Abstract
Purpose:
To evaluate the antimicrobial activity of different compositions of Gutta-percha points and calcium hydroxide (Ca(OH)2) pastes, used in endodontic therapy.
Materials and Methods:
The evaluated material consisted of Gutta-percha points containing Ca(OH)2, Gutta-percha points containing chlorhexidine (Chx), conventional Gutta-percha points and Ca(OH)2 pastes. Antimicrobial properties of Chx and CaOH paste are compared with CaOH points. Antimicrobial tests included three species of microorganisms: Escherichia coli (ATCC 25923), Staphylococcus aureus (ATCC 25922) Pseudomonas aeruginosa (ATCC BAA-427), the agar disc diffusion method was employed. The plates were kept at room temperature for 2 h for prediffusion and then incubated at 37°C for 24 h. Zones of inhibition were measured.
Results and Conclusion:
All microbial species used in the study were inhibited by the Gutta-percha points containing Chx and by the Ca(OH)2 pastes, no antimicrobial activity was observed for the other groups (conventional Gutta-percha and Ca(OH)2 group).
Keywords: Calcium hydroxide, chlorhexidine, Gutta-percha
INTRODUCTION
The success of endodontic therapy is directly related to the elimination of root canal infection. Besides the cleaning effect of biomechanical preparation, further means such as intracanal medication (calcium hydroxide [Ca(OH)2]) or the use of filling materials with antimicrobial efficacy are equally important.[1,2] Ca(OH)2 pastes, commonly used as intracanal medication, have presented antimicrobial action and ability to inactivate with endotoxins which facilitate the healing process of periapical tissues.[3,4,5] Chlorhexidine (Chx), used as an irrigating solution has antimicrobial action and is able to inhibit most endodontic bacteria including Enterococcus faecalis.[3,6] Despite of good antimicrobial effect, their distribution throughout the entire canal is problematic. Another challenge is the complete removal of intracanal medicaments as demanded by the European Society of Endodontology guidelines. Presence of residual Ca(OH)2 can impede the properties of endodontic sealer.
To overcome these drawbacks and with the aim of providing effective antimicrobial action, Gutta-percha points have been manufactured including several substances such as iodoform, Chx and Ca(OH)2. Barthel et al., evaluated the antimicrobial action of Gutta-percha points containing Chx or Ca(OH)2 when compared to Chx gel and to Ca(OH)2 paste. He observed conventional Gutta-percha points with smaller microbial inhibition.[3]
MATERIALS AND METHODS
The aim of this study was to evaluate in vitro antimicrobial activity of conventional Gutta-percha point, point containing Ca(OH)2 and Chx and Ca(OH)2 paste.
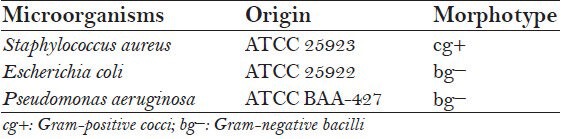
The evaluated materials were: Group A – Ca(OH)2 Gutta-percha points (Roeko Gmbh + Co., Langenau, Baden-Württemberg, Germany), Group B – gutta-percha points containing Chx (Roeko Gmbh + Co., Langenau, Baden-Württemberg, Germany), Group C – conventional Gutta-percha points and Group D – Ca(OH)2 paste (Neelkanth Medical Products, India) [Figure 1a–1d]. The morphotype and origin of the strains used as indicators of antimicrobial activity are presented in Table 1.
Figure 1.
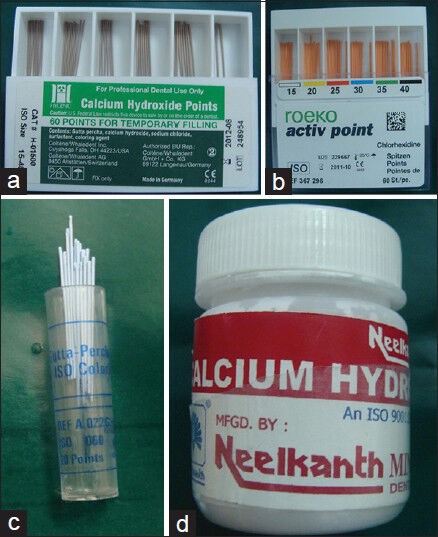
(a) Calcium hydroxide Gutta-percha points, (b) Gutta-percha points containing chlorhexidine, (c) Conventional Gutta-percha points, (d) Calcium hydroxide paste
Table 1.
Strains used as indicators of antimicrobiological activity-origin and morphotype
Pure cultures were grown on Mueller-Hinton broth and suspended to 0.5 density measured via McFarald method and followed by evaluation.
Paper points were involved with Ca(OH)2 pastes (20% concentration) and were placed on petri dishes. Afterwards the tested Gutta-percha cones were placed at equidistant. The test was made in triplicate. Plates were kept at room temperature for 2 h, so that prediffusion of the material could occur. Then plates were incubated at 37°C for 24 h. The biggest inhibition zone was measured with a millimeter ruler. Statistical evaluation was carried out using analysis of variance and Tukey test, with a level of significance at 5%.
RESULTS
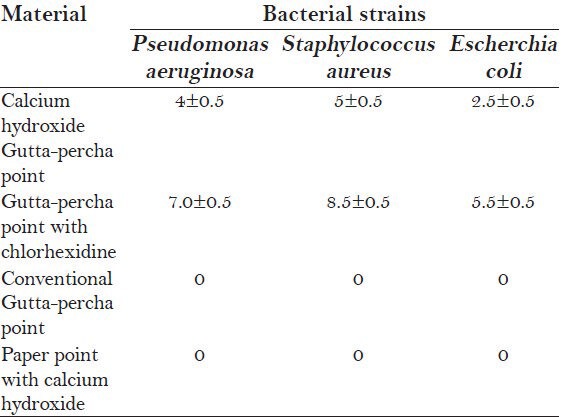
Figure 2a–2c shows the inhibition zone after 24 h of inhibition. Table 2 shows the means and standard deviations of the diameter of the inhibition zone in millimeters. The results showed that all microbial species used in the study were inhibited by the Gutta-percha points containing Chx and by the Ca(OH)2 pastes. No antimicrobial activity was observed in the other groups (Ca(OH)2 point and the conventional Gutta-percha points i.e. Groups C and D). The Gutta-percha points containing Chx showed the highest antimicrobial activity against Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli 7.0 ± 0.5, 8.5 ± 0.5 and 5.5 ± 0.5 respectively.
Figure 2.
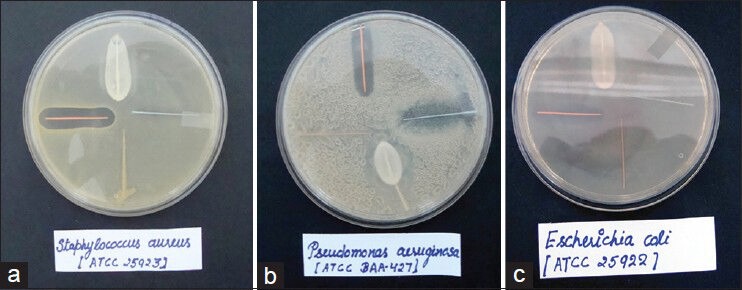
(a) Staphylococcus aureus culture plate, (b) Pseudomonas aeruginosa culture plate, (c) Escherichia coli culture plate
Table 2.
Means and standard deviation of the inhibition zones (in mm)
DISCUSSION
Incomplete decontamination of infected root canals may lead to failure of the endodontic therapy and the development of periapical lesions. Clinically, the presence of anatomic variations as well as the high number and great variety of microorganisms make it difficult to completely eliminate microorganisms from the canal.[7] Also, microorganisms can organize themselves in a biofilm, and endodontic therapy may fail in a higher percentage of these cases. In order to eliminate as many remaining bacteria as possible during debridement and to prevent their recolonization intracanal medication is highly recommended. This study was carried out to determine the antimicrobial activity of different composition of Gutta-percha points and Ca(OH)2 paste.
The bacteria evaluated in this study were mostly found in the infected root canal which included P. aeruginosa, E. coli and S. aureus. S. aureus, due to its great capacity for adaptation and resistance can be found in both pulpal and periapical infections. P. aeruginosa, E. coli are Gram-negative, aerobic and facultative anaerobic which can tolerate wide range of temperature.[7,8,9,10,11]
As an intracanal medication, Ca(OH)2 can be used in combination with distilled water, normal saline etc., to form a paste. It provides sterility to canal space until the permanent root filling is placed. Ca(OH)2 neutralizes the biological activity of bacterial polysaccide. Byström and Sundqvist reported that Ca(OH)2 was an effective intracanal medicament, rendering 34 out of 35 canals bacteria free after a 4 weeks period.[8]
Gutta-percha is desirable filling material introduced by Bowman in 1867. Composition of Gutta-percha varies with manufacturer. Friedman et al., described the approximates composition as 20% Gutta-percha, 66% zinc oxide (ZnO), 11% heavy metals sulphates (radiopacifier) and 3% waxes or resins (plasticizers).[12]
Chlorhexidine Gutta-percha points are made of Chx diacetate (approximately 5%) Gutta-percha, ZnO, BaSO4 and coloring agent. This Gutta-percha matrix allow large quantities of Chx to be released from the surface of points. Bozza et al., conducted in vitro study to evaluate antimicrobial activity of points containing Ca(OH)2, Chx points and conventional Gutta-percha points and concluded that Chx points proved to be effective against most of tested species.[13] Similar result was obtained in study conducted by Tanomaru et al.[2] Lui et al., when evaluating Gutta-percha points containing Chx in vitro, did not find enough antimicrobial activity against E. faecalis inside infected dentine tubules.[1] In the present study the Gutta-percha points containing Chx showed the highest antimicrobial activity against P. aeruginosa, S. aureus and E. coli 7.0 ± 0.5, 8.5 ± 0.5 and 5.5 ± 0.5 respectively.
Calcium hydroxide points are made of 52% Ca(OH)2, 42% Gutta-percha NaCl and coloring agent. Antimicrobial action of materials containing Ca(OH)2 depends on Ca(OH)2 ionization and on the release of hydroxil ions that promote an increase in the medium pH and its maintenance. Hydroxil ions also have some action on bacterial cell. Azabal-Arroyo et al., evaluated antimicrobial activity of materials containing Ca(OH)2 in solid media presents some difficulties as there is low solubility of the Ca(OH)2 in solid medium.[14] This fact could explain the results obtained in this study that show that the Gutta-percha points containing Ca(OH)2 did not present antimicrobial activity on tested strains. Further long term studies need to be done to confirm the same. In vivo studies are recommended.
CONCLUSION
The Gutta-percha points containing Chx and the Ca(OH)2 paste presented in vitro antimicrobial activity against all of the strains used in this study. The Ca(OH)2 points and the conventional Gutta-percha points did not present antimicrobial activity. Thus the Chx points can be employed as an intracanal medicament for an improved ionic release profile and time saving property.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lui JN, Sae-Lim V, Song KP, Chen NN. In vitro antimicrobial effect of chlorhexidine-impregnated Gutta-percha points on Enterococcus faecalis. Int Endod J. 2004;37:105–13. doi: 10.1111/j.0143-2885.2004.00734.x. [DOI] [PubMed] [Google Scholar]
- 2.Tanomaru JM, Pappen FG, Tanomaru Filho M, Spolidorio DM, Ito IY. In vitro antimicrobial activity of different Gutta-percha points and calcium hydroxide pastes. Braz Oral Res. 2007;21:35–9. doi: 10.1590/s1806-83242007000100006. [DOI] [PubMed] [Google Scholar]
- 3.Barthel CR, Zimmer S, Zilliges S, Schiller R, Göbel UB, Roulet JF. In situ antimicrobial effectiveness of chlorhexidine and calcium hydroxide: Gel and paste versus Gutta-percha points. J Endod. 2002;28:427–30. doi: 10.1097/00004770-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Tobias RS. Antibacterial properties of dental restorative materials: A review. Int Endod J. 1988;21:155–60. doi: 10.1111/j.1365-2591.1988.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 5.Tronstad L, Barnett F, Riso K, Slots J. Extraradicular endodontic infections. Endod Dent Traumatol. 1987;3:86–90. doi: 10.1111/j.1600-9657.1987.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 6.Tanomaru JM, Leonardo MR, Tanomaru Filho M, Bonetti Filho I, Silva LA. Effect of different irrigation solutions and calcium hydroxide on bacterial LPS. Int Endod J. 2003;36:733–9. doi: 10.1046/j.1365-2591.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- 7.Ebert J, Obserschachteik H, Petschelt A. A clinical study on calcium hydroxide releasing Gutta-percha points. J Dent Res. 1998;77:696. [Google Scholar]
- 8.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–8. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 9.Podbielski A, Boeckh C, Haller B. Growth inhibitory activity of Gutta-percha points containing root canal medications on common endodontic bacterial pathogens as determined by an optimized quantitative in vitro assay. J Endod. 2000;26:398–403. doi: 10.1097/00004770-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Szep S, Grumann L, Ronge K, Schriever A, Schultze M, Heidemann D. In vitro cytotoxicity of medicated and nonmedicated Gutta-percha points in cultures of gingival fibroblasts. J Endod. 2003;29:36–40. doi: 10.1097/00004770-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Siqueira JF, Jr, de Uzeda M. Intracanal medicaments: Evaluation of the antibacterial effects of chlorhexidine, metronidazole, and calcium hydroxide associated with three vehicles. J Endod. 1997;23:167–9. doi: 10.1016/S0099-2399(97)80268-3. [DOI] [PubMed] [Google Scholar]
- 12.Friedman S, Moshonov J, Trope M. Residue of Gutta-percha and a glass ionomer cement sealer following root canal retreatment. Int Endod J. 1993;26:169–72. doi: 10.1111/j.1365-2591.1993.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 13.Bozza FL, Molgatini SL, Pérez SB, Tejerina DP, Pérez Tito RI, Kaplan AE. Antimicrobial effect in vitro of chlorhexidine and calcium hydroxide impregnated Gutta-percha points. Acta Odontol Latinoam. 2005;18:51–6. [PubMed] [Google Scholar]
- 14.Azabal-Arroyo M, Menasalvas-Ruiz G, Martín-Alonso J, Arroquia JJ, Vega-del Barrio JM. Loss of hydroxyl ions from Gutta-percha points with calcium hydroxide in their composition: An in vivo study. J Endod. 2002;28:697–8. doi: 10.1097/00004770-200210000-00005. [DOI] [PubMed] [Google Scholar]


