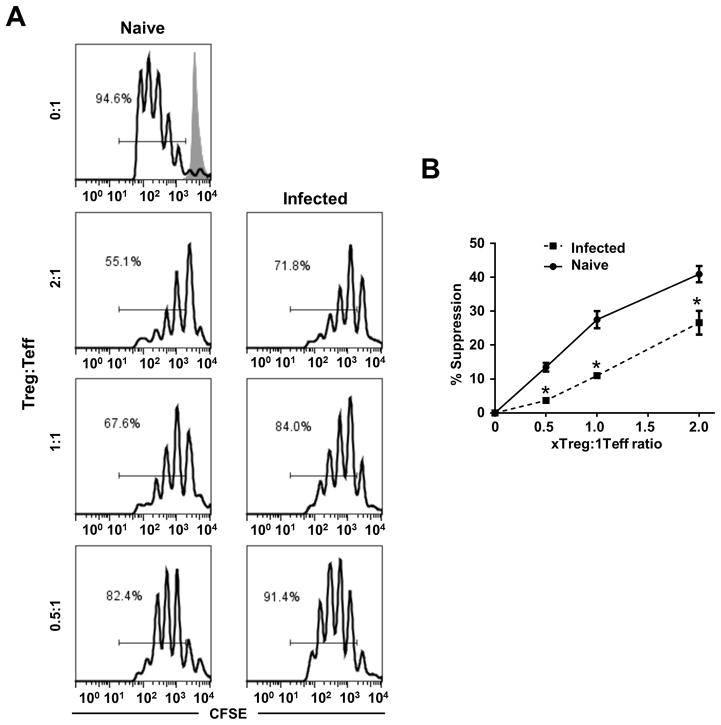
Rodriguez-Pinto, et al. demonstrated that Tregs from L. (V.) panamensis-infected patients had diminished suppressive capacity that was restored after successful drug treatment (28). Considering that a significant portion of the Treg population have an altered phenotype (Foxp3+IFN-γ+ Tbet+ cells) upon infection, this suggested that L. (V.) panamensis infection leads to diminished Treg functionality. To evaluate the regulatory capacity of Tregs from infected mice, the comparative ability of Tregs from naïve or infected mice to inhibit T effector proliferation ex vivo was assessed. Overall, the CD4+CD25+ (Tregs) cells isolated from the draining lymph node of L. (V.) panamensis infected mice were less effective than Tregs isolated from pooled peripheral lymph nodes of naïve mice (despite having equivalent Foxp3 expression) in inhibiting CD4+CD25− (Teff) proliferation in coculture with αCD3 and irradiated APCs (Figure 2A). Percentage suppression of Teff proliferation when cocultured with Tregs from infected mice was 26.6, 11.0, and 3.7 compared to 40.9, 27.5 and 13.5 when cocultured with Tregs from naïve mice at ratios of 2:1, 1:1 and 0.5:1 Treg:Teff, respectively (Figure 2B). Thus Tregs from L. (V.) panamensis-infected mice had a reduced suppressive capacity (as little as 27% that of naïve Treg cells) compared to Tregs from naïve mice. Therefore, despite maintenance of certain features consistent with Treg function (CD25, Foxp3, CTLA-4 and the surface ectonucleases CD39 and CD73), the Treg cells from L. (V.) panamensis infected mice were significantly less effective in inhibiting Teff proliferation. These results are consistent with those found for Treg cell function from human patients with active leishmaniasis cause by L. (V.) panamensis, where a reduced capacity to inhibit IFN-γ production by T effector cells was observed (28). Thus, in both human infection and the mouse model impaired Treg function is consistently observed, indicating that inflammatory response to infection may persist due to reduced Treg function.
Figure 2. Tregs from L. (V.) panamensis infected mice have reduced suppressive capacity.
(A) CFSE labeled CD4+CD25− cells (Teff) from naïve mice were cocultured with irradiated APCs, αCD3, and CD4+CD25+ cells (Treg) from chronically infected or naïve mice at the indicated ratios. Unstimulated CFSE labeled CD4+CD25− cells (shaded line) are shown; CFSE levels are plotted and % proliferated cell indicated. (B) Suppression was calculated using the following equation, (%proliferationTeff − %proliferationTreg+Teff)/%proliferationTeff. Samples were analyzed in triplicate and data are representative of two independent experiments. *, p≤0.05.
Reduction of regulatory T cells during infection exacerbates disease caused by L. (V.) panamensis
To further examine the role of T regulatory cells during infection, the effect of Treg reduction on infection was examined. Initially, the chemical inhibitor of indoleamine-2,3-deoxygenase (IDO), 1-methyltryptophan (1-MT), was utilized. IDO is known to be critical for Treg differentiation, enhancing Treg numbers and function, while suppressing T effector activation (47). It should be noted that the expression of indoleamine-2,3-dioxygenase (IDO) was significantly reduced in L. panamensis infected mice (25-fold relative to expression in peripheral lymph nodes of naïve mice; Supplementary Figure 1).
Therefore, IDO inhibition was utilized to further block the action of IDO and indirectly diminish T regulatory cell levels (48–50). Treatment of mice was initiated two days post-infection and was maintained for the duration of the experiment. As previously reported (48, 51), 1-MT treatment resulted in a significant but modest 17.3±1.7% reduction in the level of Treg cells. Further, mice treated with 1-MT showed significantly larger lesions (Figure 3A) and over 100-fold increase in parasite numbers (Figure 3B). Notably, 1-MT treatment did not appear to skew the ongoing immune response toward either a Th2 or Th17 cytokine profile. Instead 1-MT treatment significantly increased the overall cytokine production in the draining lymph node, including IFN-γ, IL-10, IL-13 and IL-17 (Figure 3C). Thus, increased levels of both inflammatory and anti-inflammatory cytokines were observed in response to IDO inhibition. An elevated cytokine response is consistent, however, with previous observations that T regulatory cells modulate both Th1 and Th2 responses (52). These results support that reduction of T regulatory cells may be detrimental to disease control and that these cells modulate the mixed, hyper-cytokine response induced upon infection.
Figure 3. Inhibition of IDO exacerbates disease.

Mice were infected 5×104 parasites then treated with 1-methyl tryptophan (2mg/ml) ad libitum in their drinking water throughout the course of infection. (A) Lesion size was evaluated throughout the course of infection. At the termination of the experiment (6 weeks) (B) parasite load in the foot was determined using the limiting dilution method and (C) draining lymph node cells were stimulated with SLA for 72 hours. Supernatants were collected and analyzed in duplicate by ELISA. Data represent two independent experiments. *, p≤0.05.
Although inhibition of IDO resulted in decreased levels of Treg cells and exacerbated disease, IDO can directly suppress the proliferation and differentiation of effector T cells, in part through tryptophan catabolism metabolites. Reducing IDO has been shown to promote the development of T effector cells in various systems (53–55). Therefore, a significant reduction in IDO is consistent both with T effector cell expansion and the findings of reduced functionality of Tregs in the L. V. panamensis infected mice. Further, the inhibitor 1-MT may potentially impact parasite survival through the buildup of tryptophan. Such a nutrient-rich environment could aid in the survival of the parasite, and has been reported that increased tryptophan levels improves trypanosome survival (a related kinetoplastid) (56). Tryptophan has been shown to be necessary for the growth of the promastigote stage of the parasite (57), although the effects on the intracellular amastigote stage are unclear and may vary dependent upon the origin (human versus mouse) of the macrophage (58). Consequently, an alternate approach was employed, using DEREG mice, where eGFP and diphtheria toxin receptor is under the control of the Foxp3 promoter, to allow the specific ablation of Foxp3+ cells (31).
As expected, Tregs from infected DEREG mice were completely depleted in response to diphtheria toxin (DT) treatment in vivo (data not shown). In response to DT-treatment, the Foxp3+ cell depleted mice developed exacerbated lesions (Figure 4A) and a 19-fold increase in parasite levels in comparison to control mice (Figure 4B). It should be mentioned that as previously reported (59, 60), infected BALB/c mice treated with diphtheria toxin developed less severe disease than either control infected mice or DT-treated DEREG mice (Supplemental Figure 2). An effect of DT toxin in vivo (although well below the known LD50 for mice (61)) has been noted recently (59, 60). This effect undoubtedly masks the level of the exacerbatory effect of Treg depletion (Figure 4A). Further, the Treg-depleted DEREG mice showed an enhanced production of the proinflammatory cytokines IL-17 and IFN-γ. However, interestingly there was no significant change in the level of IL-13 or IL-10 found at 9 weeks post-infection (Figure 4C). It has previously been shown (9) that both IL-10 and IL-13 together are necessary for parasite persistence and disease; however, the increases in Th1-Th17 responses alone in this case were not sufficient to achieve healing, but rather further exacerbated disease. Although the effects on Treg populations in the DEREG mice is temporal (31), and the decrease in Treg cell levels due to 1-MT inhibition of IDO are modest, it is notable that these treatments have significant effects on disease progression. Taken together, these Treg depletion experiments show that reduction of T regulatory cells leads to enhanced pathogenicity and increased parasite levels that are accompanied by heightened cytokine responses.
Figure 4. Depletion of T regulatory cells in DEREG mice results in disease exacerbation.
DEREG (DTR and eGFP under Foxp3 locus) mice were utilized to specifically deplete Foxp3+ cells. Three weeks post infection (after lesions developed), mice were given 0.5ug diphtheria toxin, i.p. (A) Lesion size was monitored over the course of infection and (B) parasite burden was calculated as described above. (C) At the termination of the experiment, the draining lymph node cells were stimulated with SLA for 72 hours and supernatants were collected and analyzed in duplicate by ELISA. Data represent three independent experiments. *, p≤0.05.
Transfer of regulatory T cells during chronic infection ameliorates disease and reduces parasite levels
The detrimental effects of T regulatory cell reduction, led us to hypothesize that Tregs are beneficial for disease resolution by down modulating inflammation and the mixed cytokine response. To test this, Tregs were isolated from spleens of naïve mice and adoptively transferred into infected mice with established lesions. Two days post-transfer a modest and comparable increase in Tregs was found at both the lesion site and at the draining lymph node (15–17% increase). The transfer of T regulatory cells impaired lesion progression, with significant lesion reduction evident 5 days post-transfer in comparison to control mice receiving CD4+CD25− T cells (Figure 5A). This impact upon lesion development was accompanied by a reduction in parasite burden (Figure 5B).
Figure 5. Transfer of regulatory T cells reduces leishmaniasis severity.

CD4+CD25+ and CD4+CD25− cells were isolated from the spleens of naïve mice, as described in Materials and Methods section. Three weeks post-infection, 3×105cells (CD4+CD25+ or CD4+CD25−) were injected intralesionally. (A) Lesions were measured throughout the course of infection and (B) parasite burden was analyzed as described above, at the termination of the experiment. (C) Draining lymph node cells were stimulated with SLA for 72 hours. Supernatants were collected and analyzed in duplicate by ELISA. Data represent three independent experiments. *, p≤0.05.
At the termination of the experiment, comparable reductions in the levels of production of IFN-γ, TNF-α, IL-10, IL-13 and IL-17 were observed (Figure 5C). Consistent with experiments demonstrating that depletion of T regulatory cells resulted in disease exacerbation, the reconstitution of T regulatory cells during established infection led to significant reductions in pathology and parasite levels. Overall, these results demonstrate the importance of T regulatory cells in controlling the immunopathologic state.
Treatment with rIL-2/anti-IL-2 antibody complexes induced regulatory T cells and ameliorated disease
As the transfer of CD4+CD25+ T regulatory cells mitigated disease progression, we aimed to determine whether induction/expansion of Tregs in vivo could provide an immunotherapeutic treatment strategy. As a proof of principle we utilized IL-2/anti-IL-2 antibody complex treatment (rIL-2/anti-IL-2 (JES6)) to target and promote the expansion of CD25+ Treg cells. Such treatment has been shown to preferentially amplify Tregs by blocking the medium affinity IL-2Rβγ receptor (expressed on CD8+ T cells and NK cells) binding site while the high affinity IL-2 receptor, CD25 (IL-2Rαβγ), binding site on Tregs remains exposed (33).
Following lesion development and establishment of chronic infection at 5 weeks post-infection, mice were systemically treated i.p. four times over a 9 day period (every three days) with IL-2/anti-IL-2 complex. As expected (33, 62, 63), an expansion of total cells (spleen and DLN) was observed in response to treatment (Figure 6) at day 1 and 8 post-treatment. CD4+ and CD8+ T cells as well as CD4+FoxP3+ T cells responded to IL-2/anti-IL-2-complex treatment (Figures 6B–6D). However, the overall fold-increase of CD4+FoxP3+ cells in the DLN and spleen were higher than that found for total CD4+ or CD8+ T cells, with a 3-fold Treg increase versus a 1.5-fold CD4+ and CD8+ increase in the draining lymph node and a 5-fold increase in Tregs versus a 1.3 or 2.3-fold increase in CD4+ or CD8+, respectively, in the spleen at day 2 post-treatment were found. These results indicate a change in the overall balance of these cell populations. However, whether this translates into a modulation in the level of Treg at the site of infection remains to be determined. Concomitantly, an increase in markers of T regulatory capacity increased; the MFI of CD73, CTLA4, CD39, and CD25 significantly increased with treatment. These results are consistent with previous studies (63, 64) that established an enhanced regulatory capacity of IL-2/anti-IL-2 complex induced Treg cells. The reduced level of Tregs at 8 days post-treatment are consistent with the known half-life (24 h) of the IL-2 antibody complex (62) and earlier IL-2/anti-IL-2 complex studies (63) that demonstrated that increased Treg function and levels are temporary, waning quickly after cessation of treatment.
Figure 6. IL-2 antibody complex treatment expands regulatory T cells in vivo.
Following the development of lesions (3–4 weeks post-infection), mice were given IL-2/anti-IL-2 in complex as described in Materials and Method section. (A) Representative FACS analysis (gated on CD4+ cells) comparing CD4+Foxp3+ cell populations between control and IL-2 complex treated mice two days post-treatment. (B, C) At two (left) and eight (right) days post-treatment, draining lymph node and spleens were excised and (B) cell numbers and (C) cell populations (CD4+, CD8+ and CD4+FoxP3+) were assessed. (D) Draining lymph node cells from control and treated mice (2 days post-treatment) were gated on CD4+Foxp3+ cells and analyzed for expression of CTLA-4, CD25, CD39, and CD73.
Further, IL-2/anti-IL-2 complex treatment regimen led to lesion resolution (Figure 7A) with a significant reduction (100,000-fold) in parasite burden (Figure 7B). This level of parasite reduction through rIL-2/anti-IL-2 antibody immunomodulation appears more effective than that for direct Treg cell transfer; however, this could be due to a larger, more sustained increase in Treg numbers. Interestingly, even given the short temporal enhancement of Treg cells, pronounced changes in parasite levels and pathology were found.
Figure 7. In vivo induction of regulatory T cells improves immunopathology.
Following the development of lesions (3–4 weeks post-infection), mice were given IL-2/anti-IL-2 in complex as described in Materials and Method section. (A) Lesions were measured throughout the course of infection and (B) parasite burden was analyzed at the termination of the experiment. (C) Two days following the final treatment and (D) at the termination of the experiment, lymph node cells (C, D) and splenocytes (C) were stimulated with SLA for 72 hours. Supernatants were collected and analyzed in duplicate by ELISA. Data represent two independent experiments *, p≤0.05.
The successful expansion and enhancement of Treg cells led to a significant decrease in pro-inflammatory IL-17 cytokine production at day 2 post-treatment in both the draining lymph node and spleen (Figure 7C). Previous studies have shown that IL-2/anti-IL-2 complexes lead to increased levels of p-STAT5 and a reduction in pSTAT3, which are related to the development of Treg and Th17 cells, respectively (64). In the spleen, there was also a significant reduction in IFNγ, IL-10 and IL-13 that was not observed in the dLN; this may in part reflect the higher induction of CTLA4, CD39, CD25 and CD79 of Treg cells observed in the spleen and the intraperitoneal delivery route employed.
The successful expansion of Tregs subsequently led to a significant reduction in IFN-γ, IL-10, IL-13, IL-17 at the termination of the experiment (Figure7). Consequently, there was ultimately a down-regulation of inflammatory and anti-inflammatory responses. These results are consistent with those found for the transfer of Treg cells from naïve mice (Figures 5A, 5B) and may, in part, reflect the lower number of parasites found in the treated mice. Taken together, these experiments demonstrate that increasing T regulatory cells levels and/or function, even in a temporal fashion (Treg transfer or IL-2/anti-IL-2 complexes), can lead to the resolution of disease caused by L. (V.) panamensis and targeting these cells may provide a novel approach to disease treatment.
DISCUSSION
T regulatory cells are crucial for maintaining the balance of an appropriate immune response, preventing aberrant activation and minimizing collateral damage and immunopathology (65). Although initial studies indicated these cells had a negative impact on host defense, allowing pathogens to thrive by dampening immune system activation (66), further investigations have revealed a more complex dynamic that is dependent upon the disease state and pathogen (65, 66). Recent work utilizing Foxp3-DTR (DEREG) mice has allowed for specific ablation of Foxp3+ cells and increased insight into their role in disease following infection with various pathogens (67, 68). The results from these studies show temporal effects (68–70) and indicate that evaluation of Tregs on infection should be assessed in multiple approaches during infection to fully understand the ongoing dynamic and consequent potential as targets for therapy/treatment.
The role of T regulatory cells in the spectrum of diseases caused by leishmanial parasites appears to vary. This is not surprising, as species belonging to the Leishmania genus are taxonomically diverse (1) and the immunological mechanisms regulating infection and disease vary across the genus (5, 71, 72). In the case of L. major, Tregs play variable roles in the outcome of infection that is dependent upon the genetic background and thus the susceptibility of the mouse. Although a Th1 response can lead to healing, both a Th2 as well as multiple responses that down-regulate a Th1 response can prevent disease resolution (20). For the susceptible BALB/c mouse, CD4+CD25+ cells appear to constrain excessive Th2 responses (52, 73), facilitating healing. In C57BL/6 mice, an induction of IDO is observed (74) and the induced Tregs dampen the healing Th1 response and prevent sterile cure. The induction of Tregs has a two-fold impact. The presence of persistent parasites allows for memory and immunity to reinfection; however, the absence of sterile cure can allow for disease reactivation (66). In the case of L. amazonensis, T regulatory cells ameliorate disease; the reconstitution of RAG1-deficient C57BL/6 mice with naïve spleen cells depleted of CD4+CD25+ T cells before infection results in disease exacerbation in comparison to mice receiving whole spleen cells (23). This is consistent with earlier observations (5, 72, 75, 76) that pathology associated with L. amazonensis infection is dependent upon activation of T cells, B cells and an inflammatory response that fails to develop in immunodeficient mice (nude, RAG2-, CIITA-, MHCII-deficient). Hence a dampening of the immune response and cellular recruitment provides for parasite control.
Within the L. (Viannia) subgenus, however, the role of T regulatory cells in infection had not been fully evaluated. Disease caused by this subgenus is associated with inflammatory and mixed cytokine responses that sustain the recruitment of host macrophages and persistent infection (9, 11, 35, 77, 78). Although present at the site of infection and draining lymph node in the mouse model of L. (V.) braziliensis, Treg cells do not interfere with the development of protective immunity (27). In the non-human primate macaque model, lesion resolution was ascribed to the recruitment of regulatory T cells and suppression of an effector T-cell-mediated inflammatory response (26). In patient studies of L. V. panamensis, Treg functionality was diminished during infection but was restored following successful treatment (28). Tregs with varying functionality (with suppressive or non-functional properties) have been identified in lesions of patients infected with L. (V.) braziliensis (24, 25). In contrast for L. (V.) guyanensis (35), although FoxP3 and IDO expression in lesions decrease during disease, higher levels are found for patients with chronic disease, suggesting a role for Tregs in pathogenesis. Herein, we show evidence that regulatory T cell responses are impaired during infection with Leishmania (Viannia) panamensis and that restoration of these cells (through cell transfer or therapeutically through the use of rIL-2/anti-IL-2 antibody complexes) or their depletion has either a beneficial or negative effect, respectively in disease healing.
Indoleamine 2,3-dioxygenase (IDO) is important for the development of T regulatory cells and functions through both its signaling and enzymatic activities (47, 79, 80); tryptophan catabolism products are also known to suppress T effector cell function. One of the striking results found in response to L. (V.) panamensis infection is the reduction in IDO expression in the draining lymph node in chronically infected mice. This is in contrast to findings in vitro or in vivo for L. major infection (DCs; C57Bl/6 mice), where parasites induce the up-regulation of this enzyme (74, 81). Chemical inhibition of IDO by 1-MT enhanced expression of IL-17 in L. major infected mice; this corresponded with reduced parasite burden and lesion size (74). Notably, in IL-2/anti-IL-2 complex treated L. V. panamensis infected mice, an early response (2 days) to treatment in the healing animals was the down-regulation of IL-17. These findings are consistent with other studies employing IL-2 or IL-2/anti-IL-2 complexes (64, 82), showing decreased Th17/IL-17 responses. The role of IL-17 in Leishmania (Viannia) infection (72) is not well understood, but warrants further investigation. The contrasting relationship between L. (V.) panamensis and L. major with IDO (and consequent T regulatory cells) may be a result of evolutionary advantages/disadvantages associated with an inflammatory response. While in both infectious states IDO inhibition enhanced local inflammation, a heightened inflammatory response counteracts L. major survival but it appears to be advantageous for L. (V.) panamensis persistence. Overall, Leishmania (Viannia) parasites are notable for inflammation associated with disease pathology (9, 11, 35, 77, 78, 83). Thus, increased levels of T regulatory cells in L. (V.) panamensis infection lead to control and counteract this species survival strategy.
In addition a portion of CD4+Foxp3+ T regulatory cells expressed Tbet+ following infection. Treg plasticity in response to changes in the inflammatory microenvironment has been widely described in patients and in disease model systems (84). T regulatory cells capable of producing both pro-inflammatory and anti-inflammatory cytokines have been observed (85); these cells have been hypothesized to be targeted to distinct immune environments during diverse types of inflammatory responses. Notably, the ability for Foxp3+ cells to co-express Tbet, in the presence of IFN-γ and/or IL-12, redirects the migratory and functional properties of this regulatory subset (84, 86). A low level expression of Tbet aids in the cellular recruitment of Foxp3+ Tregs to sites of Th1 inflammation, through the upregulation of CXCR3, without impacting regulatory function (87). Conversely, following lethal Toxoplasma infection, the cytokine storm induces Foxp3+ cells with equivalent Tbet expression levels to non-Treg effector cells. The Foxp3+Tbethi cells acquired the ability to produce IFN-γ, allowing these cells to contribute to the pro-inflammatory phenotype (46). This finding is consistent with in vitro studies showing that Foxp3+ cells skewed toward a Th1-like phenotype had reduced ability to suppress T effector cell proliferation (38). Moreover, recent studies of M. tuberculosis found that Tbet+Tregs expanded but subsequently underwent selective culling and elimination in response to IL-12. The mechanisms sustaining Treg populations during an inflammatory response are not fully understood; there are many factors that can impact Treg proliferation and abundance (84). In the chronic L. (V.) panamensis model, there is a sustained and elevated level of IFN-γ (9), which potentially could induce T regulatory cells; however, this occurs with the concomitant expression of IL-10 and IL-13. How this might impact on Treg development is unclear. However, the population of Foxp3+ cells in infected mice has high expression of Tbet, produces IFN-γ and exhibits a reduction in regulatory capacity. This together with the suppression of IDO expression appears to lead to an overall reduction in the regulation of the ongoing response to infection which is deleterious to the host.
Previous work has shown that BALB/c mice deficient in either IL-10 or Il-13 are resistant to L. (V.) panamensis infection (9), suggesting that immune responses counteracting the IFN-γ response prevent disease resolution. However interestingly in DEREG mouse experiments, following Foxp3+ cell depletion, disease exacerbation was found in the absence of a change in either IL-10 or IL-13. Instead, increases in the IFN-γ and IL-17 responses were observed. IFN-γ is typically associated with intracellular pathogen killing; however, in this case increased numbers of parasites were found, which corresponded with the elevated cytokine levels. L. (V.) braziliensis parasites are less susceptible to IFN-γ mediated NO induced macrophage killing compared to L. major (65). Additionally, and consistent with our findings, this study showed that TLR9 deficient mice had increased parasite loads and increased IFN-γ expression in comparison to wild type mice. Persistence and thriving within the presence of an inflammatory environment is not unique to L. (V.) panamensis. Previous work with L. amazonensis revealed parasite control (lesion size and parasite numbers) in immunodeficient (nude or RAG-2) mice and control of infection by Treg cells (5, 72, 75, 76). The absence of T effector cells in these immunodeficient models correlates with a reduction in monocytes/macrophages at the lesion site, and thus reduces the number of available cellular hosts for the parasite. Therefore, the balance between the T cell response, macrophage activation and cellular recruitment is important for parasite containment. In the case of L. (V.) panamensis infection, T regulatory cells ameliorate disease through the down regulation of inflammatory cytokines (Th1/Th2/Th17) and appear to limit the recruitment of target host cells (as observed by the reduction in lesion size). However, this hypothesis (relation of Treg function and cellular recruitment in L. V. panamensis infection) needs to be further explored.
Together, these studies have demonstrated the beneficial role of regulatory T cells in controlling excessive immunopathology and parasite numbers following infection with L. (V.) panamensis. These experiments were focused on the modulation of an established infection, with the goal of determining whether an intervention manipulating T regulatory cells would have potential therapeutic value. Indeed, we have shown that the in vivo induction of CD4+Foxp3+ T regulatory cells can lead to resolution of active disease. Thus, our data provide support for future research to focus on treatment (local delivery) expanding regulatory T cells as an immunotherapeutic approach for L. (V.) panamensis infection.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Nancy Saravia and Daniel Rodriguez for helpful discussions and insight concerning the human disease caused by L. (V.) panamensis.
This work was supported by the National Institutes of Health (NIAID) grant number 1R01AI093775; AE was supported in part through training grant T32AI07404.
Abbreviations used in this article
- L
Leishmania
- V
Viannia
- Treg
regulatory T cell
- SLA
soluble leishmanial antigen
- DT
diphtheria toxin
- IDO
indoleamine 2,3-dioxygenase
- 1-MT
1-methyl tryptophan
References
- 1.Banuls AL, Hide M, Prugnolle F. Leishmania and the leishmaniases: a parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans. Adv Parasitol. 2007;64:1–109. doi: 10.1016/S0065-308X(06)64001-3. [DOI] [PubMed] [Google Scholar]
- 2.Lipoldova M, Demant P. Genetic susceptibility to infectious disease: lessons from mouse models of leishmaniasis. Nat Rev Genet. 2006;7:294–305. doi: 10.1038/nrg1832. [DOI] [PubMed] [Google Scholar]
- 3.Varela MR, Munoz DL, Robledo SM, Kolli BK, Dutta S, Chang KP, Muskus C. Leishmania (Viannia) panamensis: an in vitro assay using the expression of GFP for screening of antileishmanial drug. Exp Parasitol. 2009;122:134–139. doi: 10.1016/j.exppara.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osorio LE, Castillo CM, Ochoa MT. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: clinical characteristics. Am J Trop Med Hyg. 1998;59:49–52. doi: 10.4269/ajtmh.1998.59.49. [DOI] [PubMed] [Google Scholar]
- 5.McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev. 2004;201:206–224. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed S, Colmenares M, Soong L, Goldsmith-Pestana K, Munstermann L, Molina R, McMahon-Pratt D. Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infect Immun. 2003;71:401–410. doi: 10.1128/IAI.71.1.401-410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nylen S, Gautam S. Immunological perspectives of leishmaniasis. J Glob Infect Dis. 2010;2:135–146. doi: 10.4103/0974-777X.62876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 9.Castilho TM, Goldsmith-Pestana K, Lozano C, Valderrama L, Saravia NG, McMahon-Pratt D. Murine model of chronic L. (Viannia) panamensis infection: role of IL-13 in disease. Eur J Immunol. 2010;40:2816–2829. doi: 10.1002/eji.201040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faria DR, Souza PE, Duraes FV, Carvalho EM, Gollob KJ, Machado PR, Dutra WO. Recruitment of CD8(+) T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite Immunol. 2009;31:432–439. doi: 10.1111/j.1365-3024.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novoa R, Bacellar O, Nascimento M, Cardoso TM, Ramasawmy R, Oliveira WN, Schriefer A, Carvalho EM. IL-17 and Regulatory Cytokines (IL-10 and IL-27) in L. braziliensis Infection. Parasite Immunol. 2011;33:132–136. doi: 10.1111/j.1365-3024.2010.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faria DR, Gollob KJ, Barbosa J, Jr, Schriefer A, Machado PR, Lessa H, Carvalho LP, Romano-Silva MA, de Jesus AR, Carvalho EM, Dutra WO. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–7859. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, Gollob KJ, Carvalho EM. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho LP, Passos S, Bacellar O, Lessa M, Almeida RP, Magalhaes A, Dutra WO, Gollob KJ, Machado P, de Jesus AR. Differential immune regulation of activated T cells between cutaneous and mucosal leishmaniasis as a model for pathogenesis. Parasite Immunol. 2007;29:251–258. doi: 10.1111/j.1365-3024.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro de Jesus A, Luna T, Pacheco de Almeida R, Machado PR, Carvalho EM. Pentoxifylline down modulate in vitro T cell responses and attenuate pathology in Leishmania and HTLV-I infections. Int Immunopharmacol. 2008;8:1344–1353. doi: 10.1016/j.intimp.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Gollob KJ. Antigen specific correlations of cellular immune responses in human leishmaniasis suggests mechanisms for immunoregulation. Clin Exp Immunol. 2004;136:341–348. doi: 10.1111/j.1365-2249.2004.02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–148. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- 18.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med. 2004;200:201–210. doi: 10.1084/jem.20040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta G, Majumdar S, Adhikari A, Bhattacharya P, Mukherjee AK, Majumdar SB. Treatment with IP-10 induces host-protective immune response by regulating the T regulatory cell functioning in Leishmania donovani-infected mice. Med Microbiol Immunol. 2011;200:241–253. doi: 10.1007/s00430-011-0197-y. [DOI] [PubMed] [Google Scholar]
- 22.Martin S, Agarwal R, Murugaiyan G, Saha B. CD40 expression levels modulate regulatory T cells in Leishmania donovani infection. J Immunol. 2010;185:551–559. doi: 10.4049/jimmunol.0902206. [DOI] [PubMed] [Google Scholar]
- 23.Ji J, Masterson J, Sun J, Soong L. CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J Immunol. 2005;174:7147–7153. doi: 10.4049/jimmunol.174.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campanelli AP, Roselino AM, Cavassani KA, Pereira MS, Mortara RA, Brodskyn CI, Goncalves HS, Belkaid Y, Barral-Netto M, Barral A, Silva JS. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis. 2006;193:1313–1322. doi: 10.1086/502980. [DOI] [PubMed] [Google Scholar]
- 25.Costa DL, Guimaraes LH, Cardoso TM, Queiroz A, Lago E, Roselino AM, Bacellar O, Carvalho EM, Silva JS. Characterization of regulatory T cell (Treg) function in patients infected with Leishmania braziliensis. Hum Immunol. 2013;74:1491–500. doi: 10.1016/j.humimm.2013.08.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de-Campos SN, Souza-Lemos C, Teva A, Porrozzi R, Grimaldi G., Jr Systemic and compartmentalised immune responses in a Leishmania braziliensis-macaque model of self-healing cutaneous leishmaniasis. Vet Immunol Immunopathol. 2010;137:149–154. doi: 10.1016/j.vetimm.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Falcao SC, de Moura TR, Clarencio J, Brodskyn C, Barral A, de Oliveira CI. The presence of Tregs does not preclude immunity to reinfection with Leishmania braziliensis. Int J Parasitol. 2012;42:771–780. doi: 10.1016/j.ijpara.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Pinto D, Navas A, Blanco VM, Ramirez L, Garcerant D, Cruz A, Craft N, Saravia NG. Regulatory T cells in the pathogenesis and healing of chronic human dermal leishmaniasis caused by Leishmania (Viannia) species. PLoS Negl Trop Dis. 2012;6:e1627. doi: 10.1371/journal.pntd.0001627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 31.Lahl K, Sparwasser T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol Biol. 2011;707:157–172. doi: 10.1007/978-1-61737-979-6_10. [DOI] [PubMed] [Google Scholar]
- 32.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 33.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 34.Diaz-Gonzalez R, Kuhlmann FM, Galan-Rodriguez C, Madeira da Silva L, Saldivia M, Karver CE, Rodriguez A, Beverley SM, Navarro M, Pollastri MP. The susceptibility of trypanosomatid pathogens to PI3/mTOR kinase inhibitors affords a new opportunity for drug repurposing. PLoS Negl Trop Dis. 2011;5:e1297. doi: 10.1371/journal.pntd.0001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourreau E, Ronet C, Darcissac E, Lise MC, Sainte Marie D, Clity E, Tacchini-Cottier F, Couppie P, Launois P. Intralesional regulatory T-cell suppressive function during human acute and chronic cutaneous leishmaniasis due to Leishmania guyanensis. Infect Immun. 2009;77:1465–1474. doi: 10.1128/IAI.01398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters N, Sacks D. Immune privilege in sites of chronic infection: Leishmania and regulatory T cells. Immunol Rev. 2006;213:159–179. doi: 10.1111/j.1600-065X.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 37.Belkaid Y. The role of CD4(+)CD25(+) regulatory T cells in Leishmania infection. Expert Opin Biol Ther. 2003;3:875–885. doi: 10.1517/14712598.3.6.875. [DOI] [PubMed] [Google Scholar]
- 38.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, Pan F, Blazar BR, Pardoll DM, Mellor AL, Shi H, Munn DH. An inherently bifunctional subset of foxp3(+) T helper cells is controlled by the transcription factor eos. Immunity. 2013;38:998–1012. doi: 10.1016/j.immuni.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall BM, Tran GT, Verma ND, Plain KM, Robinson CM, Nomura M, Hodgkinson SJ. Do Natural T Regulatory Cells become Activated to Antigen Specific T Regulatory Cells in Transplantation and in Autoimmunity? Front Immunol. 2013;4:208. doi: 10.3389/fimmu.2013.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Connor RA, Leech MD, Suffner J, Hammerling GJ, Anderton SM. Myelin-reactive, TGF-beta-induced regulatory T cells can be programmed to develop Th1-like effector function but remain less proinflammatory than myelin-reactive Th1 effectors and can suppress pathogenic T cell clonal expansion in vivo. J Immunol. 2010;185:7235–7243. doi: 10.4049/jimmunol.1001551. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt EG, Williams CB. Generation and function of induced regulatory T cells. Front Immunol. 2013;4:152. doi: 10.3389/fimmu.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirahara K, Poholek A, Vahedi G, Laurence A, Kanno Y, Milner JD, O’Shea JJ. Mechanisms underlying helper T-cell plasticity: implications for immune-mediated disease. J Allergy Clin Immunol. 2013;131:1276–1287. doi: 10.1016/j.jaci.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wohlfert E, Belkaid Y. Plasticity of T reg at infected sites. Mucosal Immunol. 2010;3:213–215. doi: 10.1038/mi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esposito M, Ruffini F, Bergami A, Garzetti L, Borsellino G, Battistini L, Martino G, Furlan R. IL-17- and IFN-gamma-secreting Foxp3+ T cells infiltrate the target tissue in experimental autoimmunity. J Immunol. 2010;185:7467–7473. doi: 10.4049/jimmunol.1001519. [DOI] [PubMed] [Google Scholar]
- 46.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O’Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, Belkaid Y. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mellor A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem Biophys Res Commun. 2005;338:20–24. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]
- 48.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli I, Horenstein AL, Fiore F, Massaia M, Colombo MP, Baccarani M, Lemoli RM. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 49.Hoshino S, Kurishima A, Inaba M, Ando Y, Fukui T, Uchida K, Nishio A, Iwai H, Yokoi T, Ito T, Hasegawa-Ishii S, Shimada A, Li M, Okazaki K, Ikehara S. Amelioration of 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice by immunoregulatory dendritic cells. J Gastroenterol. 2011;46:1368–1381. doi: 10.1007/s00535-011-0460-4. [DOI] [PubMed] [Google Scholar]
- 50.O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu T, Rowswell-Turner RB, Kilinc MO, Egilmez NK. Central role of IFNgamma-indoleamine 2,3-dioxygenase axis in regulation of interleukin-12-mediated antitumor immunity. Cancer Res. 2010;70:129–138. doi: 10.1158/0008-5472.CAN-09-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394–399. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 53.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fox JM, Sage LK, Huang L, Barber J, Klonowski KD, Mellor AL, Tompkins SM, Tripp RA. Inhibition of indoleamine 2,3-dioxygenase enhances the T-cell response to influenza virus infection. J Gen Virol. 2013;94:1451–1461. doi: 10.1099/vir.0.053124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014 Apr 8; doi: 10.1007/s00262-014-1549-4. [Epub ahead of print].CII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vincendeau P, Lesthelle S, Bertazzo A, Okomo-Assoumou MC, Allegri G, Costa CV. Importance of L-tryptophan metabolism in trypanosomiasis. Adv Exp Med Biol. 1999;467:525–531. doi: 10.1007/978-1-4615-4709-9_65. [DOI] [PubMed] [Google Scholar]
- 57.Steiger RF, Steiger E. Cultivation of Leishmania donovani and Leishmania braziliensis in defined media: nutritional requirements. J Protozool. 1977;24:437–441. doi: 10.1111/j.1550-7408.1977.tb04771.x. [DOI] [PubMed] [Google Scholar]
- 58.Murray HW, Szuro-Sudol A, Wellner D, Oca MJ, Granger AM, Libby DM, Rothermel CD, Rubin BY. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989;57:845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chapman TJ, Georas SN. Adjuvant effect of diphtheria toxin after mucosal administration in both wild type and diphtheria toxin receptor engineered mouse strains. J Immunol Meth. 2013;400–401:122–126. doi: 10.1016/j.jim.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christiaansen AF, Boggiatto PM, Varga SM. Limitations of Foxp3(+) Treg depletion following viral infection in DEREG mice. J Immunol Meth. 2014;406:58–65. doi: 10.1016/j.jim.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gill DM. Bacterial toxins: a table of lethal amounts. Microbiol Rev. 1982;46:86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Letourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J, Surh CD, Boyman O. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proc Natl Acad Sci USA. 2010;107:2171–2176. doi: 10.1073/pnas.0909384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SY, Cho ML, Oh HJ, Ryu JG, Park MJ, Jhun JY, Park MK, Stone JC, Ju JH, Hwang SY, Park SH, Surh CD, Kim HY. Interleukin-2/anti-interleukin-2 monoclonal antibody immune complex suppresses collagen-induced arthritis in mice by fortifying interleukin-2/STAT5 signalling pathways. Immunology. 2012;137:305–316. doi: 10.1111/imm.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belkaid Y. Role of Foxp3-positive regulatory T cells during infection. Eur J Immunol. 2008;38:918–921. doi: 10.1002/eji.200738120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rowe JH, Ertelt JM, Way SS. Foxp3(+) regulatory T cells, immune stimulation and host defence against infection. Immunology. 2012;136:1–10. doi: 10.1111/j.1365-2567.2011.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berod L, Puttur F, Huehn J, Sparwasser T. Tregs in infection and vaccinology: heroes or traitors? Microb Biotechnol. 2012;5:260–269. doi: 10.1111/j.1751-7915.2011.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veiga-Parga T, Suryawanshi A, Mulik S, Gimenez F, Sharma S, Sparwasser T, Rouse BT. On the role of regulatory T cells during viral-induced inflammatory lesions. J Immunol. 2012;189:5924–5933. doi: 10.4049/jimmunol.1202322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steeg C, Adler G, Sparwasser T, Fleischer B, Jacobs T. Limited role of CD4+Foxp3+ regulatory T cells in the control of experimental cerebral malaria. J Immunol. 2009;183:7014–7022. doi: 10.4049/jimmunol.0901422. [DOI] [PubMed] [Google Scholar]
- 70.Abel S, Luckheide N, Westendorf AM, Geffers R, Roers A, Muller W, Sparwasser T, Matuschewski K, Buer J, Hansen W. Strong impact of CD4+ Foxp3+ regulatory T cells and limited effect of T cell-derived IL-10 on pathogen clearance during Plasmodium yoelii infection. J Immunol. 2012;188:5467–5477. doi: 10.4049/jimmunol.1102223. [DOI] [PubMed] [Google Scholar]
- 71.Alexander J, Brombacher F. T helper1/T helper2 cells and resistance/susceptibility to Leishmania infection: is this paradigm still relevant? Front Immunol. 2012;3:80. doi: 10.3389/fimmu.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soong L, Henard CA, Melby PC. Immunopathogenesis of non-healing American cutaneous leishmaniasis and progressive visceral leishmaniasis. Semin Immunopathol. 2012;34:735–751. doi: 10.1007/s00281-012-0350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aseffa A, Gumy A, Launois P, MacDonald HR, Louis JA, Tacchini-Cottier F. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J Immunol. 2002;169:3232–3241. doi: 10.4049/jimmunol.169.6.3232. [DOI] [PubMed] [Google Scholar]
- 74.Makala LH, Baban B, Lemos H, El-Awady AR, Chandler PR, Hou DY, Munn DH, Mellor AL. Leishmania major attenuates host immunity by stimulating local indoleamine 2,3-dioxygenase expression. J Infect Dis. 2011;203:715–725. doi: 10.1093/infdis/jiq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soong L, Chang CH, Sun J, Longley BJ, Jr, Ruddle NH, Flavell RA, McMahon-Pratt D. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J Immunol. 1997;158:5374–5383. [PubMed] [Google Scholar]
- 76.Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, Shlomchik MJ, McMahon-Pratt D. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191:1063–1068. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomes-Silva A, de Cassia Bittar R, Dos Santos Nogueira R, Amato VS, da Silva Mattos M, Oliveira-Neto MP, Coutinho SG, Da-Cruz AM. Can interferon-gamma and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin Exp Immunol. 2007;149:440–444. doi: 10.1111/j.1365-2249.2007.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morgado FN, Schubach A, Rosalino CM, Quintella LP, Santos G, Salgueiro M, Conceicao-Silva F. Is the in situ inflammatory reaction an important tool to understand the cellular immune response in American tegumentary leishmaniasis? Br J Dermatol. 2008;158:50–58. doi: 10.1111/j.1365-2133.2007.08255.x. [DOI] [PubMed] [Google Scholar]
- 79.Fallarino F, Grohmann U, Puccetti P. Indoleamine 2,3-dioxygenase: from catalyst to signaling function. Eur J Immunol. 2012;42:1932–1937. doi: 10.1002/eji.201242572. [DOI] [PubMed] [Google Scholar]
- 80.Orabona C, Pallotta MT, Grohmann U. Different partners, opposite outcomes: a new perspective of the immunobiology of indoleamine 2,3-dioxygenase. Mol Med. 2012;18:834–842. doi: 10.2119/molmed.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Donovan MJ, Tripathi V, Favila MA, Geraci NS, Lange MC, Ballhorn W, McDowell MA. Indoleamine 2,3-dioxygenase (IDO) induced by Leishmania infection of human dendritic cells. Parasite Immunol. 2012;34:464–472. doi: 10.1111/j.1365-3024.2012.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 83.Ramirez C, Diaz-Toro Y, Tellez J, Castilho TM, Rojas R, Ettinger NA, Tikhonova I, Alexander ND, Valderrama L, Hager J, Wilson ME, Lin A, Zhao H, Saravia NG, McMahon-Pratt D. Human macrophage response to L. (Viannia) panamensis: microarray evidence for an early inflammatory response. PLoS Negl Trop Dis. 2012;6:e1866. doi: 10.1371/journal.pntd.0001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119:4430–4440. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood. 2013;121:2647–2658. doi: 10.1182/blood-2012-08-443473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.