Abstract
Purpose:
The objective of the present study was to assess the oral hygiene and gingival health status among Yemeni children with Down syndrome.
Materials and Methods:
The study sample comprised 101 children with Down syndrome attending special needs schools in Sana’a, Yemen. The calculus index (CI), plaque index (PI), and the gingival index (GI) were used to assess oral hygiene and gingival health status.
Results:
All subjects had gingivitis; the mean CI, PI, and GI scores were 0.58 ± 0.61, 1.45 ± 0.57, and 1.54 ± 0.64, respectively, with no significant difference found across gender. Stepwise linear regression analysis revealed that the best predictors in the descending order for CI were age and mother's education, and the best predictors for PI were IQ level, age, and father's education. Having severe mental retardation, older age, less educated parents were the most important predictors for poor gingival health status.
Conclusions:
These findings show that children with Down syndrome have poor oral hygiene and high levels of periodontal diseases. Hence, appropriate oral health education should be tailored to the needs of these children with the support of their teachers and parents.
Keywords: Down syndrome, gingival health, oral hygiene, Yemen
INTRODUCTION
Down syndrome (DS), or trisomy 21, represents the most common chromosomal abnormality associated with intellectual impairment.[1,2] Clinically, it is characterized by generalized hypotonia, neurological changes, structural cardiopathy, respiratory problems and a greater risk of infection, dental anomalies, and orofacial dysmorphology, which therefore requires special attention in the dental treatment of these patients.[1,3,4]
Periodontal disease is the most significant oral health problem in people with DS. Manual dexterity difficulties may lead to oral hygiene problems, which may result in accumulation of plaque and debris, hence favoring development of gingivitis and periodontal disease. Consequently, a large number of young people with DS lose their permanent anterior teeth in their early teens.[5] Although children and young adults with DS tend to have fewer caries because of some associated conditions such as delayed eruption of primary and permanent teeth, congenitally missing teeth, and microdontia, some may have increased risk of periodontal disease due to cariogenic food choices and reduced food clearance from the mouth.[5]
Individuals with DS need more assistance from caretakers with their daily oral health care. A 3-month supervised toothbrushing program conducted twice a week on Kuwaiti children with DS was evaluated; results showed that the mean plaque score decreased from 1.93 to 0.95 (P < 0.001) and the mean gingival score decreased from 2.00 to 0.83 (P < 0.001).[6]
In Yemen, there is no information available regarding oral health status of children with DS. Such information would enable pediatric dentists to plan and provide appropriate preventive protocol as well as effective treatment for these underprivileged children. Therefore, this study was planned and conducted to assess the oral hygiene and gingival health status of children with DS attending special needs schools in Sana’a, and also to determine the association between these outcomes and socio-demographic and clinical variables.
MATERIALS AND METHODS
This descriptive cross-sectional study, conducted between November 2013 and January 2014, included all children with DS attending institutions for individuals with special needs in Sana’a, Yemen. At the time this study was carried out, there were 115 children with DS attending three schools for mentally disabled individuals. The inclusion criteria implemented were: (1) cytogenetic diagnosis of trisomy 21, (2) adequate cooperation from the children, and (3) informed consent from the children's legal representatives. The exclusion criteria were detrimental systemic diseases, compound disability, and extremely uncooperative children. The total eligible sample comprised 101 DS children aged between 6 and 16 years.
Ethical approval for conducting the study was obtained from the Research and Ethics Committee of the Faculty of Medicine and Health Sciences, Sana’a University. Consent for participating in the study was obtained from parents and head teachers of the schools.
Clinical examination was carried out by a single pre-calibrated examiner (Al-Sufyani) in the respective institutions using artificial light, plain mouth mirror, and periodontal probe. The gingival health status of the subjects was evaluated using the gingival index (GI) of Loe and Silness,[7] while the calculus index (CI) of Ramfjord[8] and the plaque index (PI) of Silness and Loe[9] were used to ascertain oral hygiene status. Prior to conducting dental examination, demographic information was obtained from each subject on the following: Date of birth, age, school, gender, and residence. Information regarding oral hygiene practices, dietary habits, and the parent's educational background was provided by participants’ parents. Intelligence Quotient (IQ) level was recorded for each subject. This IQ had been determined prior to placing the children in schools by educational diagnosticians involved in the assessment of mentally handicapped children. According to their IQ value, children were classified into three groups: Mild (IQ 50-70), moderate (IQ 35-50), and severe (IQ ≤ 35).
SPSS version 20.0 was used for data entry and analysis. Qualitative data were presented as frequencies and percentages, while quantitative data were presented as means and standard deviations. The categorical outcomes were analyzed by Chi-square tests, while the quantitative outcomes (CI, GI, and PI) were analyzed by either t-test or analysis of variance (ANOVA), as appropriate.
Stepwise multiple linear regression analyses were executed to analyze the association between various socio-demographic and clinical variables (age, sex, frequency of teeth brushing, parents’ education background, IQ level) and the oral hygiene indicators (CI, PI, GI). A significance level of P < 0.05 was considered.
RESULTS
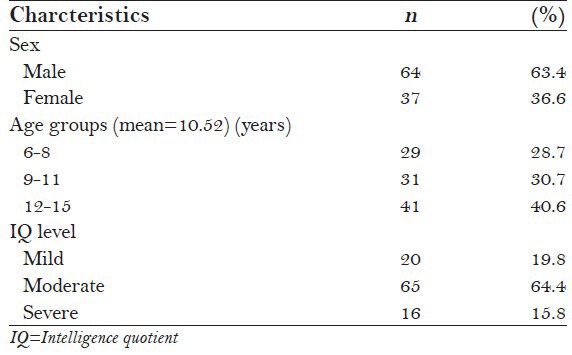
A total of 101 participants aged 6-16 years with a mean age of 10.52 ± 2.77 were included in this study. About two-thirds (63.4%) of the subjects were boys. The majority of subjects (64.4%) were moderately mentally retarded [Table 1].
Table 1.
General profile of the study subjects (N=101)
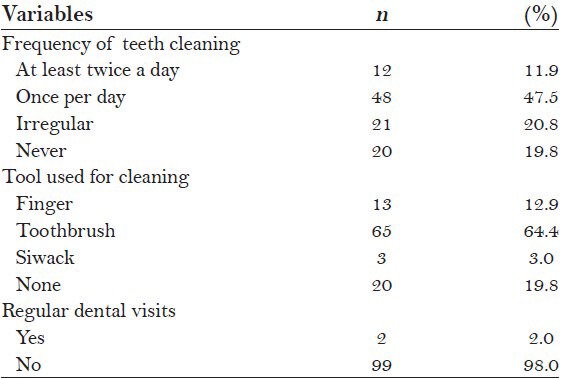
Approximately 59.4% of subjects (n = 60) reported regularly brushing their teeth either once a day (n = 48) or at least twice a day (n = 12). The most commonly used cleaning tool was toothbrush (64.4%), followed by finger (12.9%) and Siwack (3.0%). Only 2.0% of subjects (n = 2) reported regular dental visits [Table 2].
Table 2.
Oral hygiene practices of the subjects (N=101)
All subjects had gingivitis. Around 28.7% of subjects (n = 29) had severe gingivitis, 47.5% (n = 48) had moderate gingivitis, and 23.8% (n = 24) had mild gingivitis [Figure 1]. Of all the participants, only 15 subjects (14.9%) showed good oral hygiene [Figure 2].
Figure 1.
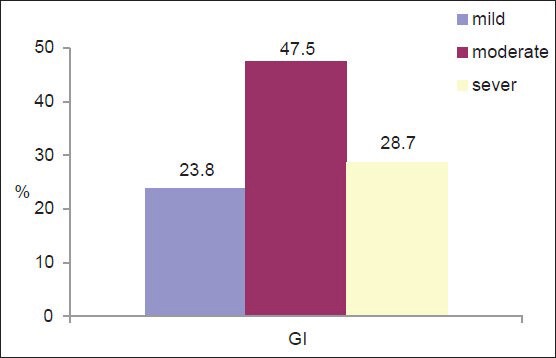
Severity of gingivitis among the subjects
Figure 2.
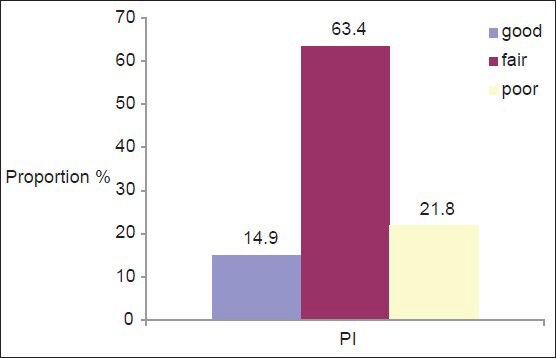
Oral hygiene status among the subjects
Table 3 presents the oral hygiene indicators with different grouping factors. Overall, CI, PI, and GI of the total population were 0.58 ± 0.61, 1.45 ± 0.57, and 1.54 ± 0.64, respectively, with no significant difference across gender (P > 0.05). The 12-16 year age group had significantly higher CI, GI, and PI scores than younger age groups (P < 0.01). Subjects with severe mental retardation had significantly higher PI (P < 0.01) and GI (P < 0.05) scores than those in mild and moderate retardation groups; however, no significant differences regarding CI were detected.
Table 3.
Calculus index (CI), plaque index (PI), and gingival index (GI) according to gender, age, and IQ groups
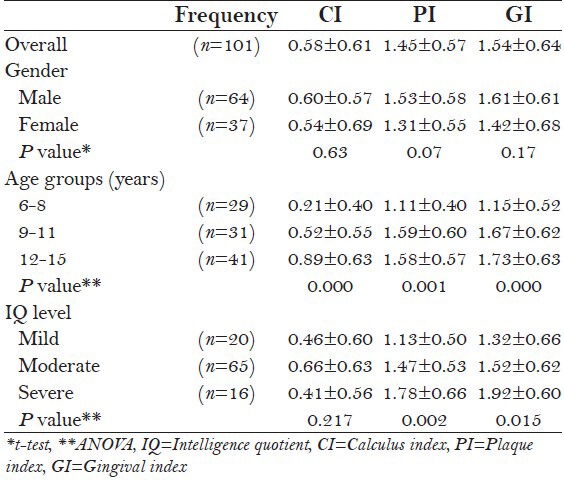
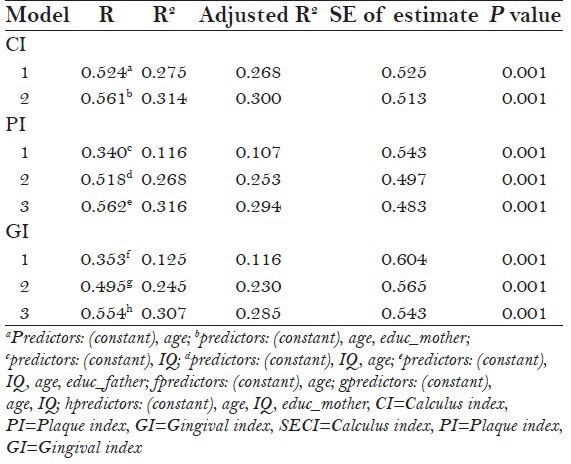
Stepwise multiple linear regressions revealed that the best predictors for CI were age and mother's education, which explained 30% of the variance. IQ level, age, and father's education were the independent predictors of PI which explained 29.4% of the variance. The best predictors for GI were age, IQ level, and mother's educational level, which explained a variance of 28.5%. It is evident from Table 4 that age was significantly associated with all oral hygiene indicators [Table 4].
Table 4.
Stepwise multiple linear regression with CI, PI, and GI as dependent variables
DISCUSSION
The current study gives a comprehensive overview of the oral hygiene practices and status, as well as gingival health condition of children with DS attending special needs schools, which is, to the best of our knowledge, the first study to explore these issues among DS individuals in Yemen.
In our study, two-thirds of the participants with DS were boys, a percentage higher than that found by Asokan et al.[10] in their study on children with DS in India in which half of the participants were boys. This was not a surprise, as in Yemen, families (due to social and cultural barriers) tend to send their sons to schools of special needs more often than their daughters.
In general, most of participants showed poor oral hygiene practices. Of total, only 59.4% reported brushing their teeth regularly (i.e. once or more in a day). This rate is lower than that reported among children with DS in Saudi Arabia[11] (75%), Jordan[12] (77.7%), and Australia[13] (90%). Lack of oral health education in both parents and children might explain this poor finding in the present study.
The present study also showed a very poor dental attendance in which only 2% of the subjects reported regular dental visits, though the lack of access to care is not limited to those with DS. Healthier people in Yemen still attend dental facilities only when there is an emergency dental problem. There are many factors contributing to poor utilization of dental services: Lack of knowledge about good oral hygiene practices among caretakers and concerned authorities, lack of motivation, low priority given to dental care in the society, lack of facility for early and regular oral health check-up and prompt treatment, and cost of treatment are among these factors.
The present study showed that children with DS had poor oral hygiene and gingival health. The mean CI, PI, and GI scores of the total population were 0.58 ± 0.61, 1.45 ± 0.57, and 1.54 ± 0.64, respectively. These values are higher than those reported for similarly aged normal children in Sana’a.[14] This is consistent with many previous studies which reported poorer standard of oral hygiene and greater prevalence of periodontal diseases in DS children than their normal counterparts.[5,12,15,16,17,18,19,20] A multitude of factors may be involved in the increased susceptibility to gingival and periodontal diseases among individuals with DS.[21] Factors previously investigated include mental retardation, subgingival plaque composition, immune/inflammatory responses, and others.[20,21,22] Microbiological studies showed that individuals with DS have significantly higher levels of periodontopathic bacteria including Porphyromonas gingivalis and Tannerella forsythensis.[17] Hence, optimal oral hygiene is of particular importance in this group in order to limit the disease onset.
The present study revealed that the overall oral hygiene status of the study population was poor with prevalence rates of 14.9%, 63.4%, and 21.8% for good, fair, and poor components, respectively, [Figure 2] which are similar to the values reported in a previous study conducted on mentally disabled children attending special schools in Udaipur, India.[22] The reasons for this may include the reduced manual dexterity of the participants, joint laxity, and lack of comprehension of oral hygiene needs due to mental difficulties. They, therefore, need help to carry out routine oral hygiene measures.
No significant difference was found in the oral hygiene scores between male and female subjects, which is in agreement with previous studies.[5] This finding implies that disability in DS children is the dominantly influential factor affecting oral hygiene, rather than gender.
The present study also showed steady increase in oral hygiene scores with age, which conforms to previous reports.[5,14,22] This is due to the accumulative effect of plaque and calculus with age.[22] There was also a definite trend for gingival disease as age increased, which could be explained by the same reason.
In multivariate analysis, it was found that the education level of the mother and father was significantly associated with oral hygiene and gingival disease. This could be explained by the findings from a study on DS children in Saudi Arabia which revealed that a higher percentage of children of illiterate mothers used water only as a method of cleaning their teeth compared to other children, and this could influence the oral hygiene status.[11] Moreover, a high correlation between poor oral hygiene and development of periodontal disease has been well documented and the role of poor oral hygiene as a risk factor of periodontal diseases is well established.[23]
In addition, the stage of mental retardation based on IQ levels was found to be associated with both oral hygiene and gingival health status, which is in accordance with earlier reports.[22,24,25] Martens et al. observed that children who were mildly mentally retarded had significantly better manual dexterity skills than the severely mentally retarded, which explains the findings in the present study that subjects with mild retardation had better oral hygiene status than those with severe retardation.[25]
The main limitation in the present study is the fact that it included no control subjects. Nevertheless, the research group will take this issue into consideration in future studies. Additionally, this study was conducted on DS children attending special needs schools. Also, it should be noted that majority of DS children in Yemen still reside at home with no educational or vocational plan from parents or guardians as they are often stigmatized by the society. Therefore, generalization must be made carefully as this study population may not reflect the DS population in Yemen.
In conclusion, the oral hygiene status of DS children in the present study was poor and influenced by age, IQ level, and parents’ level of education. Oral health promotion programs should be aimed specifically at special needs schools and parents of DS children.
ACKNOWLEDGMENT
The authors are grateful to the subjects, parents, and teachers in the special needs schools that participated in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Abanto J, Ciamponi AL, Francischini E, Murakami C, de Rezende NP, Gallottini M. Medical problems and oral care of patients with Down syndrome: A literature review. Spec Care Dentist. 2011;31:197–203. doi: 10.1111/j.1754-4505.2011.00211.x. [DOI] [PubMed] [Google Scholar]
- 2.Macho V, Palha M, Macedo AP, Ribeiro O, Andrade C. Comparative study between dental caries prevalence of Down syndrome children and their siblings. Spec Care Dentist. 2013;33:2–7. doi: 10.1111/j.1754-4505.2012.00297.x. [DOI] [PubMed] [Google Scholar]
- 3.Hennequin M, Faulks D, Veyrune JL, Bourdiol P. Significance of oral health in persons with Down syndrome: A literature review. Dev Med Child Neurol. 1999;41:275–83. doi: 10.1017/s0012162299000596. [DOI] [PubMed] [Google Scholar]
- 4.Shore S, Lightfoot T, Ansell P. Oral disease in children with Down syndrome: Causes and prevention. Community Pract. 2010;83:18–21. [PubMed] [Google Scholar]
- 5.Oredugba FA. Oral health condition and treatment needs of a group of Nigerian individuals with Down syndrome. Downs Syndr Res Pract. 2007;12:72–6. doi: 10.3104/reports.2022. [DOI] [PubMed] [Google Scholar]
- 6.Shyama M, Al-Mutawa SA, Honkala S, Honkala E. Supervised toothbrushing and oral health education program in Kuwait for children and young adults with Down syndrome. Spec Care Dentist. 2003;23:94–9. doi: 10.1111/j.1754-4505.2003.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 7.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 8.Ramfjord SP. Indices for prevalence and incidence of periodontal disease. J Periodontol. 1959;30:51–9. [Google Scholar]
- 9.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 10.Asokan S, Muthu MS, Sivakumar N. Dental caries prevalence and treatment needs of Down syndrome children in Chennai, India. Indian J Dent Res. 2008;19:224–9. doi: 10.4103/0970-9290.42955. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hussyeen A, Al-Sadhan SA. Oral hygiene practices and dietary habits among children with Down's syndrome in Riyadh, Saudi Arabia. Saudi Dent J. 2006;18:141–8. [Google Scholar]
- 12.Al Habashneh R, Al-Jundi S, Khader Y, Nofel N. Oral health status and reasons for not attending dental care among 12- to 16-year-old children with Down syndrome in special needs centres in Jordan. Int J Dent Hyg. 2012;10:259–64. doi: 10.1111/j.1601-5037.2012.00545.x. [DOI] [PubMed] [Google Scholar]
- 13.Randell DM, Harth S, Seow WK. Preventive dental health practices of non-institutionalized Down syndrome children: A controlled study. J Clin Pediatr Dent. 1992;16:225–9. [PubMed] [Google Scholar]
- 14.Al-Haddad KA, Al-Hebshi NN, Al-Ak’hali MS. Oral health status and treatment needs among school children in Sana’a City, Yemen. Int J Dent Hyg. 2010;8:80–5. doi: 10.1111/j.1601-5037.2009.00398.x. [DOI] [PubMed] [Google Scholar]
- 15.Ulseth JO, Hestnes A, Stovner LJ, Storhaug K. Dental caries and periodontitis in persons with Down syndrome. Spec Care Dentist. 1991;11:71–3. doi: 10.1111/j.1754-4505.1991.tb00819.x. [DOI] [PubMed] [Google Scholar]
- 16.López-Pérez R, Borges-Yáñez SA, Jiménez-García G, Maupomé G. Oral hygiene, gingivitis, and periodontitis in persons with Down syndrome. Spec Care Dentist. 2002;22:214–20. doi: 10.1111/j.1754-4505.2002.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 17.Sakellari D, Arapostathis KN, Konstantinidis A. Periodontal conditions and subgingival microflora in Down syndrome patients. A case-control study. J Clin Periodontol. 2005;32:684–90. doi: 10.1111/j.1600-051X.2005.00737.x. [DOI] [PubMed] [Google Scholar]
- 18.Khocht A, Janal M, Turner B. Periodontal health in Down syndrome: Contributions of mental disability, personal, and professional dental care. Spec Care Dentist. 2010;30:118–23. doi: 10.1111/j.1754-4505.2010.00134.x. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira JS, Prado RR, Júnior, de Sousa Lima KR, de Oliveira Amaral H, Moita Neto JM, Mendes RF. Intellectual disability and impact on oral health: A paired study. Spec Care Dentist. 2013;33:262–8. doi: 10.1111/scd.12015. [DOI] [PubMed] [Google Scholar]
- 20.Subramaniam P, Girish Babu K, Mohan Das L. Assessment of salivary total antioxidant levels and oral health status in children with Down syndrome. Spec Care Dentist. 2014;34:193–200. doi: 10.1111/scd.12054. [DOI] [PubMed] [Google Scholar]
- 21.Morgan J. Why is periodontal disease more prevalent and more severe in people with Down syndrome? Spec Care Dentist. 2007;27:196–201. doi: 10.1111/j.1754-4505.2007.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Sharma J, Duraiswamy P, Kulkarni S. Determinants for oral hygiene and periodontal status among mentally disabled children and adolescents. J Indian Soc Pedod Prev Dent. 2009;27:151–7. doi: 10.4103/0970-4388.57095. [DOI] [PubMed] [Google Scholar]
- 23.Bellini HT, Arneberg P, von der Fehr FR. Oral hygiene and caries. A review. Acta Odontol Scand. 1981;39:257–65. doi: 10.3109/00016358109162287. [DOI] [PubMed] [Google Scholar]
- 24.Denloye OO. Oral hygiene status of mentally handicapped school children in Ibadan, Nigeria. Odontostomatol Trop. 1998;21:19–21. [PubMed] [Google Scholar]
- 25.Martens L, Marks L, Goffin G, Gizani S, Vinckier F, Declerck D. Oral hygiene in 12-year-old disabled children in Flanders, Belgium, related to manual dexterity. Community Dent Oral Epidemiol. 2000;28:73–80. doi: 10.1034/j.1600-0528.2000.280110.x. [DOI] [PubMed] [Google Scholar]


