Abstract
Thyroid associated ophthalmopathy is an autoimmune disorder affecting the orbital and periorbital tissues. Hyperthyroidism is commonly associated with thyroid associated ophthalmopathy, however in 5% to 10% of cases it is euthyroid. Genetic, environmental and endogenous factors play a role in the initiation of the thyroid ophthalmopathy. Smoking has been identified as the strongest risk factor for the development of the disorder. The pathogenesis involves activation of both humoral and cell mediated immunity with subsequent production of gycoaminoglycans, hyaluronic acid resulting in oedema formation, increase extraocular mass and adipogenesis in the orbit. The natural history of the disease progresses from active to inactive fibrotic stage over a period of years. Diagnosis is mainly clinical and almost all patients with ophthalmopathy exhibit some form of thyroid abnormality on further testing. Treatment is based on the clinical severity of the disease. Non-severe cases are managed by supportive measures to reduce the symptomatology and severe cases are treated by either medical or surgical decompression. Rehabilitative surgery is done for quiescent disease to reduce diplopia and improve cosmesis.
Keywords: Thyroid eye disease, autoimmunity, smoking, corticosteroids, radiotherapy, surgical decompression, rehabilitative surgery
INTRODUCTION
Thyroid associated ophthalmopathy (TAO) is also known as, thyroid eye disease (TED), Graves’ ophthalmopathy/ orbitopathy (GO), dysthyroid ophthalmopathy, thyrotoxic exophthalmos and other terms. It is an autoimmune process which affects the thyroid gland, orbital and periorbital tissue and uncommonly the pretibial skin or digits (thyroid acropachy). The individual components can occur together or separately. It is the most frequent extrathyroidal manifestation of Graves’ disease. Although TAO is often associated with hyperthyroidism, it may occur in primary hypothyroidism, Hashimoto’s thyroiditis, and sometimes in euthyroid individuals.1-3 The incidence and prevalence of Graves’ disease is 0.1% and 1% respectively. The clinical signs include widening of the palpebral fissure, eye lid retraction, lid lag, conjunctival congestion, chemosis, proptosis, corneal exposure, restrictive myopathy and optic neuropathy. In majority of cases the ocular manifestations are mild, and severe form of the disease affects 3% to 5% of individuals.4
METHODOLOGY
All our reference articles were obtained from Pubmed. The key words for search were thyroid ophthalmopathy, thyroid orbitopathy, thyroid associated ophthalmopathy, ocular manifestations of thyroid, ocular features of Graves’ disease, thyroid eye disease, and Graves’ ophthalmopathy etc. We used the MeSH database and journal database for our search and our search limits were articles in English and age above 1 year.
FREQUENCY
The exact incidence of ophthalmopathy is not clear. The prevalence of TAO (thyroid associated ophthalmopathy) in patients with GD (Graves’ disease) in Caucasian population is generally thought to be between 25% and 50%.5,6 Bartley7 reported, in a population- based setting in USA, an annual incidence rate of 16 cases per 100,000 population per year for women, and 2.9 cases for men. In Malaysia, Lim et al8 reported a higher prevalence rate (34.7%) of thyroid associated ophthalmopathy in three populations of Asian patients with GD. Most patients without ophthalmopathy have subtle changes noted in orbital imaging.9 It is more common in females than males. The female to male ratio in one study was noted to be 9.3 in patients with mild ophthalmopathy, 3.2 in those with moderate ophthalmopathy, and 1.4 in those with severe ophthalmopathy.10 TAO presents usually in the fourth to fifth decade. In juvenile Graves disease, ophthalmopathy was reported in two-third of the patients in the age group of 11-18 years and one third of cases in the age group of less than 10 years.11 Men and older age are associated with more severe ophthalmopathy.12,13
The natural history of TAO is not clearly understood. In 90% of cases the disease runs a benign course. Untreated, TAO has a tendency to “burn itself out” within 3 to 36 months.14 Recurrences are usually uncommon and the disease rarely results in blindness.
PREDISPOSING FACTORS
Graves’ disease is an autoimmune disorder. Genetic, environmental and endogenous factors are believed to initiate or predispose for its development. Several genes, including HLA,15,16 CTLA4,17 TCR β-chain18 and Ig heavy chain have been known to increase the susceptibility for the development of Graves’ disease, however there are not much evidences to suggest the association between these susceptibility loci and the development of ophthalmopathy.
Environmental factors are thought to be the primary predisposing factors for the developmental of TAO. Among the several environmental factors blamed, smoking represents the strongest risk factor associated with the development of ophthalmopathy.19 Several studies have shown that the prevalence of smokers in patients with Graves’ disease and even more, patients with Graves’ ophthalmopathy is much higher than any other auto-immune or non-auto-immune thyroid disorder.20-23 Smoking causes partial hypoxia, which stimulates the orbital fibroblasts to synthesize glycoaminoglycans which exacerbates extra ocular muscle oedema and swelling.24 The cigarette smoke extract (CSE) is also known to increase adipogenesis.25 A systematic review on cigarette smoking and thyroid eye disease also shows a strong evidence for a causal association between smoking and the development of thyroid associated ophthalmopathy.26 Smokers have a higher risk of developing more advanced GO than non-smokers.27 Even in juvenile GD the prevalence of ophthalmopathy is higher among teenage smokers. Other factors found to be associated with thyroid associated ophthalmopathy are infection with Yersinia enterocolitica, other auto-immune disorders like myasthenia gravis, Addison disease, vitiligo and pernicious anaemia.
PATHOGENESIS
In Graves’ disease, the pathology is due to the presence of an IgG antibody called long-acting thyroid stimulator directed against the plasma membrane of the thyroid cells.28,29 However, the pathogenesis of TAO is uncertain. Antibodies directed against thyroid follicular cells recognize antigenic epitopes, which are shared by tissues contained within the orbital space.30 The effector and target cells are probably the periadipocytes and fibroblasts present in the perimysium of the extraocular muscles and the orbital connective tissues. These cells when stimulated differentiate into mature adipocytes which express the TSHr in increased levels.30 Inflammatory mediators released by the inflammatory cells stimulate the periadpocytes and the fibroblasts which results in adipogenesis, enlargement of extraocular muscles and secretion of glycoaminoglycans (GAG) and hyaluronic acid. This results in increase intraocular volume causing proptosis and elevation of the intraocular pressure. This suggest that the TSHr –directed antibodies do not probably have a direct pathogenic role, but only reflect the intensity of the orbital autoimmune response.
CLINICAL FEATURES
The clinical features of thyroid ophthalmopathy depend on the stage of the disease. The initial acute stage of the disease is characterized by active inflammation in which the eyes are red and painful and the disease later progresses to a stable or a quiescent stage in which the eyes are white and unchanging with a painless motility defect.31
Ocular symptoms
These may include increased lacrimation, sandy or gritty sensation, photophobia (increased sensitivity to light of normal intensity), puffy eyelids, bulging eyes, dry eyes, retrobulbar discomfort, ocular pain, and double vision, loss of vision, visual field loss and acquired colour vision defect due to damage of the large achromatic fibres and small chromatic fibres.32
Ocular signs
Ocular manifestations may be divided into infiltrative and noninfiltrative. Non-infiltrative signs precede infiltrative signs and include widening of palpebral fissure, upper eye lid retraction (Dalrymple’s sign), lower lid retraction (Collier’s sign) and lid lag on down gaze (von Grafe’s sign). Lid retraction is the commonest clinical feature of TAO seen in Caucasians.7,33,34 In Asians, exophthalmos was the commonest sign reported.8 Upper lid retraction is caused by several mechanisms which include: overaction of Muller’s muscle due to increase sympathetic drive, proptosis and fibrosis/dehiscence of the levator palpebrae superioris muscle. Normally, the location of the upper lid is 1-1.5 mm below the superior limbus and that of the lower lid is at the inferior limbus.
Infiltrative signs include: exophthalmos, conjunctival injection, chemosis, fullness of the eyelids, enlargement of lacrimal gland, herniation of orbital fat, infrequent blinking (Stellwag’s sign) increase intraocular pressure,35 strabismus and restriction of extraocular movements (Ballet sign). The inferior and medial rectus muscle is commonly involved in TAO.36 TAO is the commonest cause of axial proptosis in adults. It can be unilateral or bilateral. Proptosis is usually measured by Hertel exophthalmometer (Figure 3). It measures the distance between the lateral orbital rim and the anterior surface of the cornea. The upper limit of normal for whites is 18 mm and for blacks is 21 mm and for Asians the normal range is 12-18 mm. A difference in reading of more than 2 mm between the two eyes is suggestive of proptosis.
Figure 3.

Hertel exophthalmometer.
Figure 1.
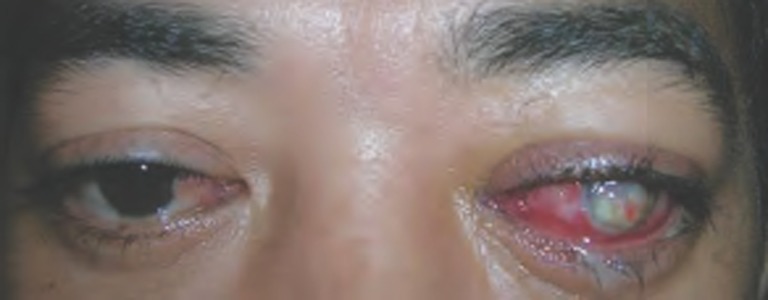
A patient with TED and corneal perforation of the left eye.
Figure 2.
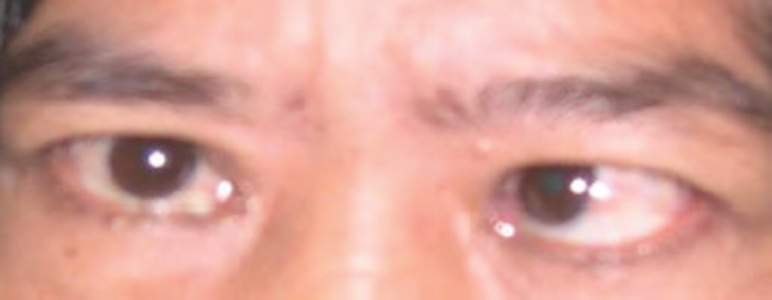
A patient with TED and strabismus of the left eye (esotropia).
THYROID DERMOPATHY / PRE-TIBIAL MYXEDEMA
It is also an autoimmune manifestation of Graves’ disease, which is usually associated with severe ophthalmopathy. Ophthalmopathy is said to occur first and dermopathy occurs later. Lesion of pre-tibial myxedema is usually asymptomatic and has only cosmetic importance. Thyroid-stimulating hormone receptor antibody in the connective tissue is believed to be the antigen initiating the immune process.37
COMPLICATIONS
Inability to close the eyelids as result of lid retraction and proptosis can lead to exposure keratopathy.38 Corneal defects in TAO also occurs because of widening of the palpebral fissure accelerating evaporation of the tear film and increase in the tear film osmolarity.39
Compression of the optic nerve or its blood supply due to enlarged extraocular muscles at the orbital apex causes optic neuropathy.40 Compressive optic neuropathy occurs in less than 5% of patients.41 The optic neuropathy produces progressive loss of visual acuity, decreased color vision and defects in the visual field, usually central scotoma.42
The prevalence of glaucoma is said to be much higher in patients with Graves’ disease than in the general population.43
CLINICAL CLASSIFICATION OF DISEASE SEVERITY
There are several classifications available to grade the severity of thyroid ophthalmopathy. The symptoms and signs of TAO are often classified with the modified NO SPECS system.44 (Table 1). The CAS system (clinical activity score) is another system which was developed for the selection of therapy, especially for deciding whether or not immunosuppressive therapy should be instituted.45
Table 1. Abridged classification of eye changes of Graves’ disease.
| Class | Definition |
|---|---|
| 0 | No physical signs and symptoms |
| I | Only signs, no symptoms (signs limited to upper eyelid retraction, stare and lid lag) |
| II | Soft tissue involvement (signs and symptoms) |
| III | Proptosis (3 mm or more) |
| IV | Extraocular muscle involvement |
| V | Corneal involvement |
| VI | Sight loss (optic nerve involvement) |
Adapted from Werner44
DIAGNOSIS
The diagnosis of TAO is clinical and is based on the triad of characteristic eye findings, thyroid dysfunction, and imaging studies.46 In majority of cases, ophthalmopathy occurs in association with hyperthyroidism. TAO is also said to occur in 5% of patients with Hashimoto’s thyroiditis. However in 5% to 10% of cases, ophthalmopathy occurs in the absence of thyroid disease (euthyroid disease). This could be due to lack of TSH control of thyroid function in patients with ophthalmic Graves’ disease. Even in patients without overt thyroid disease further tests like triiodothyronine (T3) suppression test, anti-thyroid antibodies abnormal thyroid-releasing hormone stimulation test and TSH antibody tests will demonstrate abnormalities in about 30% of patients.47,48 Patients with a euthyroid disease can even become hyper or hypothyroid after sometime. All these findings suggest that thyroid associated ophthalmopathy is heterogeneous either due to different phase of presentation or different pathogenesis in different patients.46
The most characteristic findings in TAO are enlargement of the extra ocular muscles, without involvement of their tendons. Ultrasound B-scan of the eye, CT-scan orbit and MRI can be used to show thickened muscles, however CT scan is currently the imaging study of choice. MRI is sensitive for showing compression of the optic nerve.
DIFFERENTIAL DIAGNOSIS
The clinical features of thyroid associated ophthalmopathy are usually identified easily if it is bilateral and associated with hyperthyroidism, however in patients with euthyroid disease and unilateral it is essential to rule out other causes of lid retraction and proptosis. The differential diagnosis of proptosis include vascular conditions like cavernous haemangioma, orbital varices, lymphomas, inflammatory conditions which may be pseudotumour, orbital cellulitis, sarcoidosis, primary orbital tumours like lacrimal gland tumour and metastatic tumours. The other causes of lid retraction include aberrant regeneration of third nerve, Parinaud’s syndrome and use of sympathomimetic drugs.49
TREATMENT OF GRAVES’ OPHTHALMOPATHY
The hyperthyroidism and the eye disease should be treated independently. Most of the mild to moderate TAO cases shows improvement with treatment of the underlying hyperthyroidism. The decision to treat TAO depends on the severity and the activity of the disease. The principal goals of therapy for TAO include pain relief, protection of vision and cosmetic improvement. The major therapeutic options include corticosteroids, radiotherapy and surgical intervention. TAO is categorized as severe and non-severe for treatment purposes. Severe disease can be either active or inactive. Features of severe ophthalmopathy are marked proptosis, diplopia in primary gaze or reading, exposure keratopathy, corneal ulceration or perforation and compressive optic neuropathy. Treatment options for severe, active cases include either medical decompression (steroids or radiotherapy) or surgical decompression. Majority of them prefer high doses of steroids first and surgical decompression if it fails. For severe, inactive disease, surgical decompression is the only option. Management options for non-severe cases are mainly supportive and are shown in Table 2. Lid retraction in TAO is caused by sympathetic stimulation of the Muller’s muscle. Guanethidine or â-blocker eye drops has been tried for the treatment of lid retraction with varying degree of success. In patients with severe ophthalmopathy, if the disease is active the treatment is medical decompression by either steroids or radiotherapy and if the TAO is inactive orbital decompression and rehabilitative surgery is the choice.
Table 2. Treatment of non-severe TAO.
| Signs / Symptoms | Management Options |
|---|---|
| Peri-orbital oedema | Elevation head of the bed, anti-diuretics |
| Dryness, foreign body sensation | Artificial eye drops and ointment |
| Lagophthalmos | Nocturnal eye taping, eyes shield |
| Eyelid retraction | Topical Guanethidine or β-blockers eye drops |
| Diplopia | Prisms |
| Photophobia | Sunglasses |
Glucocorticoids
Corticosteroids therapy is the mainstay and is effective against active disease, soft tissue inflammatory changes and optic neuropathy.50 It has no much role to play in long-standing and inactive disease (fibrotic stage). Favourable response rate ranges from 63% -73%. The beneficial effects of steroids are due to its anti-inflammatory, immunosuppressive.51 and ability to reduce gycoaminoglycans synthesis.52 Corticosteroid can be administered orally, locally (subconjunctival or retrobulbar) and intravenously. Intravenous steroids given by weekly pulse doses have a favourable response than daily oral steroids. Intravenous steroids have higher tolerability and success rates.53 Retrobulbar steroids are not preferred as it is associated with pain and increase in the intraocular pressure. Intravenous methylprednisolone 0.5 to 1 g every other day for 3 cycles is associated with much higher tolerability. The dose of oral steroids is 60-100 mg (7-14 days) and dose reduction over several months. The disadvantages of steroids are the frequency and severity of side-effects like Cushingoid features, glucose intolerance, gastritis, hypertension, hepatitis, depression and fatty liver.54 Recurrences after cessation or withdrawal of steroids are common.
Orbital radiotherapy
It is effective in for congestive signs, optic neuropathy and extraocular muscle involvement, not very effective against proptosis, eyelid retraction. It has a non-specific anti-inflammatory effect, and reduces the synthesis of glycoaminoglycans. Orbital lymphocytes are highly radiosensitive.55 The standard dose is 20 Gy per eye fractionated in ten daily doses for 2 weeks or 1 Gy per week for 20 weeks.56,57 Favourable responses are seen in 60% of cases. Radiotherapy is associated with increased inflammation causing temporary exacerbation of the ocular signs. This can be lessened by concomitant administration of steroids. Radiotherapy combined with intravenous steroids is more effective than other modalities of treatment.58 Cataract formation, radiation retinopathy, radiation induced optic neuropathy and carcinogenesis are some of the possible risks of radiation of the orbit. Young patients and patients with diabetic retinopathy are contraindications for radiotherapy.
Orbital decompression
It is also known as surgical decompression and involves removal of bony walls of the orbit to increase the orbital space to accommodate the orbital contents. The main indications are optic neuropathy, proptosis, severe orbital inflammation, corneal ulceration and cosmetic improvement. The orbit consists of four walls and in orbital decompression one to four walls can be removed depending on the clinical severity. There are several approaches for doing the decompression procedure. Transantral approach is the most preferred approach for most patients with optic neuropathy. Another approach, the transorbital can be used alone or in combination with transantral approach.59 Decompression can be done by either surgical techniques or endoscopically. The complications of surgical decompression are sensory disturbances, sinusitis, oroantral fistula, facial neuralgia, vision loss and diplopia.
Rehabilitative surgery
It is usually done when the TAO is stable and inactive for at least 4-6 months. It is usually done in the following order strabismus surgery, lid-lengthening surgery and blepharoplasty. Strabismus surgery is done to minimize diplopia in primary and reading positions. Lid-lengthening surgeries decreases corneal exposure and blepharoplasty is done to reduce the excess skin and soft tissue.
Other treatment modalities
Somatostatin analogues
Octreotide and lanreotide are synthetic somatostatin analogues which have shown to be beneficial in patients with TAO.60 TAO patients with somatostatin-receptor-bearing cells can be identified by orbital scintigraphy and can be subjected to treatment with the somatostatin analogues.
Immunosupressive agents
Cyclosporin, cyclophosphamide, azathioprine and ciamexone have been tried for the management of TAO due to its auto-immune nature. The results are varying with no clear conclusive evidence for their role. Cyclosporine along with glucocorticoids was found to be effective in patients with either persistent disease or steroid resistance.61
Intravenous immunoglobulins
Immumoglobulins have better side-effect profile than steroids. It has a beneficial role to play in many autoimmune disorders including TAO, due to its effect on autoantibodies, complement and phagocytes.62
Plasmapheresis
It has shown beneficial effect in patients with severe disease, especially severe progressive ophthalmopathy.
PREVENTION
Refraining from smoking plays a very vital role in primary, secondary and also tertiary prevention of TAO. Smoking influences almost all stages of thyroid eye disease. Stopping smoking prevents Graves’ ophthalmopathy (primary prevention).63 In patients who already have TAO, the chances of remission is much less in patients who quit smoking (secondary prevention) and the outcome of immunosuppressive treatment is beneficial in non-smokers than smokers63 (tertiary prevention).
CONCLUSION
Thyroid eye disease even in its mildest form affects the quality of life of the individual considerably. All the currently available treatment modalities for TAO are associated with serious side-effects and complications. Identification of the risk factors and its elimination can help to modify the disease outcome. As smoking is one of the strongest risk factor found to be associated with TAO, refraining from smoking can help to reduce the magnitude of this problem both in developed and developing countries.
Footnotes
CONFLICTS OF INTEREST
None
Contributor Information
Ps Mallika, MS.
AK Tan, MD.
S Aziz, MS.
SAR Syed Alwi, MMed.
MS Chong, MS.
R Vanitha, MS.
G Intan, MS.
Lumbar imaging for low-back pain without indications of serious underlying conditions does not improve clinical outcomes.
Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
This is a meta-analysis of 6 randomised controlled trials that compared immediate lumbar imaging (radiography, MRI, or CT) versus usual clinical care without immediate imaging for low-back pain. There were no significant differences between immediate lumbar imaging and usual care without immediate imaging for primary outcomes at either short-term or long-term follow-up.
REFERENCES
- 1.Gleeson H, Kelly W, Toft A. et al. Severe thyroid eye disease associated with primary hypothyroidism and thyroid-associated dermopathy. Thyroid. 1999;9((11)):1115–8. doi: 10.1089/thy.1999.9.1115. [DOI] [PubMed] [Google Scholar]
- 2.Salvi M, Zhang Z-G, Haegert D. et al. Patients with endocrine ophthalmopathy not associated with overt thyroid disease have multiple thyroid immunologic abnormalities. J Clin Endocrinol Metab. 1990;70((1)):89–94. doi: 10.1210/jcem-70-1-89. [DOI] [PubMed] [Google Scholar]
- 3.Kemp EH, Ridgway JN, Smith KA. et al. Autoantibodies to the flavoprotein subunit of succinate dehydrogenase: Analysis of specificity in autoimmune thyroid disease. Clin Endocrinol (Oxf) 2000;53((3)):291–9. doi: 10.1046/j.1365-2265.2000.01072.x. [DOI] [PubMed] [Google Scholar]
- 4.Burch HB, Wartofsky L. Graves’ ophthalmopathy: Current concepts regarding pathogenesis and management. Endocr Rev. 1993;14((6)):747–93. doi: 10.1210/edrv-14-6-747. [DOI] [PubMed] [Google Scholar]
- 5.Larsen PR, Davies TF, Hay ID. In: Williams textbook of Endocrinology. Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Philadelphia: WB Saunders Company; 1998. The thyroid gland; pp. 389–515. [Google Scholar]
- 6.Bahn RS, Heufelder AE. Mechanisms of disease: Pathogenesis of Graves’ ophthalmopathy. N Eng J Med. 1993;329((20)):1468–75. doi: 10.1056/NEJM199311113292007. [DOI] [PubMed] [Google Scholar]
- 7.Bartley GB. The epidemiological characteristics and clinical course of ophthalmology associated with autoimmune thyroid disease in Olmsted Country, Minnesota. Trans Am Ophthalmol Soc. 1994;92:477–588. [PMC free article] [PubMed] [Google Scholar]
- 8.Lim SL, Lim AK, Mumtaz M, Hussein E, Wan Bebakar WM, Khir AS. Prevalence, risk factors, and clinical features of thyroid-associated ophthalmopathy in multiethnic Malaysian patients with Graves’ disease. Thyroid. 2008;18((12)):1297–301. doi: 10.1089/thy.2008.0044. [DOI] [PubMed] [Google Scholar]
- 9.Forbes G, Gorman CA, Brennan MD. et al. Ophthalmopathy of Graves’ disease: Computerized volume measurements of the orbital fat and muscle. AJNR Am Neuroradiol. 1986;7((4)):651–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Perros P, Crombie AL, Matthews JNS. et al. Age and gender influence the severity of the the thyroid-associated ophthalmopathy: A study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol (Oxf) 1993;38((4)):367–72. doi: 10.1111/j.1365-2265.1993.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 11.Krassas GE, Segni M, Wiersinga WM. Childhood Graves’ ophthalmopathy: Results of a European questionnaire study. Eur J Endocrinol. 2005;153((4)):515–21. doi: 10.1530/eje.1.01991. [DOI] [PubMed] [Google Scholar]
- 12.Kendall –Taylor P, Perros P. Clinical presentation of thyroid associated orbitopathy. Thyroid. 1998;8((5)):427–8. doi: 10.1089/thy.1998.8.427. [DOI] [PubMed] [Google Scholar]
- 13.Marcocci C, Bartalena L, Bogazzi F. et al. Studies on the occurrence of ophthalmopathy in Graves’ disease. Acta Endocrinol (Copenh) 1989;120((4)):473–8. doi: 10.1530/acta.0.1200473. [DOI] [PubMed] [Google Scholar]
- 14.Day RM. In: The thyroid: a fundamental and clinical text. 4th ed. Werner SC, Ingbar SH, editors. New York: Har-Row; 1978. Hyperthyroidism: clinical manifestations of eye changes; pp. 663–70. [Google Scholar]
- 15.Inoue D, Sato K, Maeda M. et al. Genetic differences shown by HLA typing among Japanese patients with euthyroid Graves’ ophthalmopathy, Graves’ disease and Hashimoto’s thyroiditis: Genetic characteristics of euthyroid Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 1991;34((1)):57–62. doi: 10.1111/j.1365-2265.1991.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 16.Weetman AP, Zhang L, Webb S. et al. Analysis of HLA-DQB and HLA-DPB alleles in Graves’ disease by oligonucleotide probing of enzymatically amplified DNA. Clin Endocrinol (Oxf) 1990;33((1)):65–71. doi: 10.1111/j.1365-2265.1990.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 17.Payami H, Joe S, Farid NR. et al. Relative predispositional effects (RPEs) of marker allele with disease: HLA-DR alleles and Graves’ disease. Am J Hum Genet. 1989;45((4)):541–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Weetman AP, So AK, Warner CA. et al. Immunogenetics of Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 1988;28((6)):619–28. doi: 10.1111/j.1365-2265.1988.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 19.Hagg E, Asplund K. Is endocrine ophthalmopathy related to smoking? Br Med J. 1987;295:634–5. doi: 10.1136/bmj.295.6599.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prummel MF, Wiersinga WM. Smoking and risk of Graves’disease. JAMA. 1993;269((4)):479–82. [PubMed] [Google Scholar]
- 21.Bartalena L, Bogazzi F, Tanda ML. et al. Cigarette smoking and thyroid. Eur J Endocrinol. 1995;133((5)):507–12. doi: 10.1530/eje.0.1330507. [DOI] [PubMed] [Google Scholar]
- 22.Pfeilschifter J, Ziegler R. Smoking and endocrine ophthalmopathy: Impact of smoking severity and current vs lifetime cigarette consumption. Clin Endocrinol (Oxf) 1996;45((4)):477–81. doi: 10.1046/j.1365-2265.1996.8220832.x. [DOI] [PubMed] [Google Scholar]
- 23.Bartalena L, Martina E, Marcocci C. et al. More on smoking habits and Graves’ ophthalmopathy. J Endocrinol Invest. 1989;12((10)):733–7. doi: 10.1007/BF03350047. [DOI] [PubMed] [Google Scholar]
- 24.Metcalfe RA, Weetman AP. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia: Possible role in thyroid associated ophthalmopathy. Clin Endocrinol (Oxf) 1994;40((1)):67–72. doi: 10.1111/j.1365-2265.1994.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 25.Cawood TJ, Moriarty P, O’Farrelly C. et al. Smoking and thyroid-associated ophthalmopathy: A novel explanation of the biological link. J Clin Endocrinol Metab. 2007;92((1)):59–64. doi: 10.1210/jc.2006-1824. [DOI] [PubMed] [Google Scholar]
- 26.Thornton J, Kelly SP, Harrison RA. et al. Cigarette smoking and thyroid eye disease: a systematic review. Eye. 2007;21((9)):1135–45. doi: 10.1038/sj.eye.6702603. [DOI] [PubMed] [Google Scholar]
- 27.Hegediüs L, Brix TH, Vestergaard P. Relationship between cigarette smoking and Graves’ ophthalmopathy. J Endocrinol Invest. 2004;27((3)):265–71. doi: 10.1007/BF03345276. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie JM. Pathogenesis of Graves’ disease: Role of the long –acting thyroid stimulator. J Clin Endocrinol Metab. 1965;25:424–61. doi: 10.1210/jcem-25-3-424. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman MJ, Hetzel BS. The clinical significance of plasma thyroid-stimulating activity in hyperthyroidism. Australas Ann Med. 1966;15((3)):204–9. [PubMed] [Google Scholar]
- 30.Heufelder AE, Joba W. Thyroid-associated eye disease. Strabismus. 2000;8((2)):101–11. [PubMed] [Google Scholar]
- 31.Van Dyk HJ. Orbital Graves’ disease: A modification of the “NO SPECS” classification. Ophthalmology. 1981;88((6)):479–83. [PubMed] [Google Scholar]
- 32.Sharanjeet-Kaur, Dickinson CM, O’Donoghue E. et al. Spectral sensitivity in patients with dysthyroid eye disease. Ophthalmic Physiol Opt. 1997;17((3)):232–8. [PubMed] [Google Scholar]
- 33.Bartley GB. Evolution and classification systems for Graves’ ophthalmopathy. Ophthal Plast Reconstr Surg. 1995;11((4)):229–37. doi: 10.1097/00002341-199512000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Bartley GB. The differential diagnosis and classification of eyelid retraction. Ophthalmology. 1996;103((1)):168–76. doi: 10.1016/s0161-6420(96)30744-6. [DOI] [PubMed] [Google Scholar]
- 35.Danesh-Meyer HV, Savino PJ, Deramo V. et al. Intraocular pressure changes after treatment for Graves’ orbitopathy. Ophthalmology. 2001;108((1)):145–50. doi: 10.1016/s0161-6420(00)00477-2. [DOI] [PubMed] [Google Scholar]
- 36.Jordan DR, Anderson RL. Orbital decompression. Ophthalmic Plast Reconstr Surg. 2000;16((2)):167–8. doi: 10.1097/00002341-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6((5)):295–309. doi: 10.2165/00128071-200506050-00003. [DOI] [PubMed] [Google Scholar]
- 38.Garrity JA, Fatourechti V, Bergstralh EJ. et al. Results of transantral orbital decompression in 428 patients with severe Graves’ophthalmopathy. Am J Ophthalmol. 1993;116((5)):533–47. doi: 10.1016/s0002-9394(14)73194-0. [DOI] [PubMed] [Google Scholar]
- 39.Gilbard JP, Farris RL. Ocular surface drying and tear film osmolarity in thyroid eye disease. Acta Ophthalmol (Copenh) 1983;61((1)):108–16. doi: 10.1111/j.1755-3768.1983.tb01401.x. [DOI] [PubMed] [Google Scholar]
- 40.Trobe JD, Glaser JS, Laflamme P. Dysthyroid optic neuropathy. Clinical profile and rationale for management. Arch Ophthalmol. 1978;96((7)):1199–209. doi: 10.1001/archopht.1978.03910060033007. [DOI] [PubMed] [Google Scholar]
- 41.Trobe JD. Optic nerve involvement in dysthyroidism. Ophthalmology. 1981;88((6)):488–92. doi: 10.1016/s0161-6420(81)34997-5. [DOI] [PubMed] [Google Scholar]
- 42.Warren JD, Spector JG, Burde R. Long-term follow up and recent observations on 305 cases of orbital decompression for dysthyroid orbitopathy. Laryngoscope. 1989;99((1)):35–40. doi: 10.1288/00005537-198901000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Behrouzi Z, Rabei HM, Azizi F. et al. Prevalence of open-angle glaucoma, glaucoma suspect, and ocular hypertension in thyroid-related immune orbitopathy. J Glaucoma. 2007;16((4)):358–62. doi: 10.1097/IJG.0b013e31802e644b. [DOI] [PubMed] [Google Scholar]
- 44.Werner SC. Modification of the classification of the eye changes of Graves’ disease. Am J Ophthalmol. 1977;83((5)):725–7. doi: 10.1016/0002-9394(77)90140-4. [DOI] [PubMed] [Google Scholar]
- 45.Wiersinga WM, Prummel MF, Mourits MP. et al. Classification of the eye changes of Graves’ disease. Thyroid. 1991;1((4)):357–60. doi: 10.1089/thy.1991.1.357. [DOI] [PubMed] [Google Scholar]
- 46.Wall JR, Henderson J, Strakosch CR. et al. Graves’ ophthalmopathy. Can Med Assoc J. 1981;124((7)):855–62. [PMC free article] [PubMed] [Google Scholar]
- 47.Teng CS, Smith BR, Clayton B. et al. Thyroid-stimulating immunoglobulins in ophthalmic Graves’ disease. Clin Endocrinol (Oxf) 1977;6((3)):207–11. doi: 10.1111/j.1365-2265.1977.tb03316.x. [DOI] [PubMed] [Google Scholar]
- 48.Wall JR, Odgers RJ, Hetzel BS. Immunological studies of the eye changes of thyrotoxicosis. Aust NZ J Med. 1973;3((2)):162–8. doi: 10.1111/j.1445-5994.1973.tb03970.x. [DOI] [PubMed] [Google Scholar]
- 49.Bahn RS, Garrity JA, Gorman CA. Diagnosis and management of Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1990;71((3)):559–63. doi: 10.1210/jcem-71-3-559. [DOI] [PubMed] [Google Scholar]
- 50.Bahn RS, Gorman CA. Choice of therapy and criteria for assessing treatment outcome in thyroid-associated ophthalmopathy. Endocrinol Metab Clin North Am. 1987;16((2)):391–407. [PubMed] [Google Scholar]
- 51.Bartalena L, Marcocci, Bogazzi F. et al. Glucocorticoid therapy of Graves’ ophthalmopathy. Exp Clin Endocrinol. 1991;97((2-3)):320–7. doi: 10.1055/s-0029-1211086. [DOI] [PubMed] [Google Scholar]
- 52.Wiersinga WM. Graves’ ophthalmopathy. Thyroid Int. 1997;3:1–15. [Google Scholar]
- 53.Marcocci C, Bartalena L, Tanda ML. et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: Results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001;86((8)):3562–7. doi: 10.1210/jcem.86.8.7737. [DOI] [PubMed] [Google Scholar]
- 54.Hiromatsu Y. Steroid therapy for Graves’ ophthalmopathy. Nippon Rinsho. 2006;64((12)):2279–85. [PubMed] [Google Scholar]
- 55.Bartalena L, Marcocci C, Manetti L. et al. Orbital radiotherapy for Graves’ ophthalmopathy. Thyroid. 1998;8((5)):439–41. doi: 10.1089/thy.1998.8.439. [DOI] [PubMed] [Google Scholar]
- 56.Pinchera A, Bartalena L, Chiovato L, In: The eye and the orbit in thyroid disease. Gorman CA, Waller RR, Dyer JA, editors. New York: Raven Press; 1984. Radiotherapy of Graves’ ophthalmopathy; pp. 301–16. [Google Scholar]
- 57.Kahaly G, Roesler HP, Pitz S. et al. Low-versus high dose radiotherapy for Graves’ ophthalmopathy: A randomized single blind trial. J Clin Endocrinol Metab. 2000;85((1)):102–8. doi: 10.1210/jcem.85.1.6257. [DOI] [PubMed] [Google Scholar]
- 58.Wei RL, Cheng JW, Cai JP. The use of orbital radiotherapy for Graves’ ophthalmopathy: Quantitative review of the evidence. Ophthalmologica. 2008;222((1)):27–31. doi: 10.1159/000109275. [DOI] [PubMed] [Google Scholar]
- 59.Kulwin DR, Cotton RT, Kersten RC. Combined approach to orbital decompression. Otolaryngol Clin North Am. 1990;23((3)):381–90. [PubMed] [Google Scholar]
- 60.Krassas GE, Doumas A, Kaltsas T. et al. Somatostatin receptor scintigraphy before and after treatment with somatostatin analogues in patients with thyroid eye disease. Thyroid. 1999;9((1)):47–52. doi: 10.1089/thy.1999.9.47. [DOI] [PubMed] [Google Scholar]
- 61.Prummel MF, Mouritis MP, Berghout A. et al. Prednisolone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N Engl J Med. 1989;321((20)):1353–9. doi: 10.1056/NEJM198911163212002. [DOI] [PubMed] [Google Scholar]
- 62.Leibe A, Levy Y, Shoenfeld Y. Intravenous immunoglobulins treatment of patients with Graves’ ophthalmopathy. Harefuah. 2001;140((5)):392–4. [PubMed] [Google Scholar]
- 63.Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12((10)):855–60. doi: 10.1089/105072502761016476. [DOI] [PubMed] [Google Scholar]


