Abstract
Objective
Recent studies have demonstrated that lymphovascular space invasion (LVSI) is associated with increased risk of hematogenous and lymphatic metastasis and poor clinical outcome of women with epithelial ovarian cancer. Given the suspected role of estrogen in promoting ovarian cancer metastasis, we examined potential links between estrogen receptor and LVSI in high-grade serous ovarian carcinoma.
Methods
Tumoral expression of ER, PR, p53, MDR1, EGFR, HER2, DNA ploidy, and S-phase fraction was examined for 121 cases of stage I-IV high-grade serous ovarian carcinoma samples obtained at primary cytoreductive surgery. Biomarker expression was correlated to LVSI and survival outcomes.
Results
LVSI was observed in 101 (83.5%) of all cases. Immunohistochemistry of tested biomarkers showed ER (86.7%) to be the most commonly expressed followed by p53 (71.4%), HER2 (68.3%), EGFR (52.1%), MDR-1 (14.3%), and PR (8.9%). ER expression was positively correlated to PR expression (r=0.31, p=0.001). LVSI was only correlated with ER (odds ratio 6.27, 95%CI 1.93-20.4, p=0.002) but not with other biomarkers. In multivariate analysis, ER remained significantly associated with LVSI (p=0.039). LVSI remained a significant prognostic factor for decreased progression-free survival (HR 3.01, 95%CI 1.54-5.88, p=0.001) and overall survival (HR 2.69, 95%CI 1.18-6.23, p=0.021) while ER-expression did not remain as a significant variable in multivariate analysis.
Conclusion
Our data demonstrated that estrogen receptor was positively correlated with LVSI that was an independent prognostic indicator of poor survival outcomes of high-grade serous ovarian carcinoma. This study emphasizes the importance of estrogen pathway in promoting lymphatic or vascular spread of high-grade serous ovarian carcinoma.
Keywords: ovarian cancer, high-grade serous ovarian carcinoma, lymphovascular space invasion, estrogen receptor
INTRODUCTION
In 2013, approximately 22,240 women in the United States were estimated to be diagnosed with ovarian cancer and 14,030 were from this disease, making it the most fatal gynecologic malignancy [1]. While a large proportion of ovarian cancer patients go into remission after surgical resection and systemic chemotherapy, most eventually develop recurrence and succumb to their disease [2,3]. Therefore, any predictor to identify the subset of patients at increased risk of recurrence could be useful in the management of ovarian cancer. Recently, tumoral lymphovascular space invasion (LVSI), defined as the presence of tumor cells inside the capillary lumens of either lymphatic or microvascular system within ovarian tumor, has been reported as a new biomarker of ovarian cancer progression [4,5]. Specifically, tumoral LVSI was significantly associated with high-grade serous ovarian carcinoma (HGSOC) and increased risk of hematogenous and lymphatic metastasis resulting in poor clinical outcome [4]. However, the exact mechanism by which LVSI drives progression and metastasis of ovarian cancer is not fully understood.
Various studies have investigated the role of the estrogen-pathway in ovarian cancer. Generally, the incidence of ovarian cancer starts to elevate in the peri-menopausal period, a time in which circulating estrogen levels tend to rise relative to the earlier reproductive stages, as shown in recent meta-analysis of observational data [6]. HGSOC, the most common histologic subtype of ovarian cancer and known to have aggressive tumor biology, expresses estrogen receptor in 60-85% of the samples [7,8]. Estrogen replacement therapy has been related to increased ovarian cancer incidence and mortality related to disease [9-12]. In particular, this increased risk of ovarian cancer with estrogen use was seen for serous histology [13,14]. Mechanistically, two molecular pathways have been proposed to explain ovarian cancer growth, metastasis, and progression related to estrogen: (i) tumor production of vascular endothelial growth factor (VEGF) via estrogen receptor signaling (direct pathway); and (ii) increased tumor-endothelial cell migration via mitogen-activated protein kinases (MAPK) signaling (in direct pathway) [15-18]. In contrast, a recent large-size multicenter consortium study concluded that estrogen receptor expression in HGSOC did not impact survival outcomes [8]. This discrepancy between population-based and translational studies strongly suggests that there is a possibility of an unproven factor linking estrogen and ovarian cancer. Given the suspected role of estrogen in promoting ovarian cancer metastasis, we examined potential links between estrogen receptor and LVSI in HGSOC.
MATERIAL AND METHODS
Clinical information
After Institutional Review Board (IRB) approval was obtained in Mercy Medical Center in Baltimore, a previously established ovarian cancer database for in vitro drug resistance assay (EDR Assay, Oncotech, Inc., Tustin, CA) was utilized for this study [19]. In this assay, evaluation of estrogen receptor alpha (ER) is included as part of a standard package of biomarker testing. Inclusion criteria were cases with stage I-IV HGSOC who underwent primary cytoreductive surgery between January 1995 and January 2009. Cases with metastatic disease from sites other than ovarian primary, synchronous cancer types, and tumors of low malignant potential were excluded from the study. Among the eligible cases identified in the database for the analysis, medical records were examined to abstract the following variables: (i) patient demographics including age, race, preoperative CA-125; (ii) histopathology results for histology subtype, grade, FIG O stage, tumoral LVSI, and nodal metastasis (pelvic and/or para-aortic); and (iii) extent of residual disease at the time of cytoreductive surgery, and type of postoperative chemotherapy with response; and (iv) survival outcomes for progression-free survival (PFS) and overall survival (OS).
Evaluation
For the evaluation of tumoral LVSI, archived histopathology sides for hematoxylin and eosin (H&E) stain were pulled and examined by gynecologic pathologists who were blinded to the clinical information, as previously described [4]. Briefly, slides of the primary ovarian tumors and metastatic tumor implants were examined and cluster of tumor cells within lymphovascular spaces (except for the area of potential artifact or tumor cell contamination - torn tissue, free tumor fragments along the edge of the tissue) was determined as tumoral LVSI being present or absent (Figure S1) [4]. Based on our prior study, quantity of LVSI did not impact on survival outcome of epithelial ovarian cancer and thus qualification of LVSI was scored in a dichotomized fashion [4]. Two independent pathologists examined LVSI for 25 randomly picked samples that had an inter-observer agreement in 24 (96%) samples with a kappa statistic of 0.65. The results of biomarker testing by immunohistochemistry and flow cytometry on the primary ovarian tumor were abstracted from the database [19]. These biomarker evaluations for immunohistochemistry and flow cytometry were performed by Oncotech Inc. as part of routine clinical testing by using the fresh tumor samples obtained by pathologist at the time of primary cytoreductive surgery as described previously [20-22]; tumor tissues were also processed for formalin fixation for H&E staining. The H&E slides from the primary ovarian tumor specimens and metastatic sites were used for evaluation of tumoral LVSI. In addition to ER expression, the following key biomarkers known to affect ovarian cancer biology were examined: p53, multi-drug resistance 1 (MDR1), epidermal growth factor receptor (EGFR), Human Epidermal Growth Factor Receptor 2 (HER2), and progesterone receptor (PR). DNA ploidy and S-phase fraction (%) were also evaluated.
Definition
Proportional expression (0-100%) and staining intensity (0-3+) were examined, and positive expression was defined as expression in ≥5% and staining intensity of ≥1+ in our study when the results were dichotomized into expression versus non-expression. For composition scores of immunohistochemistry results, proportional expression was multiplied with the results of staining intensity (composition score, ranged 0-300). DNA ploidy was divided into aneuploidy or non-aneuploidy. The cutoff of 11% was used to examine DNA S-phase fraction results based on previous work that demonstrated increased risk of recurrence in ovarian cancer [20]. Immunohistochemistry panel of biomarkers for EDR assay were evaluated and scored per Oncotech Inc. at each submission of tumor specimens in a blinded manner with regard to clinical information. Optimal cytoreductive surgery was defined as no residual tumor measuring greater than 1 cm in maximal dimension at the end of cytoreductive surgery. Platinum resistance was defined as the first recurrence or tumor progression within 6 months from the last platinum administration while platinum sensitive was defined as the first recurrence or tumor progression >6 months from the last platinum-based therapy. The date of progression was determined by clinical examination, imaging studies, and/or CA-125 levels. PFS was defined as the time interval from the date of primary cytoreductive surgery to the date of documented first recurrence or progression of disease. If there was no recurrence, PFS was determined as the date of last follow-up. OS was defined as the interval between the primary cytoreductive surgery and the date of death or last follow-up.
Statistical analysis
Continuous variables were assessed for normality (Kolmogorov-Smirnov test) and expressed as appropriate (mean with SD or median with range). Composition scores of biomarker results for immunohistochemistry were examined with Spearman’s correlation coefficient. Risk factor associated with tumoral LVSI was assessed with binary logistic regression test, expressed with odds ratio (OR) and 95% confidence interval (CI). Multivariate analysis with logistic regression test was further performed with conditional backward method to identify the independent risk factor for tumoral LVSI. For survival data analysis, to determine the significance of variables for the survival outcomes for PFS and OS, univariate (Log-rank) and multivariate (Cox proportional hazard regression test with conditional backward method) analyses were performed as appropriate expressed with hazard ratio (HR) and 95%CI. Survival curves were constructed with Kaplan-Meier method. P-values of less than 0.05 were considered as statistically significant (all, 2-tailed). The Statistical Package for Social Science software (SPSS, version 21.0, IL) was used for all analyses.
RESULTS
There were 221 cases of HGSOC identified for the current study. Of those, 5 cases had failed the in vitro drug resistance assay and 20 cases had no additional biomarker testing. The remaining 196 cases were examined for the availability of histology slides. In 75 cases, the histology slides could not be located, leaving 121 evaluable cases. Patient demographics are shown in Table 1. Mean age of the study patients was 62.6 (SD ± 10.6). The majority of HGSOC patients were Caucasian (84.3%) and had advanced-stage disease (FIGO stage III-IV, 95.0%). Pelvic and para-aortic lymphadenectomy was performed in 66.9% (60.5% positive) and 33.1% (65.0% positive), respectively. Tumoral LVSI was observed in 101 (83.5%, 95%CI 76.9-90.1) cases. In tumors with LVSI, there was a significantly increased risk of nodal metastasis (LVSI versus no LVSI, pelvic lymph node metastasis, 98.0% versus 71.9%, OR 18.8, 95%CI 2.24-157, p=0.01; para-aortic lymph node metastasis, 100% versus 71.4%, OR 3.60, 95%CI 2.13-6.10, p=0.011; and any lymph node metastasis (pelvic and/or para-aortic), 98.2% versus 67.9%, OR 25.6, 95%CI 3.04-215, p=0.003). Carboplatin with paclitaxel (46.3%) and carboplatin with docetaxel (39.7%) were the two most common postoperative chemotherapy regimens.
Table 1. Patient demographics of high-grade serous ovarian carcinoma.
| Cases | n=121 |
|---|---|
| Age | 62.6 (±10.6) |
| < 70 | 90 (74.4%) |
| ≥ 70 | 31 (25.6%) |
|
| |
| Race | |
| Caucasian | 102 (84.3%) |
| African American | 17 (14.0%) |
| Asian | 1 (0.8%) |
| Hispanic | 1 (0.8%) |
|
| |
| CA-125 (IU/L) | 631 (24-13408) |
| < 35 | 2 (3.2%) |
| ≥ 35 | 60 (96.8%) |
|
| |
| FIGO Stage | |
| I | 2 (1.7%) |
| II | 4 (3.3%) |
| III | 102 (84.3%) |
| IV | 13 (10.7%) |
|
| |
| Nodal dissection | |
| Pelvic lymphadenectomy | 81 (66.9%) |
| No-metastasis | 32 (39.5%) |
| Metastasis | 49 (60.5%) |
| Para-aortic lymphadenectomy | 40 (33.1%) |
| No-metastasis | 14 (35.0%) |
| Metastasis | 26 (65.0%) |
| Any lymphadenectomy* | 83 (68.6%) |
| No-metastasis | 28 (33.7%) |
| Metastasis | 55 (66.3%) |
|
| |
| LVSI presenting tumor | |
| No | 20 (16.5%) |
| Yes | 101 (83.5%) |
|
| |
| Cytoreduction | |
| Optimal | 50 (41.3%) |
| Sub-optimal | 71 (58.7%) |
|
| |
| Postoperative chemotherapy | |
| Carboplatin + paclitaxel | 56 (46.3%) |
| Carboplatin + docetaxel | 48 (39.7%) |
| Others | 3 (2.5%) |
| No chemotherapy | 14 (11.6%) |
Mean (±SD), median (range), or number (%) is shown. CA-125 was recorded in 62 cases.
any lymphadenectomy: indicate pelvic and/or para-aortic lymph nodes. Abbreviations; CA- 125, cancer antigen 125; FIGO, the International Federation of Gynecology and Obstetrics; and LVSI, lympho-vascular space invasion.
Characteristics of biomarker expression are shown in Table 2. Immunohistochemistry and DNA assay results were available in >90% of all tested markers including ER (93.4%). Among tested biomarkers, ER had the highest expression (86.7%) followed by DNA aneuploidy (83.5%) and p53 (71.4%). HER2 (68.3%), EGFR (52.1%), and S-phase fraction ≥11% (40.9%) showed moderate positivity, and MDR1 (14.3%) and PR (8.9%) were associated with low expression in HGSOC. By using a composite score of immunohistochemistry, correlations of biomarkers were examined (Table 3). Statistically significant correlations among the tested biomarkers were mainly ER-pathway: positive correlations existed between ER and PR (r=0.31, p=0.001, Figure S2A), and negative correlation between (i) ER and DNA S-phase fraction (r=−0.30, p=0.001, Figure S2B) and (ii) DNA S-phase fraction and PR (r=−0.22, p=0.025, Figure S2C). p53, MDR1, EGFR, and HER2 did not correlate with ER expression in HGSOC (all, p>0.05).
Table 2. Immunohistochemistry results in high-grade serous ovarian carcinoma.
| p53 | MDR1 | EGFR | HER2 | ER | PR | |
|---|---|---|---|---|---|---|
| Examined | 119 (98.3%) |
112 (92.6%) |
111 (91.7%) |
120 (99.2%) |
113 (93.4%) |
112 (92.6%) |
| Positivity | 85 (71.4%) |
16 (14.3%) |
63 (52.1%) |
82 (68.3%) |
98 (86.7%) |
10 (8.9%) |
| Staining intensity | ||||||
| 0 | 34 (28.6%) |
96 (85.7%) |
48 (43.2%) |
38 (31.7%) |
15 (13.3%) |
101 (90.2%) |
| 1+ | 1 (0.8%) |
11 (9.8%) |
15 (13.5%) |
50 41.7%) |
23 (20.4%) |
1 (0.9%) |
| 2+ | 7 (5.9%) |
2 (1.8%) |
33 (29.7%) |
26 (21.7%) |
51 (45.1%) |
4 (3.6%) |
| 3+ | 77 (64.7%) |
3 (2.7%) |
15 (13.5%) |
6 (5.0%) |
24 (21.2%) |
6 (5.4%) |
| Percent expression | ||||||
| 0% | 34 (28.6%) |
96 (85.7%) |
48 (43.2%) |
38 (31.7%) |
15 (13.3%) |
101 (90.2%) |
| 1-25% | 10 (8.4%) |
10 (8.9%) |
37 (33.3%) |
37 (30.8%) |
29 (25.7%) |
9 (8.0%) |
| 26-50% | 4 (3.4%) |
4 (3.6%) |
16 (14.4%) |
20 (16.7%) |
27 (23.9%) |
0 (0%) |
| 51-75% | 5 (4.2%) |
1 (0.9%) |
3 (2.7%) |
13 (10.8%) |
22 (19.5%) |
2 (1.7%) |
| 76-100% | 66 (55.5%) |
1 (0.9%) |
7 (6.3%) |
12 (10.0%) |
20 (17.7%) |
0 (0%) |
Positivity of immunohistochemistry results was defined as proportional expression of ≥5% and staining intensity of ≥1+. Abbreviations: MDR1, multi-drug resistance 1; EGFR, epidermal growth factor receptor, HER2, human epidermal growth factor receptor 2; ER, estrogen receptor alpha; and PR, progesterone receptor.
Table 3. Biomrreltin high-grade serous ovarian carcinoma.
| p53 | MDR1 | EGFR | HER2 | ER | PR | S-phase | |
|---|---|---|---|---|---|---|---|
| p53 | p=0.45 | p=0.24 | p=0.32 | p=0.052 | p=0.51 | p=0.73 | |
| MDR1 | r= −0.07 | p=0.54 | p=0.09 | p=0.97 | p=0.13 | p=0.88 | |
| EGFR | r= −0.11 | r= 0.06 | p=0.25 | p=0.40 | p=0.38 | p=0.40 | |
| HER2 | r= 0.09 | r= 0.16 | r= −0.11 | p=0.82 | p=0.75 | p=0.15 | |
| ER | r= 0.19 | r= −0.01 | r= −0.08 | r= 0.02 | p=0.001 | p=0.001 | |
| PR | r= 0.06 | r= 0.15 | r= 0.09 | r= 0.03 | r= 0.31 | p=0.025 | |
| S-phase | r= 0.03 | r= −0.02 | r= 0.08 | r= 0.14 | r= −0.30 | r= −0.22 |
P-values for Spearman’s correlation using composition scores in each biomarker. Abbreviations: MDR1, multi-drug resistance 1; EGFR, epidermal growth factor receptor, HER2, human epidermal growth factor receptor 2; ER, estrogen receptor alpha; and PR, progesterone receptor.
Potential risk factors associated with tumoral LVSI in HGSOC were examined (Table 4). Advanced-stage was significantly associated with increased presence of tumoral LVSI (stage III-IV versus I-II, 87.0% versus 16.7%, OR 33.3, 95%CI 3.64-305, p=0.002). Amongst tested biomarkers, only ER was significantly positively correlated with tumoral LVSI (proportion of tumors exhibiting LVSI, ER positive versus negative, 87.8% versus 53.5%, OR 6.27, 95%CI 1.93-20.4, p=0.002). p53, MDR1, EGFR, HER2, and PR were not associated with tumoral LVSI (all, p>0.05). Proportion of ER-expressing tumors per stage were 50% in stage I, 50% in stage II, 85.7% in stage III, and 84.6% in stage IV, respectively. Advanced-stage HGSOC was significantly correlated with high ER expression (stage III-IV versus I-II, 88.8% versus 50%, OR 7.92, 95%CI 1.43-43.7, p=0.018) but none of the other tested biomarkers (all, p>0.05). Age was inversely associated with ER expression (Spearman’s correlation r= −0.21, p=0.029, Figure S3) but not with PR expression (r= −0.05, p=0.58). In addition, older age was significantly associated with decreased risk of LVSI-positive tumors (age ≥70 versus <70, 64.5% versus 90.0%, OR 0.20, 95%CI 0.07-0.55, p=0.002). In a multivariate model, ER expression remained significantly correlated with tumoral LVSI (adjusted OR for ER 4.48, 95%CI 1.08-18.6, p=0.039, Table 4) as well as advanced-stage disease (p=0.006) and older age (p=0.007). There were 83 (68.6%) women with HGSOC who underwent primary cytoreductive surgery with either pelvic and/or para-aortic lymphadenectomy, and 55 (66.3%) were found to have nodal metastasis. When this subset of study population was examined for LVSI status, ER remained a significant variable associated with increased risk of LVSI (OR 27.5, 95%CI 1.72-434, p=0.019) after controlling for lymph node metastasis (OR 71.0, 95%CI 3.62-1393, p=0.005) and optimal cytoreduction (OR 0.09, 95%CI 0.01-0.94, p=0.044) in multivariate analysis (Supplemental Table S1).
Table 4.
Risk factors for LVSI in high-grade serous ovarian carcinoma
| Univariate | Multivariate* | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. | LVSI (%) | OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Age | 0.002 | 0.007 | ||||
| < 70 | 90 | 90.0% | 1 | 1 | ||
| ≥ 70 | 31 | 64.5% | 0.20 (0.07-0.55) | 0.19 (0.06-0.63) | ||
|
| ||||||
| Race | 0.57 | |||||
| Non-White | 19 | 78.9% | 1 | |||
| White | 102 | 84.3% | 1.43 (0.42-4.88) | |||
|
| ||||||
| CA125 | 0.20 | |||||
| <35 | 2 | 50% | 1 | |||
| ≥35 | 60 | 86.7% | 6.50 (0.37-114) | |||
|
| ||||||
| Stage | 0.002 | 0.006 | ||||
| I and II | 6 | 16.7% | 1 | 1 | ||
| III and IV | 115 | 87.0% | 33.3 (3.64-305) | 38.7 (3.40-439) | ||
|
| ||||||
| Cytoreduction | 0.08 | |||||
| Sub-optimal | 71 | 88.7% | 1 | |||
| Optimal | 50 | 76.0% | 0.40 (0.15-1.07) | |||
|
| ||||||
| p53 | 0.81 | |||||
| No | 34 | 85.3% | 1 | |||
| Yes | 85 | 83.5% | 0.87 (0.29-2.65) | |||
|
| ||||||
| MDR1 | 0.83 | |||||
| No | 96 | 85.4% | 1 | |||
| Yes | 16 | 87.5% | 1.20 (0.25-5.84) | |||
|
| ||||||
| EGFR | 0.91 | |||||
| No | 48 | 83.3% | 1 | |||
| Yes | 63 | 82.5% | 0.95 (0.35-2.57) | |||
|
| ||||||
| HER2 | 0.55 | |||||
| No | 88 | 83.0% | 1 | |||
| Yes | 32 | 87.5% | 1.44 (0.44-4.71) | |||
|
| ||||||
| ER | 0.002 | 0.039 | ||||
| No | 15 | 53.3% | 1 | 1 | ||
| Yes | 98 | 87.8% | 6.27 (1.93-20.4) | 4.48 (1.08-18.6) | ||
|
| ||||||
| PR | 1.0 | |||||
| No | 102 | 81.4% | 1 | |||
| Yes | 10 | 100% | na | |||
|
| ||||||
| DNA anuploid | 0.42 | |||||
| No | 19 | 89.5% | 1 | |||
| Yes | 99 | 81.8% | 0.53 (0.11-2.50) | |||
|
| ||||||
| S-phase fraction | 0.95 | |||||
| <11% | 70 | 82.9% | 1 | |||
| ≥11% | 48 | 83.3% | 1.03 (0.39-2.76) | |||
P-values for binary logistic regression test.
multivariate analysis with conditional backward method. Abbreviations: No., number of cases; LVSI, lymphovascular space invasion; OR, odds ratio; 95%CI, 95% confidence interval; MDR1, multi-drug resistance 1; EGFR, epidermal growth factor receptor, HER2, human epidermal growth factor receptor 2; ER, estrogen receptor alpha; and PR, progesterone receptor; na not available.
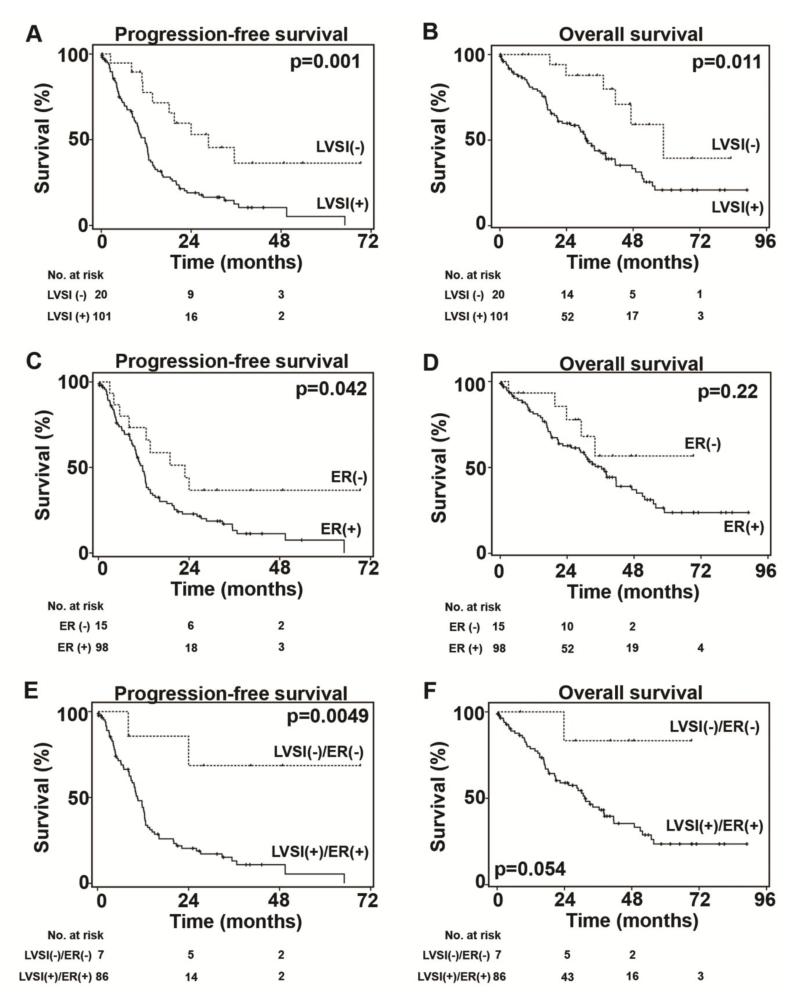
Survival analyses for HGSOC patients were performed (Table 5). In univariate analysis, tumoral LVSI was associated with decreased PFS (median time, LVSI-positive versus -negative tumor, 11.7 versus 28.6 months, HR 2.81, 95%CI 1.45-5.44, p=0.001, Figure 1A) and decreased OS (31.2 versus 58.9 months, HR 2.84, 95%CI 1.23-6.59, p=0.01, Figure 1B). ER-expressing tumors were also associated with decreased PFS (median time, ER positive versus negative, 11.7 versus 22.9 months, HR 2.03, 95%CI 1.01-4.06, p=0.042, Figure 1C), but not OS (p=0.22, Figure 1D). In multivariate analysis, tumoral LVSI and suboptimal cytoreduction remained statistically significant indicators for decreased PFS (LVSI, HR 3.01, 95%CI 1.54-5.88, p=0.001; and optimal cytoreduction, HR 0.39, 95%CI 0.25-0.62, p<0.001) and OS (LVSI, HR 2.69, 95%CI 1.18-6.23, p=0.021; and optimal cytoreduction, HR 0.33, 95%CI 0.19-0.58, p<0.001). When LVSI and ER status are combined, it gives greater magnitude of survival difference in HGSOC. Tumors expressing both LVSI and ER were significantly associated with poorer PFS when compared to tumors expressing neither of the two (median time, 10.5 months versus not reached to median, p=0.005, Figure 1E) and was marginally associated with poorer OS (31.2 months versus not reached to median, p=0.05, Figure 1F). Among ER-expressing tumors, presence of LVSI was associated with a trend toward decreased PFS (LVSI versus no LVSI, 10.5 versus 19.5 months, p=0.10) and OS (31.2 versus 47.2 months, p=0.12). There were 107 patients who received platinum-based postoperative chemotherapy (Table 1). Tumoral LVSI was not statistically associated with the timing of ovarian cancer recurrence after postoperative chemotherapy (proportion of LVSI-positive tumor, platinum resistance, platinum sensitive, and no recurrence, 88.6%, 86.0%, and 68.9%, respectively, p=0.09). Similarly, ER expression was not associated with timing of ovarian cancer recurrence after postoperative chemotherapy (proportion of ER-positive tumor, 91.2%, 89.7%, and 81.5%, respectively, p=0.47).
Table 5. Prognostic factors of high-grade serous ovarian carcinoma.
| Progression-free survival | Overall survival | ||||
|---|---|---|---|---|---|
|
| |||||
| No. | HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Age | 0.38 | 0.90 | |||
| < 70 | 90 | 1 | 1 | ||
| ≥ 70 | 31 | 0.80 (0.49-1.32) | 0.97 (0.55-1.70) | ||
|
| |||||
| Race | 0.74 | 0.81 | |||
| Non-White | 19 | 1 | 1 | ||
| White | 102 | 0.91 (0.51-1.61) | 0.92 (0.48-1.76) | ||
|
| |||||
| CA125 (IU/L) | 0.18 | 0.66 | |||
| <35 | 2 | 1 | 1 | ||
| ≥35 | 60 | 3.57 (0.48-26.4) | 1.56 (0.21-11.5) | ||
|
| |||||
| LVSI | 0.001* | 0.01* | |||
| No | 20 | 1 | 1 | ||
| Yes | 101 | 2.81 (1.45-5.44) | 2.84 (1.23-6.59) | ||
|
| |||||
| Stage | 0.006 | 0.09 | |||
| I and II | 6 | 1 | 1 | ||
| III and IV | 115 | 9.78 (1.34-71.3) | 4.71 (0.65-34.0) | ||
|
| |||||
| Cytoreduction | <0.001* | <0.001* | |||
| Suboptimal | 71 | 1 | 1 | ||
| Optimal | 50 | 0.39 (0.25-0.61) | 0.32 (0.19-0.56) | ||
|
| |||||
| p53 | 0.45 | 0.79 | |||
| No | 34 | 1 | 1 | ||
| Yes | 85 | 0.84 (0.53-1.32) | 0.93 (0.54-1.59) | ||
|
| |||||
| MDR1 | 0.58 | 0.89 | |||
| No | 96 | 1 | 1 | ||
| Yes | 16 | 1.18 (0.66-2.10) | 0.95 (0.48-1.88) | ||
|
| |||||
| EGFR | 0.18 | 0.16 | |||
| No | 48 | 1 | 1 | ||
| Yes | 63 | 1.36 (0.87-2.13) | 1.47 (0.85-2.53) | ||
|
| |||||
| HER2 | 0.89 | 0.57 | |||
| No | 88 | 1 | 1 | ||
| Yes | 32 | 1.04 (0.63-1.71) | 1.19 (0.67-2.11) | ||
|
| |||||
| ER | 0.042 | 0.22 | |||
| No | 15 | 1 | 1 | ||
| Yes | 98 | 2.03 (1.01-4.06) | 1.76 (0.70-4.40) | ||
|
| |||||
| PR | 0.42 | 0.83 | |||
| No | 102 | 1 | 1 | ||
| Yes | 10 | 0.71 (0.31-1.63) | 0.90 (0.36-2.25) | ||
|
| |||||
| DNA aneuploid | 0.19 | 0.99 | |||
| No | 19 | 1 | 1 | ||
| Yes | 99 | 0.69 (0.40-1.20) | 1.01 (0.53-1.93) | ||
|
| |||||
| S-phase fraction | 0.87 | 0.65 | |||
| <11% | 70 | 1 | 1 | ||
| ≥11% | 48 | 0.97 (0.63-1.48) | 1.12 (0.67-1.83) | ||
P-values for Log-rank test (univariate).
LVSI and optimal cytoreduction remained significant variables in multivariate analysis (Cox proportional hazard regression test with conditional backward method). Abbreviations: No., number of cases; HR, hazard ratio; 95%CI, 95% confidence interval; MDR1, multi-drug resistance 1; EGFR, epidermal growth factor receptor, HER2, human epidermal growth factor receptor 2; ER, estrogen receptor alpha; and PR, progesterone receptor.
Figure 1. Survival outcomes of high-grade serous ovarian carcinoma.
Survival curves with Kaplan-Meier method and Log-rank test for p-values. A) PFS and B) OS for LVSI. C) PFS and D) OS for ER. E) PFS and F) OS for combination patters of LVSI and ER. Abbreviations: LVSI, lymphovascular space invasion; ER, estrogen receptor; PFS, progression-free survival; and OS, overall survival.
DISCUSSION
The key findings in this paper are that in HGSOC (i) tumoral LVSI and ER are commonly expressed, (ii) tumoral LVSI was independently associated with poor prognosis, and (iii) ER correlated positively to LVSI, suggesting that the estrogen-pathway might play a pivotal role in tumor spread via lymphatic or microvasculature channels. This triad of advanced-stage disease, ER, and LVSI, represent unique characteristics of HGSOC that deserve further discussion.
HGSOC has clinically and biologically distinct characteristics when compared to other subtypes of epithelial ovarian cancer [23]. Histologically, HGSOC has a significantly higher prevalence of LVSI positivity than other histologic types [4]. As in other gynecologic tumors in which LVSI is known to be a poor prognostic indicator [24-26], it is likely that the establishment of LVSI plays an important role in tumor progression and metastasis in HGSOC. In our analysis, the ER expression was independently associated with tumoral LVSI in HGSOC, implicating a potential mechanistic role.
Anti-angiogenic therapy with a monoclonal antibody against VEGF (bevacizumab) is an effective therapeutic approach in solid tumors such as ovarian cancer [27-29]. The efficacy of bevacizumab in ovarian cancer is more prominent in those with large residual tumor volume at the end of cytoreduction [29], suggesting that the microvasculature in the residual tumor is an important target. Whether an anti-angiogenic inhibitor is more effective in the treatment of LVSI-positive HGSOC is not known and should be examined.
Our data showed that estrogen receptor is commonly expressed in HGSOC. These findings support the recent population-based study and other meta-analyses that imply prognostic impact of estrogen as a pro-oncogenic factor in ovarian cancer [10,13]. Molecularly, our data also support previous pre-clinical data that showed that the mechanisms of tumor progression in women with estrogen exposure occur as a result of direct tumor growth via VEGF induction and indirect growth via MAPK signaling in the tumor microenvironment [15-18]. However, a recent study including 1,742 women with HGSOC did not find that estrogen receptor expression itself was a significant predictor of survival [8]. This discrepancy implies the possibility of an unknown factor linking estrogen signaling and progression of HGSOC. In our view, LVSI could be this potential factor: indeed, our data showed that estrogen receptor expression was no longer significant for decreased PFS after controlled for LVSI status. Further study is warranted with larger sample size to validate our findings.
Cross-talk of downstream of estrogen receptor and other receptor tyrosine kinase (RTK) may also explain the possibility that estrogen receptor expression itself was not associated with survival outcome in HGSOC [12]. In this cross-talk mechanism, regulation of tumor growth by the ER-pathway could be activated by another type of RTK signaling that is commonly seen in other types of hormonal sensitive malignancies such as breast and endometrial cancer [30,31]. Our data showed that there was no correlation between estrogen receptor and other RTK expressions (EGFR and HER2) but this was only determined by protein expression in immunohistochemistry study and was limited with regard to the number of RTKs analyzed. Therefore, further study will be needed to determine if there is a cross-talk of ER signaling and RTK in HGSOC.
A strength of our study is that this is one of the first studies to examine the significance of tumoral LVSI in HGSOC with multiple immunohistochemistry panels. Potential weakness of the study is that this is a retrospective study and possible confounding factors might have been missed. In addition, the sample size is relatively small that might have a risk of type II error: this was especially noted in small number of early-stage cases in our study. An additional limitation of the study is that this was a database search and pattern of estrogen receptor staining (nucleus versus cytoplasm) was not available.
In summary, tumoral LVSI is significantly associated with advanced HGSOC. In addition, the possibility that ER signaling could be directly related to tumoral LVSI merits further investigation.
Supplementary Material
Acknowledgement
Part of the manuscript was presented at 44th Annual Meeting of Society of Gynecologic Oncology, Los Angeles, CA, March 9-12, 2013.
Footnotes
Disclosure: There is no conflict of interest related to this study for all the authors.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013:63–1. 11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo K, Lin YG, Roman LD, Sood AK. Overcoming platinum resistance in ovarian carcinoma. Expert Opin Investig Drugs. 2010;19(11):1339–54. doi: 10.1517/13543784.2010.515585. PMCID: 2962713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tummala MK, McGuire WP. Recurrent ovarian cancer. Clin Adv Hematol Oncol. 2005;3(9):723–36. [PubMed] [Google Scholar]
- 4.Matsuo K, Sheridan TB, Yoshino K, Miyake T, Hew KE, Im DD, et al. Significance of lymphovascular space invasion in epithelial ovarian cancer. Cancer Med. 2012;1(2):156–64. doi: 10.1002/cam4.31. PMCID: 3544453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo K, Yoshino K, Hiramatsu K, Banzai C, Hasegawa K, Yasuda M, et al. Effect of lymphovascular space invasion on survival of stage I epithelial ovarian cancer. Obstet Gynecol. 2014 doi: 10.1097/AOG.0000000000000240. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26(3):297–300. doi: 10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- 7.Escobar J, Klimowicz AC, Dean M, Chu P, Nation JG, Nelson GS, et al. Quantification of ER/PR expression in ovarian low-grade serous carcinoma. Gynecol Oncol. 2013;128(2):371–6. doi: 10.1016/j.ygyno.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Sieh W, Kobel M, Longacre TA, Bowtell DD, deFazio A, Goodman MT, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013:14–9. 853–62. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hempling RE, Wong C, Piver MS, Natarajan N, Mettlin CJ. Hormone replacement therapy as a risk factor for epithelial ovarian cancer: results of a case-control study. Obstet Gynecol. 1997;89(6):1012–6. doi: 10.1016/s0029-7844(97)00118-x. [DOI] [PubMed] [Google Scholar]
- 10.Mahavni V, Sood AK. Hormone replacement therapy and cancer risk. Curr Opin Oncol. 2001;13(5):384–9. doi: 10.1097/00001622-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez C, Calle EE, Coates RJ, Miracle-McMahill HL, Thun MJ, Heath CW., Jr. Estrogen replacement therapy and fatal ovarian cancer. Am J Epidemiol. 1995;141:828–35. doi: 10.1093/oxfordjournals.aje.a117518. [DOI] [PubMed] [Google Scholar]
- 12.Simpkins F, Garcia-Soto A, Slingerland J. New insights on the role of hormonal therapy in ovarian cancer. Steroids. 2013;78(6):530–7. doi: 10.1016/j.steroids.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beral V, Bull D, Green J, Reeves G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369(9574):1703–10. doi: 10.1016/S0140-6736(07)60534-0. [DOI] [PubMed] [Google Scholar]
- 14.Risch HA. Estrogen replacement therapy and risk of epithelial ovarian cancer. Gynecol Oncol. 1996;63(2):254–7. doi: 10.1006/gyno.1996.0315. [DOI] [PubMed] [Google Scholar]
- 15.Armaiz-Pena GN, Mangala LS, Spannuth WA, Lin YG, Jennings NB, Nick AM, et al. Estrous cycle modulates ovarian carcinoma growth. Clin Cancer Res. 2009(9):15, 2971–8. doi: 10.1158/1078-0432.CCR-08-2525. PMCID: 2743312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullinan-Bove K, Koos RD. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology. 1993;133(2):829–37. doi: 10.1210/endo.133.2.8344219. [DOI] [PubMed] [Google Scholar]
- 17.Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Makela S, Chiappetta C, et al. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. 2000;108(Suppl 5):785–90. doi: 10.1289/ehp.00108s5785. [DOI] [PubMed] [Google Scholar]
- 18.Hyder SM, Nawaz Z, Chiappetta C, Stancel GM. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res. 2000;60(12):3183–90. [PubMed] [Google Scholar]
- 19.Matsuo K, Bond VK, Eno ML, Im DD, Rosenshein NB. Low drug resistance to both platinum and taxane chemotherapy on an in vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian and peritoneal cancer. Int J Cancer. 2009;125(11):2721–7. doi: 10.1002/ijc.24654. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo K, Eno ML, Ahn EH, Shahzad MM, Im DD, Rosenshein NB, Sood AK. Multidrug resistance gene (MDR-1) and risk of brain metastasis in epithelial ovarian, fallopian tube, and peritoneal cancer. Am J Clin Oncol. 2011;34:488–93. doi: 10.1097/COC.0b013e3181ec5f4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poola I, Yue Q. Estrogen receptor alpha (ER alpha) mRNA copy numbers in immunohistochemically ER alpha-positive-, and negative breast cancer tissues. BMC Cancer. 2007 Mar 28;7:56. doi: 10.1186/1471-2407-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.d’Amato TA, Landreneau RJ, Ricketts W, Huang W, Parker R, Mechetner E, Yu IR, Luketich JD. Chemotherapy resistance and oncogene expression in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2007;133:352–63. doi: 10.1016/j.jtcvs.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164(5):1511–8. doi: 10.1016/s0002-9440(10)63708-x. PMCID: 1615664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guntupalli SR, Zighelboim I, Kizer NT, Zhang Q, Powell MA, Thaker PH, et al. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol Oncol. 2012;124(1):31–5. doi: 10.1016/j.ygyno.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raspagliesi F, Hanozet F, Ditto A, Solima E, Zanaboni F, Vecchione F, et al. Clinical and pathological prognostic factors in squamous cell carcinoma of the vulva. Gynecol Oncol. 2006;102(2):333–7. doi: 10.1016/j.ygyno.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Roman LD, Felix JC, Muderspach LI, Varkey T, Burnett AF, Qian D, et al. Influence of quantity of lymph-vascular space invasion on the risk of nodal metastases in women with early-stage squamous cancer of the cervix. Gynecol Oncol. 1998;68(3):220–5. doi: 10.1006/gyno.1998.4943. [DOI] [PubMed] [Google Scholar]
- 27.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 28.Miyake TM, Sood AK, Coleman RL. Contemporary use of bevacizumab in ovarian cancer. Expert Opin Biol Ther. 2013;13(2):283–94. doi: 10.1517/14712598.2012.745508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 30.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11(2 Pt 2):865s–70s. [PubMed] [Google Scholar]
- 31.Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res. 2012;18(21):5856–64. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.