Abstract
Melanocortin- 4 receptor (MC4R) and agouti- related peptide (AgRP) are involved in energy homeostasis in rats. According to MC4R and AgRP effects on luteinizing hormone (LH) secretion, they may influence the estrous cycle of rats. Therefore, the aim of this study was to investigate the expression of MC4R and AgRP mRNAs at different stages of estrous cycle in the rat’s hypothalamus. The estrous cycle stages (proestrus, estrus, metestrus and diestrus) were determined in 20 adult female rats using vaginal smears. The rats were divided into four equal groups (n=5). Four ovariectomized rats were selected as controls two weeks after surgery. Using real- time PCR, relative expressions (compared to controls) of MC4R and AgRP mRNAs in the hypothalamus of rats were compared in four different groups of estrous cycle. The relative expression of MC4R mRNA in the hypothalamus of female rats during proestrus stage was higher than those in other stages (P=0.001). Despite a lower mean of relative expression of AgRP mRNA at proestrus stage, the relative expression of AgRP mRNA of the four stages of estrous cycle did not differ (P>0.05). In conclusion, changes in the relative expression of MC4R and AgRP mRNAs in four stages of rat estrous cycle indicated a stimulatory role of MC4R in the proestrus and preovulatory stages and an inhibitory role of AgRP in gonadotropin releasing hormone (GnRH) and LH secretions.
Key Words: Melanocortin- 4 receptor, agouti- related peptide, hypothalamus, estrous cycle, rat
Melanocortin-4 receptor (MC4R) is the cognate receptor for α- melanocyte stimulating hormone (α-MSH) (1). This receptor belongs to the melanocotrin receptors and is one of the members of G protein-coupled receptors that activates adenylate cyclase and can be a mediator between appetite and reproduction (2). MC4R is expressed in arcuate nuclei (ARC), periventricular nuclei (PVN), medial preoptic area (MPO) and preoptic area (POA). These regions are involved in reproduction and regulate appetite (3, 4). Alpha-melanocyte stimulating hormone is effective in erection (5) and decreases luteinizing hormone (LH) in rats (6). Melanotan-II (MT-II) -agonist of MC4R- is effective in the erection of men and treatment of its abnormalities (7).
Agouti-related peptide (AgRP) is the neuropeptide with 132 amino acids that is expressed in the ARC of mice (8) and sheep (9). In some species, AgRP neurons are involved in energy balance (10). AgRP is an appetite stimulus (11) and increases during the lack of energy (12). Intracerebroventricular (ICV) injection of AgRP, increases food intake and inhibits α-MSH in mice (13). Mice deficient in the MC4R expression (14) or high expression of AgRP (15) are fat. Changes in the relative expression of MC4R and AgRP mRNAs during long term malnutrition of rat indicate a stimulatory role of MC4R and AgRP in regulating energy balance in ARC of rat hypothalamus (16). Various hormones such as insulin, leptin and glucocorticoids can change AgRP expression during homeostasis (11). Moreover, AgRP in pregnancy and MC4R in lactation in ARC of rats control the energy homeostasis (17).
Many neurons in the ARC co-express AgRP and Neuropeptide Y (NPY) which is another appetizer peptide (18). NPY is also effective in reproduction (19). It has been shown that NPY neurons have synaptic contact with gonadotropin releasing hormone (GnRH) neurons in the POA (20). ICV injection of NPY, inhibits LH secretion in rats (21). Moreover, NPY neuron termini which synthesized AgRP, synapse with GnRH neurons (22). Both NPY and AgRP inhibit LH surge (3, 23). Estradiol decreases NPY and AgRP expression in the ARC as well as NPY expression in the PVN of ovariectomized (OVX) rats (24). In addition, the low level of estradiol in OVX rat, leads to the up-regulation of NPY and AgRP in the hypothalamus (25). AgRP termini are located in the MPO which consists of GnRH neurons and ICV injection of AgRP increases GnRH, LH, follicle stimulating hormone (FSH) and testosterone in male rat but do not have any direct effect on anterior pituitary (26). AgRP inhibitor also has effect on reproductive axis and ICV injection of AgRP inhibitor leading to LH decline in OVX monkey (23).
According to the effect of MC4R and AgRP on LH secretion, there are likely to affect estrous cycle in rats. Therefore, the aim of the present study was to investigate MC4R and AgRP mRNAs expression in the hypothalamus during the estrous cycle of rat.
Materials and Methods
Twenty-four adult (3-4 months old) female Sprague-Dawley rats (Rattus norvegicus) weighing 170-220 g were used in this study. The rats were randomly selected and housed in the laboratory animal center of Shiraz University of Medical Sciences, Shiraz, Iran under controlled temperature (22 °C) and light (12:12 light to dark ratio; lights on at 7:30 am) conditions. Rats were treated humanely and in compliance with the recommendations of the Animal Care Committee of the Shiraz University of Medical Sciences. All experimental procedures were carried out between 12.00-2.00 pm. Vaginal smears were prepared for the identification of the phases of the estrous cycles of the 20 intact rats. Five rats were assigned to each phase of the cycle.
The control group comprised four randomly selected ovariectomized rats. The rats were anesthetized by an intraperitoneal injection of ketamine (100 mg/kg, Woerden, Netherlands) and xylazine (7 mg/kg, Alfazyne, Woerden, Nether-lands), then ovariectomized through a ventral midline incision. Further procedures were under-taken after a two-week recovery period. The cyclic and ovariectomized rats were decapitated, brains dissected out immediately, and the entire hypo-thalamuses were dissected. The hypothalamus samples were frozen in liquid nitrogen and stored at-80 °C. RNA extraction, DNase treatment, cDNA synthesis and relative real-time PCR procedure were performed as described elsewhere (27). Primers were designed with Allele ID 7 software for the reference gene, AgRP (NM_033650.1) and MC4R (NM_013099.2). The rat glyceraldehyde-3- phosphate dehydrogenase (GAPDH) gene (M32599) was used as reference gene for data normalization (Table 1).
Table 1.
Sequences of PCR primers used to evaluate relative expression of AgRP and MC4R genes in rat
| Amplicon length (bp) | Primer sequence | Primer |
|---|---|---|
| 181 | 5` TGGGTGTCATAAGCCTGTTGG 3` 5` GCGTCCGTGTCCGTACTG 3` |
MC4R-F MC4R-R |
| 189 | 5` TGAAGAAGACAGCAGCAGACC 3` 5` TGAAGAAGCGGCAGTAGCAC 3` |
AgRP-F AgRP-R |
| 112 | 5` AAGAAGGTGGTGAAGCAGGCATC 3` 5` CGAAGGTGGAAGAGTGGGAGTTG 3` |
GAPDH-F GAPDH-R |
For quantitative real-time PCR data, the relative expression of AgRP and MC4R was calculated based on the threshold cycle (CT) method. CT for each sample was calculated using Line-gene K software (28). Fold expression of the target mRNAs over reference values was calculated by the equation 2-ΔΔCT (29), where ΔCT is determined by subtracting the corresponding GAPDH CT value (internal control) from the specific CT of the target (AgRP or MC4R). ΔΔCT was obtained by subtracting the ΔCT of each experimental sample from that of the calibrator sample (ovariectomized rats). Data on the relative expression of AgRP and MC4R genes were subjected to the test of normality. Analysis of variance for both variables were performed using Proc GLM (SAS, 2002) followed by mean comparison by Duncan`s multiple range test. P<0.05 was considered as significant. The mean of the group and standard errors have been reported.
Results
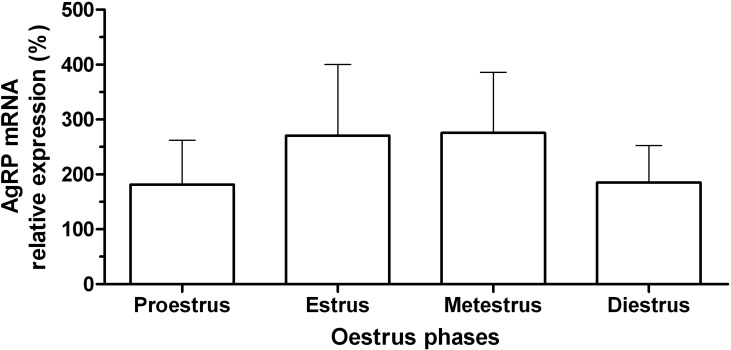
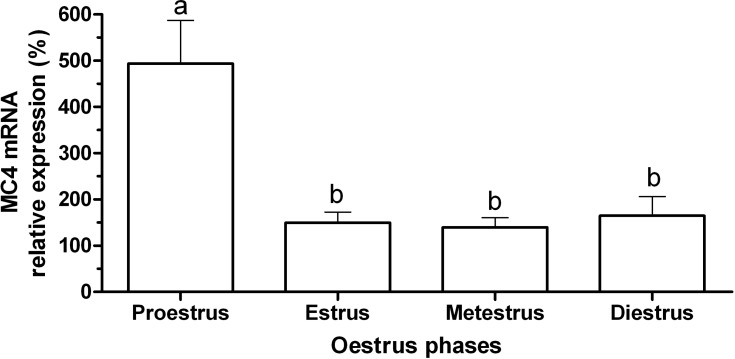
The expression of MC4R mRNA in the hypothalamus of female rats at different phases of the estrous cycle is shown in figure 1. There was higher expression of MC4R mRNA during the proestrus phase compared to other phases of the cycle (P=0.001). The expression of MC4R mRNA during the estrus, metestrus and diestrus phases did not differ significantly. The expression of AgRP mRNA in the hypothalamus of female rats in the different phases of the estrous cycle is shown in figure 2. The expression of AgRP mRNA did not significantly differ during the estrous cycle (P>0.05). Negative correlations between AgRP mRNA and MC4R mRNA during the estrous cycle were not significantly different (r= -0.14, P> 0.05).
Fig. 1.
Mean (± standard error) of the relative expression of MC4R gene in the hypothalamus of rats (n=5) during the estrous cycle. Different letters indicate significant difference (P<0.05).
Fig. 2.
Mean (± standard error) of the relative expression of AgRP gene in the hypothalamus of rats (n=5) during the estrous cycle
Discussion
In the present study, we investigated the expression of AgRP and MC4R mRNAs during the estrous cycle in the rat hypothalamus for the first time. We also showed that MC4R mRNA in proestrus was greater (3.2 fold) than the other phases of the cycle. In rats during the estrus, metestrus, diestrus, and early proestrus phases, the concentration of GnRH is at its basal level. At mid-proestrus phase, the GnRH surge center is activated (30). In the afternoon of proestrus phase, the circulating levels of LH begin to rise rapidly and reaches its peak in the evening, resulting in ovulation. The blood LH level then decreases and reaches basal levels during the rest of the cycle (31). Therefore, increase in MC4R mRNA that we observed in the present study might be involved in preovulatory GnRH/ LH surge. Most of the GnRH neurons are located in the vicinity of α-MSH expressing fiber (32) and GT1-1 hypothalamic cells express MC4R and the stimulation of these cells with Nle4 D-Phe7-α-MSH leads to GnRH secretion (33, 34).
In rats, ovarian estradiol secretion reaches its maximum level in the mid of the proestrus phase (31). Estradiol injection led to the up-regulation of MC4R in the PVN of OVX rats (35). Ovariec-tomized rats that had been treated with estrogen and progesterone indicated partial prolactin and LH surge that was inhibited during hunger (36). MC4R antagonists decrease prolactin and LH surge in the rats fed with normal diet and inhibit the effect of leptin on these hormones in hungry rats. Thus, MC4R could be a mediator of leptin effects on prolactin and LH surge (36). MT-II led to prolactin surge in hungry rats. Accordingly, MC4R may be important for prolactin surge during the preovulatory period (37). Leptin stimulates GnRH (38) and LH secretion in the rat hypothalamus and MC4R is a mediator of leptin effects during homeostasis (39). Therefore, the high level of MC4R mRNA during the proestrus phase could be involved in prolactin preovulatory surge.
In the present study, there was no significant difference in AgRP mRNA levels during the estrous cycle but the mean of AgRP mRNA in proestrus phase was 40% lower than estrus and metestrus phases and 10% lower than diestrus phase. In many mammals, GnRH secretion is regulated by ovarian steroid feedback mechanisms. In rats, ovarian estradiol secretion during the estrus phase is low, while at the end of metestrus phase, estrogen secretion begins to increase and is high during the diestrus phase; it reaches its peak in the proestrus evening, thereafter, declining to its basal level (31). In OVX rats, estradiol injection led to the decrease of AgRP expression in the ARC (25). Therefore, the low level of AgRP mRNA in proestrus and diestrus phases could be due to the increase of estradiol secretion during these phases.
In rodents, GnRH neurons do not express alpha- estradiol receptor (ERα) that is essential for positive and negative estradiol feedback (40). Increase in tonic LH secretion leads to the increase in estradiol synthesis from follicles and the peak of estradiol leads to LH surge (41). AgRP inhibits prolactin and LH surge in female rats while the antiserum against AgRP reverses this effect (42). ICV injection of AgRP, inhibits pulsatile LH secretion in monkey (23). Thus, AgRP could inhibit GnRH pulses and regulate estrous cycle.
In conclusion, our finding showed that in proestrus phase during the preovulatory period, MC4R might have excitatory effects and AgRP might have inhibitory effects on GnRH/ LH secretion in rats.
Acknowledgments
This research was financially supported by the Vice-Chancellery for Research of Shiraz University of Medical Sciences, Shiraz, Iran.
Conflict of interest
The authors declared no conflict of interests.
References
- 1.Martin WJ, McGowan E, Cashen DE, et al. Activation of melanocortin MC(4) receptors increases erectile activity in rats ex copula. Eur J Pharmacol. 2002;454:71–9. doi: 10.1016/s0014-2999(02)02479-2. [DOI] [PubMed] [Google Scholar]
- 2.Fredriksson R, Lagerstrom MC, Lundin LG, et al. The G-protein-coupled receptors in the human genome form five main families Phylogenetic analysis, paralogon groups, and fingerprints. . Mol Pharmacol. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 3.Krasnow SM, Steiner RA. Physiological mechanisms integrating metabolism and reproduction. In: Neill JD, editor. Knobil and Neills Physiology of reproduction. 3 ed. USA: Elsevier Academic Press Inc; 2006. p. 2587. [Google Scholar]
- 4.Evans JJ, Anderson GM. Balancing ovulation and anovulation: integration of the reproductive and energy balance axes by neuropeptides. Hum Reprod Update. 2012;18:313–32. doi: 10.1093/humupd/dms004. [DOI] [PubMed] [Google Scholar]
- 5.Hull EM, Wood RI, McKenna KE. Neurobiology of male sexual behavior. In: Neil JD, editor. Knobil and Neill’s Physiology of Reproduction. 3 ed. USA: Elsevier Academic press Inc; 2006. p. 1757. [Google Scholar]
- 6.Berberian V, Sanchez S, Sanchez-Borzone M, et al. Effect of alpha-melanotropin hormone on serum levels of luteinizing hormone and progesterone in experimental rat autoimmune oophoritis. Peptides. 2006;27:2295–9. doi: 10.1016/j.peptides.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Wessells H, Blevins JE, Vanderah TW. Melanocortinergic control of penile erection. Peptides. 2005;26:1972–7. doi: 10.1016/j.peptides.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shutter JR, Graham M, Kinsey AC, et al. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 9.Jafarzadeh Shirazi MR, Tamadon A, Namavar MR. Coexpression of gonadotropin inhibitory hormone with Agouti-related peptide in the neurons of arcuate nucleus of ewe hypothalamus. Physiol Pharmacol. 2011;15:201–9. [Google Scholar]
- 10.Luquet S, Perez FA, Hnasko TS, et al. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–5. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 11.Stutz AM, Morrison CD, Argyropoulos G. The agouti-related protein and its role in energy homeostasis. Peptides. 2005;26:1771–81. doi: 10.1016/j.peptides.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Kaushik S, Rodriguez-Navarro JA, Arias E, et al. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011;14:173–83. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada-Goto N, Katsuura G, Ebihara K, et al. Intracerebroventricular administration of C-type natriuretic peptide suppresses food intake via activation of the melanocortin system in mice. Diabetes. 2013;62:1500–4. doi: 10.2337/db12-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinelli CE, Keogh JM, Greenfield JR, et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab. 2011;96:E181–8. doi: 10.1210/jc.2010-1369. [DOI] [PubMed] [Google Scholar]
- 15.Krashes MJ, Koda S, Ye C, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–8. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabet Sarvestani F, Tamadon A, Hematzadeh A. Expression of melanocortin-4 receptor and agouti-related peptide mRNAs in arcuate nucleus during long term malnutrition of female ovariec-tomized rats. Iran J Basic Med Sci. 2014 In press. [PMC free article] [PubMed] [Google Scholar]
- 17.Asadi-Yousefabad SL, Sabet Sarvestani F, Tamadon A. Expression of agouti-related peptide and melanocortin-4 receptor mRNAs in arcuate nucleus of rats during the pregnancy and lactation. Iran J Biotechnol. 2014 In press. [Google Scholar]
- 18.Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–32. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acosta-Martinez M, Horton T, Levine JE. Estrogen receptors in neuropeptide Y neurons: at the crossroads of feeding and reproduction. Trends Endocrinol Metab. 2007;18:48–50. doi: 10.1016/j.tem.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Roa J. Role of GnRH Neurons and Their Neuronal Afferents as Key Integrators between Food Intake Regulatory Signals and the Control of Reproduction. Int J Endocrinol. 2013;2013:10. doi: 10.1155/2013/518046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen CD, Waser B, Korner M, et al. Neuropeptide Y acts within the rat testis to inhibit testosterone secretion. Neuropeptides. 2011;45:55–61. doi: 10.1016/j.npep.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turi GF, Liposits Z, Moenter SM, et al. Origin of neuropeptide Y-containing afferents to gonadotropin-releasing hormone neurons in male mice. Endocrinology. 2003;144:4967–74. doi: 10.1210/en.2003-0470. [DOI] [PubMed] [Google Scholar]
- 23.Vulliemoz NR, Xiao E, Xia-Zhang L, et al. Central infusion of agouti-related peptide suppresses pulsatile luteinizing hormone release in the ovariectomized rhesus monkey. Endocrinology. 2005;146:784–9. doi: 10.1210/en.2004-1093. [DOI] [PubMed] [Google Scholar]
- 24.Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) alpha to ERbeta in clonal hypothalamic neurons. Mol Endocrinol. 2006;20:2080–92. doi: 10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- 25.Clegg DJ, Brown LM, Zigman JM, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–8. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 26.Stanley SA, Small CJ, Kim MS, et al. Agouti related peptide (Agrp) stimulates the hypothalamo pituitary gonadal axis in vivo & in vitro in male rats. Endocrinology. 1999;140:5459–62. doi: 10.1210/endo.140.11.7248. [DOI] [PubMed] [Google Scholar]
- 27.Salehi MS, Jafarzadeh Shirazi MR, Zamiri MJ, et al. Hypothalamic Expression of KiSS1 and RFamide-related Peptide-3 mRNAs during The Estrous Cycle of Rats. Int J Fertil Steril. 2013;6:304–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6:62. doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Jaroenporn S, Horii Y, Akieda-Asai S, et al. Endocrine mechanisms responsible for different follicular development during the estrous cycle in Hatano high- and low-avoidance rats. J Reprod Dev. 2011;57:690–9. doi: 10.1262/jrd.10-160s. [DOI] [PubMed] [Google Scholar]
- 31.Freeman ME. Neuroendocrine control of the ovarian cycle of the rat. In: Neill JD, editor. Knobil and Neill`s physiology of reproduction. 3 ed. USA: Elsevier Academic Press Inc; 2006. pp. 2327–88. [Google Scholar]
- 32.Ward DR, Dear FM, Ward IA, et al. Innervation of gonadotropin-releasing hormone neurons by peptidergic neurons conveying circadian or energy balance information in the mouse. Plos One. 2009;4:e5322. doi: 10.1371/journal.pone.0005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai B, Li JY, Zhang W, et al. Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides. 2006;27:2846–57. doi: 10.1016/j.peptides.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Khong K, Kurtz SE, Sykes RL, et al. Expression of functional melanocortin-4 receptor in the hypothalamic GT1-1 cell line. Neuroendocrinology. 2001;74:193–201. doi: 10.1159/000054686. [DOI] [PubMed] [Google Scholar]
- 35.Silva LE, Castro M, Amaral FC, et al. Estradiol-induced hypophagia is associated with the differential mRNA expression of hypothalamic neuropeptides. Braz J Med Biol Res. 2010;43:759–66. doi: 10.1590/s0100-879x2010007500059. [DOI] [PubMed] [Google Scholar]
- 36.Donato J Jr, Cravo RM, Frazao R, et al. Hypothalamic sites of leptin action linking metabolism and reproduction. . Neuroendocrinology. 2011;93:9–18. doi: 10.1159/000322472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanobe H, Yoneda M, Kakizaki Y, et al. Further evidence for a significant participation of the melanocortin 4 receptor in the preovulatory prolactin surge in the rat. Brain Res Bull. 2001;54:521–5. doi: 10.1016/s0361-9230(01)00442-7. [DOI] [PubMed] [Google Scholar]
- 38.Watanobe H. Leptin directly acts within the hypothalamus to stimulate gonadotropin-releasing hormone secretion in vivo in rats. J Physiol. 2002;545:255–68. doi: 10.1113/jphysiol.2002.023895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Israel DD, Sheffer-Babila S, de Luca C, et al. Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology. 2012;153:2408–19. doi: 10.1210/en.2011-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herbison AE. Physiology of the gonadotropin-releasing hormone neuronal network. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3 ed. USA: 2006. pp. 1418–9, 36. [Google Scholar]
- 41.Rawlings NC, Bartlewski PM. Clinical reproductive physiology of ewes. In: Youngquist RS, Threlfall WR, editors. Current therapy in large animal theriogenology. 2 ed. Missouri: Saunders Inc; 2007. pp. 642–9. [Google Scholar]
- 42.Schioth HB, Kakizaki Y, Kohsaka A, et al. Agouti-related peptide prevents steroid-induced luteinizing hormone and prolactin surges in female rats. Neuroreport. 2001;12:687–90. doi: 10.1097/00001756-200103260-00014. [DOI] [PubMed] [Google Scholar]