Abstract
Objectives:
Vitamin D has been shown to hamper the growth of Mycobacterium tuberculosis in macrophages. The actions of vitamin D are exerted through a vitamin D receptor (VDR). The genetic variant TaqI of VDR has been implicated in tuberculosis (TB) risk in several case-control studies. However, these studies have shown inconsistent results. Hence, a meta-analysis was conducted to investigate the potential relationship between VDR TaqI polymorphism and risk of developing TB.
Materials and Methods:
We performed a quantitative synthesis for published studies based upon the relationship between TaqI polymorphism and TB risk from PubMed (Medline) and Embase databases. The meta-analysis was performed and pooled odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for all genetic models.
Results:
A total of 21 studies including 2,960 TB cases and 3,894 controls were included in this study. The pooled analysis demonstrated no evidence of association between VDR TaqI genotypes and risk of TB in any of the genetic models; variant (t vs T: P = 0.618; OR = 1.051, 95% CI = 0.864–1.278), homozygous (tt vs TT: P = 0.120; OR = 1.336, 95% CI = 0.927–1.924), heterozygous (Tt vs TT: P = 0.925; OR = 0.988, 95% CI = 0.774–1.262), dominant model (tt + Tt vs TT: P = 0.805; OR = 1.032, 95% CI = 0.805–1.322), and recessive model (tt vs TT + Tt: P = 0.180; OR = 1.229, 95% CI = 0.909–1.660). No publication bias was detected during the analysis.
Conclusions:
Overall findings of this meta-analysis suggest that genetic polymorphism TaqI of VDR gene may not contribute to the risk of TB. However, future larger studies with group of populations are warranted to analyze this relationship.
Keywords: Meta-analysis, tuberculosis, vitamin D receptor
INTRODUCTION
Tuberculosis (TB) is one of the leading chronic infectious disease worldwide and cause great harm to human beings. TB is mainly caused by Mycobacterium tuberculosis (M. tuberculosis), an intracellular pathogen that dwells within macrophages, leading cause of death worldwide annually.[1] About one-third of the world's population is thought to be infected with M. tuberculosis, but only small fraction (5-15%) of population develops an active TB disease during their lifetime.[2] Earlier studies have been shown that susceptibility to TB at different rates indicate that host genetic risk factors play an important role in the susceptibility to TB.[3] Therefore, it is predicted that the identification of host genetic factors for susceptibility to TB would greatly assist the global control and therapeutic strategies of this infectious disease.
Vitamin D is an immune-regulatory hormone, exerts its actions by vitamin D receptor (VDR), commonly involve in macrophages activation and kill the bacteria inside the macrophages cells.[4,5] The human VDR is a nuclear receptor gene of 75 kb size (located on 12q12–14) and consists of 11 exons and 11 introns.[6] VDR gene polymorphisms are occurred in several restriction enzyme sites, among them TaqI (rs731236) polymorphism located in exon 9 is the well-known polymorphism of VDR gene.[7] Previously it has been reported that mutant t/t genotype of TaqI occurred more frequently in TB patients and has been shown to affect transcription and diminished VDR functions.[8]
Having known the functional significance of this genetic variant and extensive role of VDR gene and its immune response against TB provided indication of vitamin D-related gene-environment interactions in the host response to TB.[9] Mutations in the VDR gene that impair VDR functions are associated with frequent and severe episodes of infection. Several epidemiological studies have been done in recent past in various ethnic populations to investigate the relationship between TaqI polymorphism and TB risk,[10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] but, they yielded inconsistent and conflicting results. Inconsistency in the results of those studies was mainly attributed to ethnicity of the population, sample size, and individual studies that have low power to examine the overall effect. The answer of these limitations is a meta-analysis, which is a powerful method for investigating the risk factors associated with genetic diseases. It employs quantitative method to pool the data from individual studies where individual sample sizes are small and bears low statistical power, and delivers reliable and appropriate conclusion.[31,32] In the present study, a meta-analysis has been performed to evaluate the association of TaqI variant of VDR gene with the risk of human TB.
MATERIALS AND METHODS
Literature search strategy
We performed a PubMed (Medline) and Embase web databases search covering all research articles published with a combination of the following key words: ‘VDR, Vitamin D receptor gene (polymorphism OR mutation OR variant) AND tuberculosis susceptibility or TB (last updated on October 2013). Potentially relevant genetic association studies were evaluated by examining their titles and abstracts, and all published studies matching with the eligible criteria were retrieved.
Inclusion and exclusion criteria
To minimize heterogeneity and facilitate the proper understanding of this study, published articles included in the current meta-analysis had to fulfill all the following criteria: (a) Must have assessed the association between VDR TaqI polymorphism and TB risk, (b) used a case-control design of the study, (c) recruited pathologically confirmed TB patients and TB free controls, (d) have available genotype frequency in case and control, (e) published in the English language. Also, when the case-control design study was included by more than one research article using the same case series, we selected the research study that included the largest number of individuals. The major reasons for study exclusion were: Data overlapping, review articles, case-only studies, and genotype frequencies or number not reported. The flow chart of the study selection procedure showing the inclusion and exclusion criteria have been appended as Figure 1.
Figure 1.
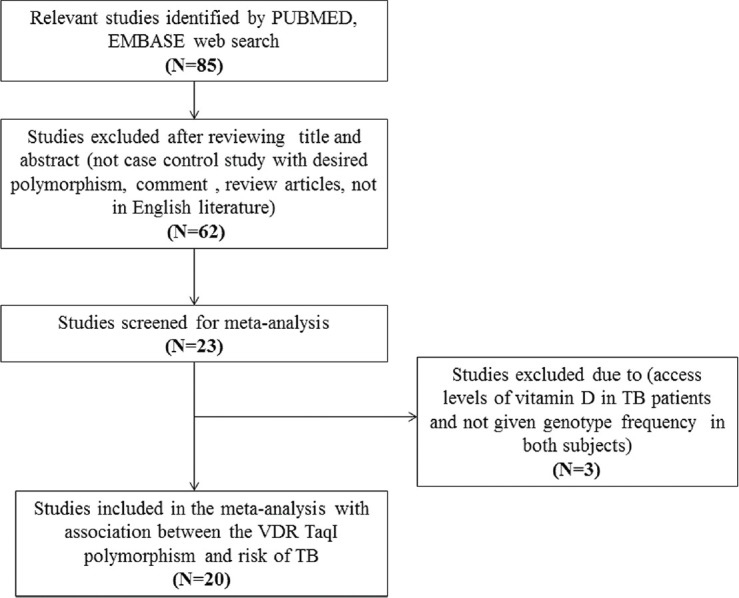
Flow chart of selection of studies. VDR = Vitamin D receptor, TB = tuberculosis
Data extraction and quality assessment
For each retrieved article, the methodological quality assessment and data extraction were independently abstracted in duplicate by two independent investigators following a standard protocol. Data-collection sheet was used to ensure the accuracy of the collected data by stringently following the inclusion-exclusion criteria as mentioned above. The major characteristics abstracted from the retrieved studies were, the name of first author, year of publication, the country of origin, the number of cases and controls, source of cases and controls, study design, and genotype frequencies. Cases related with disagreement on any item of the collected data from the selected studies were thoroughly debated with investigators to reach a final consensus.
Statistical analysis
In order to evaluate the relationship between VDR TaqI polymorphism and TB risk, pooled odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were calculated. Heterogeneity assumption was inspected by the Chi-square-based Q-test.[33] Heterogeneity was considered significant at P < 0.05. In case of non-heterogeneity, the data obtained from single comparison was combined using the fixed effects model.[34] Otherwise, the random effects model[35] was used for pooling of the data. Moreover, I2 statistics was used to quantify inter-study variability and larger values suggested an increasing degree of heterogeneity.[36] Hardy-Weinberg equilibrium (HWE) in the controls was calculated by Chi-square test. Funnel plot asymmetry was estimated by Egger's linear regression test. Egger's regression is a type of linear regression approach to measure the funnel plot asymmetry on the natural logarithm scale of the OR. The significance of the intercept was determined by the t-test (statistically significant publication bias was considered at P < 0.05).[37] A comparative assessment of ‘meta-analysis’ software programs was done by using uniform resource locator address http://www.meta-analysis.com/pages/comparisons.html. The Comprehensive Meta-Analysis (CMA) V2 program (Biostat, USA) was utilized to carry out this meta-analysis.
RESULTS
Characteristics of the published studies
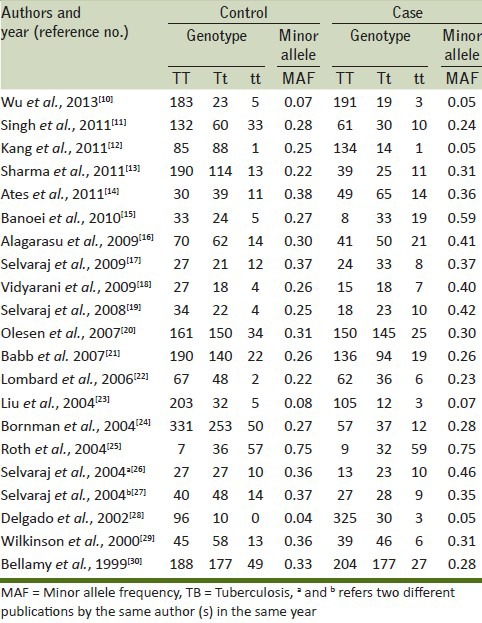
Following the inclusion and exclusion criteria of the study selection, a total of 21 research articles were finally retrieved through literature search from the PubMed (Medline) and Embase online web databases. All selected articles were examined carefully by reading the titles and abstracts, and the full texts for the potentially relevant published articles were further checked for their aptness for this meta-analysis. Publications either considering VDR variants as an indicator for response to therapy or showing VDR polymorphism to predict survival in TB patients were excluded straightaway from this study. Also, studies showing the investigations of levels of VDR mRNA or protein expression or pertinent review articles were also excluded from this meta-analysis. Only case-control or cohort design studies having frequency of all three genotypes were incorporated in this study. Besides the online web database search, the references listed in the selected articles were also checked for other potential studies [Table 1]. Distributions of genotypes and minor allele frequencies (MAFs) of controls and cases for the selected studies have been shown in Table 2.
Table 1.
Main characteristics of the selected studies included in the meta-analysis
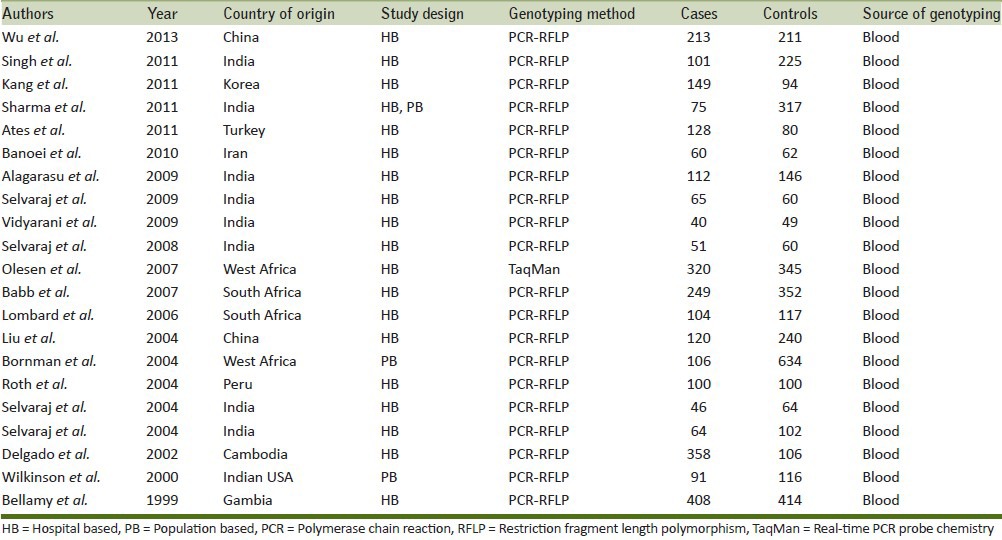
Table 2.
Distribution of TaqI gene polymorphism among controls and TB cases of all included studies
Publication bias
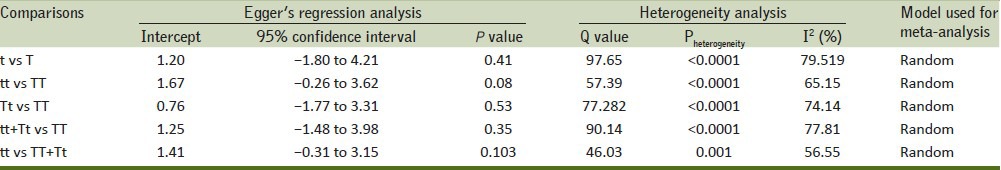
Begg's funnel plot and Egger's test were performed to review the publication bias among the selected studies for the meta-analysis. The appearance of the shape of funnel plots was seemed to be symmetrical in all genetic models. The Egger's test was done to provide the statistical confirmation of funnel plot. The outcomes of above tests resulted into lack of publication bias among all comparison models [Table 3].
Table 3.
Statistics to test publication bias and heterogeneity in the meta-analysis
Test of heterogeneity
In order to test heterogeneity among all selected studies, Q-test and I2 statistics were utilized. Heterogeneity was noted in all the models, that is, allele (t vs T), homozygous (tt vs TT), heterozygous (Tt vs. TT), dominant (tt + Tt vs TT), and recessive (tt vs TT + Tt) genotype models, which were included for this meta-analysis. Therefore, the random effects model was applied to synthesize the data [Table 3].
TaqI polymorphism of VDR gene and TB susceptibility
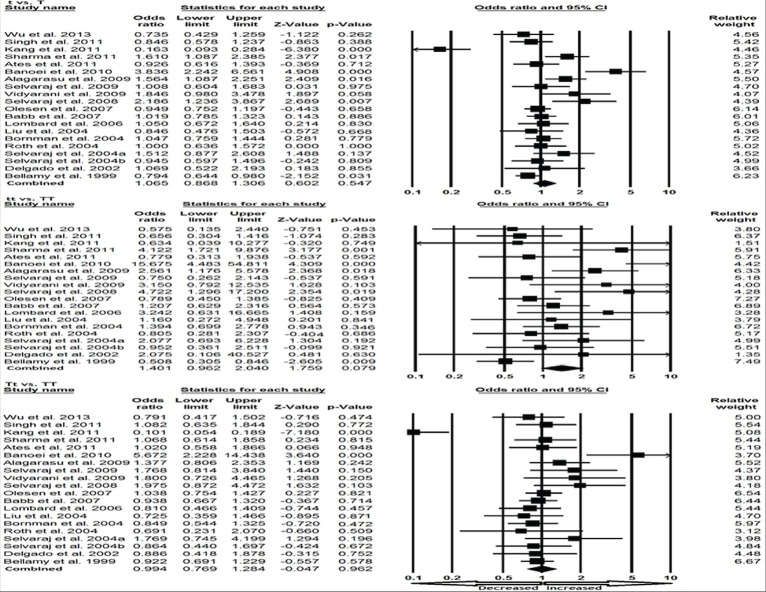
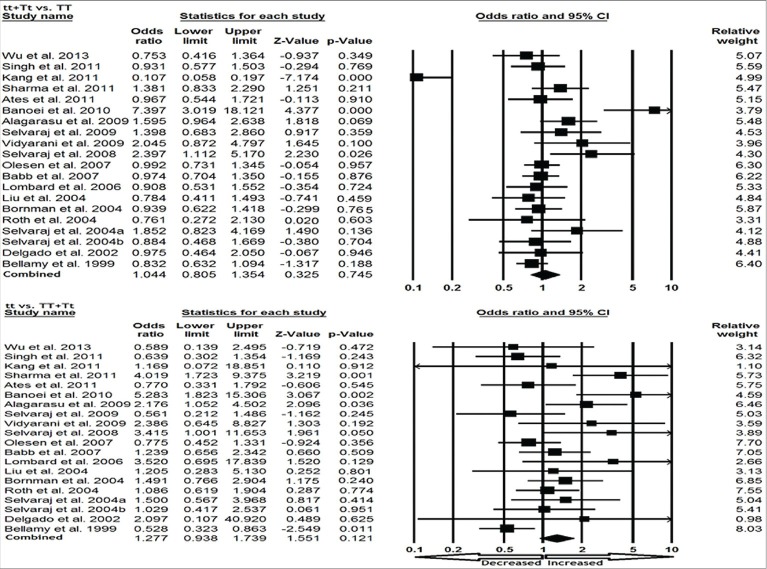
We pooled all 21 studies together and it resulted into 2,960 confirmed TB cases and 3,894 controls for assessment of overall association between the VDR TaqI polymorphism and risk of TB. The pooled results suggested that individuals who carry variant allele (t vs T: P = 0.618; OR = 1.051, 95% CI = 0.864–1.278), homozygous (tt vs TT: P = 0.120; OR = 1.336, 95% CI = 0.927-1.924), heterozygous (Tt vs TT: P = 0.925; OR = 0.988, 95% CI = 0.774–1.262) may not have an increased/decreased TB risk compared with the homozygote TT carriers [Figure 2]. Likewise, dominant (tt + Tt vs. TT: P = 0.805; OR = 1.032, 95% CI = 0.805 to 1.322) and recessive (tt vs. TT + Tt: P = 0.180; OR = 1.229, 95% CI = 0.909–1.660) models also failed to show any association with the risk for TB occurrence [Figure 3].
Figure 2.
Forest plot and ORs with 95% CI of TaqI variant of VDR gene and TB risk (t vs T; Tt vs TT; tt vs TT). OR = Odds ratio, CI = Confidence interval. The squares and horizontal lines correspond to the study-specific OR and 95% CI
Figure 3.
Forest plot and ORs with 95% CI of TaqI variant of VDR gene and TB risk (tt + Tt vs TT; tt vs TT + Tt). The squares and horizontal lines correspond to the study-specific OR and 95% CI
DISCUSSION
Vitamin D acts by triggering a chain of events via binding to the VDR that can influence cellular differentiation, inflammation, the immune and endocrine systems, insulin resistance, and lipid metabolism.[38] The VDR affects both innate and adaptive immunity, and especially innate immunity genes play significant role in the modulation of host susceptibility towards TB infection because the first line defense against M. tuberculosis involves the identification and uptake of the bacterium by macrophages and dendritic cells.[39] Previous studies in mouse models reported that vitamin D-deficient mice were more prone for increased sensitivity towards autoimmune diseases.[40] The significance of polymorphic variant genotypes of VDR gene has been emphasized on the resistance or susceptibility to various infectious diseases including TB.[41] Earlier reports demonstrated that susceptibility to TB and risk of progression from infection to the disease tends to occur more often in patients with low vitamin D levels.[42,43]
An increasing number of epidemiological studies investigated the association of the VDR TaqI polymorphism with TB risk and published inconclusive and contradictory results, because they were underpowered and impossible to reach any conclusion by examining the alleles separately. Hence, in order to conclude more precisely, we performed this meta-analysis to appraise whether an association exists between the VDR TaqI polymorphism and risk of developing TB. Pool ORs generated from large sample size and population can enhance the statistical power and pooling data from various studies has the advantage of less random errors.[44]
The pooled results of this meta-analysis revealed that VDR TaqI genetic polymorphism is not significantly associated with the risk of TB. Individuals carried the mutant allele t of TaqI polymorphism of VDR gene did not have risk of developing TB. One possible explanation is that several other single nucleotide polymorphisms (SNPs) have been reported in 3’- untranslated region (UTR) of the VDR gene and previous studies proved that these SNPs are associated with the risk of TB. It is possible that the analyzed variant does not act as primary susceptibility polymorphism and may be inhibiting VDR functions by linking with other functional polymorphism alleles found in linkage disequilibrium (LD). Previous meta-analyses of Gao et al., and Lewis et al., reported no significant association between TB risk and VDR TaqI gene polymorphism.[45,46] Similarly, Cao et al., also did not observe significant risk to TB in association with VDR TaqI polymorphism among Chinese population.[47] Additionally, susceptibility to TB is polygenic in nature; multicandidate genes are likely to be involved in the process of active disease development.[48] Due to the multifactorial nature of TB infection and complex design of the immune system,[49] single genetic variant is usually inadequate to predict the risk of TB.
Heterogeneity between studies is very common in the genetic association studies related to the meta-analysis. In the present meta-analysis we found inter-study heterogeneity in overall analysis. Several factors are responsible for such heterogeneity, for example, the genetic backgrounds for cases and controls, diverse genotype distribution of TaqI in different ethnic groups suggest that they are almost/always subjected to natural selection.[50] Moreover, the uneven selection criteria for the cases and the controls in different studies contribute for this heterogeneity.
Despite the findings from this meta-analysis, we still have to acknowledge some limitations of our study. First, we only included studies published in the English language, abstracted and indexed by the selected electronic web databases were included for data analysis; it is possible that some pertinent studies published in other languages and indexed in other electronic databases may have been missed. Although, many meta-analyses have been done considering various case-control studies analyzing this relationship selected from various databases and concluded accordingly.[51] But, due to limited database selection, each study misses some of the pertinent publications. The research article available in one database is not necessarily available in another one, so, every meta-analysis has certain limitations of database selection. Similarly, in this meta-analysis we considered most reliable databases (i.e. Pubmed/Embase) and tried to include some of the missing studies which were not included in other studies and concluded accordingly. However, possibilities are there that certain articles have been missed in this study, possibly due to database or language limitation. Second, the findings of this meta-analysis were based on unadjusted ORs because not all eligible studies reported adjusted ORs. Third, the impacts of gene-environment interactions were not considered, which may influence the risk of TB. Also, it is worthwhile to mention several strengths of this study. First, we did not detect publication bias, which suggests that our results were statistically robust. Second, we included significantly more number of cases and controls in comparison to previous meta-analysis based studies by using effective and efficient searching strategy. Third, strict inclusion/exclusion criteria were followed to limit the potential bias among studies.
In conclusion, a meta-analysis is an extremely valuable and reasonable approach of data analysis, which pools both statistically significant and nonsignificant results from individual studies and generates an absolute conclusion. This meta-analysis evaluated the relationship of TaqI polymorphism of VDR gene with the risk of TB and suggested that the TaqI polymorphism of VDR gene is not associated with susceptibility to TB. Thus, screening application of this genetic polymorphism in asymptomatic individuals is not warranted. However, well-designed large-scale association studies incorporating with consideration of environmental factors in different populations might be needed to authenticate our current findings and to enhance understanding of the underlying pathophysiology, and such future research studies might eventually lead to deliver deep insight and absolute understanding of the possible relationship between the VDR TaqI polymorphism and TB risk. Here, we only analyzed the VDR TaqI polymorphism in relation with TB risk; in our future study we will try to further explore the other interactions (as stated above) to facilitate the understanding of the pathophysiology of TB.
ACKNOWLEDGMENTS
Authors sincerely acknowledge the software related support for statistical analysis provided by the Institute of Life Sciences (Bhubaneswar, India) and Jazan University (Jazan, Saudi Arabia).
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990 – 2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Rosman MD, Oner-Eyupoglu AF. Clinical presentation and treatment of tuberculosis. In: Fishman AP, editor. Fishman's Pulmonary Diseases and Disorders. New York: McGraw-Hill; 1998. pp. 2483–502. [Google Scholar]
- 3.Nava-Aguilera E, Andersson N, Harris E, Mitchell S, Hamel C, Shea B, et al. Risk factors associated with recent transmission of tuberculosis: Systematic review and meta-analysis. Int J Tuberc Lung Dis. 2009;13:17–26. [PubMed] [Google Scholar]
- 4.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, et al. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–49. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 5.Deluca HF, Cantorna MT. Vitamin D: Its role and uses in immunology. FASEB J. 2001;15:2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 6.Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000;22:203–17. doi: 10.1093/oxfordjournals.epirev.a018033. [DOI] [PubMed] [Google Scholar]
- 7.Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, et al. Prediction of bone density from vitamin D receptor alleles. Nature. 1994;367:284–7. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- 8.Whitfield GK, Remus LS, Jurutka PW, Zitzer H, Oza AK, Dang HT, et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol. 2001;177:145–59. doi: 10.1016/s0303-7207(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy R. Evidence of gene-environment interaction in development of tuberculosis. Lancet. 2000;355:588–9. doi: 10.1016/S0140-6736(99)00426-2. [DOI] [PubMed] [Google Scholar]
- 10.Wu F, Zhang W, Zhang L, Wu J, Li C, Meng X, et al. NRAMP1, VDR, HLA-DRB1, and HLA-DQB1 gene polymorphisms in susceptibility to tuberculosis among the Chinese Kazakh population: A case-control study. Biomed Res Int 2013. 2013:484535. doi: 10.1155/2013/484535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh A, Gaughan JP, Kashyap VK. SLC11A1 and VDR gene variants and susceptibility to tuberculosis and disease progression in East India. Int J Tuberc Lung Dis. 2011;15:1468–74. doi: 10.5588/ijtld.11.0089. [DOI] [PubMed] [Google Scholar]
- 12.Kang TJ, Jin SH, Yeum CE, Lee SB, Kim CH, Lee SH, et al. Vitamin D Receptor Gene TaqI, BsmI and FokI Polymorphisms in Korean Patients with Tuberculosis. Immune Netw. 2011;11:253–7. doi: 10.4110/in.2011.11.5.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma PR, Singh S, Jena M, Mishra G, Prakash R, Das PK, et al. Coding and non-coding polymorphisms in VDR gene and susceptibility to pulmonary tuberculosis in tribes, castes and Muslims of Central India. Infect Genet Evol. 2011;11:1456–61. doi: 10.1016/j.meegid.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Ates O, Dolek B, Dalyan L, Musellim B, Ongen G, Topal-Sarikaya A. The association between BsmI variant of vitamin D receptor gene and susceptibility to tuberculosis. Mol Biol Rep. 2011;38:2633–6. doi: 10.1007/s11033-010-0404-8. [DOI] [PubMed] [Google Scholar]
- 15.Banoei MM, Mirsaeidi MS, Houshmand M, Tabarsi P, Ebrahimi G, Zargari L, et al. Vitamin D receptor homozygote mutant tt and bb are associated with susceptibility to pulmonary tuberculosis in the Iranian population. Int J Infect Dis. 2010;14:e84–5. doi: 10.1016/j.ijid.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Alagarasu K, Selvaraj P, Swaminathan S, Narendran G, Narayanan PR. 5’ regulatory and 3’ untranslated region polymorphisms of vitamin D receptor gene in south Indian HIV and HIV-TB patients. J Clin Immunol. 2009;29:196–204. doi: 10.1007/s10875-008-9234-z. [DOI] [PubMed] [Google Scholar]
- 17.Selvaraj P, Prabhu Anand S, Harishankar M, Alagarasu K. Plasma 1,25 dihydroxy vitamin D3 level and expression of vitamin D receptor and cathelicidin in pulmonary tuberculosis. J Clin Immunol. 2009;29:470–8. doi: 10.1007/s10875-009-9277-9. [DOI] [PubMed] [Google Scholar]
- 18.Vidyarani M, Selvaraj P, Raghavan S, Narayanan PR. Regulatory role of 1,25-dihydroxy vitamin D3 and vitamin D receptor gene variants on intracellular granzyme A expression in pulmonary tuberculosis. Exp Mol Pathol. 2009;86:69–73. doi: 10.1016/j.yexmp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Selvaraj P, Vidyarani M, Alagarasu K, Prabhu Anand S, Narayanan PR. Regulatory role of promoter and 3’ UTR variants of vitamin D receptor gene on cytokine response in pulmonary tuberculosis. J Clin Immunol. 2008;28:306–13. doi: 10.1007/s10875-007-9152-5. [DOI] [PubMed] [Google Scholar]
- 20.Olesen R, Wejse C, Velez DR, Bisseye C, Sodemann M, Aaby P, et al. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun. 2007;8:456–67. doi: 10.1038/sj.gene.6364410. [DOI] [PubMed] [Google Scholar]
- 21.Babb C, van der Merwe L, Beyers N, Pheiffer C, Walzl G, Duncan K, et al. Vitamin D receptor gene polymorphisms and sputum conversion time in pulmonary tuberculosis patients. Tuberculosis (Edinb) 2007;87:295–302. doi: 10.1016/j.tube.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Lombard Z, Dalton DL, Venter PA, Williams RC, Bornman L. Association of HLA-DR, -DQ, and vitamin D receptor alleles and haplotypes with tuberculosis in the Venda of South Africa. Hum Immunol. 2006;67:643–54. doi: 10.1016/j.humimm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Cao WC, Zhang CY, Tian L, Wu XM, Habbema JD, et al. VDR and NRAMP1 gene polymorphisms in susceptibility to pulmonary tuberculosis among the Chinese Han population: A case-control study. Int J Tuberc Lung Dis. 2004;8:428–34. [PubMed] [Google Scholar]
- 24.Bornman L, Campbell SJ, Fielding K, Bah B, Sillah J, Gustafson P, et al. Vitamin D receptor polymorphisms and susceptibility to tuberculosis in West Africa: A case-control and family study. J Infect Dis. 2004;190:1631–41. doi: 10.1086/424462. [DOI] [PubMed] [Google Scholar]
- 25.Roth DE, Soto G, Arenas F, Bautista CT, Ortiz J, Rodriguez R, et al. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J Infect Dis. 2004;190:920–7. doi: 10.1086/423212. [DOI] [PubMed] [Google Scholar]
- 26.Selvaraj P, Chandra G, Jawahar MS, Rani MV, Rajeshwari DN, Narayanan PR. Regulatory role of vitamin D receptor gene variants of Bsm I, Apa I, Taq I, and Fok I polymorphisms on macrophage phagocytosis and lymphoproliferative response to Mycobacterium tuberculosis antigen in pulmonary tuberculosis. J Clin Immunol. 2004;24:523–32. doi: 10.1023/B:JOCI.0000040923.07879.31. [DOI] [PubMed] [Google Scholar]
- 27.Selvaraj P, Kurian SM, Chandra G, Reetha AM, Charles N, Narayanan PR. Vitamin D receptor gene variants of BsmI, ApaI, TaqI, and FokI polymorphisms in spinal tuberculosis. Clin Genet. 2004;65:73–6. doi: 10.1111/j..2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 28.Delgado JC, Baena A, Thim S, Goldfeld AE. Ethnic-specific genetic associations with pulmonary tuberculosis. J Infect Dis. 2002;186:1463–8. doi: 10.1086/344891. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: A case-control study. Lancet. 2000;355:618–21. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 30.Bellamy R, Ruwende C, Corrah T, McAdam KP, Thursz M, Whittle HC, et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721–4. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 31.Cohn LD, Becker BJ. How meta-analysis increases statistical power. Psychol Methods. 2003;8:243–53. doi: 10.1037/1082-989X.8.3.243. [DOI] [PubMed] [Google Scholar]
- 32.Areeshi MY, Mandal RK, Panda AK, Bisht SC, Haque S. CD14 -159 C>T gene polymorphism with increased risk of tuberculosis: Evidence from a meta-analysis. PLoS One. 2013;8:e64747. doi: 10.1371/journal.pone.0064747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu R, Li B. A multiplicative-epistatic model for analyzing interspecific differences in outcrossing species. Biometrics. 1999;55:355–65. doi: 10.1111/j.0006-341x.1999.00355.x. [DOI] [PubMed] [Google Scholar]
- 34.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rostand SG, Warnock DG. Introduction to vitamin D symposium, March 14, 2008. Clin J Am Soc Nephrol. 2008;3:1534. doi: 10.2215/CJN.01130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998;66:5314–21. doi: 10.1128/iai.66.11.5314-5321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill AV. The genomics and genetics of human infectious disease susceptibility. Annu Rev Genomics Hum Genet. 2001;2:373–400. doi: 10.1146/annurev.genom.2.1.373. [DOI] [PubMed] [Google Scholar]
- 42.Gibney KB, MacGregor L, Leder K, Torresi J, Marshall C, Ebeling PR, et al. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008;46:443–6. doi: 10.1086/525268. [DOI] [PubMed] [Google Scholar]
- 43.Sita-Lumsden A, Lapthorn G, Swaminathan R, Milburn HJ. Reactivation of tuberculosis and vitamin D deficiency: The contribution of diet and exposure to sunlight. Thora×. 2007;62:1003–7. doi: 10.1136/thx.2006.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ioannidis JP, Boffetta P, Little J, O’Brien TR, Uitterlinden AG, Vineis P, et al. Assessment of cumulative evidence on genetic associations: Interim guidelines. Int J Epidemiol. 2008;37:120–32. doi: 10.1093/ije/dym159. [DOI] [PubMed] [Google Scholar]
- 45.Gao L, Tao Y, Zhang L, Jin Q. Vitamin D receptor genetic polymorphisms and tuberculosis: Updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14:15–23. [PubMed] [Google Scholar]
- 46.Lewis SJ, Baker I, Davey Smith G. Meta-analysis of vitamin D receptor polymorphisms and pulmonary tuberculosis risk. Int J Tuberc Lung Dis. 2005;9:1174–7. [PubMed] [Google Scholar]
- 47.Cao S, Luo PF, Li W, Tang WQ, Cong XN, Wei PM. Vitamin D receptor genetic polymorphisms and tuberculosis among Chinese Han ethnic group. Chin Med J (Engl) 2012;125:920–5. [PubMed] [Google Scholar]
- 48.Bellamy R. Genome-wide approaches to identifying genetic factors in host susceptibility to tuberculosis. Microbes Infect. 2006;8:1119–23. doi: 10.1016/j.micinf.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 49.Möller M, Hoal EG. Current findings, challenges and novel approaches in human genetic susceptibility to tuberculosis. Tuberculosis (Edinb) 2010;90:71–83. doi: 10.1016/j.tube.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Bamshad M, Wooding SP. Signatures of natural selection in the human genome. Nat Rev Genet. 2003;4:99–111. doi: 10.1038/nrg999. [DOI] [PubMed] [Google Scholar]
- 51.Chen C, Liu Q, Zhu L, Yang H, Lu W. Vitamin Dreceptor gene polymorphisms on the risk of tuberculosis, a meta-analysis of 29 case-control studies. Plos One. 8(12):e83843. doi: 10.1371/journal.pone.0083843. doi: 10.1371/journal.pone. 0083843. [DOI] [PMC free article] [PubMed] [Google Scholar]