Abstract
In this article, we review the clinical applications of diffusion MR imaging in the radiotherapy treatment of several key clinical sites, including those of the CNS, the head and neck, the prostate and cervix. Diffusion-weighted MRI (DWI) is an imaging technique that is rapidly gaining widespread acceptance due to its ease and wide availability. DWI measures the mobility of water within tissue at the cellular level without the need of any exogenous contrast agent. For radiotherapy treatment planning, DWI improves upon conventional imaging techniques, by better characterization of tumor tissue properties required for tumor grading, diagnosis and target volume delineation. Because diffusion weighted MRI is also a sensitive marker for alterations in tumor cellularity, it has potential clinical applications in the early assessment of treatment response following radiation therapy.
Introduction
Diffusion MRI is a technique that measures the mobility of water within tissue at the cellular level1,2 without the need of any exogenous contrast agent and is sensitive to cellular changes in the microenvironment that alter molecular mobility. The thermally-driven random movement of water molecules, or Brownian motion, along a magnetic field gradient induces signal attenuation and can be quantified by a diffusion coefficient (mm2/s). These principles can also be applied to tissues in biologic systems where the movement of water molecules occurs in both the intra and extracellular domains and is impeded by cell membranes, extracellular tortuosity and macromolecules.3–5 In most tissues, the intracellular compartment contributes most of the MRI signal by volume, but the relatively high mobility in the extracellular space has a strong influence on the net measured mobility. Diffusion MRI is sensitive to complex biophysical processes mediated by the volume fraction of water in the intra-/extra-cellular domains, water interaction with intracellular constituents, and the degree to which extracellular water is “free” versus hindered by tortuosity. In addition, non-thermal, semi-random motions are manifest as diffusion-like signal attenuation. A clear example of this is cardiovascular-driven blood perfusion through the (semi)random capillary network. Given these complexities, the term “apparent” diffusion coefficient (ADC) is used to reflect the fact that a singular pure diffusion coefficient in tissues is not measurable by MRI, and that ADC values are influenced by acquisition conditions.
Despite these caveats, diffusion weighted (DW) imaging is a fast, simple, and readily available MR imaging technique. In comparison to other functional MR imaging techniques, it is practical for clinical use in a variety of applications. Diffusion weighted MRI can be used for lesion detection, diagnosis, grading and further characterization of tumor tissue properties. Disruption of the normal tissue structure, e.g., the disruption of the prostate capsule and fluid flow by the presence of tumor cells can lead to alterations in the diffusion coefficient. A simplistic, though conveniently useful concept is that ADC values are inversely related to tumor cellularity. That is, tissues having relatively high cell density tend to exhibit lower ADC values due to the impeded water movement amongst the cell-packed milieu. Diffusion weighted MRI is also a sensitive marker for alterations in tumor cellularity and the early assessment of treatment response. Successful treatment leads to necrosis, alters cell membrane permeability and water homeostasis, leading to changes in tumor cell density. Increased ADC values following effective cytotoxic therapy reflects a decrease in tumor cellularity. These cellular changes can be detected early, prior to changes in tumor size and therefore is a potential, early non-invasive imaging biomarker of response and overall survival.6,7
Acquisition and Technical Issues
Diffusion MRI is obtained by inserting additional strong magnetic field gradient pulses within an MR imaging sequence to create diffusion sensitive (weighted) images. Typically a pair of additional pulses is used where the first pulse “encodes” locations of the ensemble of water molecules and the second “decodes” location. Any molecular movement between encode-decode events creates signal loss that is analyzed as a function of diffusion encoding gradient strength. More specifically, the diffusion gradient has “direction” (eg. along right/left axis), so random molecular movement along the given direction is being probed. The extent of diffusion weighting (or MR signal loss) depends upon the duration, time and amplitude of applied diffusion gradients, which is often composited into a scalar factor, called the b-value (s/mm2). If no additional diffusion gradient pulses are applied, the b-value equals zero. The signal intensities in diffusion weighting images decrease with increasing b-value and the diffusion coefficient. While signal decreases with b-value in diffusion-weighted MRI (DWI), tissue contrast attributable to differences in water mobility increases with b-value, therefore heavily diffusion-weighted images are widely used to accentuate (detect) cellular dense tissues/lesions by reading qualitative DWI. Quantitative ADC maps derived from DWI acquired with two (or more) b-values reflects the local mobility of water molecules calculated on a pixel-by-pixel basis.8
There are several technical issues to consider in obtaining DWI imaging. As previously indicated, diffusion weighted MRI is not only sensitive to diffusion of water molecules, but can also reflect capillary perfusion.1,2,9 Biologic processes including edema and inflammation can also affect the measured apparent diffusion coefficient (ADC) values. An accurate estimation of the measured ADC therefore depends upon the proper choice of b values and analysis method. To reduce the effect of perfusion–related signals from the measured diffusion coefficients, the ADC should be calculated from at least two diffusion weighted images with one at a modest low b-value (~50–200 s/mm2) other than 0 and another at a high b value (~800 – 1000 s/mm2).8 There is a potential advantage to even higher b-value (>1000) diffusion weighted images that may improve the delineation of tumors with highly restricted diffusion from benign tissue but at the price of poor signal to noise which can be compensated by longer scan times. Further studies to determine its value compared to conventional diffusion weighted imaging or apparent diffusion coefficient (b < 1000) are still needed.10–12
Diffusion weighted MR images are not only sensitive to random motion of water molecules, but also to bulk movement of the organ, e.g., due to cardiac and respiratory motion.4,13 These motions will degrade the quality of diffusion weighted images and can create artifacts and quantitative errors. All of these acquisition issues can create additional challenges for obtaining body diffusion imaging.
As indicated, diffusion gradients are directional and diffusion of water molecules in tissue is a three-dimensional process not necessarily equal in all directions. Directional mobility of tissue (i.e. anisotropy) is typically mathematically modeled as a tensor, and diffusion tensor imaging (DTI) measures the diffusion along multiple gradient directions (at least 6, and typically >16) to detail tissue microstructure patterns, particularly for highly anisotropic tissue such as white matter. To greatly mitigate influence of tissue anisotropy on mean diffusivity measures, DWI from three orthogonal gradient directions are combined for ADC calculation.
Clinical Applications for Diffusion MRI
In the following section, we review the potential role for diffusion MRI in radiotherapy treatment planning specifically in regards to target volume delineation, tumor response assessment, and prediction of normal tissue toxicity in several key clinical sites including brain tumors, head and neck cancer, prostate cancer, and cervical cancer.
Brain Tumors
Diffusion weighted MRI is helpful for the grading of malignant gliomas. Low ADC values are used as indicators for high-grade gliomas and correlate with poor survival in malignant astrocytomas independent of tumor grade. 14,15 Several studies have also demonstrated that diffusion MRI is a sensitive and early indicator of both treatment response and overall survival in brain tumors. 6,16–19
Malignant gliomas are heterogeneous tumors. Due to the complexity of tissue changes following therapy, tumor heterogeneity hinders the use of change in whole-ROI/VOI mean apparent diffusion coeffiecent (ADC) as an indicator of tumor response. Alterations in the tumor following therapy may involve cell swelling (secondary to loss of cellular water homeostasis) followed by subsequent necrotic or apoptotic cell death. In addition, there may be changes in the free extra-cellular water, e.g. increase in edema. The interplay of all of these factors may produce transient decreases and increases in regional diffusion values, which may be underestimated using the ROI/VOI mean ADC. An alternative voxel-wise analysis was devised to help mitigate the heterogeneity issue20–22. In this voxel-wise approach for image analysis, parametric response maps (PRM) retain regional changes in apparent diffusion coefficients (both increasing and decreasing) that can be further quantified. When this PRM approach was applied to a population of patients with high-grade gliomas, the percentage of the tumor volume undergoing a significant increase in ADC 3 weeks after the initiation of treatment was predictive of both response and survival. Patients stratified based upon PRM (ADC) analysis at 3 weeks were found to have significantly different time to progression and overall survival. 16
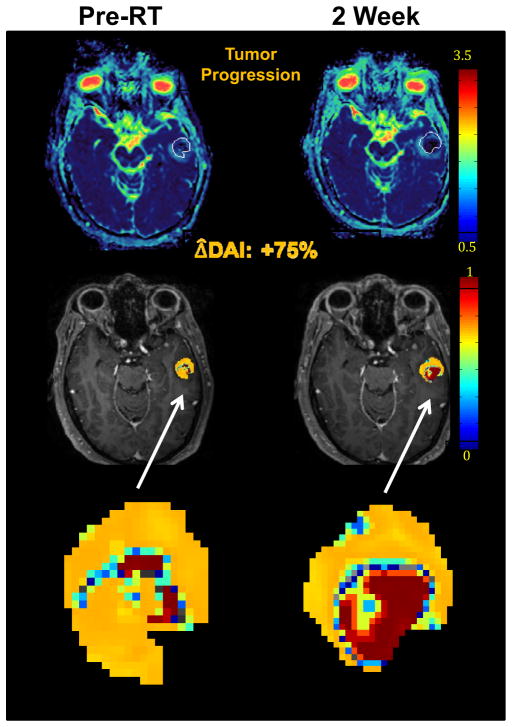
Another image analysis approach applied to brain metastases has been developed. Evaluating the entire ADC histogram may be important in assessing tumor response in brain metastases to therapy by reflecting changes in both low ADC (high cellularity) and high ADC (possible edema and necrosis). Therefore, a diffusion abnormal probability accounting for both of these changes was generated for each tumor voxel and then summed altogether in a new index, termed the diffusion abnormality index (DAI). In a preliminary study of 24 patients, early changes in the DAI were analyzed to predict response of brain metastases in patients receiving whole brain radiotherapy (WBRT). (Fig 1) The results demonstrated that early changes in the DAI measured from pre-RT to the end of RT was a significantly better predictor of post-treatment response than changes in gross tumor volume observed during the same time interval.23 There is a potential role for DWI in improving early response assessment in brain metastases.
Fig 1.
Diffusion abnormality index (DAI) of a patient with a brain metastasis treated with whole brain radiation therapy. DW MRI was obtained both pre RT (left column) and at the end of RT (right column). Top row: color-coded ADC maps; middle row: DAI maps; bottom row: zoomed DAI maps of the tumor. The tumor volume showed no significant change from pre RT to the end of RT. However, the DAI increased by 75% from pre RT to end of RT, suggesting tumor progression which was confirmed by post-Gd T1-weighted MRI one month post RT.
Multi-parametric imaging biomarkers may provide further improvement in the early assessment of treatment response. PRM approach was used to quantify hemodynamic alterations in relative cerebral blood volume (rCBV) in high-grade gliomas at 1 and 3 weeks during chemo-radiation and was found to be a strong predictor of early cancer treatment outcome.24 Further analyses demonstrate that the combination of both PRM (ADC) as well as PRM (rCBV) obtained at 3 weeks during treatment had a stronger correlation in prediction of overall survival than any baseline clinical or single treatment response imaging metric. 21 PRM is an imaging biomarker that provides an opportunity for radiation oncologists to identify early patients with regional responses that may be resistant to therapy and may benefit from alternative treatment strategies such as an adaptive conformal radiation boost plan.
Conclusions
Clinical data demonstrates the potential for diffusion-weighted MRI to provide a non-invasive method of monitoring early therapeutic response to treatment in malignant brain tumors.37–38 Accurate response assessment in malignant gliomas has significant implications for patient management, particularly in treatment decision-making and determination of treatment efficacy in clinical trials. Early response assessment may allow the future development of adaptive radiation treatment plans to identify patients who may require further treatment intensification prior to completion of treatment. However, further prospective multi-institutional trials validating these analyses are warranted prior to its incorporation into routine clinical practice.
Diffusion MRI Applications in Identifying Radiation-induced Brain Injury
While radiation is an effective treatment for many cerebral tumors, late neurocognitive dysfunction is a devastating clinical problem.26 Late delayed CNS associated radiation effects are characterized by demyelination, vascular abnormalities and ultimately radiation necrosis. Cognitive dysfunction is typically observed more than 6 months post-irradiation25 and can occur in the absence of any apparent structural lesions. Diffusion tensor MRI provides a non-invasive method of analyzing changes to the normal tissue prior to the development of irreversible late CNS toxicity. Imaging biomarkers, such as DTI indices potentially provide a mechanism to identify a window of opportunity for therapeutic intervention.
A prospective diffusion tensor imaging (DTI) study was performed in high-grade gliomas (n=19), low-grade gliomas (n=3), and benign tumors (n=3) in patients receiving partial brain irradiation with DTI obtained before, during, and after radiation.27 During the initial three months post-RT, dose-dependent demyelination was noted predominantly in regions receiving high RT doses. However, diffuse demyelination was noted at 6 months following completion of radiation even in areas receiving lower RT doses. This study confirms that DTI indices can detect early changes in the normal appearing white matter of CNS patients receiving partial brain irradiation. The potential latency suggests that imaging may play a role in identifying high-risk patients in whom early drug intervention may help to avoid permanent late radiation-induced white matter deficits.28
Radiation-associated changes in the hippocampus region due to demyelination or axonal injury may lead to long-term deficits in learning, memory, and executive function. Fractional anisotropy (FA) is a diffusion tensor index that reflects overall white matter fiber integrity. In patients receiving whole brain radiation, there was a significant decrease in FA noted in the para-hippocampal cingulum (Fig 2), along with an increase in radial diffusivity (i.e. perpendicular to fiber axis) suggesting radiation induced early demyelination. In this preliminary study, there was a correlation between early diffusivity changes in the para-hippocampal cingulum and associated late declines in verbal recall.29 These results suggest that longitudinal studies using DTI indices may be a useful non-invasive biomarker to monitor patients receiving therapeutic interventions, such as hippocampal sparing WBI during RT and may predict for long-term cognitive outcomes.30
Fig 2.
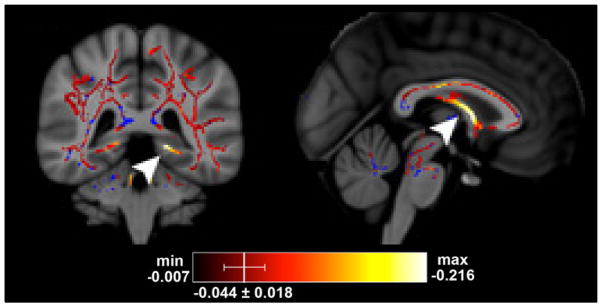
Tract-based statistical analysis (TBSS) of diffusion tensor images in patients receiving whole brain radiation therapy. There was a significant decrease in FA in the left inferior cingulum (left) and fornix columns (right) from pre RT to end of RT. Hot colors indicate a significant decrease in FA on the TBSS skeleton, and blue represents the TBSS skeleton without significant changes.
Conclusions
Diffusion tensor imaging provides a potential opportunity for early drug interventions including novel therapies such as pioglitazone, a peroxisome proliferator-activated receptor agonist and angiotensin-converting enzyme inhibitors such as ramipril that may limit the effects of radiation damage to normal brain tissue and potentially reverse the initial radiation injury without diminishing RT tumor control. 25 The rate and degree of radiation damage to cognition likely varies depending on which pathways are affected and may not be limited to the sparing of one specific region, such as the hippocampus.
Head and Neck Tumors
Diffusion weighted imaging may be helpful in the detection and diagnosis of nodal metastases in the radiotherapy treatment planning of head and neck cancer. Accurate lymph node staging with CT and MRI scans can be difficult in distinguishing benign reactive adenopathy with metastatic adenopathy and identifying nodal metastases in clinically undetectable lymph nodes. FDG-PET scans can detect regional lymph nodes with an overall sensitivity of 80% and specificity of 86%; however, the sensitivity is lowered to 50% in clinically undetectable lymph nodes. Therefore, additional physiologic parameters obtained from MR imaging may provide additional information.
Several studies have investigated DW-MRI obtained prior to surgical resection and pathologic tumor tissue specimens. 31–33 These results reported improved accuracy in the detection of metastatic lymph nodes using DWI compared to conventional turbo-spin echo imaging. There was also added value with DWI in the detection of subcentimeter nodal metastases with a higher sensitivity at 76% but further comparisons with other imaging techniques are required. The common observation is that malignant lymph nodes have lower ADC than in benign adenopathy. However, the presence of necrotic lymph nodes can falsely reflect increasing ADC values while benign nodal infiltration can cause decreases in ADC similar to metastatic tumor level. Standardization of the appropriate sequence and ADC threshold values as well as validation in prospective, large multi-center trials are needed.
There are several technical considerations in obtaining DWI in head and neck tumors such as the increased susceptibility to motion artifact relative to brain due to swallowing, breathing, jaw movement, and coughing. There is also the proximity of the sinuses with the air-tissue boundaries as well as metallic dental amalgams and irregular shape of the head and neck anatomy that can increase magnetic field inhomogeneity thereby hindering effective fat signal suppression that is essential for DWI. Both bulk motion, poor magnetic field homogeneity and presence of unsuppressed fat signal can lead to spatial distortion, spurious signal artifact and quantitative ADC errors.
DW-MRI in Response Assessment
Volumetric analysis using CT scans yield variable results in predicting outcome. FDG-PET scans have been extensively investigated and correlate with late tumor response. However, post-treatment induced inflammatory changes can lead to difficulties in data interpretation.
DW-MRI has the ability to detect tissue microstructural changes prior to macroscopic tumor changes. A preclinical study using a head and neck squamous cell carcinoma mouse model demonstrated DW-MRI as an early imaging biomarker of response.34 Longitudinal DWI studies obtained during chemo-radiation show that early changes in ADC noted at 1–2 weeks following treatment were predictive of increased tumor control. Multivariate analysis demonstrated that increases in ADC noted at both 2 and 4 weeks during chemo-radiation were independent predictors of 2-year locoregional disease control. Galban et al demonstrated that regions of increasing PRM (ADC) in locally advanced head and neck squamous cell carcinomas were more likely to be found in complete responders compared to partial responders.35 DWI MRI may also have a role in evaluating response post chemo-radiation by identifying residual and recurrent tumor from post therapeutic treatment changes. Using an ADC threshold of 1.3x10−3 mm2/s, there was a 95% specificity and 96% sensitivity in identifying tumor recurrence.
Conclusions
Due to the heterogeneous response of tumors to head and neck cancer treatment, the ability to identify early regions of poor response to chemo-radiation that may benefit from an adaptive radiation treatment plan with focal dose escalation may further improve local control. The ability of DW imaging to improve target volume delineation, early tumor response assessment and differentiate between normal post-treatment changes suggests an important clinical role in radiotherapy. However, further validation is required prior to its routine clinical use.
Prostate Cancer
Target volume definition based upon CT scans is proving to be a limiting factor in advancing the precision of radiation treatment of prostate cancer. Superior soft tissue contrast provided by MRI has resulted in the investigation of the roles of multi-parametric MRI for the detection, localization and delineation of prostatic cancer. To this end, T2-weighted, diffusion-weighted (or ADC), DCE, and H1 spectroscopy images all have been investigated. T2-weighted MRI is the primary modality used in prostate MR imaging and can delineate tumors in the peripheral zone. However, the transition zone is more variable with increasing age and many benign lesions including post-radiation, hormonal treatment, post-hemorrhage, chronic prostatitis, hyperplasia can all resemble tumor.
There is emerging evidence suggesting that multi-parametric MRI is superior to CT scans, and C11-choline PET may not provide any additional value for the detection and delineation of prostate cancer.36 The current, key question is whether diffusion imaging (or ADC) plays a critical or a supplementary role for the detection and delineation of prostate cancer. Further the sensitivity and specificity of using MR diffusion imaging to delineate the target volumes for external beam radiation therapy and brachytherapy needs to be determined.
In the last several years, clinical investigations have been performed to evaluate the capability of diffusion weighted imaging to detect and to delineate prostate cancer. Systematic random prostate biopsy is prone to sampling errors which can often lead to inaccurate Gleason score grading and prostate cancer detection. DW imaging is a reflection of tumor cellularity and tumor aggressiveness and therefore can be used to determine suspicious regions for MR-guided prostate biopsy. Overall, the results based on the evaluation of pathological tumor specimens demonstrate that diffusion imaging can detect prostate cancer with a sensitivity and specificity of 80% or greater, which is superior to results usingT2-weighted images alone. (Figure 3)37–39 Similar findings are also reported in identifying recurrent prostate cancers after radiation therapy.40,41
Fig 3.
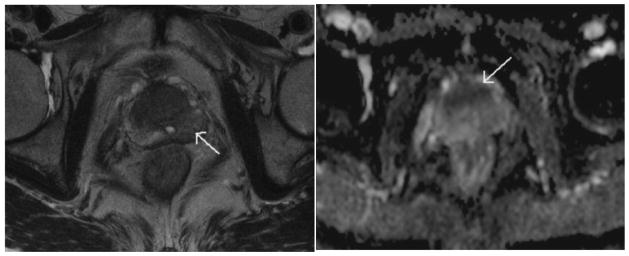
A 72-year-old patient with a serum PSA level of 4.1 ng/mL, the T2W imaging (left panel) showed a hypointense area in the left peripheral zone (arrow); discrimination of cancer from BPH in the transition zone was difficult. The ADC map (right panel) depicted a cancer focus in the anterior prostate as a hypointense area (arrow). Pathologic results revealed a cancer focus well correlated with the finding of the ADC map, but no malignancy was found in the left peripheral zone. Reprinted with permission from Miao et al in Eur J Radiol 2007;61:297–302.
A recent study investigated the appropriate ADC cut-off threshold required to delineate prostate cancer in the peripheral zone (PZ) with the use of an endorectal receiver coil at 1.5 T.38 The radiologist was able to detect 43 out of 60 lesions (size > 0.1 cm) in the PZ of 52 patients. Among the 43 cancers, an ADC threshold of 1.4x10−3 mm2/s missed 4 lesions, while a cutoff threshold of 1.6x10−3 mm2/s missed one. At the voxel-level, the low cutoff threshold had a sensitivity of 82% and a specificity of 85%, while the higher threshold resulted in 95% of sensitivity but a specificity of only 65%. (Figure 4) The addition of DW imaging to T2 weighted imaging significantly improved the accuracy of tumor volume delineation of PZ prostate cancers. 42 Overall, MR diffusion imaging has been recognized as providing additional complementary information compared to T2-weighted images alone.
Fig 4.
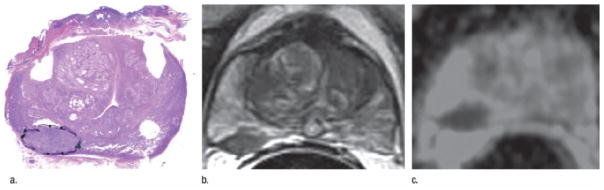
A 66-year-old patient with prostate cancer: presurgical PSA, 5.52 ng/mL, clinical stage, T1C; surgical Gleason score, 3+4; and pathologic tumor volume, 4.77cm3. Whole-mount step-section histopathologic map shows prostate gland (a) Only one (of 12) slices shown; tumor was present on seven slices. (b) Closest transverse T2-weighted image corresponding to matching pathologic slice. (c) ADC map of slice in b. Reprinted with permission from Mazaheri et al in Radiology 2009;252:449–57.
Dynamic contrast-enhanced MR imaging consists of a series of fast T1-weighted sequences covering the entire prostate before and after rapid injection (2–4 mL/sec) with a bolus of a low-molecular-weight gadopentetate dimeglumine (concentration, 0.1–0.2 mmol/kg) that measures tumor vascularity. Several studies have investigated the incremental benefit of T2-weighted, ADC, and DCE images, by adding them sequentially to increase specificity for prostate cancer localization.43–45 Results regarding the additional information provided by DCE imaging toT2-weighted and ADC images have remained inconsistent.43,46 Standardized techniques and analytic tools for DCE MRI need to be further developed.
Using multi-parametric MRI is also challenging to differentiate prostate cancer from stromal hyperplasia (SH) and glandular hyperplasia (GH). A recent study showed that the average ADCs and associated confidence intervals in central gland carcinoma, SH foci, and GH foci had little overlap, as 1.05x10−3 mm2/s (95% CI:0.97, 1.11), 1.27 (95% CI: 1.20, 1.33), and 1.73 (95% CI:1.64, 1.83), respectively.47 The areas under the ROC curve were 0.99 and 0.78 for differentiation of carcinoma from GH and SH, respectively.
There are several technical issues that are important to note with different image technologies, lesion size, tumor cell density, and the location of the lesion all which can significantly alter the DWI imaging results. Diffusion-weighted images using 1.5 T without the use of an endo-rectal coil was limited by the signal-to-noise ratio and produced noisy ADC images. However, the use of an external phase-coil array at 3T seems to yield image quality that is equivalent to that obtained with an endo-rectal coil at 1.5 T.48
There is limited data on the early assessment of treatment response to radiation therapy using DWI. An initial study has detected significant changes in ADC at week 6 during a fractionated RT course.49 However, the value of DWI imaging obtained during RT for early assessment of response to prostate cancer by radiation needs to be further evaluated.
Conclusions
In summary, considering the available imaging modalities for prostate cancer radiation therapy, emerging evidence supports that multi-parametric MRI, including diffusion imaging, is superior for prostatic cancer detection and localization. Whether using multi-parametric MRI to define a boost target for intensified radiation therapy can lead to better outcomes, or reduce toxicity has to yet be demonstrated in prospective clinical trials.
Gynecological Cancer
The prognostic and predictive value of dynamic contrast enhanced (DCE) MRI for radiation therapy outcomes of cervical cancers has already been demonstrated by several studies.50,51 These studies suggested that well oxygenated and better perfused tumors were more sensitive to radiation while tumors with increased non-enhancing volumes attributable to necrotic or hypoxic tumor at baseline and persisting during the early course of RT were associated with worse overall and progression free survival. The key question is whether diffusion weighted imaging can provide any additional complimentary information for guiding radiation therapy in cervical cancers.
Several studies have shown the potential of ADC measurements in the diagnosis and treatment response assessment of cervical cancers to radiotherapy.52–57 An initial study of 49 patients with cervical cancer has shown that the median ADC value (1.1x10−3 mm2/s) in cervical cancer is significantly lower than in normal cervical tissue (2.1x10−3 mm2/s),56 indicating a robust discrimination of cervical cancer from normal tissue using ADC. In another study, the ADC defined tumor volume of cervical cancer was compared to tumor volumes defined by 40% of max SUV of FDG PET, the latter is the standard functional imaging for pre and post RT evaluation.52 There was good spatial overlap noted between the two tumor volumes, and the mean SUV on the contours of the ADC defined tumor volumes was approximately 34% of the max SUV, suggesting that tumor sub-volumes with an increase in metabolic activity on FDG PET also had a greater cell density (or low ADC). Another study investigated the ADC values in the GEC ESTRO target volumes for image guided adaptive brachytherapy of locally advanced cervical cancer.55 Using an ADC of 1.2x10−3 mm2/s as the threshold to define low diffusion, they found that 37%, 20% and 11% of respective brachytherapy gross tumor volume, high-risk clinical target volume, and intermediate-risk target volume had low diffusion. These studies suggest that it is worthwhile to further investigate using ADC imaging as a means to define a boost target volume for advanced cervical cancers by either a highly conformal external beam radiation therapy boost plan or with brachytherapy. Early ADC changes during a course of chemoradiation therapy for cervical cancers have been evaluated in two studies.54,57 In both studies, diffusion MRI was obtained prior to chemo-radiation therapy, and at 2 weeks during concurrent cisplatin and radiation delivered for 5 weeks. The response was determined by the tumor volume change at the end of the treatment. All patients except one had a partial response. The ADC values in the cervical cancers increased robustly after receiving 2 weeks of chemo-RT. The early ADC changes were significantly correlated with tumor volume response at the end of RT. However, whether this early ADC response noted during in chemo-radiation treatment in cervical cancer predicts for improved overall survival requires further investigation.
Conclusions
Diffusion MR imaging is a promising imaging biomarker for cervical cancer. Further investigation in its clinical application to radiotherapy treatment planning is warranted. Robust changes in ADC are noted early during the course of RT in cervical cancer indicating its potential as a predictive marker. DW imaging may provide complementary information to DCE MRI for the early assessment and prediction of clinical outcome.
Summary
Diffusion weighted MR imaging is a functional imaging technique that requires no contrast agent and is also rapidly and easily obtained as part of routine MR imaging evaluations. Diffusion weighted and diffusion tensor imaging has extensive clinical applications in radiation oncology. DWI provides important information regarding early assessment of treatment response. Other emerging applications include improvement in diagnosis and staging by defining areas of tumor aggressiveness based on tumor cellularity, determination of target volumes for biopsy, quantitative assessment of changes to normal appearing white matter following radiation and in defining potential non-responding tumor regions that may benefit from an additional radiotherapy boost. However, standardization of the imaging protocols and validation in large multi-center trials is still required prior to its routine clinical use.
Acknowledgments
Grant Support: RO1 NS064973 (Cao, Tsien)
RO1 CA132834 (Cao)
RO1 EB016079 (Cao, Tsien)
P01 CA085878 (Chenevert)
U01 CA166104 (Chenevert)
The authors would like to thank Chris Chapman, B.A. and Reza Farjam, PhD. for their work in creating the figures in the brain imaging section for this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 2.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–7. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 3.Szafer A, Zhong J, Gore JC. Theoretical model for water diffusion in tissues. Magn Reson Med. 1995;33:697–712. doi: 10.1002/mrm.1910330516. [DOI] [PubMed] [Google Scholar]
- 4.Norris DG. Implications of bulk motion for diffusion weighted imaging experiments: effects, mechanisms, and solutions. J Magn Reson Imaging. 2001;13:486–95. doi: 10.1002/jmri.1072. [DOI] [PubMed] [Google Scholar]
- 5.Kauppinen RA. Monitoring cytotoxic tumour treatment response by diffusion magnetic resonance imaging and proton spectroscopy. NMR Biomed. 2002;15:6–17. doi: 10.1002/nbm.742. [DOI] [PubMed] [Google Scholar]
- 6.Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–36. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 7.Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3:1457–66. [PubMed] [Google Scholar]
- 8.Chenevert TL. Principles of Diffusion Weighted Imaging (DW RMI) as Applied to Body Imaging. In: Koh DM, Thoeny HC, editors. Diffusion Weighted MR Imaging Application in the Body in Medical Radiology: Diagnostic Imaging and Raidation Oncology. Heidelberg: Springer; 2010. [Google Scholar]
- 9.Norris DG. The effects of microscopic tissue parameters on the diffusion weighted magnetic resonance imaging experiment. NMR Biomed. 2001;14:77–93. doi: 10.1002/nbm.682. [DOI] [PubMed] [Google Scholar]
- 10.Van Cauter S, Veraart J, Sijbers J, et al. Gliomas: diffusion kurtosis MR imaging in grading. Radiology. 2012;263:492–501. doi: 10.1148/radiol.12110927. [DOI] [PubMed] [Google Scholar]
- 11.Raab P, Hattingen E, Franz K, Zanella FE, Lanfermann H. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010;254:876–81. doi: 10.1148/radiol.09090819. [DOI] [PubMed] [Google Scholar]
- 12.Kwee TC, Galban CJ, Tsien C, et al. Comparison of apparent diffusion coefficients and distributed diffusion coefficients in high grade gliomas. J Magn Reson Imaging. 2010;31:531–7. doi: 10.1002/jmri.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chenevert TL, Pipe JG. Effect of bulk tissue motion on quantitative perfusion and diffusion magnetic resonance imaging. Magn Reson Med. 1991;19:261–5. doi: 10.1002/mrm.1910190212. [DOI] [PubMed] [Google Scholar]
- 14.Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion weighted MRI with echo planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9:53–60. doi: 10.1002/(sici)1522-2586(199901)9:1<53::aid-jmri7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Zulfiqar M, Yousem DM, Lai H. ADC values and prognosis of malignant astrocytomas: does lower ADC predict a worse prognosis independent of grade of tumor? a meta analysis. AJR Am J Roentgenol. 2013;200:624–9. doi: 10.2214/AJR.12.8679. [DOI] [PubMed] [Google Scholar]
- 16.Hamstra DA, Galban CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol. 2008;26:3387–94. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross BD, Moffat BA, Lawrence TS, et al. Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol Cancer Ther. 2003;2:581–7. [PubMed] [Google Scholar]
- 18.Hall DE, Moffat BA, Stojanovska J, et al. Therapeutic efficacy of DTI 015 using diffusion magnetic resonance imaging as an early surrogate marker. Clin Cancer Res. 2004;10:7852–9. doi: 10.1158/1078-0432.CCR-04-1218. [DOI] [PubMed] [Google Scholar]
- 19.Rehemtulla A, Hall DE, Stegman LD, et al. Molecular imaging of gene expression and efficacy following adenoviral mediated brain tumor gene therapy. Mol Imaging. 2002;1:43–55. doi: 10.1162/15353500200200005. [DOI] [PubMed] [Google Scholar]
- 20.Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A. 2005;102:5524–9. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galban CJ, Chenevert TL, Meyer CR, et al. Prospective analysis of parametric response map derived MRI biomarkers: identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin Cancer Res. 2011;17:4751–60. doi: 10.1158/1078-0432.CCR-10-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamstra DA, Chenevert TL, Moffat BA, et al. Evaluation of the functional diffusion map as an early biomarker of time to progression and overall survival in high grade glioma. Proc Natl Acad Sci U S A. 2005;102:16759–64. doi: 10.1073/pnas.0508347102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farjam R, Tsien CI, Feng FY, et al. Diffusion Abnormality Index: a New Imaging Biomarker for Early Assessment of Tumor Response to Therapy. Neuro Oncology. 2013 doi: 10.1016/j.prro.2013.01.018. (in press) [DOI] [PubMed] [Google Scholar]
- 24.Galban CJ, Chenevert TL, Meyer CR, et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat Med. 2009;15:572–6. doi: 10.1038/nm.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins ME, Brunso Bechtold JK, Peiffer AM, Tsien CI, Bailey JE, Marks LB. Imaging radiation-induced normal tissue injury. Radiat Res. 2012;177:449–66. doi: 10.1667/rr2530.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–9. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 27.Nagesh V, Tsien CI, Chenevert TL, et al. Radiation induced changes in normal appearing white matter in patients with cerebral tumors: a diffusion tensor imaging study. Int J Radiat Oncol Biol Phys. 2008;70:1002–10. doi: 10.1016/j.ijrobp.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole brain irradiation in rats. Radiat Res. 2005;164:662–8. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- 29.Chapman CH, Nagesh V, Sundgren PC, et al. Diffusion tensor imaging of normal appearing white matter as biomarker for radiation induced late delayed cognitive decline. Int J Radiat Oncol Biol Phys. 2012;82:2033–40. doi: 10.1016/j.ijrobp.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haris M, Kumar S, Raj MK, et al. Serial diffusion tensor imaging to characterize radiation induced changes in normal appearing white matter following radiotherapy in patients with adult low grade gliomas. Radiat Med. 2008;26:140–50. doi: 10.1007/s11604-007-0209-4. [DOI] [PubMed] [Google Scholar]
- 31.Abdel Razek AA, Soliman NY, Elkhamary S, Alsharaway MK, Tawfik A. Role of diffusion weighted MR imaging in cervical lymphadenopathy. Eur Radiol. 2006;16:1468–77. doi: 10.1007/s00330-005-0133-x. [DOI] [PubMed] [Google Scholar]
- 32.Vandecaveye V, De Keyzer F, Vander Poorten V, et al. Head and neck squamous cell carcinoma: value of diffusion weighted MR imaging for nodal staging. Radiology. 2009;251:134–46. doi: 10.1148/radiol.2511080128. [DOI] [PubMed] [Google Scholar]
- 33.Holzapfel K, Duetsch S, Fauser C, Eiber M, Rummeny EJ, Gaa J. Value of diffusion weighted MR imaging in the differentiation between benign and malignant cervical lymph nodes. Eur J Radiol. 2009;72:381–7. doi: 10.1016/j.ejrad.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 34.Hamstra DA, Lee KC, Moffat BA, Chenevert TL, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an imaging treatment response biomarker to chemoradiotherapy in a mouse model of squamous cell cancer of the head and neck. Transl Oncol. 2008;1:187–94. doi: 10.1593/tlo.08166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galban CJ, Mukherji SK, Chenevert TL, et al. A feasibility study of parametric response map analysis of diffusion weighted magnetic resonance imaging scans of head and neck cancer patients for providing early detection of therapeutic efficacy. Transl Oncol. 2009;2:184–90. doi: 10.1593/tlo.09175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van den Bergh L, Isebaert S, Koole M, et al. Does C choline PET CT contribute to multiparametric MRI for prostate cancer localisation? Strahlenther Onkol. 2013 doi: 10.1007/s00066-013-0359-5. [DOI] [PubMed] [Google Scholar]
- 37.Miao H, Fukatsu H, Ishigaki T. Prostate cancer detection with 3 T MRI: comparison of diffusion-weighted and T2 weighted imaging. Eur J Radiol. 2007;61:297–302. doi: 10.1016/j.ejrad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Mazaheri Y, Hricak H, Fine SW, et al. Prostate tumor volume measurement with combined T2-weighted imaging and diffusion weighted MR: correlation with pathologic tumor volume. Radiology. 2009;252:449–57. doi: 10.1148/radiol.2523081423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kajihara H, Hayashida Y, Murakami R, et al. Usefulness of diffusion weighted imaging in the localization of prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:399–403. doi: 10.1016/j.ijrobp.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 40.Hara T, Inoue Y, Satoh T, et al. Diffusion weighted imaging of local recurrent prostate cancer after radiation therapy: comparison with 22 core three dimensional prostate mapping biopsy. Magn Reson Imaging. 2012;30:1091–8. doi: 10.1016/j.mri.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Liauw SL, Pitroda SP, Eggener SE, et al. Evaluation of the prostate bed for local recurrence after radical prostatectomy using endorectal magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2013;85:378–84. doi: 10.1016/j.ijrobp.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Rischke HC, Nestle U, Fechter T, et al. 3 Tesla multiparametric MRI for GTV definition of Dominant Intraprostatic Lesions in patients with Prostate Cancer an interobserver variability study. Radiat Oncol. 2013;8:183. doi: 10.1186/1748-717X-8-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donati OF, Jung SI, Vargas HA, et al. Multiparametric Prostate MR Imaging with T2 weighted, Diffusion weighted, and Dynamic Contrast enhanced Sequences: Are All Pulse Sequences Necessary to Detect Locally Recurrent Prostate Cancer after Radiation Therapy? Radiology. 2013;268:440–50. doi: 10.1148/radiol.13122149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akin O, Gultekin DH, Vargas HA, et al. Incremental value of diffusion weighted and dynamic contrast enhanced MRI in the detection of locally recurrent prostate cancer after radiation treatment: preliminary results. Eur Radiol. 2011;21:1970–8. doi: 10.1007/s00330-011-2130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim CK, Park BK, Lee HM. Prediction of locally recurrent prostate cancer after radiation therapy: incremental value of 3T diffusion weighted MRI. J Magn Reson Imaging. 2009;29:391–7. doi: 10.1002/jmri.21645. [DOI] [PubMed] [Google Scholar]
- 46.Kim CK, Park BK, Park W, Kim SS. Prostate MR imaging at 3T using a phased arrayed coil in predicting locally recurrent prostate cancer after radiation therapy: preliminary experience. Abdom Imaging. 2010;35:246–52. doi: 10.1007/s00261-008-9495-2. [DOI] [PubMed] [Google Scholar]
- 47.Oto A, Kayhan A, Jiang Y, et al. Prostate cancer: differentiation of central gland cancer from benign prostatic hyperplasia by using diffusion weighted and dynamic contrast enhanced MR imaging. Radiology. 2010;257:715–23. doi: 10.1148/radiol.10100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sosna J, Pedrosa I, Dewolf WC, Mahallati H, Lenkinski RE, Rofsky NM. MR imaging of the prostate at 3 Tesla: comparison of an external phased array coil to imaging with an endorectal coil at 1. 5 Tesla. Acad Radiol. 2004;11:857–62. doi: 10.1016/j.acra.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Foltz WD, Wu A, Chung P, et al. Changes in apparent diffusion coefficient and T2 relaxation during radiotherapy for prostate cancer. J Magn Reson Imaging. 2013;37:909–16. doi: 10.1002/jmri.23885. [DOI] [PubMed] [Google Scholar]
- 50.Mayr NA, Huang Z, Wang JZ, et al. Characterizing tumor heterogeneity with functional imaging and quantifying high risk tumor volume for early prediction of treatment outcome: cervical cancer as a model. Int J Radiat Oncol Biol Phys. 2012;83:972–9. doi: 10.1016/j.ijrobp.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayr NA, Wang JZ, Zhang D, et al. Longitudinal changes in tumor perfusion pattern during the radiation therapy course and its clinical impact in cervical cancer. Int J Radiat Oncol Biol Phys. 2010;77:502–8. doi: 10.1016/j.ijrobp.2009.04.084. [DOI] [PubMed] [Google Scholar]
- 52.Olsen JR, Esthappan J, DeWees T, et al. Tumor volume and subvolume concordance between FDG PET/CT and diffusion weighted MRI for squamous cell carcinoma of the cervix. J Magn Reson Imaging. 2013;37:431–4. doi: 10.1002/jmri.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Somoye G, Harry V, Semple S, et al. Early diffusion weighted magnetic resonance imaging can predict survival in women with locally advanced cancer of the cervix treated with combined chemoradiation. Eur Radiol. 2012;22:2319–27. doi: 10.1007/s00330-012-2496-0. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Chen JY, Xie CM, et al. Diffusion weighted magnetic resonance imaging for prediction of response of advanced cervical cancer to chemoradiation. J Comput Assist Tomogr. 2011;35:102–7. doi: 10.1097/RCT.0b013e3181f6528b. [DOI] [PubMed] [Google Scholar]
- 55.Haack S, Pedersen EM, Jespersen SN, Kallehauge JF, Lindegaard JC, Tanderup K. Apparent diffusion coefficients in GEC ESTRO target volumes for image guided adaptive brachytherapy of locally advanced cervical cancer. Acta Oncol. 2010;49:978–83. doi: 10.3109/0284186X.2010.500619. [DOI] [PubMed] [Google Scholar]
- 56.McVeigh PZ, Syed AM, Milosevic M, Fyles A, Haider MA. Diffusion weighted MRI in cervical cancer. Eur Radiol. 2008;18:1058–64. doi: 10.1007/s00330-007-0843-3. [DOI] [PubMed] [Google Scholar]
- 57.Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusion weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111:213–20. doi: 10.1016/j.ygyno.2008.07.048. [DOI] [PubMed] [Google Scholar]