Abstract
Structural knowledge of the cystic fibrosis transmembrane conductance regulator (CFTR) requires developing methods to purify and stabilize this aggregation-prone membrane protein above 1 mg/ml. Starting with green fluorescent protein- and epitope-tagged human CFTR produced in mammalian cells known to properly fold and process CFTR, we devised a rapid tandem affinity purification scheme to minimize CFTR exposure to detergent in order to preserve its ATPase function. We compared a panel of detergents, including widely used detergents (maltosides, neopentyl gycols (MNG), C12E8, lysolipids, Chaps) and innovative detergents (branched alkylmaltosides, facial amphiphiles) for CFTR purification, function, monodispersity and stability. ATPase activity after reconstitution into proteoliposomes was 2–3 times higher when CFTR was purified using facial amphiphiles. ATPase activity was also demonstrated in purified CFTR samples without detergent removal using a novel lipid supplementation assay. By electron microscopy, negatively stained CFTR samples were monodisperse at low concentration, and size exclusion chromatography showed a predominance of monomer even after CFTR concentration above 1 mg/ml. Rates of CFTR aggregation quantified in an electrophoretic mobility shift assay showed that detergents which best preserved reconstituted ATPase activity also supported the greatest stability, with CFTR monomer half-lives of 6–9 days in MNG or Chaps, and 12–17 days in facial amphiphile. Cryoelectron microscopy of concentrated CFTR in MNG or facial amphiphile confirmed mostly monomeric protein, producing low resolution reconstructions in conformity with similar proteins. These protocols can be used to generate samples of pure, functional, stable CFTR at concentrations amenable to biophysical characterization.
Keywords: cystic fibrosis, ATP-binding cassette, ABC transporter, maltoside neopentyl glycol detergents, facial amphiphiles
1. Introduction
The cystic fibrosis transmembrane conductance regulator (CFTR) is a chloride channel essential to fluid regulation in a number of epithelial tissues, including lung and intestine. Mutations causing CFTR dysfunction lead to cystic fibrosis (CF), a genetic disease common in Europe, USA, and other populations of European origin [1]. CF represents a severe affliction for which palliative treatment has been the only option, with the recent exception of Ivacaftor, a drug that improves CFTR function in patients with the G551D mutation [2]. Ivacaftor shows promise for other channel gating mutations as well, but only 4–5% of CF patients harbor these gating mutations [3]. The most common mutation accounting for 70% of patients, the F508 deletion, results in CFTR misfolding and its premature degradation [4, 5]. At this writing, over 1960 other CFTR mutations have been identified (www.genet.sickkids.on.ca) that vary broadly in phenotypic effect, complicating both diagnosis and treatment [1]. Expanding effective drug treatment to greater numbers of CF patients will require intensive drug development effort which can be facilitated by knowledge of CFTR structure. However, even wild-type CFTR exhibits conformational instability that renders it prone to misfold in vivo, as well as to aggregate and lose activity when solubilized in detergents [5–8]. This has been a major roadblock for structural and biophysical studies that require mg/ml concentrations of pure protein.
CFTR belongs to the ATP-binding cassette (ABC) transporter superfamily of transporters, which possess two transmembrane domains and two nucleotide binding domains (NBDs) which interact, forming composite sites of ATP hydrolysis. In CFTR the two nonequivalent NBDs hydrolyze ATP at different rates [9], and ATP hydrolysis mediates channel gating [5, 10–12]. Another unique feature of CFTR is the R region whose phosphorylation regulates channel gating through an unknown mechanism [13]. CFTR NBDs exhibit conformational sensitivity to many detergents, likely contributing to the great difficulty in recovering active purified protein [14]. Purification of active CFTR from insect and yeast cells using perfluorooctanoate [15] or lysolipid [16–18] has been reported, but CFTR purified with these detergents in other laboratories has proven too unstable for detailed biophysical characterization. Although human CFTR purified from mammalian (BHK) cells in dodecylmaltoside remained functional [19], the preparations were prone to aggregation and had a concentration limit of 0.3 mg CFTR/ml [7]. Therefore, identification of detergents effective in preserving CFTR structural and functional integrity remains an urgent priority. Effort in recent years to develop novel detergents has brought many notable successes in structural biology of challenging membrane proteins [20], and it is worthwhile to include these in a systematic trial of detergents for CFTR purification.
The expression of modified human CFTR in human embryonic kidney (HEK) cell line D165 is described in depth elsewhere [EH, A Mulky, HD, QD, A Aleksandrov, B Bajrami, PA Diego, X Wu, M Ray, AP Naren, JR Riordan, X Yao, LJD, ILU and JCK, manuscript in preparation]. The recombinant CFTR protein comprises an N-terminal His10 tag linked to a SUMOstar domain, full length human CFTR with a Flag epitope inserted proximal to amino acid N901 in the fourth extracellular loop, and a C-terminal enhanced green fluorescent protein (GFP) domain. Post-translational modifications of CFTR that are essential to its maturation and trafficking to the cell surface [5] have been well-characterized in this expression system [21], and the inserted domains were shown not to interfere with normal gating of the channel [EH, A Mulky, HD, QD, A Aleksandrov, B Bajrami, PA Diego, X Wu, M Ray, AP Naren, JR Riordan, X Yao, LJD, ILU and JCK, manuscript in preparation]. With CFTR expressed at levels exceeding 1 mg per 109 cells, this cell line provides an ideal starting material for isolation of functional protein. Here we have devised a simple, rapid protocol for CFTR purification from D165 cell microsomal membranes, utilizing the His10 and Flag epitopes in a tandem affinity scheme that delivers functionally pure CFTR in 8 to 10 h. Because the protein is also GFP-tagged, recoveries in each step were readily quantifiable. We show that purified CFTR retains ATPase activity with or without reconstitution into proteoliposomes, and compare a panel of detergents for effectiveness in CFTR solubilization, purification, ATPase activity, monodispersity, and stability when concentrated above 1 mg/ml. Because of their crucial importance in structural biology, cutting edge amphiphiles continue to be developed [20]. Among the detergents tested, our study shows that neopentyl maltoside detergents (MNGs) [22] are best overall among the detergents currently available commercially, and that novel, soon to be available facial amphiphiles outperform MNGs with regard to CFTR activity and long-term stability.
2. Materials and methods
2.1 CFTR-expressing cell lines
The inducible HEK293 cell line D165, expressing His10-SUMOstar-human CFTR901Flag-GFP at the cell surface, is described in detail elsewhere [EH, A Mulky, HD, QD, A Aleksandrov, B Bajrami, PA Diego, X Wu, M Ray, AP Naren, JR Riordan, X Yao, LJD, ILU and JCK, manuscript in preparation]. The G551D CFTR cell line was generated in the same way after standard QuikChange mutagenesis. Cells were expanded to a scale of ~ 1 liter and to a density of 3–5 × 106 cells per ml. CFTR expression was induced with 1 μg/ml doxycyclin for 36 h.
2.2 Cell lysis and membrane isolation
Frozen HEK cell pellets were lysed hypotonically in buffer H (8 mM Hepes Na pH 7.2/0.8 mM EDTA/1 mM dithiothreitol) containing a mixture of protease inhibitors at the following final concentrations: 1 mM phenylmethylsulfonylfluoride (PMSF), 2.5 μg/ml each E64 and chymostatin, 10 μg/ml each leupeptin and pepstatin A [19, 23]. After douncing, the lysate was promptly mixed with 1/7th vol Buffer H/2 M sucrose, and centrifuged twice at 1000 × g, 10 min. The 1000 × g supernatant was ultracentrifuged 1 h at 100,000 × g. The resulting pellet was washed by resuspension in 50 mM Tris Cl pH 7.5/0.5 M NaCl/10% glycerol/1 mM dithiothreitol (DTT) containing protease inhibitor mixture and ultracentrifugation for 1 h at 100,000 × g. All centrifugation steps used slow braking. The final membrane pellet was resuspended in Buffer S (50 mM Tris Cl pH 8.0/0.2 M NaCl/10% glycerol/1 mM DTT) with protease inhibitor mixture. Aliquots were frozen at −80 °C. The procedure yielded 30 ± 8 mg microsomal protein per 109 cells.
2.3 CFTR quantitation by in-gel GFP fluorescence
CFTR quantitation by in-gel GFP fluorescence is based on an established method [24]. Samples were separated on 8% SDS-PAGE gels, and fluorescent images recorded using a scanner or digital camera with FITC filter set. Each gel included known amounts of an external standard, either Sumo-GFP (LifeSensors, 39 kD) or mouse P-glycoprotein-GFP fusion protein (172 kD) purified in our lab. Exposure times were adjusted so that fluorescence intensity fell within the linear range. Fluorescent bands were quantitated by densitometry using the public domain application Image J (http://imagej.nih.gov/ij/index.html). Amounts of CFTR (212 kD) were calculated from the linear standard curve, taking into account its molecular weight difference. After image capture, gels were silver stained to assess sample purity [25].
2.4 Tandem affinity purification
Membranes were adjusted to 2 mg/ml in Buffer S (section 2.2) also containing 10 mM imidazole, 2.5 mM MgCl2, 1 mM ATP, and protease inhibitor mixture. Detergent was added to give 0.5% (w/v). After 30 min incubation on ice with periodic mixing, the suspension was centrifuged 30 min at 100,000 × g with slow deceleration. The detergent extract was diluted 5-fold in Buffer S containing 10 mM imidazole, 2.5 mM MgCl2, 1 mM ATP, and 0.1 mM PMSF. If necessary, detergent was included in this mixture to keep detergent at the working concentration; these working concentrations are stated in Table 1. All subsequent buffers contained detergent at the working concentration. NiNTA resin (Qiagen Superflow) was added to give 1% (v/v), and after 3 h gentle rotation at 4 °C the binding mixture was packed into a chromatography column under gravity flow. The resin was washed with 30 vol Buffer W (50 mM Tris Cl pH 7.5/10% glycerol/2.5 mM MgCl2/1 mM ATP) containing 0.5 M NaCl and 60 mM imidazole, then 10 vol Buffer W with 0.15 M NaCl and 60 mM imidazole. The column was eluted over 30 min with 4–5 vol Buffer W containing 0.15 M NaCl and 350 mM imidazole. NiNTA eluate was mixed with 1/5th vol of a 50% antiFlag gel suspension (Sigma A2220) at 4 °C for 90 min, then transferred to a column. The antiFlag column was washed with 10 vol Buffer W with 0.5 M NaCl, then 10 vol Buffer W with 0.15 M NaCl. CFTR was eluted over 25 min with 5 vol of the final wash solution containing 0.1 mg/ml DYKDDDDK. Upon completion, 1 mM DTT was added to the final eluate.
Table 1.
Detergents surveyed, and CFTR recoveries in solubilization and tandem affinity purification
| overall CFTR recovery | ||||||
|---|---|---|---|---|---|---|
| detergent | abbrev | CMC (w/v) | working conc (w/v) | CFTR solubilized | method 1 | method 2 |
| 1-myristoyl-2-hydroxy-sn-glycero-3-phosphocholine | lysoPC14 | 0.003%b | 0.05% | nd | nd | nd |
| 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine | lysoPC16 | 0.0002%a | 0.05% | 83 ± 24% | 1.1 ± 0.6% | nd |
| 1-myristoyl -2-hydroxy-sn-glycero-3-phospho-(1′-rac-glycerol) | lysoPG14 | 0.0003%b | 0.05% | nd | nd | nd |
| dodecyl octaethylene glycol ether | C12E8 | 0.005%a | 0.05% | 78 ± 20% | 5.7 ± 0.9% | nd |
| 1-decyl-β-D-maltoside | DM | 0.09%a | 0.3% | 78 ± 19% | 6.2 ± 1.8% | nd |
| 1-undecyl-β-D-maltoside | UDM | 0.03%a | 0.1% | 66 ± 14% | 4.9 ± 1.4% | nd |
| 1-dodecyl-β-D-maltoside | DDM | 0.01%a | 0.05% | 71 ± 14% | 5.0 ± 1.2% | nd |
| 1-tridecyl-β-D-maltoside | TDM | 0.001%a | 0.05% | 62% d | nd | nd |
| 3-tridecyl-β-D-maltoside | Mal 11-2 | 0.006% b | 0.05% | 58 ± 11% | 2.4 ± 1.0% | nd |
| 2-tetradecyl-β-D-maltoside | Mal 12-1 | 0.004% b | 0.05% | 62 ± 17% | 6.0 ± 1.9% | nd |
| 3-tetradecyl-β-D-maltoside | Mal12-2 | 0.001% [31] | 0.05% | 28 ± 7% | 1.1 ± 0.6% | nd |
| 5-cyclohexyl-1-pentyl-β-D-maltoside | Cymal 5 | 0.12%a | 0.4% | 57 ± 23% | 5.1 ± 1.2% | nd |
| 6-cyclohexyl-1-hexyl-β-D-maltoside | Cymal 6 | 0.03%a | 0.1% | 60 ± 10% | 4.4 ± 1.4% | nd |
| 7-cyclohexyl-1-heptyl-β-D-maltoside | Cymal 7 | 0.01%a | 0.05% | 71 ± 15% | 4.8 ± 0.9% | nd |
| decyl maltose neopentyl glycol | MNG10 | 0.003% [22] | 0.05% | 53 ± 9% | 3.8 ± 1.3% | nd |
| lauryl maltose neopentyl glycol | MNG12 | 0.001% [22] | 0.05% | 45 ± 13% | 4.4 ± 1.7% | nd |
| 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate | Chaps | 0.5% | 1% | 35 ± 8% | nd | 6.3 ± 1.5% |
| 3α,7α,12α-tri((O-β-D-maltopyranosyl)ethyloxy)-17-(pentan-2-yl)-cholane | FA-4 | 0.028% [32] | 0.05% | 6% d | 0.3% d | 7.4 ± 2.5% |
| 3α,7α,12α-tri((O-β-D-maltopyranosyl)ethyloxy)-17-(hexan-2-yl)-cholane | FA609 | 0.009% c | 0.05% | 12% d | 0.2% d | 6.5 ± 1.1% |
| 3α,7α,12α-tri((O-β-D-maltopyranosyl)ethyloxy)-17-(heptan-2-yl)-cholane | FA611 | 0.004% c | 0.05% | 20% d | 1.1% d | 6.9 ± 2.7% |
| 3α,7α,12α-tri((O-β-D-maltopyranosyl)ethyloxy)-17-(6-methylheptan-2-yl)-cholane | FA613 | 0.001% c | 0.05% | 22% d | 1.6% d | 7.9 ± 3.1% |
Generalized structures for detergent families are shown in figure 3. CFTR was extracted from microsomes at 0.5% detergent. Chromatography was conducted at the stated working concentrations, which were selected to be just above the CMC or no less than 0.05%. In method 1, the same detergent was used for solubilization and for chromatography. In method 2, UDM was used for solubilization and a second detergent was used for chromatography. Overall recoveries refer to amount of CFTR recovered from the final affinity step compared to CFTR present in the starting microsomal membranes. Values represent mean ± standard deviation for three or more trials, except as noted.
nd: not done
determined as described in section 2.11
result in a single trial
2.5 Reconstitution
We chose Biobeads for reconstitution because this detergent removal method is applicable even with very low CMC detergents [26]. Several elements of this protocol were systematically varied, including lipid composition, lipid:protein ratio, and rate of detergent removal; we found the factor most strongly influencing downstream results to be the use of C12E8 for pre-destabilizing liposomes. Sonicated liposomes were prepared from a 5:3:1:1 or 5:1:3:1 mixture by weight of 1-palmitoyl-2-oleoyl-phosphatidylethanolamine, brain phosphatidylserine, egg phosphatidylcholine (all phospholipids from Avanti Polar Lipids), and cholesterol (Anatrace) [16, 18, 19]. Liposomes were destabilized on the day of use by addition of C12E8 at a detergent:lipid ratio of 1:4 w/w, incubated at room temperature for 1 h, then chilled until use. Purified CFTR (150–200 μl, ~5 μg) was mixed with 400 μg of destabilized liposomes at 4 °C for 30 min, then four equal additions of polystyrene beads (Biobeads SM2, Biorad) were made at intervals over the next 15–20 h [26]. A total 30-fold bead excess was used, assuming a detergent capacity of 100 μg/mg [27]. Mock proteoliposomes were prepared in parallel using column elution buffer without Flag peptide, which had no effect on assay background. Proteoliposome and mock samples were then diluted in 20 vol 20 mM Tris Cl pH 7.5/0.15 M NaCl/2.5 mM MgCl2/1 mM DTT, filtered to remove beads, collected by ultracentrifugation at 200,000 × g, and resuspended in 1/5th–1/10th the original eluate volume of Buffer A (25 mM Tris Cl pH 7.5/0.2 M NaCl/2.5 mM MgCl2/1 mM DTT).
2.6 ATP activity measurements
2.6.1 ATPase assay
Proteoliposomes and mock samples were incubated with 0.3 mM [α32P]-ATP at 33 °C for 2 h. If necessary, samples were diluted with Buffer A (section 2.5) so that substrate conversion did not exceed 10%. A 1 μl portion of each incubation was quenched with 1 μl of 10% SDS containing 10 mM ATP and 10 mM ADP, and half was spotted onto polyethyleneimine-cellulose TLC plates with fluorescent indicator [17]. After development in 1 M HCOOH/0.5 M LiCl, the ATP and ADP spots were cut and counted in 4 ml Ecolume (MP Biomedicals), and product formation was calculated from the observed ADP/ATP ratio. Blank rates (nmol/h) measured in mock samples under each test condition were subtracted before calculating specific enzymatic activities.
To directly assay antiFlag eluates without reconstitution, ATP was omitted from antiFlag chromatography buffers. Samples or mock samples were supplemented with 2 mg/ml C12E8-destabilized liposomes (described in section 2.5) and preincubated on ice for 45–60 min prior to substrate addition as above. Control experiments showed that product formation was proportional to CFTR concentration.
2.6.2 Michaelis-Menten kinetic measurements
CFTR was phosphorylated or dephosphorylated while NiNTA-bound. In control experiments, silver staining showed that this approach allowed complete removal of the modification enzymes from purified CFTR prior to its reconstitution, and CFTR bands demonstrated the appropriate mobility shifts. After the first 0.5 M NaCl wash (section 2.4), the NiNTA column was washed with 10 vol 50 mM Tris Cl pH 7.5/0.15 M NaCl/10% glycerol/5 mM MgCl2/1 mM ATP, then resuspended in 1 vol of the same solution containing either 500 U/ml protein kinase A catalytic subunit (PKA, Promega) or 4 mM MnCl2 and 4000 U/ml lambda protein phosphatase (New England Biolabs). Enzymatic treatments were for 20 min at 4 °C, followed by NiNTA washing with 10 vol Buffer W at 0.15 M NaCl and 60 mM imidazole. Elution, antiFlag chromatography and reconstitution were completed as described in sections 2.4 and 2.5. Washed proteoliposomes were resuspended in Buffer A containing 6 mM MgCl2 and assayed at ATP concentrations varying from 0.3 to 2.4 mM. Km and Vmax were obtained by least squares fitting of the Michaelis-Menten equation. Allowing Hill coefficients to differ from unity did not alter the curve fits.
2.7 Photoaffinity labeling
Samples of microsomes, proteoliposomes, or mock proteoliposomes in DTT-free buffer A were incubated on ice for 5 min with 0.1 mM 8-azidoadenosine 5′-triphosphate 2′,3′-biotin-long chain hydrazone (8-azido-ATP-biotin, Affinity Photoprobes) in the presence or absence of 10 mM competitor ATP. The mixtures were irradiated on ice for 40 sec at 254 nm in a UV Products crosslinker set at 120,000 Joule/cm2, then quenched by addition of 50 mM DTT in SDS-gel sample buffer. After resolution on an 8% SDS gel, proteins were transferred to nitrocellulose. Photoaffinity nucleotide labeled protein was detected with horseradish peroxidase-conjugated goat antibiotin (1:6000 in 1% milk, 1 h), with chemiluminescent detection.
2.8 Electron microscopy (EM)
Upon completion of tandem affinity-purification, samples of CFTR in various detergents were brought to 1 mM DTT, and rapidly frozen for shipment. These low concentration samples were examined by negatively stained EM as described previously [28]. For cryoelectron microscopy, PKA-phosphorylated, tandem affinity purified CFTR samples were concentrated as described in section 2.9, then buffer-exchanged on the ultrafilter into modified Buffer W containing 8% glycerol, 0.15 M NaCl, 1 mM DTT, and detergent at working concentration. Samples were rapidly frozen by plunge-freezing into liquid ethane and examined using an FEI Polara transmission electron microscope operated at 200 kV as described previously [7] but with strong underfocus (> 4 μm) employed during imaging because of the relatively small mass of the particles. Particles were selected from 4k × 4k images using the autoboxing function of the EMAN2 software suite and subsequently processed using the same software [29]. Resolution of the final 3D reconstructions was estimated at 3.5–4.0 nm.
2.9 Concentration and size exclusion chromatography
NiNTA-purified CFTR (at least 80% pure by silver stain) was reduced with 1 mM DTT, then gradually concentrated by successive spins in 100,000 MWCO spin concentrators (Amicon Ultra, Millipore), sampling the retentate at stages along the way. These samples were promptly frozen, and later analyzed for the presence of monomer, multimer, and void volume aggregates by size exclusion chromatography (SEC) on a 3.2 mm × 30 cm Superose 6 column (GE Healthcare) [30]. SEC buffer was 50 mM Tris Cl pH 7.5, 0.15 M NaCl, 10% glycerol, 2.5 mM MgCl2, 0.05% MNG10, 0.2 mM tris(2-carboxyethyl)phosphine, flowing at 0.04 ml/min. GFP fluorescence was monitored at 488 nm/509 nm using a Jasco FP2020 Plus fluorimetric detector, with gain set at 100 and attenuation at 64.
2.10 Measurement of CFTR aggregation rates by electrophoretic mobility shift
To compare CFTR stability after concentration in detergents, NiNTA-purified CFTR was concentrated as in section 2.9. To match buffer conditions in cryoEM and SEC analyses, the retentate was washed twice in Buffer W (section 2.4) with 0.15 M NaCl, 1 mM DTT, and detergent at working concentration, then concentrated to minimum volume. The retentate was sampled immediately and after 1, 4, and 7 days of 4 °C storage. These samples were diluted 15-fold into SDS-gel sample buffer for quantitation of CFTR monomer band by in-gel fluorescence (section 2.3). The method therefore measures CFTR that has not entered into SDS-resistant complexes.
2.11 Reagents
Branched-chain maltosides used in this work were synthesized as previously described [31]. Mal 11-2 and 12-1 have recently become commercially available from Avanti Polar Lipids. Facial amphiphiles FA 609, 611, and 613 were prepared similarly to FA-4 [32], starting from 3α, 7α, 12α-trihydroxycholane bearing different alkyl chains that were prepared by one-step Kolbe electrolysis reaction of cholic acid and aliphatic carboxylic acids [32]. Facial amphiphiles are currently under commercial development by Avanti Polar Lipids. Their critical micelle concentrations (CMC) were determined by a standard fluorescent dye binding protocol using 8-anilino-1-naphthalenesulfonic acid [33]. Other maltoside detergents, Chaps, and C12E8 were from Anatrace, and lysolipids were from Avanti Polar Lipids.
Inhibitor sources were sodium azide (>99%, ThermoFisher), ouabain (Sigma), and CFTR Inhibitor 172 (Calbiochem).
2.12 Statistical comparisons
Statistical comparisons were made using Student’s two-tailed t-test.
3. Results
In selecting detergents for this survey we focused on mild detergents with a record of success in conserving membrane protein structure and function. Detergents were tested for effectiveness in CFTR purification and for retention of ATPase activity. Those which performed well in the functional assessment were evaluated for CFTR stability, which included concentration of CFTR, assessments of monodispersity, and protein aggregation rate.
3.1 Rapid CFTR purification scheme
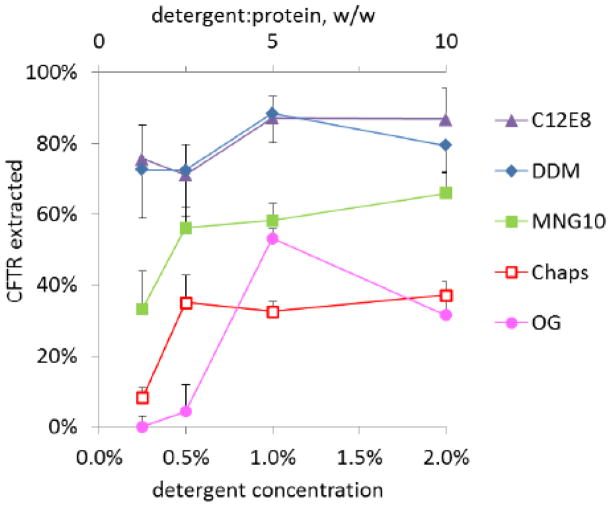
In this study we have prioritized the quality of purified protein over quantity. The strategy behind our purification scheme was to minimize both detergent concentration and duration of exposure, throughout initial CFTR solubilization and chromatography. We began by titrating a limited set of detergents commonly used for membrane protein purifications to determine the lowest effective detergent:protein ratios for CFTR solubilization from microsomes, utilizing C-terminal GFP domain fluorescence for quantitation. This analysis (figure 1) showed the effectiveness of mild detergents such as DDM or C12E8 in extracting 70–90% of CFTR even at low detergent:protein ratios. On the other hand, octylglucoside or Chaps extracted no more than 30–50% of CFTR even at detergent concentrations as high as 2%. The neopentyl maltoside detergent MNG10 [22] demonstrated intermediate effectiveness. In general, increasing the detergent:protein ratio from 2.5 (w/w) to 5 or even 10 extracted little additional CFTR, and so a low detergent:protein ratio of 2.5 was selected for CFTR solubilization throughout all subsequent studies. In addition, we carried out solubilizations at low protein concentration (2 mg/ml) to allow the use of low detergent concentration (0.5%) while maintaining that ratio. Immediately after clarification of extraction mixtures by ultracentrifugation, extracts were diluted 5-fold, bringing the detergent to 0.1% during batchwise NiNTA binding. This binding period was limited to 3 h, because while overnight binding did increase CFTR recovery, this came at the expense of function (data not shown). NiNTA affinity purification achieved ~80% CFTR purity (figure 2A), which we considered insufficient for measurement of ATPase activity because CFTR cannot be selectively assayed. We therefore introduced an antiFlag immunoaffinity chromatography step that improved purity to ~95% as judged by silver staining (figure 2A). Time elapsed from membrane solubilization to antiFlag elution was typically 8–10 h.
Figure 1. Minimum effective detergent concentrations for CFTR solubilization.
CFTR-expressing D165 cell microsomes (2 mg/ml) were incubated with the indicated concentrations of detergent (w/v) on ice for 30 min, then centrifuged 20 min at 100,000 × g. CFTR concentrations in supernatants or in microsomes dissolved in SDS sample buffer were determined by in-gel fluorescence (section 2.3).
OG: octylglucoside
Figure 2. Functional purity of purified, reconstituted CFTR.
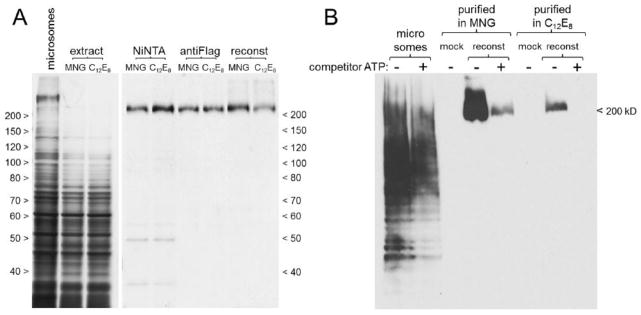
CFTR (212 kD) was purified in parallel either in MNG10 or in C12E8, then reconstituted into proteoliposomes.
Panel A Samples of CFTR at the following stages of purification were resolved on an 8% SDS-gel and silver stained: starting material (microsomes, 5 μl), detergent extracts (5 μl), NiNTA column and antiFlag column eluates (5 μl), and reconstituted proteoliposomes (0.2–0.3 μg CFTR). Sizes of flanking molecular weight markers, in kD, are indicated at left and right. For clarity, staining of lanes containing microsomes and extract was terminated sooner than the purified samples.
Panel B The proteoliposome samples shown in panel A, or mock proteoliposomes, were used for photoaffinity nucleotide labeling in the presence or absence of 10 mM competitor ATP, as described in section 2.7. A fresh aliquot of microsomes from the same batch used as starting material in CFTR purification was included as a labeling control. Sample load per lane was 60 μg (total protein) microsomes, 1.7 μg CFTR (MNG10) or 1.2 μg (C12E8 ), or the equivalent volume of mock proteoliposomes. The arrowhead at right shows the position of the uppermost prestained marker band in an adjacent lane of the Western blot.
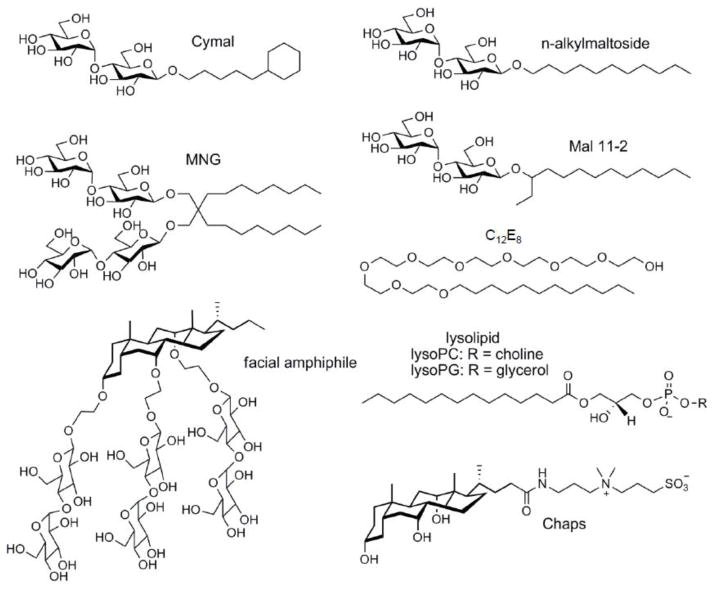
We utilized this tandem affinity scheme to assess the effectiveness of a panel of mild detergents for CFTR purification (Table 1). General detergent structures are shown in figure 3. Purity of CFTR preparations throughout the study was verified by silver staining. Regardless of detergent choice, ~95% purity was consistently achieved using the rapid tandem affinity purification scheme (figures S1, 2A, and other data not shown). With limited opportunity for binding to affinity matrices, CFTR recoveries averaged 23–33% for each affinity step which, in combination with partial solubilization, gave overall recoveries (Table 1) in the 5% range (2–3 μg CFTR per mg starting membrane protein, or 75 μg per 109 cells). Use of mild detergents such as C12E8 or a broad variety of maltoside detergents led to much higher recovery of purified CFTR than was obtained using lysolipids, despite the latter’s effectiveness for CFTR solubilization (Table 1, and other data not shown). CFTR recoveries were somewhat lower with neopentyl (MNG) detergents than with n-alkylmaltosides or Cymals. With branched alkylmaltosides Mal 11-2, 12-1, and 12-2, recoveries varied quite strongly with alkyl chain length, the 12-1 configuration providing the most satisfactory recovery of CFTR. Like Chaps, sterol-based facial amphiphiles FA-4, FA609, FA611 and FA613 proved to be ineffective CFTR solubilizers, extracting <22%. Because these new facial amphiphiles exhibit unique favorable properties [20, 32], we tested them by carrying out solubilization in UDM, then exchanging into the facial amphiphile or Chaps detergent during column washing (Table 1, method 2). In this approach, CFTR recoveries were significantly higher using facial amphiphile for chromatography than using UDM (P < 0.015), and were the highest CFTR recoveries achieved in the study. Exchange into Chaps gave only marginal improvement (P = 0.028).
Figure 3. Detergent types compared in this study.
Chemical names of individual detergents, their CMC values, and working concentrations used in this study are specified in Table 1. Most feature the canonical polar head/apolar tail arrangement, except facial amphiphiles which combine side polarity with a hydrophobic rigid steroid moiety.
3.2 Control experiments to validate function
To assess functional integrity of CFTR obtained with our rapid purification procedure, we reconstituted CFTR into proteoliposomes and compared its functional characteristics to those previously reported.
3.2.1 Photoaffinity labeling of nucleotide binding sites
ATP binding and hydrolysis regulate CFTR channel activity [11, 12, 34]. To demonstrate functional purity, two preparations of CFTR purified in either C12E8 or MNG10 were reconstituted into proteoliposomes, then labeled with 8-azido-ATP-biotin (figure 2B). Whereas the photoaffinity reagent labeled a great number of ATP-binding proteins in microsomal membranes, only the CFTR band was labeled in purified, reconstituted preparations. Based on the absence of other ATP-binding entities, ATPase activity detected in purified, reconstituted CFTR (sections 3.2.2 and 3.3) would be unlikely to arise from contaminant proteins. Greater labeling of reconstituted CFTR was observed following purification in MNG10 than in C12E8, reflecting better preservation of NBD function which was also manifest in greater ATPase activity (section 3.3).
3.2.2 Validation of CFTR-specific ATP hydrolysis
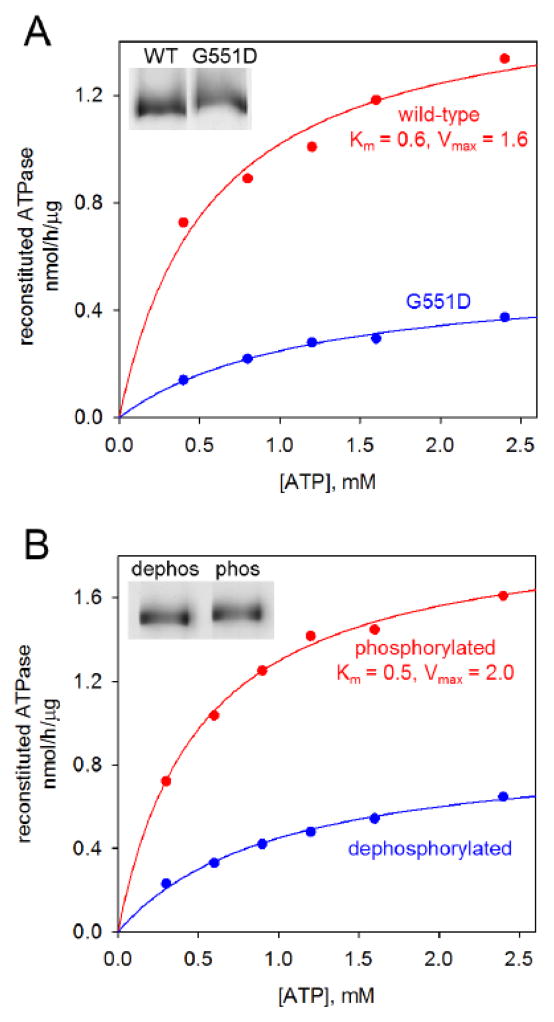
G551D is a cystic fibrosis-causing CFTR mutation that reduces ATP binding and hydrolysis [35, 36]. As a second check that ATP hydrolysis was CFTR-specific, we expressed G551D CFTR in HEK cells, and affinity purified and reconstituted the mutant protein in parallel with wild-type CFTR. ATPase activities were measured over a range of ATP concentrations using protein that had been PKA-phosphorylated, as explained below. Figure 4A shows a representative result. Wild-type CFTR exhibited Km = 0.4 ± 0.1 mM and Vmax = 1.4 ± 0.5 nmol/h/μg (n=4). ATPase activity of the G551D mutant was markedly reduced compared to parallel wild-type preparations, such that kinetic parameters could not be reliably determined. In triplicate experiments, the hydrolysis rate at 2.4 mM ATP for G551D was 14 ± 12% of the wild-type rate.
Figure 4. CFTR modifications that alter rates of ATP hydrolysis.
CFTR was purified in MNG10 and reconstituted into proteoliposomes. Rates of ATP hydrolysis were measured vs. ATP concentration to derive Michaelis-Menten parameters. Each experiment shown was replicated in three independent trials with comparable results.
Panel A Wild-type CFTR and the mutant G551D were PKA-phosphorylated in vitro, purified, and reconstituted. Equal volumes were assayed in parallel. The CFTR mutant showed markedly reduced rates of ATP hydrolysis. Inset: silver stain of proteoliposome samples, equal volumes loaded in each lane.
Panel B Portions of wild-type CFTR were either PKA phosphorylated or dephosphorylated in vitro. After purification and reconstitution, these modifications were shown to alter ATP hydrolysis rates. Inset: silver stain showing difference in electrophoretic mobility of purified CFTR due to alterations in the degree of phosphorylation.
Protein kinase A (PKA) regulates CFTR gating in intact cells [5, 11], but reports differ as to the influence of PKA phosphorylation on CFTR’s in vitro ATPase activity [17, 36]. To investigate this, parallel CFTR samples in MNG10 were either dephosphorylated or PKA-phosphorylated while NiNTA-bound, an approach that allowed complete washout of the modification enzymes which were undetectable by silver staining. Appropriate SDS gel mobility shifts due to changes in CFTR phosphorylation state were observed (figure 4B, inset). After immunoaffinity purification and reconstitution, we determined ATPase activity vs. ATP concentration for the phosphorylated and dephosphorylated samples; figure 4B shows a representative result. In accord with PKA regulation of CFTR gating, ATPase activity of purified reconstituted CFTR was strongly enhanced by PKA phosphorylation compared to the dephosphorylated state, for which kinetic parameters could not be reproducibly determined. In triplicate experiments, dephosphorylated CFTR hydrolyzed 2.4 mM ATP at 30 ± 8% the rate for PKA-phosphorylated CFTR. CFTR is highly phosphorylated in HEK cells [21], and we later found that ATPase activity could be readily measured in these reconstituted and rapidly purified preparations without in vitro phosphorylation (sections 3.3, 3.4).
3.3 Survey of detergents for preservation of CFTR function
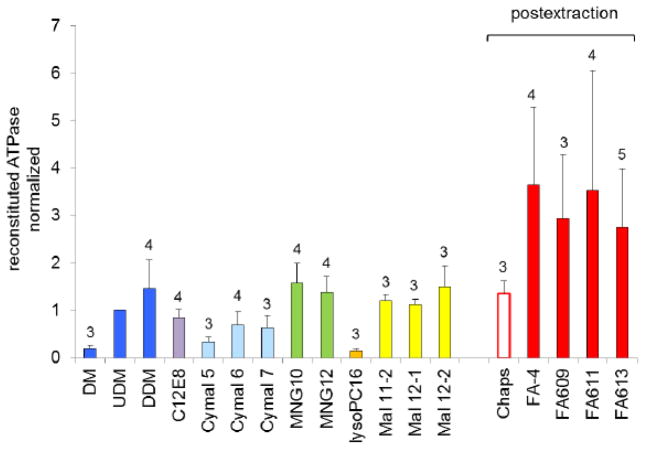
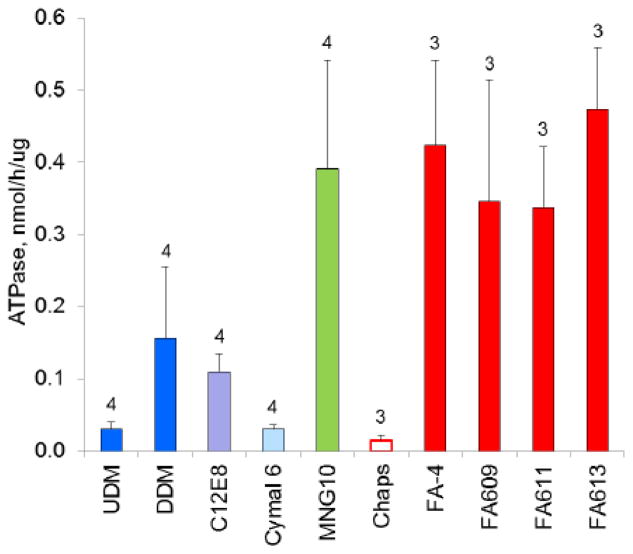
To assess retention of function for a panel of detergents, we compared ATPase activity among reconstituted CFTR preparations (figure 5). Among conventional linear alkylmaltoside detergents, significantly higher ATPase activity was recovered using UDM or DDM for purification compared to their shorter-chain counterpart DM, a trend also observed among cyclohexylmaltosides (Cymal 6 and 7 vs. Cymal 5). The shorter chain detergents have higher CMC values, and observed differences in ATPase activity might be due to increased detergent exposure at the necessarily higher detergent concentrations used during chromatography. Purification of CFTR in lysoPC, despite its low CMC, resulted in quite low reconstituted ATPase activity, suggesting loss of conformation such as that demonstrated when isolated NBDs of CFTR were exposed to this detergent [14]. Among widely used detergents, MNGs, DDM, and Chaps preserved the highest CFTR ATPase activities. They were rivaled by novel branched maltosides (Mal11-2, Mal12-1, Mal12-2) for ATPase retention, and two of these compounds recently became available commercially. Highest reconstituted ATPase activity, by a factor of 2–3, was seen with CFTR purified in facial amphiphiles (FA-4, FA609, FA611, FA613). These novel amphiphiles (currently undergoing commercialization) are structurally unique (figure 3) and are often less denaturing, attributed to their rigid hydrophobic steroid moiety [32].
Figure 5. The detergent used for CFTR purification influences reconstituted ATPase activity.
When CFTR was purified in facial amphiphiles or Chaps, UDM was used for initial solubilization; in all other trials, a single detergent was used for both solubilization and chromatography. After reconstitution into proteoliposomes, ATPase activities were measured in fixed-time assays at 0.3 mM ATP, controlling the amount of material assayed so that conversion of substrate to product did not exceed 10%. Each trial included duplicate samples from four to six parallel purifications/reconstitutions, each with matching mock samples serving as assay blanks. The complete analysis entailed combining data from a large number of trials, and so to manage day-to-day variability all trials included a reference condition, namely use of UDM throughout solubilization and purification, and specific ATPase activities were normalized to these reference samples. Specific activity for reference samples averaged 0.19 ± 0.04 nmol/h/μg (n=16). Combined data from three or more independent trials of each detergent are shown as mean ± standard deviation, with the number of trials shown in the figure.
3.4 Conditions for direct measurement of ATPase without reconstitution
We discovered conditions permitting assay of CFTR ATPase activity directly in detergent solution, without laborious reconstitution. This required supplementation with C12E8-destabilized liposomes (Table 2). Other destabilizing detergents failed to substitute for C12E8 in making the lipid supplement effective (data not shown), perhaps owing to the unique bilayer-destabilizing properties of polyoxyethylene detergents [26, 37]. With detergent removal omitted, detergent effects on activity are possible, and therefore this expedient assay is more suited for studies in a single detergent. For example, we used the direct assay to measure ATPase activity of purified samples before and after freezing at −80 °C, in each case supplementing with destabilized lipid 45 min prior to assay. We found that CFTR purified in MNG10 or in FA 4 retained 94% and 95% of initial ATPase activity through the freeze/thaw cycle. This simple observation would have been considerably more arduous using non-simultaneous reconstitutions.
Table 2.
Conditions for measurement of ATPase activity directly in detergent solution
| ATPase, nmol/h/μg | ||||
|---|---|---|---|---|
| purified in FA-4 | purified in MNG10 | |||
| supplement added | wild-type | G551D | wild-type | G551D |
| buffer | 0.005 | 0.012 | 0.024 | 0.006 |
| untreated liposomes | 0.013 | 0.015 | 0.094 | 0.040 |
| C12E8-destabilized liposomes | 0.150 | 0.040 | 0.143 | 0.054 |
| C12E8 only | 0.001 | 0.012 | 0.009 | 0.005 |
Wild-type and G551D CFTR were tandem affinity-purified in parallel in the indicated detergents. AntiFlag eluates were supplemented with 2 mg/ml untreated liposomes, with 2 mg/ml C12E8-destabilized liposomes (section 2.5), or an equivalent amount of C12E8 (0.5 mg/ml) 45 min prior to assay at submaximal ATP (0.3 mM), as described in section 2.6.1. Values shown are the average of duplicates that differed by no more than 6%.
In a second application, we used selective inhibitors to test for the presence of contaminating ATPase activities at successive stages of CFTR purification in FA-4 (Table 3). Samples purified by NiNTA only or by tandem NiNTA/antiFlag chromatography were preincubated with inhibitors in the presence of destabilized liposomes, then ATPase activities measured in the direct assay. Neither CFTR preparation was inhibited by ouabain, indicating the absence of plasma membrane Na/K ATPase. NiNTA-purified CFTR retained significant (P<0.003) sensitivity to azide, an inhibitor of mitochondrial F-type ATPase, whereas the antiFlag step removed this azide-sensitive contaminant (P<0.0005). CFTR Inhibitor 172 (Inh172) [38–40] was reported to partially inhibit CFTR ATPase function [41]. Preincubation with Inh172 significantly inhibited CFTR ATPase activity in the direct assay (P<0.002). Partially purified CFTR was significantly less sensitive to Inh172 (P<0.01), as would be expected for an impure sample.
Table 3.
Differential inhibitor sensitivities of NiNTA-purified and tandem affinity-purified CFTR preparations
| partially purified (NiNTA only) | tandem affintiy purified (NiNTA/antiFlag) | ||||
|---|---|---|---|---|---|
| inhibitor | ATPase, % of control | P vs vehicle | ATPase, % of control | P vs vehicle | P vs partially purified |
| 2 mM NaN3 {water} | 78 ± 5% (4) | 0.003 | 98 ± 3% (4) | ns | 0.0005 |
| 5 mM NaN3 {water} | 80 ± 4% (4) | 0.002 | 98 ± 3% (4) | ns | 0.0004 |
| 2 mM ouabain {0.5% DMSO} | 98 ± 4% (4) | ns | 101 ± 2% (4) | ns | ns |
| 5 mM ouabain {1.2% DMSO} | 93 ± 4% (4) | ns | 95 ± 2% (4) | ns | ns |
| 20 μM Inh172 {1% DMSO} | 90 ± 1% (4) | ns | 81 ± 5% (5) | 0.002 | 0.01 |
| 50 μM Inh172 {2.5% DMSO} | 74 ± 5% (4) | 0.008 | 64 ± 5% (5) | 0.0001 | 0.02 |
| 1% DMSO | 97 ± 6% (4) | 94 ± 2% (4) | ns | ||
| 2.5% DMSO | 91 ± 7% (4) | 85 ± 2% (4) | ns | ||
CFTR was purified from UDM extract in FA-4 by NiNTA chromatography or by tandem NiNTA/antiFlag chromatography. 1 mM ATP was included during binding to affinity matrices but omitted from chromatography buffers. Samples of NiNTA eluate (diluted 1:5 with buffer) or antiFlag eluate were mixed with 2 mg/ml C12E8-destabilized liposomes (section 2.5), then with inhibitor, and preincubated on ice for 60 min prior to substrate addition and assay as in section 2.6.1. Ouabain and Inh172 stocks were prepared in DMSO; vehicle concentrations in the assay are stated in curly brackets. Combined results of n replicate assays are shown. Control ATPase activity (no inhibitor) averaged 0.34 ± .04 nmol/h/μg for partially purified CFTR and 0.16 ± .02 nmol/h/μg for tandem affinity purified CFTR, under these submaximal assay conditions.
ns: not statistically significant
Not all detergents worked equally well in the direct assay, and this depended strongly on both detergent structure and alkyl chain length. CFTR purified in MNG10 or in facial amphiphiles showed the highest ATPase activities in this assay, while lower activities measured in the presence of other detergents suggested inhibitory effects in those cases (figure 6).
Figure 6. Purified CFTR hydrolyzes ATP in the presence of detergents.
Tandem affinity-purified CFTR samples, as eluted from antiFlag columns, were preincubated with destabilized liposomes. ATPase activities were then assayed at 0.3 mM ATP. Combined data from replicate experiments are shown as mean ± sd, with the number of repeat experiments for each detergent shown in the figure. A single batch of microsomes was used as source material for this group of experiments.
3.5 Monodispersity of CFTR purified in select detergents before and after concentration
While retention of CFTR function is important, structural characterization will require additional favorable properties such as monodispersity at high protein concentration. CFTR preparations purified in several detergents were evaluated initially at low concentration (20–30 μg/ml) by negative staining and transmission EM (figure S2). For all of the purification detergents tested, these images demonstrated little evidence of major aggregates. CFTR particle widths were similar in all detergents tested and ranged from 5–12 nm. Particles of 10–12 nm diameter have previously been identified as CFTR dimers [7, 28].
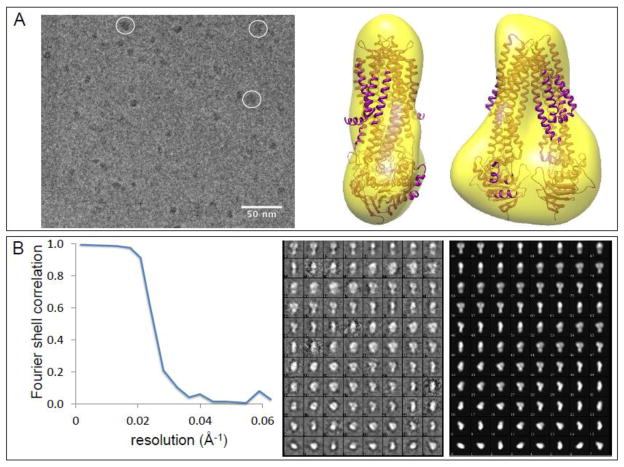
To assess behavior of CFTR at higher protein concentrations, samples of CFTR purified in MNG10 or FA-4 were concentrated by ultrafiltration to 1.8 and 3.1 mg CFTR/ml, respectively. Concentration proceeded smoothly without overt evidence of aggregation, such as cloudiness or blocking of the ultrafilters. CryoEM of these samples likewise showed little evidence of aggregation; figure 7 shows results for the sample in FA-4, and results for MNG10 were comparable. A monomeric CFTR particle, including mass of bound detergent, would be approximately 250 kD. Assuming that CFTR has a typical ABC transporter structure, monomers would approximate to cylinders 11 nm long and 6 nm in diameter. Automated particle selection and classification revealed a relatively uniform size distribution (figure 7B), with some classes having the appearance expected for monomers, whilst a few classes displayed what appeared to be two adjacent CFTR monomers. These may represent transient but favored CFTR particle associations, and were excluded from the subsequent analysis. Reconstruction of 3D volume for each dataset by standard iterative procedures generated maps (figure 7A, right) with low resolution because of the strong underfocus employed and the small particle size. In both detergents, CFTR dimensions and shape were consistent with expectations for an ABC transporter such as the structurally well characterized mitochondrial ABC transporter ABCB10 (the superimposed ribbon diagram in figure 7A) [42].
Figure 7. Purified, concentrated CFTR is predominantly monomeric.
Panel A Cryo-electron micrographs of CFTR samples purified in FA-4 were concentrated to 3.1 mg/ml and imaged in vitreous buffer. CFTR particles show predominantly monomeric particles (darker grey particles). A few examples of larger particles (circled) may represent aggregates or oligomers, and were excluded from the single particle analysis. To the right are orthogonal views of the final 3D map (yellow transparent surface) into which has been fitted the structural model of an ABC protein (ABCB10, PDB: 4ayw).
Panel B The quality of the map was assessed using Fourier shell correlation between two subsets of the particles, giving a resolution estimate of about 25 Å. To the right are shown the projection class averages derived from the raw data with no symmetry applied (center panel). These compare closely with reprojections of the final 3D map (rightmost panel).
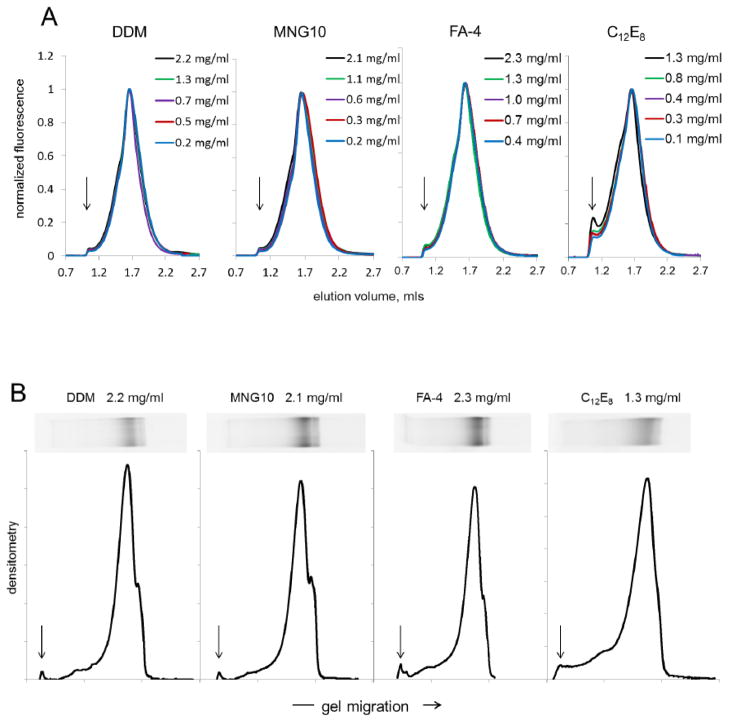
Size exclusion chromatography (SEC) detects high molecular weight complexes with high sensitivity, provided they remain in solution. CFTR partially purified in DDM, MNG10, FA-4, or C12E8 was progressively concentrated by ultrafiltration, sampling the retentates at intermediate concentrations along the way. SEC analysis with fluorimetric detection showed that, for each detergent tested, CFTR eluted predominantly in a single peak (figure 8A), with small amounts eluting as higher molecular weight multimers (leading edge shoulder) or at the void volume. This monodispersity distribution, showing only small amounts of oligomer or aggregate, was consistent with our EM results which were obtained on virtually identical samples and which clearly showed a predominance of monomer. Noticeably more CFTR appeared at the void volume when purified in C12E8, even at 0.1 mg/ml, the lowest concentration examined. With progressive concentration of this sample above 1 mg/ml, high molecular weight complexes at the void volume grew increasingly evident. In contrast, CFTR preparations purified in DDM, MNG10, or FA-4 could be concentrated above 2 mg/ml with negligible new accumulation of higher molecular weight complexes.
Figure 8. Certain detergents support concentration of CFTR above 1 mg/ml without aggregation.
Panel A Size exclusion chromatography with fluorescent detection. CFTR samples at the indicated concentrations were diluted into SEC buffer containing 0.05% MNG10 shortly before injection. The injection volume was 25 μl. All fluorescent traces were normalized to peak height for the main CFTR peak at 1.64 ml. Arrows mark the column void volume at 1.05 ml. Mouse P-glycoprotein-GFP (172 kD) purified in DDM eluted at 1.75 ml. Soluble protein standards of 669 kD and 158 kD eluted at 1.52 mls and 1.72 mls, respectively.
Panel B Portions of the most highly concentrated samples of CFTR were applied to SDS gels, and in-gel fluorescence was imaged (insets). These traces, left to right, represent densitometric profiles from the midpoint of the stacking gel to below the principal CFTR band at 212 kD. Arrows indicate the top of the separating gel. A shoulder on the right-hand side of the main peak represents under-glycosylated CFTR that copurified.
Because SEC can fail to detect severe aggregation when precipitable material does not elute from the column, we also assessed the concentrated samples using an electrophoretic mobility shift approach to reveal irreversible, SDS-resistant aggregates lodged at the top of the gel (figure 8B). Densitometric traces of fluorescent bands on the gel are shown for samples concentrated to ~1 mg/ml. Results of this mobility shift analysis largely coincided with the SEC outcome, showing pronounced CFTR monomer bands; there was significant evidence of SDS-resistant multimer or aggregated protein at the top of the separating gel only in the case of CFTR concentrated in C12E8.
3.6 Stability of CFTR above 1 mg/ml
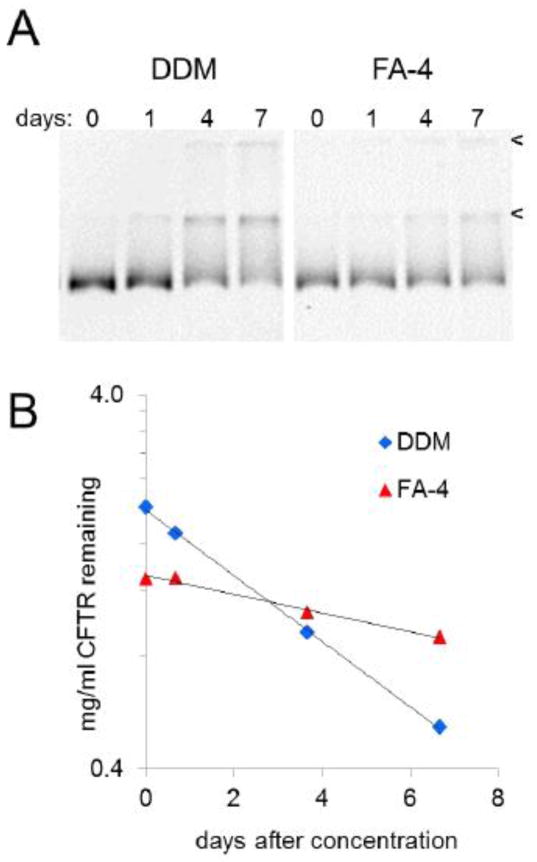
It is also important to assess stability of purified CFTR over the longer term. We implemented the facile mobility shift assay to monitor progress of CFTR aggregation over time for a larger number of detergents. Partially purified CFTR preparations in different detergents were concentrated by ultrafiltration. We took samples immediately after concentration and again at intervals during storage at 4 °C. As illustrated by the example in figure 9A, SDS-resistant complexes of CFTR accumulated with time and at different rates depending on the detergent. Evidence of proteolysis on these gels was negligible both by fluorescent imaging and by silver staining (data not shown). Using in-gel fluorescence to quantitate the CFTR monomer band vs. time, we found that its disappearance obeyed first-order kinetics (figure 9B), and this was the case for every detergent that we tested. This useful outcome provided a straightforward way to compare CFTR aggregation rates in the test detergents. Table 4 presents t1/2 values measured for aggregation of CFTR in a number of detergents. This kinetic analysis demonstrated relatively poor CFTR stability in conventional maltosides such as DDM, whereas use of MNG10 or Chaps for purification produced CFTR preparations with greater resistance to aggregation. Among the detergents tested, FA-4 provided the lowest rate of CFTR aggregation, with a t1/2 close to two weeks.
Figure 9. Apparent first-order kinetics allow measurement of CFTR aggregation rates.
CFTR was purified and concentrated in various detergents, and placed at 4 °C. Samples taken at the indicated time points were applied to SDS gels, and in-gel fluorescence was imaged. The 212 kD CFTR monomer was quantitated by densitometry. Representative results are shown for two detergents, among the detergents listed in Table 4 that were analyzed in this way.
Panel A Fluorescent image showing time-dependent conversion of CFTR monomer to higher molecular weight, SDS-resistant complexes migrating at the top of the separating gel and stacking gel, marked by arrowheads.
Panel B A semilog plot of CFTR monomer concentrations vs. time for the experiment shown in A, demonstrating apparent first order kinetics for CFTR aggregation.
Table 4.
Half-life values for aggregation of CFTR purified in various detergents
| t1/2, days (initial CFTR concentration) two experiments |
||
|---|---|---|
| 0.05% Mal 12-1 | 1.4 (0.95 mg/ml) | 2.2 (1.4 mg/ml) |
| 0.1% undecylmaltoside | 1.9 (1.2 mg/ml) | 2.7 (1.8 mg/ml) |
| 0.05% dodecylmaltoside | 3.4 (2.0 mg/ml) | 2.6 (2.0 mg/ml) |
| 0.05% tridecylmaltoside | 2.3 (1.5 mg/ml) | nd |
| 0.05% C12E8 | 3.9 (0.25 mg/ml) | 2.1 (0.70 mg/ml) |
| 0.05% Cymal 6 | 3.4 (0.51 mg/ml) | 5.0 (0.94 mg/ml) |
| 0.05% lysoPG14 | 3.1 (2.2 mg/ml) | nd |
| 0.05% lysoPC14 | 4.4 (1.5 mg/ml) | nd |
| 0.05% MNG10 | 6.4 (0.53 mg/ml) | 8.8 (1.1 mg/ml) |
| 1% Chaps | 7.4 (1.5 mg/ml) | 8.8 (1.9 mg/ml) |
| 0.05% FA-4 | 12.0 (1.3 mg/ml) | 17.0 (1.4 mg/ml) |
CFTR was partially purified by NiNTA chromatography in the indicated detergent. To improve recoveries, UDM was used for solubilization when the detergent for chromatography was lysoPG, lysoPC, Chaps or FA-4. Otherwise, a single detergent was used throughout. CFTR was then concentrated by ultrafiltration and buffer-exchanged as described in section 2.9. Samples were taken periodically over 7 days of storage at 4 °C for determination of CFTR monomer concentration by in-gel fluorescence.
nd: not done
4. Discussion
In structural and biophysical characterization of membrane proteins, detergent choice is critical. We devised a simple, rapid tandem affinity purification scheme and used this to evaluate function, monodispersity, and stability of CFTR purified in various detergents. This detergent survey focused primarily on mild detergents, including recently developed detergents and amphiphiles, and identified MNGs and novel facial amphiphiles as the most favorable for purification of active CFTR and for achieving the higher protein concentrations that will be required for its biophysical and structural analysis.
CFTR was originally purified to homogeneity from insect cells using SDS, and after lipid addition and extensive dialysis against cholate, single channel recordings were demonstrated [43]. ATP hydrolysis was later demonstrated in CFTR purified and reconstituted using the same scheme, with supporting mutant controls even though purity was not shown in these reports [36, 44]. In a direct comparison, O’Riordan et al. found similar function of CFTR prepared in that purification scheme to CFTR purified ~70% from Chinese hamster ovary (CHO) cells using lysoPC for solubilization and chromatography [16]. Introduction of the polyhistidine affinity tag enabled efficient purification of recombinant CFTR from insect cells [15, 17] or yeast [18], but still required harsh detergents like lysolipids or PFO that have not generally been found useful in structural analyses. Recombinant CFTR expression in baby hamster kidney cells or CHO cells allowed modest amounts of CFTR to be purified in active form with preferred detergents such as DDM [19, 45], but the purified protein proved aggregation-prone [28].
The development of a mammalian expression system providing high levels of human CFTR at the cell surface [EH, A Mulky, HD, QD, A Aleksandrov, B Bajrami, PA Diego, X Wu, M Ray, AP Naren, JR Riordan, X Yao, LJD, ILU and JCK, manuscript in preparation] underpinned our success in purifying active, monodisperse CFTR, for multiple reasons: (1) CFTR-enriched starting material, (2) native protein phosphorylation and glycosylation that influence CFTR folding, stabilize conformation and regulate function [13, 46, 47], (3) proven channel function and proper gating properties of the engineered protein, (4) multiple strategically placed affinity tags, and (5) a GFP domain enabling CFTR quantitation even in microsomes, crude extracts, and proteoliposomes. Here we have shown that CFTR can be readily extracted from this starting material using any of a variety of mild detergents at modest detergent:protein ratios. Minimizing detergent exposure formed the basis of our purification strategy, which prioritizes function rather than recovery. Yields of pure CFTR with MNG10 or with FA-4 averaged 71 μg or 100 μg per 109 cells, respectively, and with bioreactor cell production it should be possible to purify sufficient amounts of CFTR for biophysical characterizations using these procedures. Higher CFTR recoveries obtained using facial amphiphiles for chromatography than MNGs or other maltoside detergents suggest more facile interaction of epitope tags with affinity matrices and/or greater short term stability.
Because CFTR hydrolyzes ATP rather slowly, and because it cannot be selectively assayed, CFTR purity is essential in characterizing this function. Inhibition experiments demonstrated that the comparatively rapid antiFlag step removed contaminating azide-sensitive ATPase activity present in NiNTA-purified CFTR preparations. The combination of NiNTA with immunoaffinity chromatography produces CFTR of sufficient purity for ATPase measurement in the absence of inhibitors. Upon CFTR reconstitution into proteoliposomes, ATPase activity was 2- to 3-fold higher using facial amphiphiles for chromatography compared to MNG10.
Functional assessments of proteoliposome preparations provide limited information about the state of the protein prior to reconstitution. We also showed that CFTR purified in FA-4 or in MNG10 exhibits robust ATPase activity in the presence of lipid, without removal of detergent. This finding suggests that the conformation of the catalytic site, believed to be a composite of NBD1 and NBD2 domains [5, 10–12], remains largely intact in these detergents. Certain other detergents (e.g. UDM, Chaps) appeared to exert inhibitory effects. The direct assay is therefore most suited to carefully chosen detergents. This new streamlined assay procedure affords the opportunity to measure activity promptly, and is readily adaptable to multiwell applications, for example in testing whether mutations stabilize the protein.
Others have established the utility of SEC with protein fluorescence monitoring for profiling membrane protein polydispersity [30, 48] and to detect aggregation triggered by thermal treatment [48, 49], detergent exposure [30, 50], or other challenges to protein stability. We applied SEC in an analogous test of CFTR stability in response to the challenge of concentration by ultrafiltration in select detergents. CFTR purified in DDM, MNG10 or FA-4 exhibits good short-term stability above 1 mg/ml, but somewhat more aggregation takes place in C12E8 during the process of concentration. Results obtained by examining these samples for aggregation in our gel mobility shift assay were congruent with SEC results. Employing electron microscopic techniques, we further showed that purified CFTR preparations in MNG or in FA-4 appear predominantly monomeric and free of large protein aggregates. Unlike SEC columns that unavoidably dilute protein samples approximately 10-fold during analysis, cryoEM analysis provided information about CFTR monodispersity at original protein concentration. CryoEM also produced monomeric CFTR particle image galleries compatible with known ABC transporter structures.
The gel mobility shift assay provided a simple method to quantitate CFTR resistance to aggregation on the timescale of days. Long-term CFTR stability varies more widely among detergents, with facial amphiphiles providing the best long-term stability, followed by MNG or Chaps. Complex multistep and/or multipathway processes such as protein aggregation can and often do present apparent first order kinetics [51, 52], and indeed CFTR entry into SDS-resistant aggregates obeyed first order kinetics in many detergents. Because CFTR aggregation rates vary widely with detergent, it is reasonable to propose that aggregation may be rate-limited by destabilizing interactions with detergent. Biophysical studies showed the individual NBD domains to be highly sensitive to detergent, and nucleotide photoaffinity labeling indicated this also to be the case with full-length CFTR [14]. Nonetheless, other solution components that might contribute to instability were not investigated in the present study. Manipulation of buffer conditions should be explored to further stabilize CFTR against aggregation over the long term.
5. Conclusions
Affinity-tagged CFTR expressed in HEK293 cells can be solubilized and rapidly purified to homogeneity using mild detergents. Conditions have been identified that allow measurement of ATPase activity of purified CFTR without detergent removal. Novel facial amphiphiles and neopentyl maltoside detergents support CFTR function, monodispersity, and stability above 1 mg/ml for many days – an achievement expected to advance the field. These protocols can be used to generate samples of pure, active CFTR at concentrations amenable to biophysical characterization.
Supplementary Material
Highlights.
Detergents were screened for human CFTR purification, function and stability
Purified CFTR hydrolyzes ATP without detergent removal if supplemented with lipid
CFTR is monodisperse and can be concentrated above 1 mg/ml without aggregation
Activity and stability are greatest in neopentyl maltoside or facial amphiphiles
Acknowledgments
We thank Marjorie Ray and other members of the CFTR 3D Structure Consortium for continual insight, support and feedback. Peter C. Maloney provided encouragement and guidance in the earliest stages of this project. Doug Swartz kindly provided purified P-glycoprotein-GFP. This work was supported by Cystic Fibrosis Foundation Therapeutics grants URBATS13XX0, FORD13XX0 and DELUCA03G0; and by National Institutes of Health grants R01GM098538, P50GM073197 and R01GM095639. This study was also supported by the Virology, Genetic Sequencing and Flow Cytometry Cores of the UAB Center for AIDS Research (P30-AI-27767).
abbreviations
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CMC
critical micelle concentration
- DTT
dithiothreitol
- EM
electron microscopy
- GFP
green fluorescent protein
- HEK
human embryonic kidney cells
- Inh172
CFTR Inhibitor 172
- NBD
nucleotide binding domain
- PKA
protein kinase A
- PMSF
phenylmethylsulfonylfluoride
- SDS
sodium dodecyl sulfate
- SEC
size exclusion chromatography
Footnotes
Author contributions
EH conceived the study, devised and conducted all purification, reconstitution and ATPase assay procedures in the laboratory of ILU, and wrote the bulk of the manuscript. QZ designed amphiphiles, suggested detergents to be tested, and contributed to the manuscript. LP and YF synthesized facial amphiphiles and branched alkylmaltoside detergents. RF recorded and processed cryoelectron micrographs, generated three dimensional maps, and wrote sections of the manuscript. NC captured negatively stained electron micrographs. LJD and JCK developed, optimized, and established validity of the CFTR expressing cell lines and assisted manuscript revision. HD and QD contributed to cell line development and grew and harvested cells. ILU arranged collaborations and suggested experiments and revisions to the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strausbaugh SD, Davis PB. Cystic fibrosis: a review of epidemiology and pathobiology. Clinics in chest medicine. 2007;28:279–288. doi: 10.1016/j.ccm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Kotha K, Clancy JP. Ivacaftor treatment of cystic fibrosis patients with the G551D mutation: a review of the evidence. Therapeutic advances in respiratory disease. 2013;7:288–296. doi: 10.1177/1753465813502115. [DOI] [PubMed] [Google Scholar]
- 3.Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP, Jr, Urrutia A, Joubran J, Seepersaud S, Sussky K, Hoffman BJ, Van Goor F. Ivacaftor potentiation of multiple CFTR channels with gating mutations. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2012;11:237–245. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Hanrahan JW, Sampson HM, Thomas DY. Novel pharmacological strategies to treat cystic fibrosis. Trends in pharmacological sciences. 2013;34:119–125. doi: 10.1016/j.tips.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Riordan JR. CFTR function and prospects for therapy. Annual review of biochemistry. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 6.Ward CL, Kopito RR. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. The Journal of biological chemistry. 1994;269:25710–25718. [PubMed] [Google Scholar]
- 7.Zhang L, Aleksandrov LA, Zhao Z, Birtley JR, Riordan JR, Ford RC. Architecture of the cystic fibrosis transmembrane conductance regulator protein and structural changes associated with phosphorylation and nucleotide binding. Journal of structural biology. 2009;167:242–251. doi: 10.1016/j.jsb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Cant N, Pollock N, Ford RC. CFTR structure and cystic fibrosis. The international journal of biochemistry & cell biology. 2014 doi: 10.1016/j.biocel.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Aleksandrov L, Aleksandrov AA, Chang XB, Riordan JR. The First Nucleotide Binding Domain of Cystic Fibrosis Transmembrane Conductance Regulator Is a Site of Stable Nucleotide Interaction, whereas the Second Is a Site of Rapid Turnover. The Journal of biological chemistry. 2002;277:15419–15425. doi: 10.1074/jbc.M111713200. [DOI] [PubMed] [Google Scholar]
- 10.Hunt JF, Wang C, Ford RC. Cystic fibrosis transmembrane conductance regulator (ABCC7) structure. Cold Spring Harbor perspectives in medicine. 2013;3:a009514. doi: 10.1101/cshperspect.a009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang TC, Sheppard DN. Gating of the CFTR Cl- channel by ATP-driven nucleotide-binding domain dimerisation. The Journal of physiology. 2009;587:2151–2161. doi: 10.1113/jphysiol.2009.171595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozoky Z, Krzeminski M, Chong PA, Forman-Kay JD. Structural changes of CFTR R region upon phosphorylation: a plastic platform for intramolecular and intermolecular interactions. The FEBS journal. 2013;280:4407–4416. doi: 10.1111/febs.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Wang C, Zhou Q, An J, Hildebrandt E, Aleksandrov LA, Riordan JR, Urbatsch IL, Hunt JF, Brouillette CG. Membrane protein stability can be compromised by detergent interactions with the extramembranous soluble domains. Protein Science. 2014;23:769–89. doi: 10.1002/pro.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramjeesingh M, Li C, Garami E, Huan LJ, Hewryk M, Wang Y, Galley K, Bear CE. A novel procedure for the efficient purification of the cystic fibrosis transmembrane conductance regulator (CFTR) The Biochemical journal. 1997;327(Pt 1):17–21. doi: 10.1042/bj3270017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Riordan CR, Erickson A, Bear C, Li C, Manavalan P, Wang KX, Marshall J, Scheule RK, McPherson JM, Cheng SH, et al. Purification and characterization of recombinant cystic fibrosis transmembrane conductance regulator from Chinese hamster ovary and insect cells. The Journal of biological chemistry. 1995;270:17033–17043. doi: 10.1074/jbc.270.28.17033. [DOI] [PubMed] [Google Scholar]
- 17.Ketchum CJ, Rajendrakumar GV, Maloney PC. Characterization of the adenosinetriphosphatase and transport activities of purified cystic fibrosis transmembrane conductance regulator. Biochemistry. 2004;43:1045–1053. doi: 10.1021/bi035382a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang P, Liu Q, Scarborough GA. Lysophosphatidylglycerol: a novel effective detergent for solubilizing and purifying the cystic fibrosis transmembrane conductance regulator. Analytical biochemistry. 1998;259:89–97. doi: 10.1006/abio.1998.2633. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg MF, Kamis AB, Aleksandrov LA, Ford RC, Riordan JR. Purification and crystallization of the cystic fibrosis transmembrane conductance regulator (CFTR) The Journal of biological chemistry. 2004;279:39051–39057. doi: 10.1074/jbc.M407434200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Tao H, Hong WX. New amphiphiles for membrane protein structural biology. Methods. 2011;55:318–323. doi: 10.1016/j.ymeth.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClure M, DeLucas LJ, Wilson L, Ray M, Rowe SM, Wu X, Dai Q, Hong JS, Sorscher EJ, Kappes JC, Barnes S. Purification of CFTR for mass spectrometry analysis: identification of palmitoylation and other post-translational modifications. Protein engineering, design & selection: PEDS. 2012;25:7–14. doi: 10.1093/protein/gzr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chae PS, Rasmussen SG, Rana RR, Gotfryd K, Chandra R, Goren MA, Kruse AC, Nurva S, Loland CJ, Pierre Y, Drew D, Popot JL, Picot D, Fox BG, Guan L, Gether U, Byrne B, Kobilka B, Gellman SH. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nature methods. 2010;7:1003–1008. doi: 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbatsch IL, al-Shawi MK, Senior AE. Characterization of the ATPase activity of purified Chinese hamster P-glycoprotein. Biochemistry. 1994;33:7069–7076. doi: 10.1021/bi00189a008. [DOI] [PubMed] [Google Scholar]
- 24.Drew D, Lerch M, Kunji E, Slotboom DJ, de Gier JW. Optimization of membrane protein overexpression and purification using GFP fusions. Nature methods. 2006;3:303–313. doi: 10.1038/nmeth0406-303. [DOI] [PubMed] [Google Scholar]
- 25.Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- 26.Geertsma ER, Nik Mahmood NA, Schuurman-Wolters GK, Poolman B. Membrane reconstitution of ABC transporters and assays of translocator function. Nature protocols. 2008;3:256–266. doi: 10.1038/nprot.2007.519. [DOI] [PubMed] [Google Scholar]
- 27.Rigaud JL, Levy D, Mosser G, Lambert O. Detergent removal by non-polar polystyrene beads - Applications to membrane protein reconstitution and two-dimensional crystallization. Eur Biophys J Biophy. 1998;27:305–319. [Google Scholar]
- 28.Zhang L, Aleksandrov LA, Riordan JR, Ford RC. Domain location within the cystic fibrosis transmembrane conductance regulator protein investigated by electron microscopy and gold labelling. Biochimica et biophysica acta. 2011;1808:399–404. doi: 10.1016/j.bbamem.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Cong Y, Ludtke SJ. Single particle analysis at high resolution. Methods in enzymology. 2010;482:211–235. doi: 10.1016/S0076-6879(10)82009-9. [DOI] [PubMed] [Google Scholar]
- 30.Kawate T, Gouaux E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 2006;14:673–681. doi: 10.1016/j.str.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Hong WX, Baker KA, Ma X, Stevens RC, Yeager M, Zhang Q. Design, synthesis, and properties of branch-chained maltoside detergents for stabilization and crystallization of integral membrane proteins: human connexin 26. Langmuir: the ACS journal of surfaces and colloids. 2010;26:8690–8696. doi: 10.1021/la904893d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SC, Bennett BC, Hong WX, Fu Y, Baker KA, Marcoux J, Robinson CV, Ward AB, Halpert JR, Stevens RC, Stout CD, Yeager MJ, Zhang Q. Steroid-based facial amphiphiles for stabilization and crystallization of membrane proteins. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E1203–1211. doi: 10.1073/pnas.1221442110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Vendittis E, Palumbo G, Parlato G, Bocchini V. A fluorimetric method for the estimation of the critical micelle concentration of surfactants. Analytical biochemistry. 1981;115:278–286. doi: 10.1016/0003-2697(81)90006-3. [DOI] [PubMed] [Google Scholar]
- 34.Aleksandrov AA, Riordan JR. Regulation of CFTR ion channel gating by MgATP. FEBS letters. 1998;431:97–101. doi: 10.1016/s0014-5793(98)00713-3. [DOI] [PubMed] [Google Scholar]
- 35.Logan J, Hiestand D, Daram P, Huang Z, Muccio DD, Hartman J, Haley B, Cook WJ, Sorscher EJ. Cystic fibrosis transmembrane conductance regulator mutations that disrupt nucleotide binding. The Journal of clinical investigation. 1994;94:228–236. doi: 10.1172/JCI117311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Ramjeesingh M, Wang W, Garami E, Hewryk M, Lee D, Rommens JM, Galley K, Bear CE. ATPase activity of the cystic fibrosis transmembrane conductance regulator. The Journal of biological chemistry. 1996;271:28463–28468. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- 37.Poolman B, Doeven MK, Geertsma ER, Biemans-Oldehinkel E, Konings WN, Rees DC. Functional analysis of detergent-solubilized and membrane-reconstituted ATP-binding cassette transporters. Methods in enzymology. 2005;400:429–459. doi: 10.1016/S0076-6879(05)00025-X. [DOI] [PubMed] [Google Scholar]
- 38.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. The Journal of clinical investigation. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caci E, Caputo A, Hinzpeter A, Arous N, Fanen P, Sonawane N, Verkman AS, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Evidence for direct CFTR inhibition by CFTR(inh)-172 based on Arg347 mutagenesis. The Biochemical journal. 2008;413:135–142. doi: 10.1042/BJ20080029. [DOI] [PubMed] [Google Scholar]
- 40.Kopeikin Z, Sohma Y, Li M, Hwang TC. On the mechanism of CFTR inhibition by a thiazolidinone derivative. The Journal of general physiology. 2010;136:659–671. doi: 10.1085/jgp.201010518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wellhauser L, Kim Chiaw P, Pasyk S, Li C, Ramjeesingh M, Bear CE. A small-molecule modulator interacts directly with deltaPhe508-CFTR to modify its ATPase activity and conformational stability. Molecular pharmacology. 2009;75:1430–1438. doi: 10.1124/mol.109.055608. [DOI] [PubMed] [Google Scholar]
- 42.Shintre CA, Pike AC, Li Q, Kim JI, Barr AJ, Goubin S, Shrestha L, Yang J, Berridge G, Ross J, Stansfeld PJ, Sansom MS, Edwards AM, Bountra C, Marsden BD, von Delft F, Bullock AN, Gileadi O, Burgess-Brown NA, Carpenter EP. Structures of ABCB10, a human ATP-binding cassette transporter in apo- and nucleotide-bound states. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9710–9715. doi: 10.1073/pnas.1217042110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bear CE, Li CH, Kartner N, Bridges RJ, Jensen TJ, Ramjeesingh M, Riordan JR. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR) Cell. 1992;68:809–818. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- 44.Ramjeesingh M, Li C, Garami E, Huan LJ, Galley K, Wang Y, Bear CE. Walker mutations reveal loose relationship between catalytic and channel-gating activities of purified CFTR (cystic fibrosis transmembrane conductance regulator) Biochemistry. 1999;38:1463–1468. doi: 10.1021/bi982243y. [DOI] [PubMed] [Google Scholar]
- 45.Aleksandrov AA, Aleksandrov L, Riordan JR. Nucleoside triphosphate pentose ring impact on CFTR gating and hydrolysis. FEBS letters. 2002;518:183–188. doi: 10.1016/s0014-5793(02)02698-4. [DOI] [PubMed] [Google Scholar]
- 46.Chang XB, Mengos A, Hou YX, Cui L, Jensen TJ, Aleksandrov A, Riordan JR, Gentzsch M. Role of N-linked oligosaccharides in the biosynthetic processing of the cystic fibrosis membrane conductance regulator. Journal of cell science. 2008;121:2814–2823. doi: 10.1242/jcs.028951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glozman R, Okiyoneda T, Mulvihill CM, Rini JM, Barriere H, Lukacs GL. N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. The Journal of cell biology. 2009;184:847–862. doi: 10.1083/jcb.200808124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattori M, Hibbs RE, Gouaux E. A fluorescence-detection size-exclusion chromatography-based thermostability assay for membrane protein precrystallization screening. Structure. 2012;20:1293–1299. doi: 10.1016/j.str.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mancusso R, Karpowich NK, Czyzewski BK, Wang DN. Simple screening method for improving membrane protein thermostability. Methods. 2011;55:324–329. doi: 10.1016/j.ymeth.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonoda Y, Newstead S, Hu NJ, Alguel Y, Nji E, Beis K, Yashiro S, Lee C, Leung J, Cameron AD, Byrne B, Iwata S, Drew D. Benchmarking membrane protein detergent stability for improving throughput of high-resolution X-ray structures. Structure. 2011;19:17–25. doi: 10.1016/j.str.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bandstra JZ, Tratnyek PG. Central limit theorem for chemical kinetics in complex systems. J Math Chem. 2005;37:409–422. [Google Scholar]
- 52.Wang W, Nema S, Teagarden D. Protein aggregation--pathways and influencing factors. International journal of pharmaceutics. 2010;390:89–99. doi: 10.1016/j.ijpharm.2010.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.