Abstract
Objective
The purpose of the present study was to examine the acoustic features of crying demonstrated by infants who experienced apnea of infancy (AOI) and compare these features to a non-AOI group of infants. Based on past physiological descriptions of AOI, three predictions in regard to the influence of AOI on acoustic cry features were proposed: (1) the rate of crying would be significantly faster among infants with AOI, (2) the latency of crying onset would be significantly longer among infants with AOI and (3) the F0 characterizing an overall episode of crying would be significantly lower among infants with AOI
Patients and Methods
Pain-induced crying episodes were collected from a group of healthy term infants (HT) and those with AOI. One complete crying episode was obtained from each infant and analyzed acoustically with regard to durational and spectral features of the cry.
Results
Infants comprising the AOI group were found to demonstrate a significantly longer cry latency and lower F0 compared to HT infants
Conclusions
The acoustic cry features measured for the AOI infants are discussed with regard to past reports of poor arousal and decreased muscle tone. A model of AOI crying is proposed whereby the autonomic nervous system and associated pathways are slower to interpret pain stimulus compared to HT infants.
Keywords: acoustic analysis, apnea, cry, infancy
Introduction
Apnea of infancy (AOI) is defined as an unexplained cessation of breathing for 20 seconds or longer, or a shorter respiratory pause in breathing accompanied by bradycardia, cyanosis, pallor, and/or marked hypotonia [1]. There is controversy surrounding the precise relationship of AOI to sudden infant death syndrome (SIDS) [2]. There is general agreement, however, that these children are at increased risk for SIDS [3–5]. As a means of better understanding AOI, a number of clinical studies have been undertaken. The ultimate aim of this research is to identify physiological or behavioral markers that relate to better treatment and/or management of this life-threatening event [6–9].
One potential marker that has been overlooked in past research of AOI is that of infant crying. Dating back to Fairbanks [10], who examined the pitch of infant hunger wails, acoustic cry analysis has been shown to provide a noninvasive means of correlating an infant’s neurobehavioral integrity with stability of laryngeal coordination [11–12]. Interpretation of cries is based on the Source-Filter Theory of phonation proposed by Fant [13] which allows for characterization of the acoustic signal according to respiratory, laryngeal and supralaryngeal influences. This well known and accepted acoustic model of phonation has been specifically applied to infant crying behavior [14–15].
The common practice of acoustic cry analysis is evident in a recent report by LaGasse et al. [16] who indexed approximately 50 cry studies that categorized infants according to (1) cry characteristics associated with severe medical conditions, or (2) cry characteristics associated with potential central nervous system insults. More often than not, various acoustic features of the cries of unhealthy infants are clearly different than the cries of healthy infants. While the aim of acoustic cry research is to identify acoustic markers of health status, it is important to recognize that such markers are best utilized as an adjunct to the diagnosis of specific health conditions.
There have been surprisingly few studies of the crying behavior of children with AOI. A review of the literature resulted in no specific studies of AOI, although there are reports of children with asphyxia. Nearly 30 years ago, Michelsson and her colleagues [17–19] completed a series of studies examining infants with asphyxia. They found that infants with asphyxia demonstrated highly abnormal cries characterized primarily by a fundamental frequency (Fo) in excess of 1000 Hz. In addition, the prognosis for survival in these infants was poor. The condition of AOI is subtler than asphyxia yet the risks for a reduced life expectancy may be just as great.
The purpose of the present study was to examine the acoustic features of crying demonstrated by infants who experienced AOI and compare these features to a non-AOI group of infants. Based on past physiological descriptions of AOI, three predictions in regard to the influence of AOI on acoustic cry features were proposed. First, assuming infants with AOI demonstrate significantly higher mean respiratory rates than non-AOI infants [8], we predicted these infants to demonstrate a significantly faster rate of crying. The duration of phonation, for example, crying, is linked to the breathing cycle [20]. Accordingly, high respiratory rates should be revealed in the cry signal as a faster rate of crying during a bout of crying compared to non-AOI babies. Second, infants with AOI are reported to demonstrate less arousal than non-AOI babies [21–22]. We predicted that infants with AOI would demonstrate a longer latency in the initiation of crying, following the presentation of a cry-eliciting stimulus. Finally, assuming AOI is associated with a marked decrease in muscle tone [3], we predicted that this condition would be manifested as a decrease in laryngeal tension compared to non-AOI babies. Accordingly, the acoustic spectrum of cry, namely Fo, among infants with AOI should be lower compared to non-AOI babies.
Method
Data Collection
Data for the current study were collected as part of the Collaborative Home Infant Monitoring Evaluation (CHIME) Study, a program supported by the National Institute of Child Health and Human Development, National Institutes of Health.2 Objectives of the CHIME study were to (1) assess cardio respiratory events (CREs) documented by home monitoring in infants at high epidemiologic risk for SIDS, (2) to determine antecedent medical, demographic, physiologic, and behavioral characteristics that predict incidence of CREs, (3) to assess the relationship between severity of CREs and adverse neurodevelopment outcomes, and (4) to determine compliance achieved during monitoring. Five clinical sites from across the U.S. were involved in the CHIME study including respiratory physiology laboratories in Cleveland, Toledo, Chicago, Los Angeles, and Honolulu. The Institutional Review Board at each of these sites approved the study.
Participants
For this study, the cries of 23 healthy full-term (HT) and 26 infants with AOI were examined from infants delivered at Kapiolani Medical Center for Woman and Children, Honolulu, Hawaii. Informed consent was obtained from each of the 49 mothers. The pregnancy and delivery of the HT infants were normal and without complications. The mean gestational age (MGA) of the HT infants was 39 weeks with an average birth weight of 3318g (±301g). The HT infants showed no signs of apnea of idiopathic apparent life-threatening events. In addition there was no family history of SIDS in siblings and no other family history of SIDS in the last ten years. The MGA of the AOI infants was 38 week with an average birth weight of 3316g (±421g). All infants with AOI demonstrated an episode of apnea within the first two weeks of life that required mouth-to-mouth resuscitation or vigorous stimulation. The event occurred in the home and was identified by caretaker observation.
As part of the data collection protocol for the CHIME study, each infant was audio recorded within two weeks following birth in the laboratory. Cries were elicited via administration of a pain stimulus to the sole of the infant’s right foot. One complete crying episode (i.e., bout) was obtained from each infant in a supine position. All infants were in a quiet, restful state prior to cry recording and no attempts were made to elicit cries at particular phases of the infant’s respiratory breath cycle. A crying episode was defined as the total period of continuous crying activity [23]. An episode of crying commenced with the first audible cry following administration of a pain stimulus. The pain stimulus was delivered by a two inch stainless steel wire attached via a spring to a heel block. The infant’s sole of their right foot was placed in the block and the steel wire was cocked and released, providing the pain stimulus to the infant’s heel. The use of this pain-eliciting device allowed for a standard form of cry sampling across the 5 clinical sites. The completion of a crying episode was noted as the last audible cry that was followed by a minimum of 15 seconds of silence. All cries were audio recorded using a condenser microphone (Realistic 33–1052) coupled to a cassette recorder (Marantz PMD-360). The microphone was situated at a constant 15 cm from the infant’s mouth. An audible tone was time-locked to administration of the pain stimulus and served as an acoustic reference for measurement of cry latency (see below).
Acoustic Analysis and Measurements
An individual experienced in the acoustic analysis of infant crying performed all acoustic measurements (MPR). The entire crying episodes were analyzed using an integrated computer hardware-software package (KAY CSL-4300). Each audio recorded crying episode was digitized at 16 kHz and displayed as an amplitude-by-time waveform on a computer monitor. A number of acoustic measurements were preformed to help address the research questions. The specific measurements were as follows:
Number of Expiratory Cries
An expiratory cry was defined as the onset and offset of visible acoustic energy that was perceived to occur on the expiratory phase of the respiratory cycle. Cry segments were bound by visible breaks (or silent periods) in the waveform. The total number of expiratory cries was tabulated for each infant and a mean number of expiratory cries were calculated for each group. The use of both visual examination of the cry waveform and perceptual evaluation of the cry signal to determine instances of expiratory cries have previously shown to be a highly reliable method of analysis [23]. Grau et al. have noted that in the absence of such an approach, it is likely that instances of audible inspiratory phonation would be mistaken as expiratory cries. In the present study, the number of expiratory cries was used as an estimate of the rate of crying during an en tire episode [24].
Overall Cry Duration
Using a pair of vertical cursors superimposed over the cry waveform, the onset and offset of acoustic energy across the entire crying episode was determined. Overall cry duration was used as part of the calculation for estimating rate of crying [24].
Cry Latency
The time elapsed between the administration of the pain stimulus, as indicated by an audible tone, and the onset of cry phonation was determined to represent cry latency. The measure is assumed to reflect the time for the central nervous system (CNS) to interpret the pain stimulus [25].
First Spectral Peak (FSP)
Using a pair of vertical cursors superimposed over the waveform, the non-voiced portions of the waveform were systematically removed leaving a continuous display of phonatory behavior. On the basis of this edited waveform, the long-term average spectrum (LTAS) was calculated through an averaging of individual fast Fourier transform (FFT) computations performed every 23 ms across the entire crying episode. An LTAS analysis serves to average out the effects of the vocal tract on the acoustic signal, leaving a representation of the vibratory patterns of the vocal folds. The LTAS display provided a relative amplitude-by-frequency representation of the average energy concentrations evident throughout the frequency range of 0–8000 Hz. The frequency value associated with the first amplitude peak in the spectrum (FSP) was taken as the average Fo across a crying episode. While past studies tend to concentrate on estimating vocal fold behavior based on characterizations of individual cry segments, the use of LTAS served to provide a profile of the complete crying episode. This form of analysis has been used successfully in previous cry investigations proving sensitive in differentiating HT from preterm infants [26].
Reliability
Accuracy of the acoustic measurements was assessed by re-measuring the cry samples obtained from two HT infants and two infants with AOI, randomly selected from the original groups. The values obtained from the re-measurement were then compared to the values obtained from the original measurements. The average measurement difference in cry duration between the four original samples and re-measured samples was 16 ms (or less than 2% difference). The average remeasurement difference for cry latency was 6 ms (or less than 5%). The remeasurement difference for determination of FSP was 5 Hz (or less than 3%). Retabulation of the number of expiratory cries was 100% accurate.
Results
Expiratory Cries
The average number of expiratory cries occurring in the overall crying episodes of the HT and AOI groups is listed in Table 1. A two-tailed t-test was performed to determine if the HT and AOI groups differed significantly in the average number of expiratory cries. The test was not significant (p >.05)
Table 1.
Acoustic features of crying episodes obtained from infants who were healthy full term (HT) and those who experienced apnea of infancy (AOI). The features include the mean number of expiratory cries occurring during an entire crying episode, mean overall crying episode duration, mean ratio of overall cry duration-to-number of expiratory cries, mean cry latency, and the mean first spectral peak (FSP). Standard deviations are shown in parentheses.
| Cry Feature | HT | AOI |
|---|---|---|
| No. Expiratory Cries | 17.8 (6.33) | 18.0 (4.87) |
| Cry Duration (s) | 28.32 (9.55) | 28.40 (10.55) |
| Cry Ratio | 1.59 (0.43) | 1.58 (0.64) |
| Cry Latency (s) | 1.10 (0.33) | 1.29 (0.42) |
| FSP (Hz) | 463 (45) | 435 (48) |
Cry Duration and Cry Latency
The average duration of crying episodes and cry latency values for the HT and AOI groups are listed in Table 1. Results of a t-test indicated that the two groups did not differ significantly for overall cry duration (p >.05). However, a significant difference was found for cry latency with the AOI group demonstrating longer cry latency compared to the HT group (p <.04). A comparison of the cry latencies of one HT infant and one with AOI is provided in Figure 1.
Figure 1.
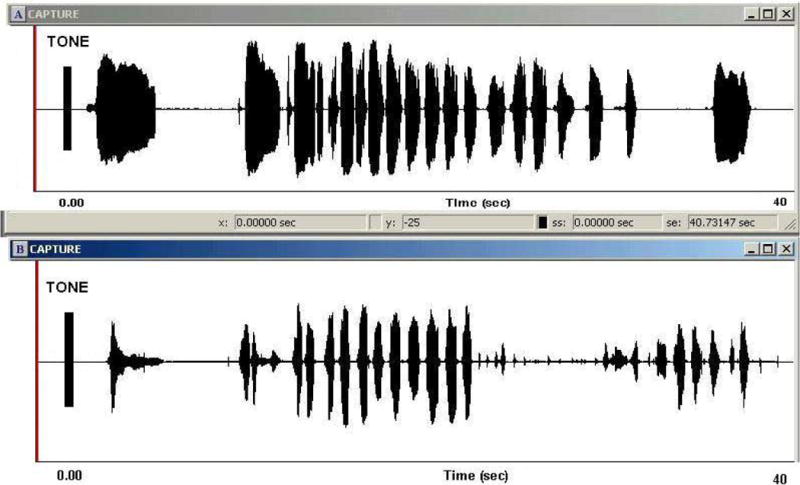
Amplitude-by-time waveform displays of the crying episode of a healthy term (HT) infant (panel A) and an infant with apnea of infancy (AOI) (panel B). The waveforms depict instances of several expiratory cries comprising the overall crying episode. Each crying episode was preceded by an audible tone that was time-locked to administration of the pain stimulus. The tone served as the acoustic reference for measurement of cry latency.
Cry Ratio
A ratio of the overall cry duration-to-number of expiratory cries was calculated for each child and a mean cry ratio was determined for each group. The ratio was assumed to provide an estimate of the proportion of time each expiratory cry consumed the entire crying episode. The cry ratio of each group is reported in Table 1. The ratio did not differ significantly between groups (p >.05), indicating that the rate of expiratory crying was similar between HT and AOI groups.
First Spectral Peak
The average FSP values obtained for the HT and AOI groups are listed in Table 1. A significant difference was found between the two groups (p <.02), indicating a significantly lower F0 across crying episodes among the AOI group.
Discussion
Three predictions were proposed and tested in the present study. The predications were that (1) the rate of crying would be significantly faster among infants with AOI, (2) the latency of crying onset would be significantly longer among infants with AOI and (3) the F0 characterizing an overall episode of crying would be significantly lower among infants with AOI. Two of the three predictions were supported by the present results. The results obtained for each prediction are discussed below.
Oren et al. [8] found infants with AOI to demonstrate significantly higher respiratory rates than non-AOI babies. Assuming this type of breathing pattern would also be apparent during moments of phonation, there should have been a greater number of expiratory cries and shorter expiratory cry durations in this group compared to HT infants. This was not the case. The average number of expiratory cries occurring per episode was similar between groups. Further, the proportion of time each expiratory cry consumed during the entire crying episode did not differ between groups The AOI group actually demonstrated slightly longer expiratory cry durations. Subsequently, the acoustic measures examined for this particular prediction did not reveal respiratory rate differences between HT and AOI groups.
The obvious explanation for the lack of difference relates to known differences in respiratory rates for quiet breathing versus breathing for phonation. During quiet breathing the total time dedicated to the inspiratory and expiratory phases of a breath cycle are roughly equal. The breath cycle of a normal newborn averages 40 to 70 cycles per minute [27]. During phonation, the breath cycle changes dramatically with approximately 90% of the cycle dedicated to the expiratory phase [27]. The alteration in breath cycle is necessary in order to accommodate prolonged phonation. In the present study, it is possible that respiratory differences between HT and AOI groups are only apparent during quiet breathing. The respiratory adjustments necessary to produce cry/phonation may be too dramatic to differentiate infant groups.
Past reports [21], have found that infants with AOI take longer to arouse from a sleep state compared to non-AOI babies. One reason offered for this difficulty is that infants with AOI may have elevated catecholamine levels, which contribute to depressed arousal [21]. This failure to arouse normally was used as the premise for examining cry latency in HT and AOI groups. In the present study, infants with AOI demonstrated significantly longer cry latencies compared to the HT infants. Cry latency is assumed to reflect the time for the nervous system to interpret the pain stimulus [25]. Therefore, the present findings support previous physiological findings documenting poor arousal among infants with AOI.
Infants with AOI are known to demonstrate a decrease in muscle tone [3]. Assuming, a pervasive decrease in muscle tone is part of this condition, it is not unreasonable to assume that laryngeal musculature would likewise be affected. The present findings found a significantly lower F0 among the AOI group compared to the HT group, thereby lending further support that decreased muscle tone is a physiological marker of AOI.
The results obtained in the present study can be interpreted according to a model of infant cry related to the role of the tenth cranial (vagus) nerve [14, 28–32]. This model emphasizes the role of the vagus, as the most proximate neural input affecting the muscles that control the lungs, vocal folds, and vocal tract. The nucleus ambiguus of the medulla provides the primary vagal input to these various organs as well as coordinating heart rate, breathing, and vocalizing. During moments of stress, such as that provided by administration of painful stimulus to the heel, there is a withdrawal of vagal output from the nucleus ambiguus, resulting in a number of physiological responses including increased respiration, heart rate, and an increase in tension of laryngeal muscles [28–32]. This type of nervous system response is typical and would be expected to influence the acoustic characteristics of normal infant crying. In the present study, AOI babies demonstrated significantly different crying behavior compared to the HT group. Therefore, it would appear as though the crying behavior of these two groups may have differed as a result of differing nervous system responses to stress. It is possible the AOI group demonstrated a slower than normal nervous system response to stress. This type of response would result in an increase in vagal output, thereby resulting in an abnormal decrease in laryngeal tension. Acoustically, this response would be revealed as a longer cry latency, lower F0, and larger cry ratio in comparison to HT infants. This was indeed the case in the present study.
In conclusion, the present study provides acoustic cry data on a unique group of children who were resuscitated for AOI. The results support the contention that these infants take longer to arouse and demonstrate a marked decrease in muscle tone compared to HT infants. As such, it is conceivable that crying behavior can serve as a potential clinical marker in the early identification of this life-threatening condition. We stress that such data are best utilized as an adjunct to the diagnosis of specific health conditions. However, improved methods of diagnosis and/or prevention of AOI will likely result from an improved understanding of the prima facie condition and to this end, the inclusion of infant crying is warranted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supported by the Department of Pediatrics. John A. Burns School of Medicine University of Hawaii and the Chun Foundation for Medical Research in Pediatrics.
The CHIME study was supported by the National Institute of Child Health and Human Development grants (NICHD) HD: 29067, 29071, 28971, 29073, 29060, 29056, and 34625.
Contributor Information
Michael P. Robb, Department of Communication Disorders University of Canterbury Christchurch, NEW ZEALAND.
David H. Crowell, Department of Pediatrics, John A. Burns School of Medicine, University of Hawaii at Manoa and Kapiolani Medical Center for Women and Children Honolulu, Hawaii USA.
Peter Dunn-Rankin, College of Education University of Hawaii at Manoa Honolulu, Hawaii USA.
References
- 1.Slye D. Meeting the challenges of neonatal cardiorespiratory event monitoring. Neonat Inten Care. 1994 Sep-Oct;:14–18. [PubMed] [Google Scholar]
- 2.Fan LL. Apnea of infancy. Prim Care. 1984;11:443–452. [PubMed] [Google Scholar]
- 3.Davis N, Sweeney LB. Apnea of infancy: A clinical problem. Western J Med. 1986;144:429–432. [PMC free article] [PubMed] [Google Scholar]
- 4.Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Cardiorespiratory patterns during SIDS. Pediatr Pulmonol. 1997;24:450. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Steinschneider A. Prolonged apnea and the sudden infant death syndrome: Clinical and laboratory observations. Pediatrics. 1970;50:646–654. [PubMed] [Google Scholar]
- 6.Guilleminault C, Stoohs R. From apnea of infancy to obstructive sleep apnea syndrome in the young child. Chest. 102:1065–1071. doi: 10.1378/chest.102.4.1065. [DOI] [PubMed] [Google Scholar]
- 7.Kiechl-Kohlendorfer U, Hof D, Pupp U, Traweger-Ravanelli B, Kiechl S. Epedimiology of apparent life threatening events. Arch Dis Child. 2005;90:297–300. doi: 10.1136/adc.2004.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oren J, Kelly DH, Shannon DC. Pneumogram recordings in infants resuscitated for apnea of infancy. Pediatrics. 1989;83:364–368. [PubMed] [Google Scholar]
- 9.Ward SL, Marcus CL. Obstructive sleep apnea in infants and young children. J Clin Neurophys. 1996;13:198–207. doi: 10.1097/00004691-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Fairbanks G. An acoustical study of the pitch of infant hunger wails. Child Dev. 1942;13:227–232. [Google Scholar]
- 11.Barr RG, Hopkins B, Green JA. Crying as a sign, a symptom, and a signal Cambridge. Cambridge University Press; 2000. [Google Scholar]
- 12.Lind K, Wermke K. Development of the vocal fundamental frequency of spontaneous cries during the first 3 months. Int J Pediatr Otorhinolaryngol. 2002;64:97–104. doi: 10.1016/s0165-5876(02)00024-1. [DOI] [PubMed] [Google Scholar]
- 13.Fant G. Acoustic theory of speech production. The Hague, Netherlands, Mouton; 1960. [Google Scholar]
- 14.Golub H, Corwin M. A physioacoustic model of the infant cry. In: Lester B, Boukydis C, editors. Infant crying: Theoretical and research perspectives. New York: Plenum Press; 1985. [Google Scholar]
- 15.Green J, Irwin J, Gustafson G. Acoustic cry analysis, neonatal status and long-term developmental outcomes. In: Barr RG, Hopkins B, Green JA, editors. Crying as a sign, a symptom, and a signal. Cambridge, Cambridge University Press; 2000. [Google Scholar]
- 16.LaGasse L, Neal A, Lester B. Assessment of infant cry: Acoustic cry analysis and parental perception. Mental Retard Dev Disabil Res Rev. 2005;11:83–93. doi: 10.1002/mrdd.20050. [DOI] [PubMed] [Google Scholar]
- 17.Michelsson K. Cry analysis of symptomless low birthweight neonates and asphyxiated newborn infants. Acta Paediatr Scan Suppl. 1971;216:9–45. [PubMed] [Google Scholar]
- 18.Michelsson K, Sirvio P, Wasz-Hockert O. Pain cry in full-term asphyxiated newborn infants correlated with late findings. Acta Paedatr Scand. 1977;66:611–616. doi: 10.1111/j.1651-2227.1977.tb07956.x. [DOI] [PubMed] [Google Scholar]
- 19.Partanen T, Wasz-Hockert O, Vuorenski V, et al. Auditory identification of pain cry signals of young infants in pathological conditions and its sound spectrographic basis. Ann Paediatr Fenn. 1967;13:56–63. [PubMed] [Google Scholar]
- 20.Lieberman P. Intonation, perception and language. Cambridge, Mass: MIT Press; 1967. [Google Scholar]
- 21.Rodgriguez AM, Warburton D, Keens TG. Elevated catecholamine levels and abnormal hypoxic arousal in apnea of infancy. Pediatrics. 1987;79:269–274. [PubMed] [Google Scholar]
- 22.Sundell HW, Hafstrom O, Poole S, Milerad J. Dopaminergic mediation of delayed hypoxic arousal in young lambs exposed to nicotine in utero. Pediatr Pulmonol. 1997;24:450. [Google Scholar]
- 23.Grau SM, Robb MP, Cacace AT. Acoustic correlates of inspiratory phonation during infant cry. J Speech Hear Res. 1995;38:373–381. doi: 10.1044/jshr.3802.373. [DOI] [PubMed] [Google Scholar]
- 24.Cacace AT, Robb MP, Saxman JH, Rismeberg HM. Acoustic features of normal-hearing pre-term cry. Int J Pediatr Otorhinolaryngol. 1995;32:100–110. doi: 10.1016/0165-5876(95)01211-7. [DOI] [PubMed] [Google Scholar]
- 25.Runefors P, Arnbjornsson E. A sound spectrogram analysis of children’s crying after painful stimuli during the first year of life. Folia Phoniatr Logop. 2005;57:90–95. doi: 10.1159/000083570. [DOI] [PubMed] [Google Scholar]
- 26.Goberman AM, Robb MP. Acoustic examination of preterm and full-term infant cries: The long-time average spectrum. J Speech Lang Hear Res. 1999;42:850–861. doi: 10.1044/jslhr.4204.850. [DOI] [PubMed] [Google Scholar]
- 27.Seikel J, Drumright D, Seikel P. Essentials of anatomy and physiology. Clifton Park NY: Thomson Delmar; 2004. [Google Scholar]
- 28.Boers J, Kirkwood PA, de Weerd H, Holstege G. Ultrastructural evidence for direct excitatory retroambiguus projections to cutaneous trunci and abdominal external oblique muscle motoneurons in the cat. Brain Res Bull. 2006;68:249–256. doi: 10.1016/j.brainresbull.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Boers J, Klop EM, Hulshoff AD, de Weerd H, Holstege G. Direct projections from the nucleus retroambiguus to cricothyroid motoneurons in the cat. Neurosci Lett. 2002;319:5–8. doi: 10.1016/s0304-3940(01)02395-3. [DOI] [PubMed] [Google Scholar]
- 30.Porter F, Porges S, Marshall R. Newborn pain cries and vagal tone: Parallel changes in response to circumcision. Child Dev. 1988;59:495–505. [PubMed] [Google Scholar]
- 31.Porges S. Cardiac vagal tone: A physiological index of stress. Neurosci Biobehav Rev. 1995;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- 32.Soltis J. The signal functions of early infant crying. Behav Brain Sci. 2004;27:443–490. [PubMed] [Google Scholar]


