Abstract
Objectives:
Deltamethrin is a synthetic pyrethroid insecticide used worldwide in agriculture, household pest control, protection of foodstuff, and disease vector control. Although initially thought to be least toxic, a number of recent reports showed its toxic effects in mammalian and non-mammalian animal species. The current study was performed to assess the dose-dependent deltamethrin toxicity on testes, liver, and kidney of male Wistar rats.
Materials and Methods:
Twenty-four rats were divided in four groups of 6 each. Group A served as normal control. Group B, C, and D were administered with different doses (2 or 3 or 6 mg/kg corresponding to 1/30th or 1/20th or 1/10th of LD50, respectively) of deltamethrin for 28 days.
Results:
Deltamethrin exposure caused a significant reduction in weight of reproductive organs, decrease in sperm count, sperm motility, serum testosterone (T), follicle stimulating hormones (FSH), and luteinizing hormones (LH) in testis. Glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione S transferase (GST), glutathione reductase (GR), glutathione peroxidase (GPx) were decreased in testis, liver and kidney of exposed rats. Deltamethrin exposure significantly increased sperm abnormalities in testis. Significant increase in lipid peroxidation (LPO) level was observed in testis, liver and kidney. Deltamethrin also caused histological alterations in testes, liver, and kidney.
Conclusions:
The results indicated that deltamethrin at a dose of 6 mg/kg exerts significant harmful effects on testes, liver and kidney as compare to 2 mg and 3 mg/kg. The study concluded that the system toxicity induced by deltamethrin was dose dependent.
Keywords: Deltamethrin, kidney, liver, oxidative stress, testes, Wistar rats
INTRODUCTION
Deltamethrin [(1R,3S) [α-cyano(3-phenoxyphenyl) methyl]-3-(2,2-dibromo-ethenyl)-2,2-dimethyl-cyclopropanecarboxylate] is a synthetic pyrethroid used worldwide in agriculture, home pest control, protection of foodstuff, and disease vector control. It is also used in the public and animal health programmes. It is rapidly absorbed and excreted in urine (19 - 47%) and feces (32 - 55%) within 24 hours of oral administration in rats. The main routes of metabolism include cleavage of the ester bond and oxidation at position 4 of the phenoxy ring of the alcohol moiety. The acid and alcohol moieties are further transformed to conjugated metabolites. Unchanged deltamethrin is the major compound in faces. During its metabolism, reactive oxygen species (ROS) are generated and result in oxidative stress in intoxicated animals.[1] The most important sources of the animal and human exposure to deltamethrin are polluted food and water, and it is readily absorbed by the oral route.[2] The production of ROS is a normal physiological event in various organs. However, overproduction of ROS can be harmful to reproductive[3] hepatic[4] and nephritic system.[5] Earlier studies have shown that deltamethrin produces dose and time-dependent increase in the cytochrome P450s (CYPs), involved in its metabolism in rat brain and liver.[6,7] Deltamethtrin has a deleterious effect of on reproductive system.[3,8] In mammals, spermplasma- membranes have extremely high concentration of polyunsaturated fatty acids and insufficient antioxidant defenses; hence they are highly susceptible to lipid peroxidation.[9]
Hence, the present study was aimed to evaluate the influence of different doses of deltamethrin on reproductive, hepatic and nephritic system of male Wistar rats.
MATERIALS AND METHODS
Animals
Male Wistar rats, weighing about 200-250 gm, were used in the experiments. Animals were obtained from Central Drug Research Institute (CSIR), Lucknow and were kept for acclimatization in the animal house at ambient temperature of 25°C and 45-55% relative humidity, with 12h each of dark and light cycles. Animals were fed pellet diet and water ad-libitum, Principles of Laboratory Animal Care (NH publication no, 85-23, revised 1985) were followed for animal care during the course of experimentation.
Chemicals
Technical grade deltamethrin (99%) was gifted by Gharda Chemicals Ltd., Mumbai. Curcumin was purchased from Sigma Aldrich, India. All other chemicals used in this study were of high purity.
Experimental design
Twenty-four male Wistar rats were divided into four groups of six rats each. Deltamethrin dissolved in dimethyl sulphoxide (DMSO) and administered orally by gavaging to Wistar rats as per given plan
Group-A Normal diet and water ad libitum for 28 days
Group-B Deltamethrin 1/10 of LD50 (6 mg/kg bw) for 28 days
Group-C Deltamethrin 1/20 of LD50 (3 mg/kgbw) for 28 days
Group-D Deltamethrin 1/30 of LD50 (2 mg/kgbw) for 28 days.
At the end of the experiment, rats were sacrificed as per institutional ethical committee guidelines (BU/Pharma/IAEC/11/042). Blood was taken out from ventricle of heart for separation of serum for analysis of reproductive hormones. Both the testis and epididymis were removed and weighted. One part of testis was used for sperm head counts and caudas for sperm motility and sperm morphological study. Part of testis, liver, and kidney were used for lipid peroxidation, enzymatic, and non-enzymatic antioxidants and other part was kept in 10% formaline for histological examinations.
Methods for estimation of various reproductive parameters
Sperm motility count
A segment of distal cauda epididymis was removed and 2 ml of Dulbecco's phosphate buffer saline maintained at 36-38°C was added. Cauda was minced sufficiently and placed in a water bath to disperse the sperm for 1-5 minutes and gently mixed using pasture pipette. Five to 10 μl sample was loaded into one slide of the hemocytometer chamber and the number of non-motile sperm count in WBC counting area of the hemocytometer was counted. Sperm was classified as motile if it exhibits any type of movement/motion. Hemocytometer was placed into ice for 10-20 seconds to rendered all the sperms immotile and count the total sperm.[10]
Sperm morphology
Cauda was minced in 1 ml of 0.9% saline with the help of razor and 1 ml of 10% neutral buffer formalin was added. The suspension was diluted with water to volume suitable for performing the assay. One percent of 1-2 ml of eosin was added to 20 ml of above suspension and incubated at room temperature for 45 to 60 minutes and one drop was taken on slide and a smear was prepared. The head and tail abnormalities were expressed as a percentage.[11]
Testicular sperm head count
Sperm head counts were determined with hemocytometer using a method described by Choi et al. (2008)[12] with necessary modifications. The testis was removed and weighed. Tunica albuginea (outer covering) was removed. The testis was minced and homogenized for 2 minutes at highest speed in 0.9% Nacl and 0.05 triton X solution (1-15) microliter of homogenate was placed on hemocytometer, after 5 minutes sperm head was counted in RBC chamber at 40X magnification.
Determination of testosterone, FSH, and LH
Testosterone level was estimated by using Labor Diagnostika Nord Gmbh ELISA kit. LH level was estimated by using Eliscan LH ELISA kit and FSH level was estimated by using Eliscan FSH ELISA kit.
Biochemical estimations
Preparation of homogenate
Tissues (testis, liver, and kidney) were homogenized separately with 10% (w/v) homogenizing buffer (0.1 M phosphate buffers, pH 7.4 + 150 mM Kcl). This is 10% homogenate which is used for LPO and GSH estimations. The remaining 10% homogenate was centrifuged at 9000 rpm for 20 min. to get supernatant(S). The supernatant(S) obtained was used for SOD, CAT, GPx, GR, GST and protein estimations.
Assay of lipid peroxidation, non-enzymatic antioxidants and enzymatic antioxidants
Lipid peroxidation was estimated by Ohkawa et al. (1979).[13] Glutathione reductase was estimated by the method of Ellman (1959).[14] The activity of Super Oxide Dismutase was estimated by the method described by Kakkar et al. (1984).[15] Catalase activity was estimated by the method described by Sinha (1972).[16] GPx, GST and GR activities were assayed by the method of Rotruck et al. 1973,[17] Habig et al. 1974[18] and Calbery and mavmermic, 1981[19] respectively. Protein content in tissue homogenate was estimated by the method of Lowry et al. (1961).[20]
Statistical calculations
Results were expressed as Mean ± SEM. Statistical significance was determined by one way analysis of variance (ANOVA). The treatment groups were compared with control group using Dunnett's test. All statistics were carried out in Graph Pad Instat software, Inc., v. 3. 06, San Digeo, USA.
RESULTS
Dose-dependent effect of deltamethrin on reproductive organs weight
Testis weight showed a significant decrease in group B (P < 0.01, 35.87%) and group C (P < 0.05, 16.26%) and non-significant (P > 0.05) decrease (1.63%) in group D as compared to group A. Epididymis weight of deltamethrin exposed male rats decreased significantly in group B (P < 0.01, 25.72%) and group C (P < 0.05, 20.20%), whereas non-significantly (P > 0.05) in group D (11.66%) as compared to group A [Figure 1].
Figure 1.
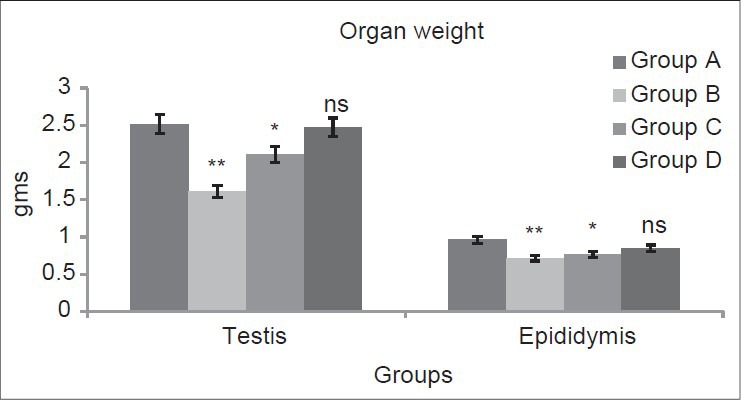
Dose-dependent effect of deltamethrin on testis and epididymis weight. The values represents as Mean ± SEM for 6 rats each. **=P < 0.01, * = P < 0.05, ns = P > 0.05 as compared with control value. Group A-Control; Group B-Deltamethrin exposure I; Group C-Deltamethrin exposure
Dose-dependent effect of deltamethrin on fertility parameters
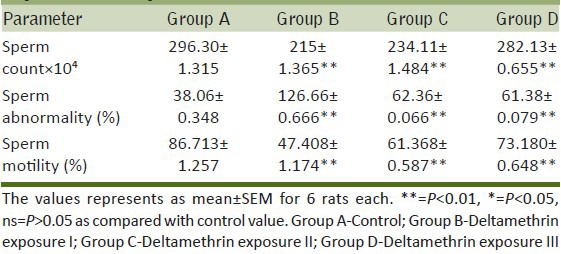
In the present investigations, sperm count, and sperm motility were significantly (P < 0.01) decreased in group B (27.36%, 45.32%), group C (20.94%, 29.22%), and group D (4.72%, 15.6%) respectively as compared to group A. Sperm abnormality showed a significant (P < 0.01) increase in group B (232.79%), group C (63.84%) and group D (61.27%) as compared to group A [Table 1].
Table 1.
Dose-dependent effect of deltamethrin on reproductive parameters
Dose-dependent effect of deltamethrin on reproductive hormone levels
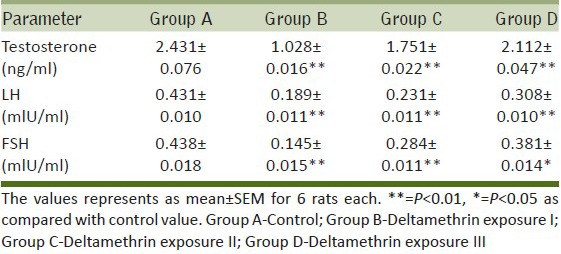
Testosterone, LH and FSH levels in the serum were observed to be significantly (P < 0.01) reduced in group B (57.71%, 56.14% and 66.89%) and group C (27.97%, 46.4%, and 35.15%) respectively. A significant (P < 0.01) fall in the level of LH (28.53%), FSH (13.01%), and testosterone (P < 0.05, 13.12%) in group D was reported when compared with group A [Table 2].
Table 2.
Dose-dependent effect of deltamethrin on reproductive hormone levels
Dose-dependent effect of deltamethrin on liver and kidney weight
Figure 2 depicted that the liver weight of Wistar rats exposed to different doses of deltamethrin increased significantly (P < 0.01) in group B (38.69%), group C (31.21%) and group D (11.53%) as compared to group A. A significant increase in group B (P < 0.01, 29.75%), group C (P < 0.05, 19.69%) and group D (P > 0.05, 4.33%) was reported in kidney weight of Wistar rats as compared to group A.
Figure 2.
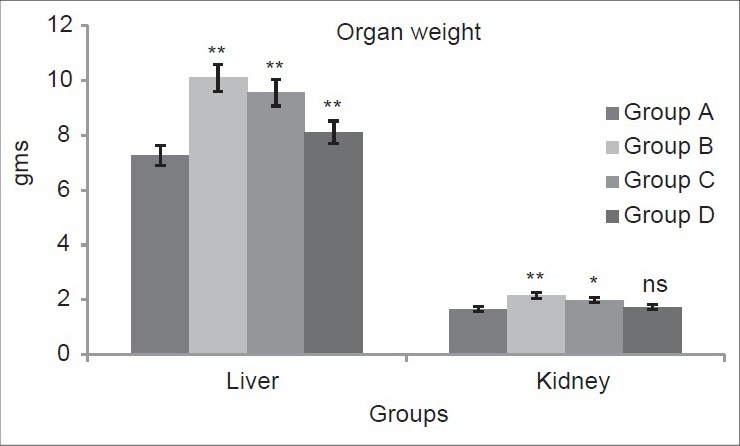
Dose-dependent effect of deltamethrin on liver and kidney weight. The values represents as Mean ± SEM for 6 rats each. **= P < 0.01 as compared with control value. Group A-Control; Group B-Deltamethrin exposure I; Group C-Deltamethrin exposure II; Group D-Deltamethrin exposure III
Dose-dependent effect of deltamethrin on lipid peroxidation, non-enzymatic, and enzymatic antioxidants
Lipid Peroxidation
In Figure 3 LPO level significantly (P < 0.01) increases in group B (Testis 128.7%, Liver 76.28%, Kidney 75.5%) group C (Testis 92.23%, Liver 42.85%, Kidney 44.4%), and group D (Testis 49.18%, Liver 21.7%, Kidney 35.79%) as compared to group A.
Figure 3.

Dose-dependent effect of deltamethrin on lipid peroxidation (LPO), non- enzymatic antioxidant (GSH) and enzymatic antioxidants (SOD, CAT, GST, GPx, GR) in testis, liver, and kidney. The values represents as Mean ± SEM for 6 rats each. ** = P < 0.01, * = P < 0.05, · = P > 0.05 as compared with control value. Group A-Control; Group B-Deltamethrin exposure I; Group C-Deltamethrin exposure II; Group D-Deltamethrin exposure III
Non- Enzymatic antioxidants
GSH level significantly (P < 0.01) decreases in group B and C (Testis 49.01%, 35.36%) (Liver 75.4%, 39.93%), and (Kidney 70.4%, 48.52%) as compared to group A. In group D, GSH level significantly (P < 0.05 and P < 0.01) decreases in Testis and Kidney (15.21%, 31.61%) respectively; however, in Liver, GSH level decreases non-significantly (P > 0.05, 18.31%) as compared to group A [Figure 4].
Figure 4.
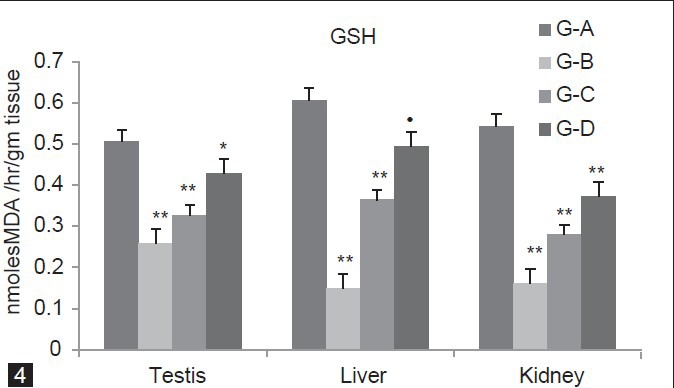
Dose-dependent effect of deltamethrin on lipid peroxidation (LPO), non- enzymatic antioxidant (GSH) and enzymatic antioxidants (SOD, CAT, GST, GPx, GR) in testis, liver, and kidney. The values represents as Mean ± SEM for 6 rats each. ** = P < 0.01, * = P < 0.05, · = P > 0.05 as compared with control value. Group A-Control; Group B-Deltamethrin exposure I; Group C-Deltamethrin exposure II; Group D-Deltamethrin exposure III
Enzymatic antioxidants
In Figure 5, SOD concentration was significantly (P < 0.01) increases in group B, C and D (Testis 29.72%, 20.38%, 11.05%) (Liver 37.17%, 21.47%, 12.3%) (Kidney 37.66%, 12.04%, 8.13%) as compared to group A. Figure 6 showed that CAT level was significantly (P < 0.01) decreases for group B, C and D (Testis 13.53%, 7.39%, 4.08%) (Liver 33.91%, 26.1%, 17.15%) (Kidney 22%, 13.3%, 8.63%) as compared to group A. In Group B and C, GST level significantly (P < 0.01) decreases (Testis 41.16%, 26.7% Liver 60.36%, 36.49%, Kidney 51.71%, 31%) whereas in Group D it is significantly (P < 0.05) decline in (Testis 8.68%, Liver 12.79%) and non-significantly (P > 0.05) in (Kidney 10.83%) as compared to group A [Figure 7].
Figure 5.
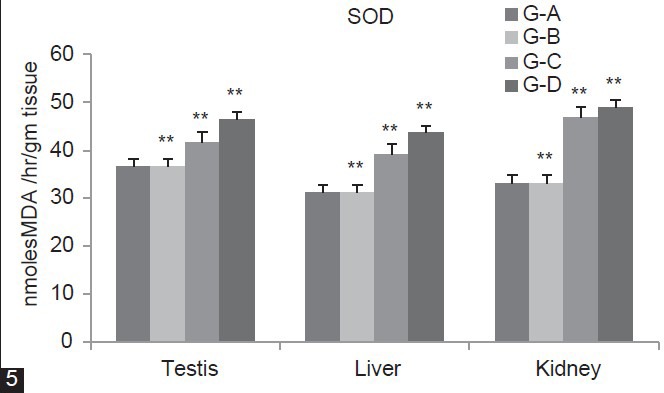
Dose-dependent effect of deltamethrin on lipid peroxidation (LPO), non- enzymatic antioxidant (GSH) and enzymatic antioxidants (SOD, CAT, GST, GPx, GR) in testis, liver, and kidney. The values represents as Mean ± SEM for 6 rats each. ** = P < 0.01, * = P < 0.05, · = P > 0.05 as compared with control value. Group A-Control; Group B-Deltamethrin exposure I; Group C-Deltamethrin exposure II; Group D-Deltamethrin exposure III
Figure 6.
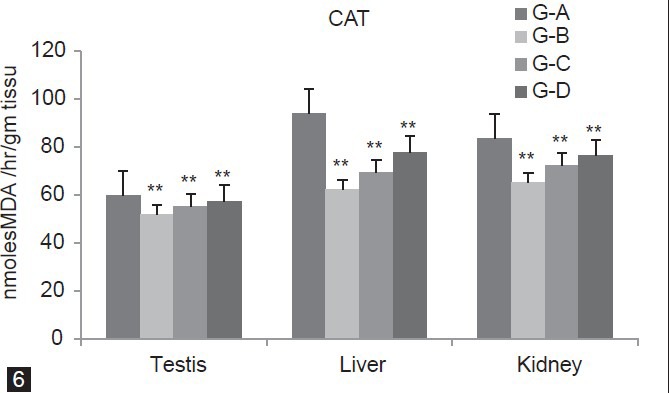
Dose-dependent effect of deltamethrin on lipid peroxidation (LPO), non- enzymatic antioxidant (GSH) and enzymatic antioxidants (SOD, CAT, GST, GPx, GR) in testis, liver, and kidney. The values represents as Mean ± SEM for 6 rats each. ** = P < 0.01, * = P < 0.05, · = P > 0.05 as compared with control value. Group A-Control; Group B-Deltamethrin exposure I; Group C-Deltamethrin exposure II; Group D-Deltamethrin exposure III
Figure 7.
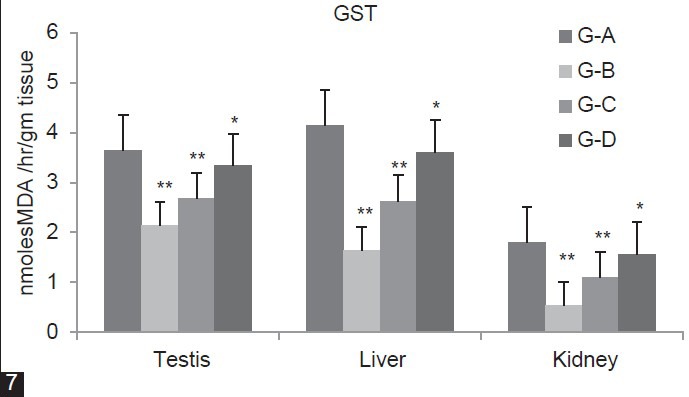
Dose-dependent effect of deltamethrin on lipid peroxidation (LPO), non- enzymatic antioxidant (GSH) and enzymatic antioxidants (SOD, CAT, GST, GPx, GR) in testis, liver, and kidney. The values represents as Mean ± SEM for 6 rats each. ** = P < 0.01, * = P < 0.05, · = P > 0.05 as compared with control value. Group A-Control; Group B-Deltamethrin exposure I; Group C-Deltamethrin exposure II; Group D-Deltamethrin exposure III
Figure 8 depicted that GPx level significantly (P < 0.01) decline in group B (Testis 38.63%, Liver 38.41%, Kidney 74.94%) as compared to group A. Group C also show significant (P < 0.01) decreases in Testis 23.94%, Liver 36.32%), Kidney (P < 0.05, 43.52%) as compared to group A. In group D, GPx concentration decreases in Testis (P < 0.05, 16.49%), Liver (P < 0.01, 18.58%) when compared with group A. The level of GR in group B, C and D was significantly (P < 0.01) decreases (Testis 55.94%, 27.88%, 15.01%) (Liver 27.05%, 16.20%, 13.65%) and (Kidney 69.87%, 38.92%,) as compared to group A. However, group D showed significant (P < 0.05) decline in Kidney (13.78%) as compared to group A [Figure 9].
Figure 8.
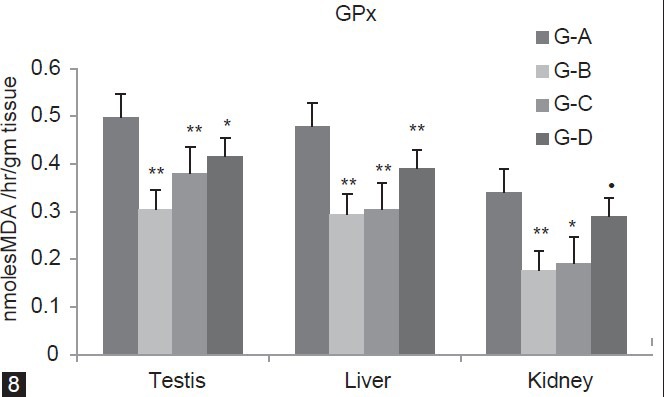
Dose-dependent effect of deltamethrin on lipid peroxidation (LPO), non- enzymatic antioxidant (GSH) and enzymatic antioxidants (SOD, CAT, GST, GPx, GR) in testis, liver, and kidney. The values represents as Mean ± SEM for 6 rats each. ** = P < 0.01, * = P < 0.05, · = P > 0.05 as compared with control value. Group A-Control; Group B-Deltamethrin exposure I; Group C-Deltamethrin exposure II; Group D-Deltamethrin exposure III
Figure 9.

Dose-dependent effect of deltamethrin on lipid peroxidation (LPO), non- enzymatic antioxidant (GSH) and enzymatic antioxidants (SOD, CAT, GST, GPx, GR) in testis, liver, and kidney. The values represents as Mean ± SEM for 6 rats each. ** = P < 0.01, * = P < 0.05, · = P > 0.05 as compared with control value. Group A-Control; Group B-Deltamethrin exposure I; Group C-Deltamethrin exposure II; Group D-Deltamethrin exposure III
Total Protein
In group B and C, total protein concentration was significantly (P < 0.01) increases (Testis 26.19%, 18.45%), [Liver 41.6%, (P < 0.05, 9.96%)], (Kidney 12.5%, 7.14%) as compared to group A. Group D reported non-significant (P > 0.05) increase in Testis (11%), Liver (9.96%) and Kidney (3.57%) when compared with group A [Figure 10].
Figure 10.
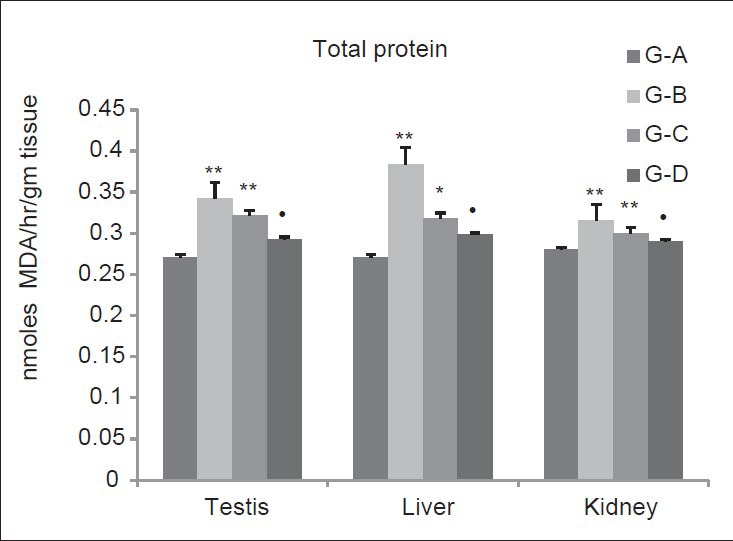
Dose-dependent effect of deltamethrin on total protein The values represents as Mean ± SEM for six rats each. ** = P < 0.01, * = P < 0.05, · = P > 0.05 as compared with control value. Group A-Control; Group B-Deltamethrin exposure I; Group C-Deltamethrin exposure II; Group D-Deltamethrin exposure III
Organs Histology
Testis
Group A represents testicular sections of the control rats with normal histoarchitecture that consisted of uniform, well-organized seminiferous tubules with complete spermatogenesis and normal interstitial connective tissue. Deltamethrin-treated group B showed highly degeneration of the seminiferous tubules. Seminiferous tubules lost its shape and appeared with irregular outline. The intertubular tissue was degenerated and showed many vacuoles with blood hemorrhage and most of the seminiferous tubules were devoid of germ cells. In group C the germ cells were degenerated and exfoliated in the lumen center. Sloughed germinal epithelium and vacuolization was observed in group C. Group D showed alterations which were not severe as in Group B and Group C due to decrease in deltamethrin dose. These changes were characterized by shrunken, disorganized seminiferous tubules [Figure 11].
Figure 11.
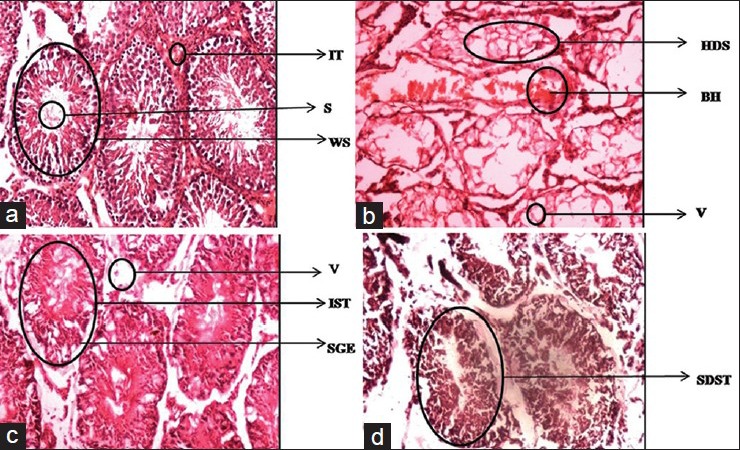
Dose-dependent effect of deltamethrin on histology of testis in Wistar rats Shrunken disorganized semineferous tubules (SDST), Irregular outlined semineferous tubules (IST), Vacuolation(V) and Sloughed germinal epithilium (SGE), Highly degenerated semineferous tubules (HDS), Vacuolation (V), Blood Hemorrhage (BH), Well organized seminiferous tubules (WS), Normal interstitial connective tissue (IT) and Sperms(S). Testis Group-A, Testis Group-B, Testis Group-C, Testis Group-D
Liver
Group A showed normal cellular architecture of liver with distinct normal hepatocytes, central vein, fat vacuoles, normal sinusoids, and hepatocyte nuclei. The histoarchitecture of liver in group B showed that the central veins were dilated and congested. Some area of central vein showed necrosis infiltrated with mononuclear cells. In addition, the hepatocytes lost their normal architecture and vacuolization appeared in the cytoplasm. In group C histo-architecture of liver also showed congestion and dilation of central veins. Cell degeneration and cellular debris were showed by the liver section. Hepatocytes were also degenerated and vacuolized and the sinusoids and nuclei also appear. Hepatic architecture of group D showed mild variations like dilation in central veins. Hepatocytes, fat vacuole sinusoids and central veins were mildly affected than group B and Group C [Figure 12].
Figure 12.
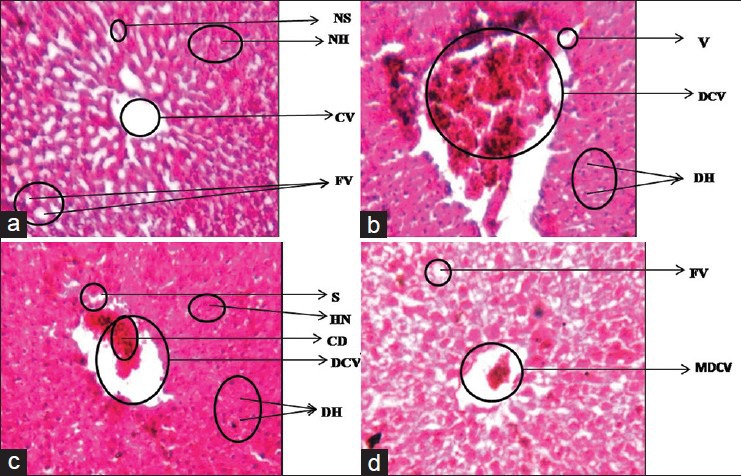
Dose-dependent effect of deltamethrin on histology of liver in Wistar rats Normal Hepatocytes (NH), Central vein (CV), Normal sinusoids (NS), and Fat vacuoles (FV), dilated and congested central vein (DCV), Vacuolization (V), degenerated hepatocytes (DH), Cellular debris (CD), Sinusoids (S), degenerated hepatocytes (DH) and hepatic nuclei also appear (HN), Mildly dilated central vein (MDCV). Liver Group-A, Liver Group-B, Liver Group-C, Liver Group-D
Kidney
In group A kidney sections of control rats showed normal histological structures of the glomeruli surrounded with Bouman's capsule, and renal tubules in the cortical and medullary portions without any inflammatory changes. Deltamethrin treatment in group B caused many histological alterations in the kidney of Wistar rats. The renal tubules were degenerated and showed intraluminal exfoliation with granular cast formation. Leucocytic inflammatory cells were spread in large areas of the intertubular tissue. Kidneys also showed congestion of the blood vessels. The tubular lumen was filled with cellular debris. The histological alterations by deltamethrin treatment in group C showed that the glomeruli were not distinguished and were degenerated. The renal veins were enlarged and congested with blood and the renal tubules showed wide lumen and separation of the epithelial cells from its membrane. Cellular debris was also present in tubular lumen. In group D the kidney architecture showed vascular congestion and tubular necrosis. Degeneration and shrinkage of glomerulus, degenerated Bowman's capsule with wide cellular space and cellular debris were also observed. These changes were severe in group B and group C than group D [Figure 13].
Figure 13.
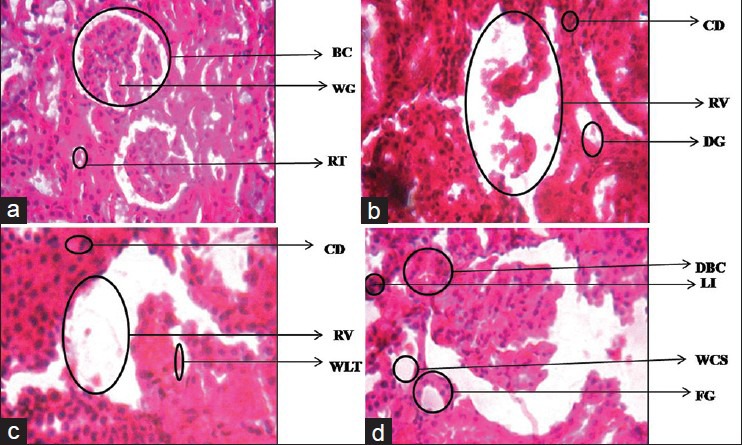
Dose-dependent effect of deltamethrin on histology of kidney in Wistar rats Enlarged Renal vein (ERV), Degenerated tubules (DG), Cellular debris (CD), Enlarged renal vein (RV), Cellular debris (CD), Wide lumen tubule (WLT), Wide cellular spaces and Leucocytic infiltrations (WCS). Kidney Group-A, Kidney Group-B, Kidney Group-C, Kidney Group-D
DISCUSSION
Usages of synthetic pyrethroids have increased in past few years owing its rapid biodegradability and target oriented insecticidal action. Widespread use deltamethrin have increased the risk of toxicity in humans. A number of studies on the side effects of deltamethrin have been reported, including neurotoxicity,[21] immunosupression,[22] and reproductive side effects.[8] In addition, hepatotoxicity[23] and nephrotoxicity[24] has also been induced. Dose-dependent system was evaluated in present study.
Monitoring of organ weight is an important parameter for the toxicological studies.[25] In the present study, liver and kidney weight increased significantly due to administration of different doses of deltamethrin as compared to control group. Liver and kidney are associated with metabolism and excretion toxicants including pesticides. Increase in weight of these organs may be a compensatory response to increase the functional demand for metabolizing and excreting higher dose of deltamethrin. Decreased in weight of testis and epididymis have been reported in this study. Testis damage on deltamethrin exposure has been reported.[5] Decrease in testis weight were also reported by Anderson et al. (2002)[26] Oda and El-Maddawy (2011)[3] in rats exposed to deltamethrin. Owing to the lipophilic nature of testicular tissue, deltamethrin may have accumulated in this tissue resulting in excessive production of ROS and tissue damage. The decrease in weight may be due to the decreased number of germ cells, inhibition of spermatogenesis and decrease in testosterone level. Recent study showed that deltamethrin depresses spermatogenesis in mammals by causing death of developing germ cells in the seminiferous tubules.[27]
Decrease in sperm count, sperm motility and increase in sperm abnormalities has been observed at different doses of deltamethrin. Induction in ROS generation has been correlated with a reduction of sperm motility.[28,29] The link between ROS and reduced motility may be due to a cascade of events that results in a decrease in axonemal protein phosphorylation and sperm immobilization, both of which are associated with a reduction in membrane fluidity that is necessary for sperm oocyte fusion.[30] The reduction in sperm count may be due to the adverse effect of different doses of deltamethrin on spermatogenesis. Testosterone is essential to maintain the structure and function of the male accessory sex gland. Lack of testosterone disrupts spermatogenesis.[31] The harmful effect of deltamethrin on spermatogenesis may be due to either reduction in serum testosterone levels or deltamethrin-induced lipid peroxidation and ROS induction. The reduction in sperm count was consistent with changes in testis and epididymal weights. Decrease in sperm concentration and sperm motility were also reported by Sakr and Al-Amoudi (2012)[5] in rats exposed to deltamethrin.
Deltamethrin showed decrease in serum testosterone, FSH and LH level in a dose-dependent manner. Dose of 6 mg/kgbw was produced more decrease compared to lesser doses. There are reports of decrease in FSH and LH on exposure to organophosphate pesticides.[32,33,34] The decrease in testosterone levels observed during the present study may be due to the effect of deltamethrin on LH and FSH and their receptors. Insecticides are considered as xenohormones and interact with natural hormones receptors. In fact, several studies revealed that androgenic hormone biosynthesis is mainly located in testicular leydig cells and the endocrine regulation of leydig cells function occurs predominantly through the action of LH/human chorionic gonadotropin (hCG), interacting with its cognate receptor and coupling to the adenylate cyclase signal transduction system.[35] Issam et al. (2009)[8] have reported reduction in FSH, LH and testosterone in male Wistar rats after deltamethrin treatment. Reduction in testosterone level were also reported by Oda and El-Maddawy (2011)[5] in rats treated with deltamethrin.
Different doses of deltamethrin significantly increased liver, kidney, brain and testis LPO level in present study. Increase in LPO level may be due to oxidation of molecular oxygen to produce superoxide radicals. This reaction is also the source of H2O2, which causes the production of Malondialdehyde (MDA) by initiating the peroxidation of unsaturated fatty acids in the membrane. The hydroxyl radical can initiate lipid per oxidation which is a free radical chain leading to loss of membrane structure and function.[1,36] The increase in LPO and generation of ROS may reduce cell viability. Thus the oxidative stress produced by deltamethrin resulted in increased LPO level in liver, kidney, and testis. Increase in LPO and ROS have been previously reported on deltamethrin exposure in liver,[8] plasma, and kidney.[5]
GSH is a tripeptide, an antioxidant and a powerful nucleophile, critical for cellular protection. It is an antioxidant, preventing damage to important cellular components caused by reactive oxygen species such as free radicals and peroxides.[37] All cells in the human body are capable of synthesizing glutathione, liver glutathione synthesis has been shown to be essential. In the present study, different doses of deltamethrin decreased the GSH level in the liver, kidney, and testis as compared with control groups. This decrease in GSH level may be due to the oxidative stress produced by deltamethrin or due to inhibition of enzymes such as GR, GPx, etc., which leads to depletion of levels of GSH. Same results were reported by Manna et al. (2005)[4] in liver of rats, Oda and El-Maddawy, 2011[5] in testis after deltamethrin administration. Parallel to this finding, deltamethrin caused a decrease in GSH content in mice and was more pronounced in kidney than the liver.[38] Decrease in non-enzymatic antioxidant GSH level were also reported by Sankar et al. 2012[39] in rats exposed to cypermethrin.
Significant decrease in SOD activity in liver, kidney, and testis was observed in rats exposed to deltamethrin. Decrease in SOD activity is likely to accumulate hydrogen peroxide in testis. Accumulated H2O2 is known to inhibit CAT activity.[40] Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of molecules of hydrogen peroxide to water and oxygen each second. Hydrogen peroxide is a harmful byproduct of many normal metabolic processes; to prevent damage to cells and tissues, it must be quickly converted into other, less dangerous substances. In the present study, CAT activity decreased in rat liver, kidney, and testis due to deltamethrin administration as compared to control group which may be due to increased hydrogen peroxides by deltamethrin exposure. Decrease in SOD and CAT were reported by Sakr and Al-Amoudi (2012)[5] in rat kidney, Manna et al. (2005)[8] in liver after deltamethrin administration. Decrease in catalase level was also reported by Sharma and Singh (2010)[41] in rat testis exposed to lindane.
In the present study, different doses of deltamethrin decreased the GPx activity in liver, kidney, and testis of rats as compared to control group. It may be due to reduced activity of glutathione (which is due to over production of free radicals) which is used as substrate for GPx. Similar results were reported by Sun et al. (2007)[42] in the rat brain exposed to deltamethrin, and Latchoumycandane and Mathur (2002)[40] in rat testis with methoxychlor.
Depletion in the GR activity in liver, kidney, and testis was also observed in rats exposed to deltamethrin, Which may be due to change in the enzyme structure caused by this pesticide. The results are also supported by the findings of Latchoumycandane and Mathur (2002)[40] who reported the decrease in the GR level in the testis of rats upon methoxychlor administration.
Total protein levels was also reported to be decreased in deltamethrin exposed groups. The decrease in the total protein may be due to depletion in the level of various antioxidative enzymes and due to the inhibition of protein synthesis.
The dose-dependent toxicity of deltamethrin was also confirmed by histological studies. In testes, degeneration of the seminiferous tubules and vacuole formation was observed. The degree of degeneration and vacuolization was more pronounced at 6 mg/kgbw as compared to lower doses. The oxidative stress generated on deltamethrin exposure may be correlated with histopathological changes in tissues. Hepatic degeneration and necrosis was also reported in this study which may be due to oxidative stress induced by deltamethrin leading to depletion of activities of enzymatic and non-enzymatic antioxidants and increase in level of LPO and ROS. Degenerated tubules and enlarged renal vein was also observed in kidney of rats exposed to deltamethrin. Necrosis in kidney may be due to oxidative stress induced by deltamethrin.
It may be concluded from the study that deltamethrin induced system toxicity in male Wistar rats in a dose-dependent manner. The higher doses produced more deleterious effect as compared to lower doses. Repeated exposure of rats for 28 day at 6mg/kgbw of the pesticide produced significant toxicity in testes, liver, and kidney. Deltamethrin decreased the activities of enzymatic and non-enzymatic antioxidants as well as increased LPO and ROS level resulting in widespread damage.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Kale M, Rathore N, John S, Bhatnagar D. Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rats erythrocytes: As possible involvement of reactive oxygen species. Toxicol Lett. 1999;105:197–205. doi: 10.1016/s0378-4274(98)00399-3. [DOI] [PubMed] [Google Scholar]
- 2.Barlow SM, Sullivan FM, Lines J. Risk assessment of the use of deltamethrin on bednets for the prevention of malaria. Food Chem Toxicol. 2001;39:407–22. doi: 10.1016/s0278-6915(00)00152-6. [DOI] [PubMed] [Google Scholar]
- 3.Oda SS, El-Maddawy ZK. Protective effect of vitamin E and selenium combination on deltamethrin-induced reproductive toxicity in male rats. Exp Toxicol Pathol. 2012;64:813–9. doi: 10.1016/j.etp.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Manna S, Bhattacharyya D, Mandal TK, Das S. Repeated dose toxicity of deltamethrin in rats. Indian J Pharmacol. 2005;37:160–4. [Google Scholar]
- 5.Sakr SA, Al-Amoudi WM. Effect of leave extract of Ocimum basilicum on deltamethrin induced nephrotoxicity and oxidative stress in albino rats. J Appl Pharma Sci. 2012;2:22–7. [Google Scholar]
- 6.Dayal M, Parmar D, Dhawan A, Dwivedi UN, Doehmer J, Seth PK. Induction of rat brain and liver cytochrome P450 1A1/1A2 and 2B1/2B2 isoenzymes by deltamethrin. Environ Toxicol Pharmacol. 1999;7:169–78. doi: 10.1016/s1382-6689(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 7.Anand SS, Bruckner JV, Haines WT, Muralidhara S, Fisher JW, Padilla S. Characterization of deltamethrin metabolism by rat plasma and liver microsomes. Toxicol Appl Pharmacol. 2006;212:156–66. doi: 10.1016/j.taap.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Issam C, Samir H, Zohra H, Monia Z, Hassen BC. Toxic responses to deltamethrin (DM) low doses on gonads, sex hormones and lipid peroxidation in male rats following subcutaneous treatments. J Toxicol Sci. 2009;34:663–70. doi: 10.2131/jts.34.663. [DOI] [PubMed] [Google Scholar]
- 9.Aitken RJ. Molecular mechanisms regulating human sperm functions. Mol Hum Reprod. 1997;3:169–73. doi: 10.1093/molehr/3.3.169. [DOI] [PubMed] [Google Scholar]
- 10.Williams J. Semen analysis and fertility assessment in rabit. In: Chapin RE, Heindel JJ, editors. Methods in Toxicology. Part A. Vol. 3. San Diego: Academic Press; 1993. pp. 344–50. [Google Scholar]
- 11.Turk G, Attesahin A, Sonmez M, Yuce A, Ceribasi AO. Lycopene protects against cyclosporine A-induced testicular toxicity in rats. Thereogenology. 2007;67:778–85. doi: 10.1016/j.theriogenology.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Choi EK, Tsunekawa N, Kanai Y, Kurohmaru M. A new protocol for measurement of testicular sperm production. J Reprod Dev. 2008;54:90–3. doi: 10.1262/jrd.19123. [DOI] [PubMed] [Google Scholar]
- 13.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Kakkar P, Das B, Viswnathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 16.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 17.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 18.Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–90. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 19.Habig WJ, Pabst M, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 21.Husain R, Malaviya M, Seth PK, Husain R. Effect of deltamethrin on regional brain polyamines and behaviour in young rats. Pharmacol Toxicol. 1994;74:211–5. doi: 10.1111/j.1600-0773.1994.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 22.Lukowicz-Ratajczak J, Krechniak J. Effects of deltamethrin on the immune system in mice Environ Res. 1992;59:467–75. doi: 10.1016/s0013-9351(05)80049-0. [DOI] [PubMed] [Google Scholar]
- 23.Chargui I, Grissa I, Bensassi F, Hrira MY, Haouem S, Haouas Z, et al. Oxidative stress, biochemical and histopathological alterations in the liver and kidney of female rats exposed to low doses of deltamethrin (DM): A molecular assessment. Biomed Environ Sci. 2012;25:672–83. doi: 10.3967/0895-3988.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 24.El-Gerbed MS. Protective effect of lycopene on deltamethrin-induced histological and ultrastructural changes in kidney tissue of rats. Toxicol Ind Health. 2014;30:160–73. doi: 10.1177/0748233712448115. [DOI] [PubMed] [Google Scholar]
- 25.Yavasoglu A, Karaaslan MA, Uyanikgil Y, Sayim F, Ates U, Yavasoglu NU. Toxic effects of anatoxin-a on testes and sperm counts of male mice. Exp Toxicol Pathol. 2008;60:391–6. doi: 10.1016/j.etp.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JM, Samanta AU, Gladys MS, Masahiko O, Paulo RD. Reproductive effects of deltamethrin on male offspring of rats exposed during pregnancy and lactation. Regul Toxicol Pharmacol. 2002;36:310–7. doi: 10.1006/rtph.2002.1586. [DOI] [PubMed] [Google Scholar]
- 27.Shivaraj, David M, Ravi KB. Spermatotoxicity evaluation of deltamethrin 1%+chlorpyriphos 35% ec by oral gavage in Wistar rats. Int J Pharma Bio Sci. 2011;2:261–3. [Google Scholar]
- 28.Lenzi A, Cualosso F, Gandini L, Lombardo F, Dondero F. Placebo controlled, double-blind, cross-over trial of glutathione therapy, in male infertility. Hum Reprod. 1993;9:2044–50. doi: 10.1093/oxfordjournals.humrep.a137909. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong IS, Rajasekaran M, Chamulitrat W, Gatti P, Hellstrom WJ, Sikka SC. Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Free Radic Biol Med. 1999;26:869–80. doi: 10.1016/s0891-5849(98)00275-5. [DOI] [PubMed] [Google Scholar]
- 30.de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum Reprod. 1995;10:15–21. doi: 10.1093/humrep/10.suppl_1.15. [DOI] [PubMed] [Google Scholar]
- 31.Broockfor FR, Blake CA. Chronic administration of 4-tert-octylphenol to adult male rats causes shrinkage to the testes and male accessory sex organs, disrupts sper- matogenesis, and increases the incidence of sperm deformities. Biol Reprod. 1997;57:267–77. doi: 10.1095/biolreprod57.2.267. [DOI] [PubMed] [Google Scholar]
- 32.Gore AC. Environmental toxicant effects on neuroendocrine function. Endocrine. 2001;14:235–46. doi: 10.1385/ENDO:14:2:235. [DOI] [PubMed] [Google Scholar]
- 33.Padungtod C, Lasley BL, Christiani DC, Ryan LM, Xu X. Reproductive hormone profile among pesticide factory workers. J Occup Med. 1998;40:1038–47. doi: 10.1097/00043764-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Guven M, Bayram F, Unluhizarc K, Kelestimur F. Endocrine changes in patients with acute organophosphate poisoning. Hum Toxicol. 1999;18:598–601. doi: 10.1191/096032799678839419. [DOI] [PubMed] [Google Scholar]
- 35.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–44. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 36.Ray DE. Pesticides derived from plants and other organism. In: Hayes WY Jr, Lows ER Jr, editors. Handbook of Pesticides Toxicology. San Diego: Academic Press Inc; 1991. pp. 585–636. [Google Scholar]
- 37.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 38.Rehman H, Ali M, Atif F, Kaur M, Bhatia K, Raisuddin S. The modulatory effect of deltamethrin on antioxidants in mice. Clin Chem Acta. 2006;369:61–5. doi: 10.1016/j.cca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Sankar P, Telang AG, Manimaran A. Protective effect of curcumin on cypermethrin-induced oxidative stress in Wistar rats. Exp Toxicol Pathol. 2012;64:487–93. doi: 10.1016/j.etp.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Latchoumycandane C, Mathur PP. Induction of oxidative stress in the rat testis after short-term exposure to the organochlorine pesticide Methoxychlor. Arch Toxicol. 2002;76:692–8. doi: 10.1007/s00204-002-0388-9. [DOI] [PubMed] [Google Scholar]
- 41.Sharma P, Singh R. Protective role of curcumin on lindane induced reproductive toxicity in male Wistar rats. Bull Environ Contam Toxicol. 2010;84:378–84. doi: 10.1007/s00128-010-9942-y. [DOI] [PubMed] [Google Scholar]
- 42.Sun M, Xu PP, Ren Y, Li YF, Zhong YF, Yan H. Protective effect of melatonin on oxidative damage by Deltamethrin in rat brain. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2007;25:155–8. [PubMed] [Google Scholar]


