Abstract
Introduction:
The use of self-administered carbamide peroxide bleaching gels has become increasingly popular for whitening of discolored vital teeth. Studies have reported that its use may induce increased levels of sensitivity and surface roughness of the tooth due to demineralization. This study evaluates the effect of fluoride addition to the bleaching agent – its remineralizing capacity and alterations in the whitening properties.
Materials and Methods:
Twenty-four extracted lower third molar teeth, with the pretreatment shade determined, were taken up in the study. Each tooth was sectioned into four and labeled as groups A, B, C, and D. The tooth quadrants in group A-C were demineralized; groups A and B were treated with 10% carbamide peroxide gel (group-A without fluoride and group-B with 0.463% fluoride addition) (no further treatment was carried out for group c) group-D remained as the control. The post-treatment shade was determined. The tooth samples were sectioned (approximately 200 μm) for evaluation under a light microscope. The depth of demineralization was analyzed at five different equidistant points. Statistical analysis was carried out with t-tests, accepting ≤0.05 as significant.
Results and Conclusion:
Addition of fluoride caused remineralization of demineralized enamel. The tooth whitening system showed that the remineralization properties did not affect the whitening properties.
Keywords: Carbamide peroxide whitening gel, demineralization, remineralization, white spot lesions
INTRODUCTION
The population demands with regard to dental appearance are greater than ever today. Treating discolored teeth by the conservative esthetic approach with non-restorative procedures is an area under rapid development. There are a plethora of causes of tooth discoloration and they are varied and complex. Tooth discolorations can be broadly classified as extrinsic or intrinsic.[1] A third category of ‘stain internalization’ has recently been described, to include those circumstances where the extrinsic stain enters the tooth through defects in the tooth structure.[2]
At present, there are several methods to treat discolored teeth such as bleaching, laminates, veneers, microabrasion, and porcelain jacket crowns. Bleaching as a treatment modality continues to hold its century old place as the simplest, most common, least invasive, and least expensive means to lighten the teeth.[3] 10-22% carbamide peroxide is the most commonly used home bleaching products.[4]
The effect of the whitening treatment on dental hard tissues has long been a concern of dentists. Bleaching with 10% carbamide peroxide decreased the mineral content of enamel calcium, phosphate, and fluoride, altering the chemical, structural, and mechanical properties, causing demineralization. There is also loss of enamel microhardness as carbamide peroxide gels are not limited to the enamel surface alone, but can also be detected in the subsurface within in the outermost layer.[5,6] Bleaching affects not only the inorganic, but also the organic components of the enamel and there is increased susceptibility to erosion, reduced fracture stability or decreased abrasion resistance of the bleached dental hard tissues.[7,8]
There is evidence that enamel surface alterations caused by whitening treatments are more susceptible to carious lesion formation. It is reasonable to suggest that a higher permeability of bleached enamel and increased number of cariogenic bacteria adhering to bleached enamel surface would promote caries formation.[9] An incidence of tooth sensitivity of 9-100% has been noted with different bleaching methods, but more commonly in the 60% range. Tooth sensitivity followed by bleaching, prevents the patient from completing the full course of treatment.[9,10]
The whitening system that also fights decay is of a higher value than the one that only whitens teeth.[11] Use of fluorides during or after bleaching has been shown to be beneficial, as it forms a calcium fluoride layer on the enamel, which inhibits demineralization and promotes remineralization.[12] The addition of fluoride has also been shown to decrease tooth sensitivity often associated with whitening, while not significantly changing the efficacy of the gel used.[13]
The effect of carbamide peroxide on white spot lesions is another area of interest. The effect of carbamide peroxide gels on white spot lesions and early erosions is not fully understood and neither is the capability of fluoridated carbamide peroxide gels in recovering the enamel microhardness and strength. The present study evaluated the effect of unfluoridated and fluoridated 10% carbamide peroxide gels on the remineraliztion of demineralized enamel (initial caries-like lesions) and its affect on whitening.
MATERIALS AND METHODS
Twenty-four freshly extracted third molars, without any restorations, fractures, dental fluorosis, detectable enamel enamel or dentinal defects like attrition, abrasion, abfraction, erosion are selected and stored in phosphate buffer saline solution (PBS). The pretreatment shade was determined by using the VITA 3D MASTER shade guide (Vita Zahnfabrick, Bad Säckingen, Germany) by one observer, using natural light, to avoid metamerism. The teeth were sectioned into quadrants both mesiodistally and labiolingually, using a slow speed dental hand piece and diamond sectioning disk [Figure 1]. Once sectioned, one quarter of each tooth was randomly assigned and designated as belonging to either group A, B, C or D. This experimental approach allowed each tooth to serve as its own control.
Figure 1.
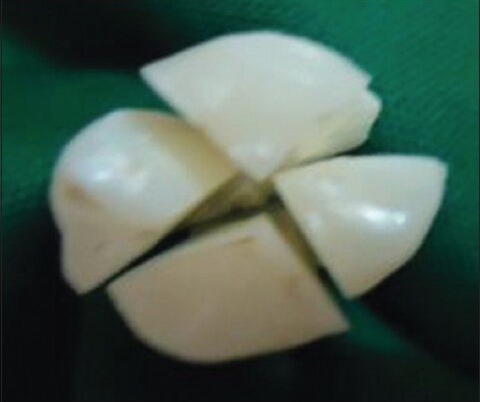
Sectioning of the tooth (Tooth sectioned into four quadrants)
Experimental groups A, B, and C were exposed to a demineralizing solution (10% (w/v) methylcellulose gel acidified with 0.1 m lactic acid-sodium lactate, at pH 4.5, having a hydroxyapatite content of 0.05%(w/v)). Group D served as a control, without any treatment. After one week, the tooth quadrants of groups A, B, and C were removed from the demineralizing solution and rinsed with deionized water. After demineralization bleaching treatment was continued using 10% carbamide peroxide (VivaStyle, Ivoclar Vivadent AC).
Group A: (Demineralized + 10% carbamide peroxide treated)
The tooth samples were treated for eight hours per day for 21 days with 10% carbamide peroxide gel.
Group B: (Demineralized + 0.463% fluoridated 10% carbamide peroxide treated)
The tooth samples were treated for eight per day for 21 days with 0.463% sodium fluoride added to 10% carbamide peroxide whitening gel.
The tooth samples were cleaned and stored in PBS solution for 16 hours, after eight hours of the whitening treatment. The whitening gel treatment was then repeated for 21 days.
Group C: (Demineralization)
After demineralization of the tooth samples for one week, they were replaced in the PBS solution, with no further treatment.
Group D: (Control group)
No treatment was done and they remained as the control group, placed in PBS solution.
The final shade determination was made after 21 days of treatment [Figure 2]. The samples were mounted in acrylic resin blocks. Next, the sections were ground to a thickness of 250 μ using a hard tissue microtome (Leica Sp 1600), for viewing under a Polarizing Microscope. The images were captured using a 4× objective and 10× eyepiece magnification. The depth of the remineralization and demineralization areas, in micrometers, were carefully measured on the enamel surface of the samples at five equidistant points and recorded with a digital image analysis system that was connected to the Polarizing Microscope and personal computer [Figures 3 and 4]. The average of the five equidistant points was taken to obtain the final measurement. The results were expressed in microns and were subjected to statistical analysis by a paired t test; P < 0.05 was considered as the level of significance.
Figure 2.
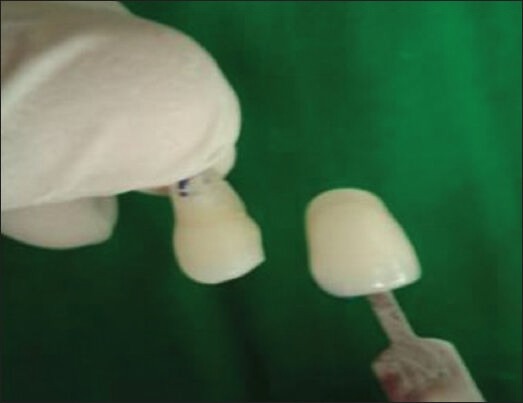
Post treatment shade determination (Shade after bleaching treatment)
Figure 3.
Control group viewed under microscope. sample without any treatment
Figure 4.

Experimental group viewed under microscope. Sample after demineralization and bleaching treatment
RESULTS
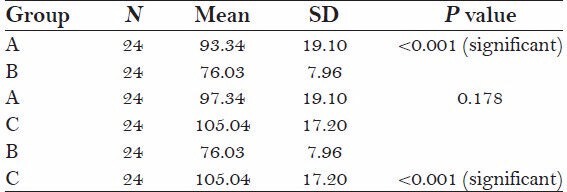
The test of significance by a paired t test showed that there was a significant difference in the mean depth of the three study groups (P < 0.001).
The study showed that the mean value of group A (97.34 ± 19.10) and C (105.04 ± 17) had a significantly higher mean depth than group B (76.03 ± 19.10) P < 0.05
There was no significant mean depth of difference between groups A (97.34 ± 19.10) and C (105.04 ± 17) P > 0.05.
The mean value in Group C (105.04 ± 17) is the highest followed by Group A (97.34 ± 19.10), Group B (76.03 ± 19.10).
DISCUSSION
Discoloration of the anterior teeth may have a psychological impact on individuals and this drives them to a dentist for esthetic treatment.[14] When using external bleaching techniques, there is no need to prepare the teeth so that the enamel and dentin structures remain largely untouched. Home bleaching was developed as a conservative and safe method to reduce chairside time and to minimize the gingival irritation and pulpal reaction, often associated with in-office bleaching.[15] Carbamide peroxide gel is used in conjunction with a soft mouth guard for overnight bleaching and this procedure is called as ‘Night-guard vital bleaching’ or ‘Home bleaching’.[16]
Comparison of mean demineralization (microns) among the different study groups
Graph.
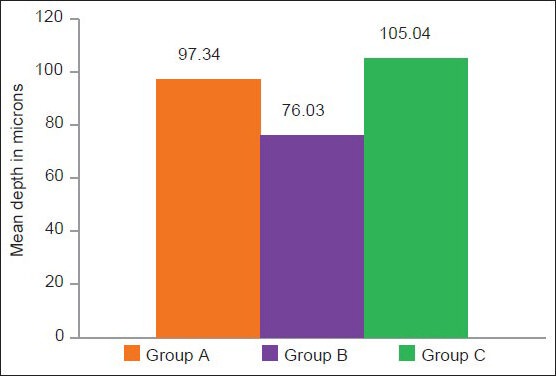
The mean demineralization in different study groups A, B, and C
Carbamide peroxide is a urea containing 3.4% hydrogen peroxide. Carbamide peroxide is an unstable compound, which breaks down into hydrogen peroxide and urea.[17] Hydrogen peroxide is a strong oxidizing agent. It generates free radicals, and these hydroxyl radicals, which are highly reactive, electrophilic, and unstable, disrupt the electron conjugation and change the absorption energy of the molecule, which can result in a change into a lighter spectrum of the compound.[18]
Ten percent carbamide peroxide has been considered as the standard for bleaching although varying concentrations of carbamide peroxide ranging from 2-30% have been tried.[19] Ten percent carbamide peroxide, apart from successfully whitening the teeth, affects the microstructure, reduces hardness, and causes demineralization of the enamel.[20] It has been reported that urea, which breaks down into carbon-di-oxide and ammonia, may affect the interprismatic regions on the enamel, which denatures the protein structure and may cause structural and morphological alterations of the enamel through degradation of the organic molecules, such as, amelogenin.[21] As a result of demineralization of the enamel, carbamide peroxide bleaching systems may cause tooth sensitivity and gingival irritation, which varies from patient to patient.[5,22]
It is reasonable to suggest that a higher permeability of bleached enamel and an increased number of cryogenic bacteria adhering to bleached enamel surface would promote caries.[8] The effect of 10% carbamide peroxide on initial enamel caries (white spot lesions) would be more detrimental. Porous enamel of the white spot lesion allows deep penetration of the bleaching gel, which affects the subsurface layers. Although saliva increases the microhardness of the bleached enamel by providing calcium and phosphate ions, rehardening is not always complete. Remineralizing agents such as Fluorides, CPP-ACP, Potassium nitrate, NovaMin, Sorbitol, and Xylitol are used after bleaching. The susceptibility of bleached enamel to demineralization is lower for enamel bleached with a fluoride-containing carbamide peroxide bleaching gel.[23,24]
In the present study 0.463% fluoride was directly added to bleaching agent, to identify its effect of remineralization on white spot lesions and its influence on tooth whitening, which would be beneficial for the patients in many aspects like reduction of post bleaching sensitivity, caries susceptibility, and saving the patient's time, with no further treatment following bleaching.
Kumar et al.,[25] analyzed the depth of demineralization and they found it to be 167-168 μ with 15% carbamide peroxide. The present study showed a demineralization depth of 97.34-105.04 μ, with 10% carbamide peroxide. The demineralization and surface alteration of the bleached enamel may be attributed to the pH and viscosity of the bleaching agent and the prolonged contact time between the bleaching agents and the enamel.
The results of the present study using fluoride as a remineralizing agent in group B, which was added to the bleaching gel, showed an improvement in the mineral content of the demineralized enamel and confirmed to the previous reported literature.[3,26] Samples with white spot lesions not only improved their shade, but also remineralized, with less depth of demineralization compared with the untreated white spot lesions. Irregular surface enamel of the white spot lesion allowed more fluoride to enter the deep layers to form calcium fluoride. The average depth of the lesion was 76.03 μm in group B, less compared to the other groups; in group A it was 97.34 μm and in group B 105.04 μm, which was statistically significant. There was no significant difference in the depth of demineralization between group A (demineralization + carbamide peroxide treatment) and group C (only demineralization).
Attin et al., found that fluoridated bleaching gels rehardened the bleached enamel faster than non-fluoridated gels. The teeth treated with a fluoridated bleaching agent were also reported to be with higher caries resistance compared to those without a fluoridated bleaching treatment. The findings of these investigations appeared to support the continued use of fluoridated bleaching agents.[24,27]
CONCLUSION
The present study supported the addition of fluoride to bleaching gel. Fluoride of 0.463%, added to 10% carbamide peroxide gel, showed a remineralizing effect on demineralized enamel (white spot lesions) compared to unfluoridated 10% carbamide peroxide gel. Fluoride added to 10% carbamide peroxide gel did not affect the whitening properties of the gel. Both fluoridated and unfluoridated gels have shown similar whitening properties.
ACKNOWLEDGMENTS
Financial interests, direct or indirect, for any of the authors do not exist for this article. Sources of outside support of the project are nil for this article.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Watts A, Addy M. Tooth discoloration and staining: A review of the literature. Br Dent J. 2001;190:309–16. doi: 10.1038/sj.bdj.4800959. [DOI] [PubMed] [Google Scholar]
- 2.Addy M, Moran J. Mechanisms of stain formation on teeth, in particular associated with metal ions and antiseptics. Adv Dent Res. 1995;9:450–6. [Google Scholar]
- 3.Bizhang M, Seemann R, Duve G, Römhild G, Altenburger JM, Jahn KR, et al. Demineralization effects of 2 bleaching procedures on enamel surfaces with and without post-treatment fluoride application. Oper Dent. 2006;31:705–9. doi: 10.2341/05-144. [DOI] [PubMed] [Google Scholar]
- 4.Hayman VB. Nightguard vital bleaching: Current information and research. Esthet Dent Update. 1990;1:20–5. [Google Scholar]
- 5.Wiegand M, Schreier M, Attin T. Effect of different fluoridation regimes on the microhardness of bleached enamel. Oper Dent. 2007;32:610–5. doi: 10.2341/06-171. [DOI] [PubMed] [Google Scholar]
- 6.Attin T, Müller A, Patyk A, Lennon AM. Influence of different bleaching systems on fracture toughness and hardness of enamel. Oper Dent. 2004;29:188–95. [PubMed] [Google Scholar]
- 7.Hegedüs C, Bistey T, Flóra-Nagy E, Keszthelyi G, Jenei A. An atomic force microscopy study on the effect of bleaching agents on enamel surface. J Dent. 1999;27:509–15. doi: 10.1016/s0300-5712(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 8.Gürgan S, Bolay S, Alaçam R. In vitro adherence of bacteria to bleached or unbleached enamel surfaces. J Oral Rehabil. 1997;24:624–7. doi: 10.1046/j.1365-2842.1997.00534.x. [DOI] [PubMed] [Google Scholar]
- 9.Tam L. Clinical trial of three 10% carbamide peroxide bleaching products. J Can Dent Assoc. 1999;65:201–5. [PubMed] [Google Scholar]
- 10.Leonard RH., Jr Efficacy, longevity, side effects, and patient perceptions of nightguard vital bleaching. Compend Contin Educ Dent. 1998;19:766–70. 772, 774, passim. [PubMed] [Google Scholar]
- 11.Gladwell J, Simmons D, Wright JT. Remineralization potential of a fluoridated carbamide peroxide whitening gel. J Esthet Restor Dent. 2006;18:206–13. doi: 10.1111/j.1708-8240.2006.00021_1.x. [DOI] [PubMed] [Google Scholar]
- 12.Attin T, Kielbassa AM, Schwanenberg M, Hellwig E. Effect of fluoride treatment on remineralization of bleached enamel. J Oral Rehabil. 1997;24:282–6. doi: 10.1046/j.1365-2842.1997.d01-291.x. [DOI] [PubMed] [Google Scholar]
- 13.Attin T, Kocabiyik M, Buchalla W, Hannig C, Becker K. Susceptibility of enamel surfaces to demineralization after application of fluoridated carbamide peroxide gels. Caries Res. 2003;37:93–9. doi: 10.1159/000069015. [DOI] [PubMed] [Google Scholar]
- 14.Kihn PW. Vital tooth whitening. Dent Clin North Am. 2007;51:319–31. doi: 10.1016/j.cden.2006.12.001. viii. [DOI] [PubMed] [Google Scholar]
- 15.Brunton PA, Ellwood R, Davies R. A six-month study of two self-applied tooth whitening products containing carbamide peroxide. Oper Dent. 2004;29:623–6. [PubMed] [Google Scholar]
- 16.Zekonis R, Matis BA, Cochran MA, Al Shetri SE, Eckert GJ, Carlson TJ. Clinical evaluation of in-office and at-home bleaching treatments. Oper Dent. 2003;28:114–21. [PubMed] [Google Scholar]
- 17.Lopes GC, Bonissoni L, Baratieri LN, Vieira LC, Monteiro S., Jr Effect of bleaching agents on the hardness and morphology of enamel. J Esthet Restor Dent. 2002;14:24–30. doi: 10.1111/j.1708-8240.2002.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 18.Seghi RR, Denry I. Effects of external bleaching of indentation and abrasion characteristics of human enamel in vitro. J Dent Res. 1992;71:1340–4. doi: 10.1177/00220345920710061201. [DOI] [PubMed] [Google Scholar]
- 19.Metz MJ, Cochran MA, Matis BA, Gonzalez C, Platt JA, Pund MR. Clinical evaluation of 15% carbamide peroxide on the surface microhardness and shear bond strength of human enamel. Oper Dent. 2007;32:427–36. doi: 10.2341/06-142. [DOI] [PubMed] [Google Scholar]
- 20.Leonard RH, Sharma A, Haywood VB. Use of different concentrations of carbamide peroxide for bleaching teeth: An in vitro study. Quintessence Int. 1998;29:503–7. [PubMed] [Google Scholar]
- 21.Cadenaro M, Breschi L, Nucci C, Antoniolli F, Visintini E, Prati C, et al. Effect of two in-office whitening agents on the enamel surface in vivo: A morphological and non-contact profilometric study. Oper Dent. 2008;33:127–34. doi: 10.2341/07-89. [DOI] [PubMed] [Google Scholar]
- 22.Yeh ST, Su Y, Lu YC, Lee SY. Surface changes and acid dissolution of enamel after carbamide peroxide bleach treatment. Oper Dent. 2005;30:507–15. [PubMed] [Google Scholar]
- 23.Attin T, Albrecht K, Becker K, Hannig C, Wiegand A. Influence of carbamide peroxide on enamel fluoride uptake. J Dent. 2006;34:668–75. doi: 10.1016/j.jdent.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Attin T, Betke H, Schippan F, Wiegand A. Potential of fluoridated carbamide peroxide gels to support post-bleaching enamel re-hardening. J Dent. 2007;35:755–9. doi: 10.1016/j.jdent.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Kumar VL, Itthagarun A, King NM. The effect of casein phosphopeptide-amorphorus calcium phosphate on remineralization of artificial caries-like lesions: An in vitro study. Aust Dent J. 2008;53:34–40. doi: 10.1111/j.1834-7819.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 26.Potocnik I, Kosec L, Gaspersic D. Effect of 10% carbamide peroxide bleaching gel on enamel microhardness, microstructure, and mineral content. J Endod. 2000;26:203–6. doi: 10.1097/00004770-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Al-Qunaian TA. The effect of whitening agents on caries susceptibility of human enamel. Oper Dent. 2005;30:265–70. [PubMed] [Google Scholar]


