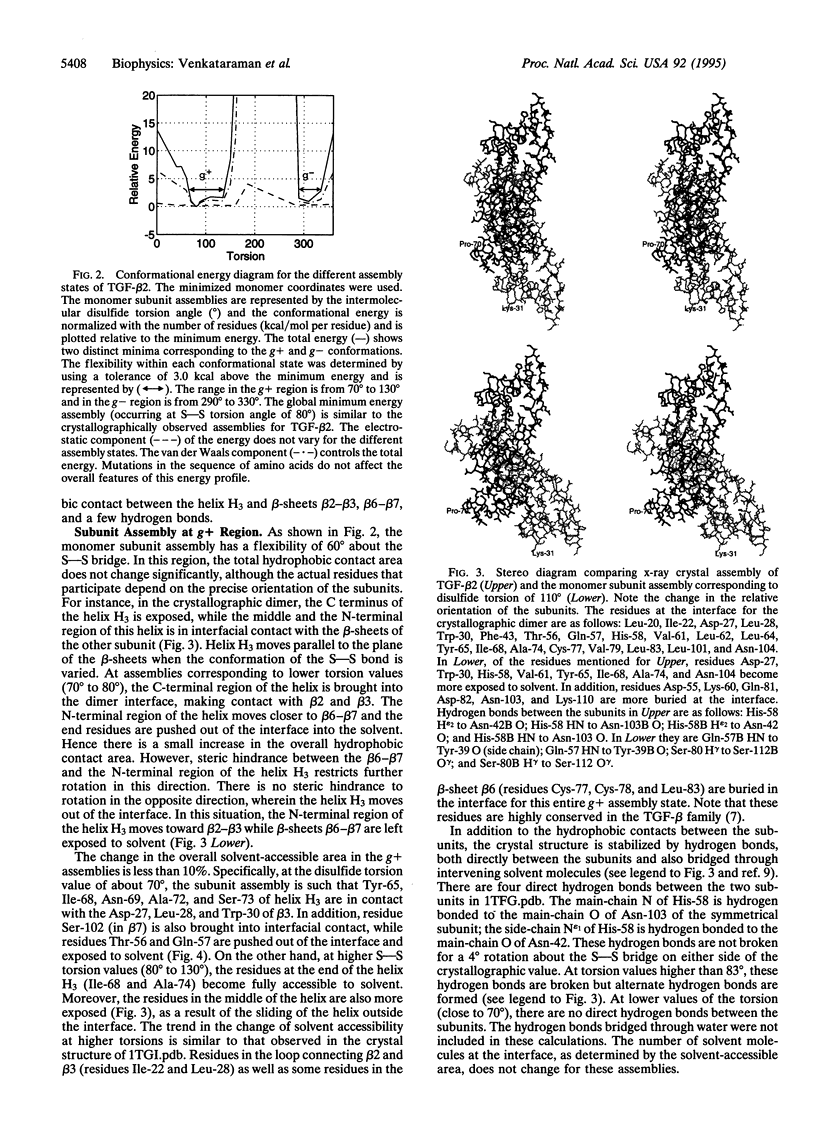
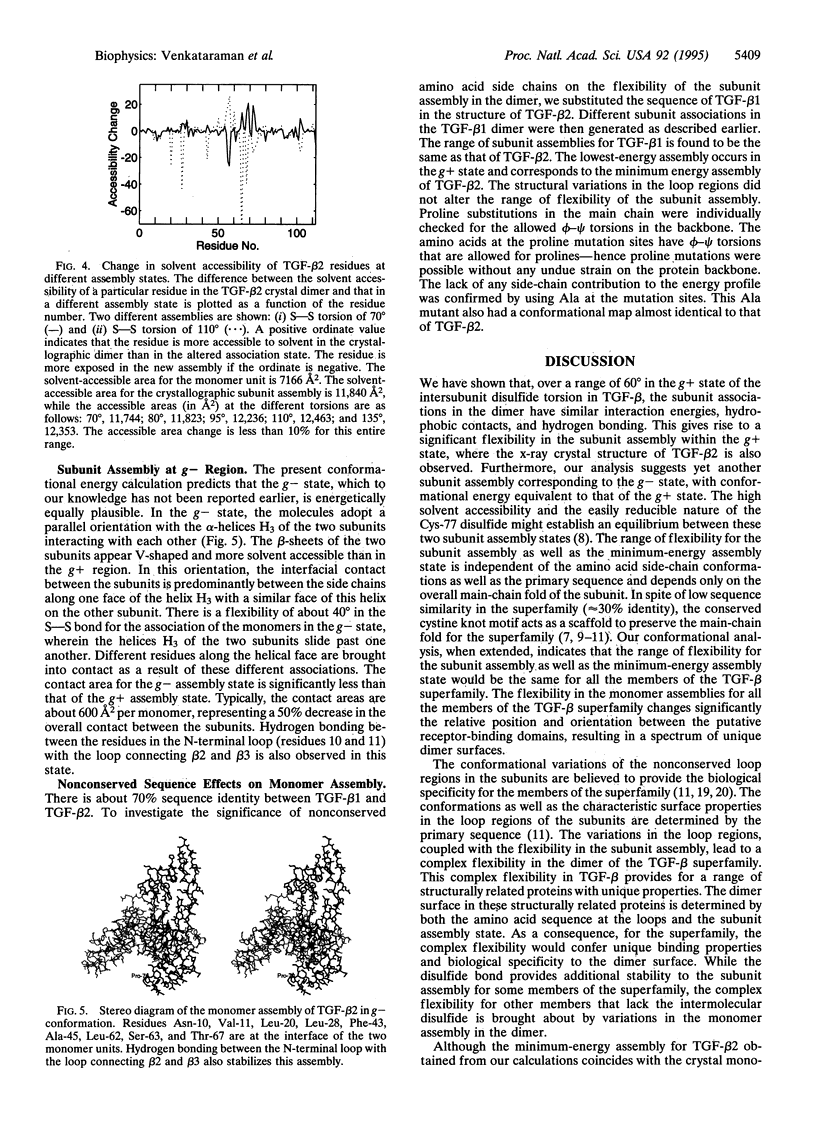
Abstract
The transforming growth factors beta (TGF-beta s) are important modulators of growth and differentiation. They are intermolecular disulfide-bonded homodimeric molecules. The monomer fold has a conserved cystine knot and lacks a hydrophobic core. The biological specificity of a given member of the family is believed to be determined by the conformational flexibility of the variable loop regions of the monomer. The monomer subunit assembly in the dimer is stabilized mainly by hydrophobic contacts and a few hydrogen bonds. Since these interactions are nondirectional, we examined subunit assemblies of TGF-beta by using conformational analysis. The different subunit assemblies in TGF-beta 2 dimer were characterized in terms of the intersubunit disulfide torsion. Our analyses show that the subunit assemblies fall into two states: the crystallographically observed gauche+conformation and the previously not reported gauche--conformation, both having almost identical interaction energies. Furthermore, there is significant flexibility in the subunit assembly within the gauche+ and the gauche- states of the disulfide bond. The monomer subunit assembly is independent of the variations about the loop regions. The variations in the loop regions, coupled with flexibility in the monomer assembly, lead to a complex flexibility in the dimer of the TGF-beta superfamily. For the TGF-beta superfamily, the cystine knot acts as a scaffold and complex flexibility provides for biological selectivity. Complex flexibility might provide an explanation for the diverse range of biological activities that these important molecules display.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer S. J., Bax A., Roberts A. B., Sporn M. B., Ogawa Y., Piez K. A., Weatherbee J. A., Tsang M. L., Lucas R., Zheng B. L. Transforming growth factor beta 1: secondary structure as determined by heteronuclear magnetic resonance spectroscopy. Biochemistry. 1993 Feb 2;32(4):1164–1171. doi: 10.1021/bi00055a022. [DOI] [PubMed] [Google Scholar]
- Burmester J. K., Qian S. W., Roberts A. B., Huang A., Amatayakul-Chantler S., Suardet L., Odartchenko N., Madri J. A., Sporn M. B. Characterization of distinct functional domains of transforming growth factor beta. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8628–8632. doi: 10.1073/pnas.90.18.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C. Hydrophobic bonding and accessible surface area in proteins. Nature. 1974 Mar 22;248(446):338–339. doi: 10.1038/248338a0. [DOI] [PubMed] [Google Scholar]
- Daopin S., Li M., Davies D. R. Crystal structure of TGF-beta 2 refined at 1.8 A resolution. Proteins. 1993 Oct;17(2):176–192. doi: 10.1002/prot.340170207. [DOI] [PubMed] [Google Scholar]
- Daopin S., Piez K. A., Ogawa Y., Davies D. R. Crystal structure of transforming growth factor-beta 2: an unusual fold for the superfamily. Science. 1992 Jul 17;257(5068):369–373. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Baase W. A., Zhang X. J., Heinz D. W., Blaber M., Baldwin E. P., Matthews B. W. Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science. 1992 Jan 10;255(5041):178–183. doi: 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994 Jan;8(2):133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Becktel W. J., Matthews B. W. Hydrophobic stabilization in T4 lysozyme determined directly by multiple substitutions of Ile 3. Nature. 1988 Aug 4;334(6181):406–410. doi: 10.1038/334406a0. [DOI] [PubMed] [Google Scholar]
- McDonald N. Q., Hendrickson W. A. A structural superfamily of growth factors containing a cystine knot motif. Cell. 1993 May 7;73(3):421–424. doi: 10.1016/0092-8674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- Ooi T., Oobatake M., Némethy G., Scheraga H. A. Accessible surface areas as a measure of the thermodynamic parameters of hydration of peptides. Proc Natl Acad Sci U S A. 1987 May;84(10):3086–3090. doi: 10.1073/pnas.84.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S. W., Burmester J. K., Merwin J. R., Madri J. A., Sporn M. B., Roberts A. B. Identification of a structural domain that distinguishes the actions of the type 1 and 2 isoforms of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6290–6294. doi: 10.1073/pnas.89.14.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S. W., Burmester J. K., Sun P. D., Huang A., Ohlsen D. J., Suardet L., Flanders K. C., Davies D., Roberts A. B., Sporn M. B. Characterization of mutated transforming growth factor-beta s which possess unique biological properties. Biochemistry. 1994 Oct 11;33(40):12298–12304. doi: 10.1021/bi00206a037. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Sasisekharan V. Conformation of polypeptides and proteins. Adv Protein Chem. 1968;23:283–438. doi: 10.1016/s0065-3233(08)60402-7. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth Factors. 1993;8(1):1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Schlunegger M. P., Grütter M. G. An unusual feature revealed by the crystal structure at 2.2 A resolution of human transforming growth factor-beta 2. Nature. 1992 Jul 30;358(6385):430–434. doi: 10.1038/358430a0. [DOI] [PubMed] [Google Scholar]
- Schlunegger M. P., Grütter M. G. Refined crystal structure of human transforming growth factor beta 2 at 1.95 A resolution. J Mol Biol. 1993 May 20;231(2):445–458. doi: 10.1006/jmbi.1993.1293. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Wieser R., Ventura F., Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994 Aug 4;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]