Abstract
Background:
The conflicting roles of quercetin against tissue pathologies associated with oxidative stress are known.
Objective:
To evaluate the effect of quercetin at doses of 5 mg/kg (Q5) or 10 mg/kg (Q10) against atrazine (120 mg/kg, ATZ)-induced oxidative stress in various tissues of rats.
Materials and Methods:
Adult male albino Wistar rats were administered ATZ, Q5, and Q10 alone or in combination for 16 days. At the end of the 16th day, the animals were sacrificed by cervical dislocation; and the blood, heart, brain, kidney and liver were collected and used for biochemical determinations and histopathological examination.
Results:
Q10 but not Q5 attenuated ATZ-induced increase in the levels of serum enzyme markers sorbitol dehydrogenase (SDH), acid phosphatase (ACP), alkaline phosphatase (ALP), and aspartate aminotransferase (AST). The heart was less susceptible to ATZ-induced oxidative stress than the liver, kidney, and brain of treated animals, and there were tendencies for synergistic effects in the heart and liver of Q5 + ATZ-treated rats. Oxidative stress-induced by ATZ in terms of increased lipid peroxidation level and superoxide dismutase (SOD) activity was decreased in the brain of the Q5 + ATZ-treated rats but not that of the Q10 + ATZ-treated rats. Conversely, histopathological changes and oxidative stress-induced by ATZ in terms of elevated lipid peroxidation level, decreased SOD, and catalase (CAT) activities were prevented in the kidney and liver of the Q10 + ATZ-treated rats but not that of the Q5 + ATZ-treated rats.
Conclusion:
Quercetin at the investigated doses and especially the low dose may not protect against ATZ-induced oxidative stress in rat tissues in an overall sense.
Keywords: Atrazine, malondialdehyde, oxidative stress, quercetin, serum marker enzymes
INTRODUCTION
More attention has been paid to the protective effects of flavonoids against drug-induced toxicities especially whenever oxidative stress is involved.[1] Quercetin, a representative flavonoid shows a variety of biological activities, including anticarcinogenic, antiinflammatory, and antiviral actions. The protective effects of quercetin against chemically induced tissue pathologies are widely known. For example, quercetin protects carbon tetrachloride-induced hepatotoxicity, 2,4-D-induced oxidative damage in spermatogonial cells, ethanol-induced oxidative stress, cyclosporin-induced nephrotoxicity, cisplatin-induced cytotoxicity in cultured tubular epithelial cells, atrazine (ATZ)-induced cytotoxicity in cultured Sertoli-germ cells and Leydig cells.[2,3,4,5,6,7,8] Many of these beneficial effects of quercetin are considered to be related to the experimental situation, antioxidant properties of quercetin, and the dose of quercetin used.[9] For instance, quercetin inhibits the deoxyribonucleic acid (DNA) damage caused by H2O2, but at high concentration it induces cellular DNA damage,[7] and also act as pro-oxidants on glutathione antioxidant system.[9,10,11]
ATZ, a triazine-based herbicide extensively used in agriculture worldwide, is a well-known environmental pollutant that is reported to induce toxic effects in several tissues of mammalian experimental models.[12] It is known to cause hepatic damage in rats and pigs;[13,14,15] disrupt the functioning of the reproductive tissues;[16,17,18] cause DNA damage and genotoxicity in the stomach, kidney, and liver of rats;[19,20] damage rat erythrocytes[21] as well as induce oxidative stress in several experimental models.[8,21,22,23] We previously reported that ATZ at a dose of 120 mg/kg induces oxidative stress in rat tissues and blood at 16 days of treatment, and that the oxidative stress was associated with increased lipid peroxidation and changes in the antioxidative system.[12,22,23,24,25] Data from our laboratories have also revealed that experimental models involving co-treatment of 120 mg/kg ATZ and 20 mg/kg quercetin resulted in increased susceptibility of hepatic tissues and reproductive organs including the testis and epididymides to ATZ-induced oxidative damage and tissue pathologies[22,24] even when there was no toxicity associated with quercetin at the investigated dose,[24,25] suggesting that the protective effect of quercetin in this experimental model depends on the dose of quercetin that interacted with ATZ. Because quercetin is widely distributed in many fruit, vegetables, and many other dietary sources, highly consumed by humans either as supplement or directly from natural sources, and have both pro-oxidant and antioxidant nature,[24] information on its appropriate dose that could be beneficial in ATZ-induced tissue pathogenesis is significant. Therefore, the present studies were initiated with lower doses of quercetin (5 mg/kg and 10 mg/kg) with the intention of selecting appropriate dose of quercetin that can protect against ATZ-induced cardio-hepatorenal and brain-oxidative damage.
MATERIALS AND METHODS
Thirty-six adult male rats (Wistar albino) with an average weight of 164 ± 7.38 gm were provided by the animal house of the Department of Biochemistry, University of Ibadan, Nigeria and housed in six groups each containing six animals. The animals were maintained under 12 light: 12 dark cycle and were supplied with water and feed ad libitum. The rats were housed for a minimum of 1 week to acclimatize before being dosed with the test substances. The experimental designs and protocols for study were in accordance with the standard guide for the care and use of laboratory animals. ATZ in the commercial product cotrazine 80WP (an 80% wettable powder) were obtained from Nantong foreign trade, Meheco, China. Quercetin was obtained from Sigma Chemicals (St. Louis, MO). All other reagents were commercially available analytical-grade chemicals. The Q5 and Q10 groups were orally administered 5 mg/kg and 10 mg/kg quercetin (QT), respectively. ATZ was dissolved in the vehicle (corn oil) and orally administered to the ATZ groups at a dose equivalent to 120 mg/kg body weight (based on the active ingredient). QT was simultaneously administered with ATZ in the Q5 + ATZ groups and Q10 + ATZ groups at the same dose of QT and ATZ in the other groups. The control groups was administered the vehicle (2 mL/kg body weight) and all the treatment continued for 16 days. A 16-day period was chosen because we established that this is the minimum period required for ATZ to cause changes in the antioxidative system in rats and consequently induce oxidative damage.[22,23] At the end of the 16th day of treatment, the rats in each group were sacrificed, and the heart, liver, brain, and kidney of each rat were quickly excised, cleared of adhering fat, rinsed with a cold 1.15% potassium chloride, and weighed. One kidney, portion of the liver, cerebrum, and cerebellum of each rat were preserved in neutral buffered formalin. Tissues were sectioned and stained routinely with hematoxylin and eosin for microscopy. The tissues were homogenized using Potter-Elvehjem homogenizer in a 4 volume of 50 mM Tris-HCl buffer (pH 7.4) containing 1.15% potassium chloride and centrifuged at 10,000× g, 4°C for 10 min. The supernatant was used for the measurement of the biochemical markers of oxidative stress. The activity of superoxide dismutase (SOD) was assayed by the method described by Misra and Fridovich[26] and expressed as units/mg protein. The catalase (CA) T activity was assayed by the method of Sinha[27] and expressed as expressed as μmol H2O2 consumed/min. The level of reduced glutathione (GSH) was determined at 412 nm using the method described by Sedlak and Lindsay[28] and was expressed as μg GSH/mg protein. Lipid peroxidation was quantified as malondialdehyde (MDA) according to the method described by Ohkawa et al.,[29] and expressed as μmol MDA/g tissue. Protein concentration was determined by the method of Lowry et al.[30] Blood collected through an eye vein was transferred into non-heparinized tubes for the recovery of serum. The serum was processed for the measurement of acid phosphatase (ACP)[31] and alkaline phosphatase (ALP)[32] using p-nitrophenylphosphate (PNPP) as the substrate. The aspartate aminotransferase (AST) activity was estimated by the method of Reitman and Frankel.[33] The sorbitol dehydrogenase (SDH) assay was based on the conversion of sorbitol to fructose.[34] The appearance of nicotinamide adenine dinucleotide (NADH) was measured spectrophotometrically at 340 nm. Data were expressed as mean ± standard deviation (SD), and the statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) Statistics 17.0 (SPSS Inc., Chicago, IL, USA). All the statistical analyses were analyzed by analysis of variance (ANOVA) followed by Dunnett's test as a post hoc test. P values less than 0.05 were considered to indicate statistical significance.
RESULTS AND DISCUSSION
The rats in the group of ATZ + Q5 showed some clinical signs such as lethargy, weight loss [Table 1] leading finally to the death of 1 animal at day 9. The animals in the other groups remained relatively in good health throughout the study and there were no exposure-related clinical observations. The effects of ATZ administration on tissue-damages index were evaluated as marker enzymes in serum samples from control and treated rats. The results showed that ATZ caused an increase in ALP, ACP, AST, and SDH. Co-administration of Q5 had no effect on ATZ-induced changes on these marker enzymes. In the Q10 + ATZ animals, the changes caused by ATZ on the serum-marker enzymes were normalised to near the control values. Q5 or Q10 alone has no effect on the serum-marker enzymes [Figure 1]. ATZ treatment caused a marked effect on MDA concentration and the key constituent of antioxidative defense system [Table 2]. According to our results, MDA level significantly increased in the brain, liver, and kidney but no alterations were observed in the heart. SOD activity significantly increased in the brain, whereas it decreased in the kidney and liver but did not change in the heart. Catalase (CAT) activity significantly decreased in the brain, liver, and kidney without significant change in the heart. Meanwhile GSH levels remain unchanged in all the tissues of rats treated with ATZ except in the liver were the level was increased. Treatments with Q5 reversed SOD activity in the brain but not in the kidney and liver, and have synergistic effects in the heart. MDA values were normalized in the brain but not in the kidney, whereas MDA levels in the heart remain unaltered in these groups of animals. Furthermore, the combine administration of Q5 + ATZ demonstrated synergistic effects on MDA level in the liver. GSH levels remain unaltered in the brain, kidney, and heart except in the liver where its level remains high. In addition, the CAT activity in the brain and kidney of the Q5 + ATZ animals remain low, and were decreased in the liver and heart comparable to the ATZ-treated rats. Treatment with Q10 could not prevent ATZ-induced increase in the MDA level in the brain, but it decreased the MDA value in the kidney and liver when compared to the ATZ value. Furthermore, the MDA level in the kidney and liver was lower in the Q10 + ATZ group than in the Q5 + ATZ animals. SOD activity was normalized to the control value in the kidney, liver, and heart of Q10 + ATZ animals except in the brain where its activity was still high compared to the control value but was decreased compared to the ATZ value [Table 2], suggesting that Q10 was not as effective in restoring to normalcy of the SOD activity in the brain than in the other tissues. Thus, the application of this dose of QT (10 mg/kg body weight) could potentially inhibit oxidative stress in the kidney and liver but not the brain of the ATZ-treated animals. Treatment with Q5 alone had no effect on any of these parameters, whereas the Q10 increased SOD activity and MDA level, and had no effect on CAT activity and GSH level in the brain. All the investigated parameters in the heart, kidney, and liver tissues were not affected by treatment with Q10 alone. Histological study as shown in Figures 2–5 demonstrates normal cerebellum [Figures 2a–d] and cerebrum [Figures 3a–d] of control and treated animals with no visible lesion. On the other hand, severe congestion with marked interstitial cellular infiltration was noted in the kidney ATZ-treated animals, whereas no visible lesions were observed in the control and the combine exposure group [Figures 4a–d]. The liver of the control [Figure 5a], Q5-treated [Figure 5b] and Q10-treated [Figure 5c] animals showed normal morphology, whereas those of the ATZ-treated rats showed mild diffuse vacuolar degeneration of hepatocytes [Figure 5d] and those of the ATZ + Q5 showed mild periportal cellular infiltration by mononuclear cells [Figure 5e]. The liver histology of the ATZ + Q10 resembles those of the control, Q5, or Q10 groups [Figure 5f].
Table 1.
Effects of atrazine and quercetin on body weights of rats
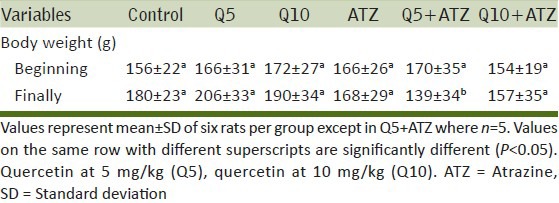
Figure 1.
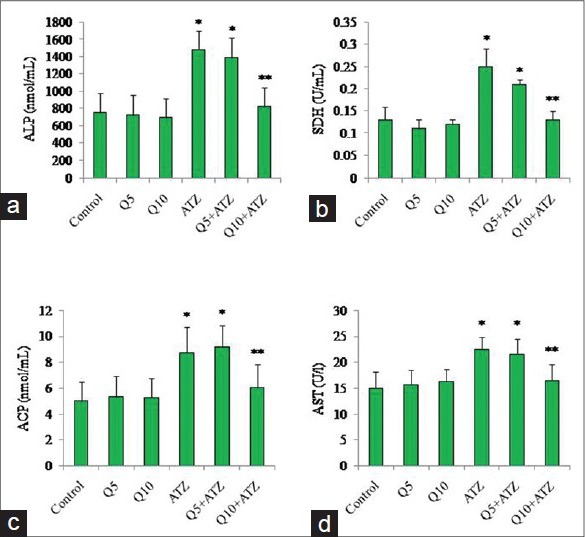
Effects of atrazine and quercetin on serum enzyme levels of rats. (a) Alkaline phosphatase (ALP), (b) Sorbitol dehydrogenase (SDH), (c) Acid phosphatase (ACP), (d) Aspartate amino transferase (AST). (Mean ± standard deviation (SD), n = 6 except in Q5 + atrazine (ATZ) where n = 5). *vs control, **vs. all groups (P < 0.05)
Table 2.
Effects of ATZ and quercetin on antioxidant defense systems and MDA levels in various tissues of rats
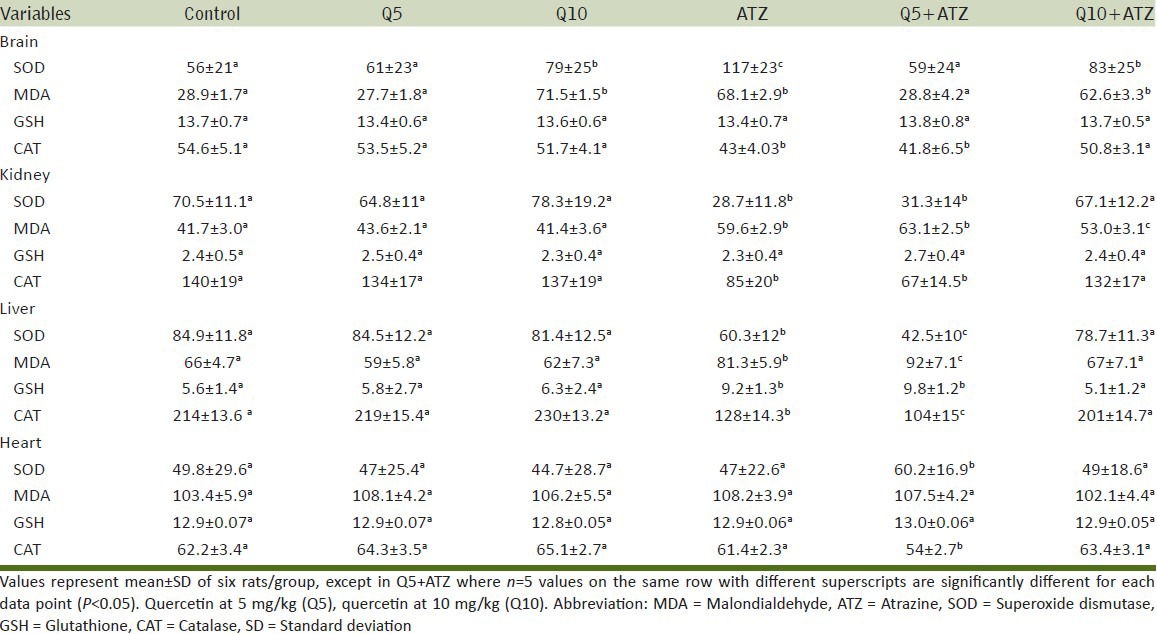
Figure 2.
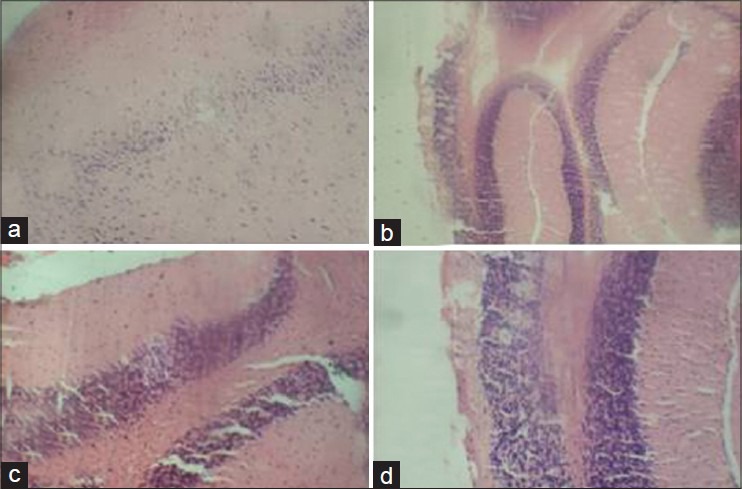
Representative photomicrograph of hematoxylin and eosin-stained sections of cerebellum of control and experimental rats showing normal morphology in all groups. (a) Control, (b) Quercetin, 5 mg/kg, (c) Atrazine, (d) Quercetin, 5 mg/kg + atrazine (×400)
Figure 5.
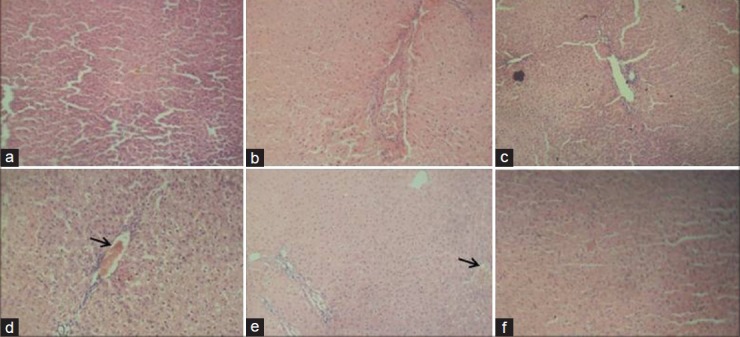
Representative photomicrograph of hematoxylin and eosin-stained sections of liver of control and experimental rats (a) Control, (b) Quercetin, 5 mg/kg, (c) Quercetin, 10mg/kg, (d) Atrazine, (e) Quercetin, 5 mg/kg + atrazine (f) Quercetin, 10 mg/kg + atrazine. (Arrows: [d] Mild diffuse vacuolar degeneration of hepatocytes, [e] mild periportal cellular infiltration by mononuclear cells).(×400)
Figure 3.
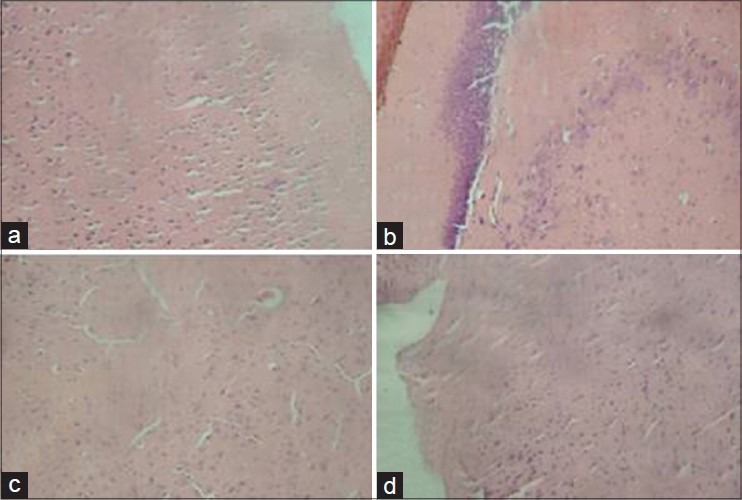
Representative photomicrograph of hematoxylin and eosin-stained sections of cerebrum of control and experimental rats showing normal morphology in all groups. (a) Control, (b) Quercetin, 5 mg/kg, (c) Atrazine, (d) Quercetin, 5 mg/kg + atrazine (×400)
Figure 4.
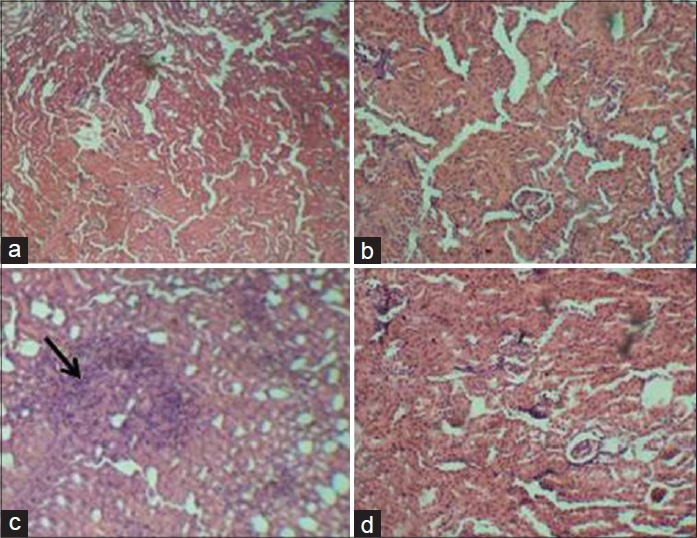
Representative photomicrograph of hematoxylin and eosin-stained sections of kidney of control and experimental rats (a) Control, (b) Quercetin, 10 mg/kg, (c) Atrazine, (d) Quercetin, 10 mg/kg + atrazine. (Arrow: Severe congestion with marked interstitial cellular infiltration (×400)
Our previous research on effects of combined administration of ATZ (120 mg/kg) and QT (20 mg/kg) to adult rats revealed potentiation of oxidative stress in the rat liver and reproductive organs (testis and epididymis) in terms of alteration of antioxidant enzymes and lipid peroxidation level.[22,24] As a follow-up, the present studies were initiated with lower doses of QT (5 or 10 mg/kg) with the intention of selecting appropriate dose of QT that can protect against ATZ-induced oxidative stress, not only in the liver but also in the brain, kidney, and heart, and eventually put the QT effects at low and high doses into connection. Liver is the first target of ingested oxidants and also very important tissue in defense against oxidative stress. Heart, kidney, and brain also posses antioxidant defense and alterations in the activity of antioxidant enzymes in these tissues in rats exposed to pesticides are known.[35,36,37,38] Therefore, in this study we also measured the antioxidant status in the different tissues and liver indirectly, by measuring the activities of CAT and SOD; GSH and MDA levels as indicators of susceptibility to oxidative stress in rats.
Our results showed that ATZ increased MDA formation in the liver, brain, and kidney but not in the heart. The activities of SOD and CAT decreased in the liver and kidney, increased and decreased, respectively, in the brain, and remains unchanged in the heart. GSH level was not affected in all the tissues except in the liver where the level was increased. These clearly support our previous studies and those of others[12,22,23,24,25,39] that ATZ was able to induce oxidative stress in these tissues. The increased activity of SOD in the brain and decreased activity in the kidney and liver with the simultaneous decrease in CAT activity and escalated MDA concentration in these tissues allow us to conclude that the brain, liver, and kidney antioxidant defense system was impaired leading to the elevated lipid peroxidation. The differential response in SOD and CAT activities in these tissues confirms the existence of an inducible antioxidant system, and is known to be an adaptive response to oxidative stress.[40,41] GSH levels were not changed in all the tissues except the liver where it was increased. The unchanged GSH levels in the kidney and brain have also been reported by us in the testis and epididymis,[23] suggesting the differential tissue responsiveness of GSH in ATZ-induced oxidative stress in the liver.[12] Because the antioxidative enzymes, GSH, and MDA levels were unchanged in the heart, it indicates that the heart tissue was completely resistant to ATZ-induced oxidative stress. Overall, the fluctuated levels in the antioxidant enzymes, GSH, and MDA concentrations in the various tissues of rats treated with ATZ may be dependent on the differences between interstitial concentrations. In other words, the tissues might have to be exposed to different ATZ concentration due to blood volume differences in the tissues.
Co-administration of Q5 did not prevent the increased levels in the serum-marker enzymes caused by ATZ. With respect to the oxidative stress parameters, Q5 normalized SOD and MDA in the brain but not CAT. This protective response on SOD and MDA was not seen in the kidney and liver. In the heart, SOD activity was enhanced, CAT activity was decreased but MDA level and both brain and heart tissues GSH remain unchanged when compared with the vehicle control or the ATZ-treated rats. Furthermore, the combine effects of Q5 + ATZ did not recover CAT activity in the kidney and further decreased the activity in the liver, suggestive of synergistic effect of ATZ and Q5 with respect to CAT activity. It may seem that Q5 effectively protect the brain against ATZ-induced lipid peroxidation but not the kidney and liver. The increased SOD activity in the heart with the simultaneous decrease in the activity of CAT along with the unchanged MDA concentration allow us to conclude that the heart antioxidant defense system still effectively protects from the action of the interactive effects of Q5 + ATZ-induced oxidative stress. Q5 alone had no effect on any of the measured biochemical variables, whereas Q10 alone increased SOD in the brain. The increase in the SOD activity, which is indicative of a protective response to oxidative stress[42] is consistent with the elevated MDA concentration in the brain. We suggest that this dose of QT might have a pro-oxidant effect in the brain. The activities of antioxidant enzymes have also been reported to be inhibited or increased by QT depending on the experimental model and dose.[9,10,11] The brain exhibits high oxygen consumption and are enriched with polyunsaturated fatty acids, so it is quite vulnerable to lipid peroxidation.[43,44] There exists, thus, a possibility in our experimental model that the brain would be more vulnerable to quercetin-induced oxidative stress even at a dose that would be incapable of inducing the same effect in other tissues.
Q10 is thought to act as effective cytoprotectant in the kidney and liver since it prevented the cellular injury, oxidative stress, and subsequently inhibited the leakage of these enzymes (SDH, ACP, ALP, and AST) into the blood circulation. The induced hepatic GSH levels; decreased CAT activities in the brain, kidney, and liver; decreased SOD activity in the kidney, liver, and heart but not the brain of the Q10 + ATZ-treated animals were comparable to the control values. The inability of Q10 to effectively restore brain SOD activity as was observed in the Q5 + ATZ-treated animals was consistent with the elevated level of MDA. It is probable that QT exhibits better antioxidant effect in the brain when its concentration is low, while in other tissues like the liver and kidney, a higher dose of QT would be required for QT to be an effective antioxidant. However, this dose would still be within a narrow therapeutic concentration range, because at doses of QT equal to 20 mg/kg body weight, QT becomes a pro-oxidant in the liver and reproductive organs when co-administered with ATZ.[22,24] Thus, ATZ and QT are good models to study in vivo combined effects, and especially synergistic effects for pesticides and flavonoids.
As most of the histological changes observed in the present study were noted in the kidney and less in the liver and also with the cerebrum and cerebellum maintaining a normal morphology similar to the control, the elevated serum-marker enzymes could be most likely due to the renal damage.[13,45] It is also possible that the slight degeneration of hepatocytes in the ATZ-treated rats and/or mild periportal cellular infiltration by mononuclear cells in the ATZ + Q5-treated rats were sufficient to induce the release of the serum markers from the liver into the systemic circulation which was completely prevented on co-administration of Q10. The susceptibility of renal tissues to the toxic effects of noxious chemicals can be attributed in part to the differences in the anatomic and physiologic features of this organ. For instance, under resting state, the renal tissue receives about 20-25% of the resting cardiac output.[46] Therefore, any drug or chemical in the systemic circulation will be delivered to these organs in relatively high amounts; consequently, a non-toxic concentration of chemical in the plasma or other tissues may reach toxic concentration in the kidney.[46] Also the presence of the blood-brain barrier could also limit the drug concentrations to level below which no histological changes would occur.[47] Therefore, different experimental models, such as a longer exposure period, increased ATZ dose may be required to induce major histopathological changes in the brain.
In conclusion, lipid peroxidation in terms of MDA levels are more intensified in renal tissues of Q5 + ATZ than those of Q10 + ATZ animals and are prevented in the hepatic tissues of Q10 + ATZ, and exacerbated in those of the Q5 + ATZ-treated rats. Furthermore, the fluctuation in the constituent antioxidative system in the tissues of the Q10 + ATZ-treated animals was markedly less advanced than in those observed in the Q5 + ATZ animals, but the brain tissues of these animals were vulnerable to lipid peroxidation. Hence Q10 is more beneficial in reversing ATZ-induced hepatic and renal damage. With regards to particular tissue, Q5 is a better effective dose for protecting against ATZ-induced lipid peroxidation in the brain, whereas Q10 is a better effective dose for preventing ATZ-induced changes in the kidney and liver antioxidant defense system while the heart seems less susceptible to ATZ- and Q5 + ATZ-induced oxidative stress. Thus, the differential protective effect of QT on the antioxidant defense system could be that the antioxidant system might have to be exposed to different concentrations of QT due to blood volume/physiologic/anatomic differences in the tissues. Regarding the symptoms shown by these animals (Q5 + ATZ) including lethargy and decreased body weight, and finally death allow us to suppose that the health conditions of these animals (Q5 + ATZ) are not normal and indicative of presence of general toxicity. Therefore, when a bioactive compound such as quercetin protects a target tissue from a chemically induced oxidative damage, it may in fact predispose or not protect another tissue to damage caused by the noxious chemical under the same experimental condition.
ACKNOWLEDGEMENT
Sunny Abarikwu acknowledges the support of the Redeemer's University, Redemption Camp, Nigeria.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J Nutr. 2003;133:3275S–84. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 2.Kuhlmann MK, Horsch E, Burkhardt G, Wagner M, Kohler H. Reduction of cisplatin toxicity in cultured renal tubular cells by the bioflavonoid quercetin. Arch Toxicol. 1998;72:536–40. doi: 10.1007/s002040050539. [DOI] [PubMed] [Google Scholar]
- 3.Satyanarayana PS, Singh D, Chopra K. Quercetin a bioflavonoid, protects against oxidative stress-related renal dysfunction by cyclosporine in rats. Methods Find Exp Clin Pharmacol. 2001;23:175–81. doi: 10.1358/mf.2001.23.4.634641. [DOI] [PubMed] [Google Scholar]
- 4.Pavanato A, Tunon MJ, Sanchez-Campos S, Marroni CA, Llesuy S, Gonzalez-Gallego J, et al. Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci. 2003;48:824–9. doi: 10.1023/a:1022869716643. [DOI] [PubMed] [Google Scholar]
- 5.Molina MF, Sanchez-Reus I, Iglesias I, Benedi J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol Pharm Bull. 2003;26:1398–402. doi: 10.1248/bpb.26.1398. [DOI] [PubMed] [Google Scholar]
- 6.Alarcon de la lastra C, Martin MJ, Motilva V. Antiulcer and gastroprotective effects of quercetin: A gross and histologic study. Pharmacology. 1994;48:56–62. doi: 10.1159/000139162. [DOI] [PubMed] [Google Scholar]
- 7.Abarikwu SO, Pant AB, Farombi EO. The protective effects of quercetin on the cytotoxicity on rat Sertoli-germ cell co-culture. Int J Androl. 2012;35:590–600. doi: 10.1111/j.1365-2605.2011.01239.x. [DOI] [PubMed] [Google Scholar]
- 8.Abarikwu SO, Pant AB, Farombi EO. Quercetin decreases steroidogenic enzyme activity, NF-κB expression and oxidative stress in cultured Leydig Cells exposed to atrazine. Mol Cell Biochem. 2013;373:19–28. doi: 10.1007/s11010-012-1471-z. [DOI] [PubMed] [Google Scholar]
- 9.Choi EJ, Lee BH, Lee K, Chee KM. Long-term combined administration of quercetin and daidzein inhibits quercetin-induced suppression of glutathione antioxidant defences. Food Chem Toxicol. 2005;43:793–8. doi: 10.1016/j.fct.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Choi EJ, Chee KM, Lee BH. Anti- and prooxidant effects of chronic quercetin administration in rats. Eur J Pharmacol. 2003;482:281–5. doi: 10.1016/j.ejphar.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 11.Guzy J, Chovanová Z, Mareková M, Chavková Z, Tomeèková V, Mojzisova G, et al. Effect of quercetin on paracetamol-induced rat liver mitochondria dysfunction. Biologia Bratislava. 2004;59:399–403. [Google Scholar]
- 12.Adesiyan AC, Oyeloja TO, Abarikwu SO, Oyeyemi MO, Farombi EO. Selenium provides protection to the liver but not the reproductive organs in an atrazine-model of experimental toxicity. Exp Toxicol Pathol. 2011;63:201–7. doi: 10.1016/j.etp.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Santa Maria C, Moreno J, Lopez-Campos JL. Hepatotoxicity induced by the herbicide atrazine in the rat. J Appl Toxicol. 1987;7:373–8. doi: 10.1002/jat.2550070605. [DOI] [PubMed] [Google Scholar]
- 14.Campos-Pereira FD, Oliveira CA, Pigoso AA, Silva-Zacarin EC, Barbieri R, Spatti EF, et al. Early cytotoxic and genotoxic effects of atrazine on Wistar rat liver: A morphological, immunohistochemical, biochemical, and molecular study. Ecotoxicol Environ Saf. 2012;78:170–7. doi: 10.1016/j.ecoenv.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Gojmerac T, Kartal B, Zuriæ M, Curiæ S, Mitak M. Serum biochemical and histopathological changes related to the hepatic function in pigs following atrazine treatment. J Appl Toxicol. 1995;15:233–6. doi: 10.1002/jat.2550150315. [DOI] [PubMed] [Google Scholar]
- 16.Ashby J, Tinwell H, Stevens J, Pastoor T, Breckendridge CB. The effects of atrazine on the sexual maturation of female rats. Regul Toxicol Pharmacol. 2002;35:468–73. doi: 10.1006/rtph.2002.1571. [DOI] [PubMed] [Google Scholar]
- 17.Kniewald J, Jakominic M, Tomljenovic A, Simic B, Romac P, Vranesic D, et al. Disorders of male reproductive tract under the influence of atrazine. J Appl Toxicol. 2000;20:61–8. [PubMed] [Google Scholar]
- 18.Trentacoste SV, Friedmann AS, Youker RT, Breckenridge CB, Zirkin BR. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J Androl. 2001;22:142–8. [PubMed] [Google Scholar]
- 19.Pino A, Maura A, Grillo P. DNA damage in stomach, kidney, liver and lung of rats treated with atrazine. Mutat Res. 1988;209:145–7. doi: 10.1016/0165-7992(88)90032-2. [DOI] [PubMed] [Google Scholar]
- 20.Singh M, Sandhir R, Kiran R. Effects on antioxidant status of liver following atrazine exposure and its attenuation by vitamin E. Exp Toxicol Pathol. 2011;63:269–76. doi: 10.1016/j.etp.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Bhatti JS, Sidhu IP, Bhatti GK. Ameliorative action of melatonin on oxidative damage induced by atrazine toxicity in rat erythrocytes. Mol Cell Biochem. 2011;353:139–49. doi: 10.1007/s11010-011-0780-y. [DOI] [PubMed] [Google Scholar]
- 22.Abarikwu SO, Adesiyan AC, Oyeloja TO, Farombi EO. Quercetin potentiates oxidative stress in the liver of rats exposed to the herbicide, atrazine. In: Justin AD, editor. Advances in Environmental Research. New York: Nova Science Publishers; 2012. pp. 487–502. [Google Scholar]
- 23.Abarikwu SO, Adesiyan AC, Oyeloja TO, Oyeyemi MO, Farombi EO. Changes in sperm characteristics and induction of oxidative stress in the testis and epididymis of experimental rats by a herbicide, atrazine. Arch Environ Contam Toxicol. 2010;58:874–82. doi: 10.1007/s00244-009-9371-2. [DOI] [PubMed] [Google Scholar]
- 24.Farombi EO, Abarikwu SO, Adesiyan AC, Oyejola TO. Quercetin exacerbates the effects of subacute treatment of atrazine on reproductive tissue antioxidant defence system, lipid peroxidation and sperm quality in rats. Andrologia. 2013;45:256–65. doi: 10.1111/and.12001. [DOI] [PubMed] [Google Scholar]
- 25.Abarikwu SO, Adedara IA, Farombi EO. Influence of quercetin on haematological indices and biomarkers of oxidative stress in the serum of rats exposed to atrazine. Afr J Med Med Sci. 2010;39:81–8. [PubMed] [Google Scholar]
- 26.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 27.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 28.Sedlak J, Lindsay RH. Estimation of total, protein-bound and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 29.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 31.Vanha-Pertula T, Nikkanen V. Acid phosphatases of the rat testis in experimental conditions. Acta Endocrinol (Copenh) 1973;72:376–90. doi: 10.1530/acta.0.0720376. [DOI] [PubMed] [Google Scholar]
- 32.Malymy M, Horecker BL. Methods in enzymology. New York: Academy Press; 1966. Alkaline phosphatase; pp. 639–42. [Google Scholar]
- 33.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 34.Gerlach U. Sorbitol dehydrogenase. In: Bergmeyer HU, Bergemeyer J, Grassi M, editors. Methods of Enzymatic Analysis. Verlag Chemie: Berlin; 1983. pp. 112–7. [Google Scholar]
- 35.Rai DK, Sharma B. Carbofuran-induced oxidative stress in mammalian brain. Mol Biotechnol. 2007;37:66–71. doi: 10.1007/s12033-007-0046-9. [DOI] [PubMed] [Google Scholar]
- 36.Sharma P, Singh R. Dichlorvos and lindane-induced oxidative stress in rat brain: Protective effects of ginger. Pharmacognosy Res. 2012;4:27–32. doi: 10.4103/0974-8490.91031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdou HM, Hussien HM, Yousef MI. Deleterious effects of cypermethrin on rat liver and kidney: Protective role of sesame oil. J Environ Sci Health B. 2012;47:306–14. doi: 10.1080/03601234.2012.640913. [DOI] [PubMed] [Google Scholar]
- 38.Jalili Sh, Farshid AA, Heydari R, Ilkhanipour M, Salehi S. The effects of vitamin E on endosulfan-induced oxidative stress in rat heart. Pak J Nutr. 2007;6:375–80. doi: 10.3923/pjbs.2007.1922.1925. [DOI] [PubMed] [Google Scholar]
- 39.Pogrmic-Majkic K, Kaisarevic S, Fa S, Dakic V, Glisic B, Hrubik J, et al. Atrazine effects on antioxidant status and xenobiotic metabolising enzymes after oral administration in peripubertal male rats. Environ Toxicol Pharmacol. 2012;34:495–501. doi: 10.1016/j.etap.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Doyotte A, Cossu C, Jacquin MC, Babut M, Vasseur P. Antioxidant enzymes, glutathione and lipid peroxidation as relevant biomarkers of experimental or field exposure in the gills and the digestive gland of the freshwater bivalve Unio tumidus. Aquat Toxicol. 1997;39:93–110. [Google Scholar]
- 41.Dimitrova MS, Tsinova V, Velcheva V. Combined effect of zinc and lead on the hepatic superoxide dismutase-catalase system in carp (Cyprinus carpio) Comp Biochem Physiol. 1994;108-C:43–6. [Google Scholar]
- 42.Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of human superoxide dismutase and catalase in vivo: A fundamentally new approach to antioxidant therapy. Free Radic Biol Med. 2006;40:341–7. doi: 10.1016/j.freeradbiomed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 43.Neely MD, Sidell KR, Graham DG, Montine TJ. The lipid peroxidation product 4-hydroxynonenal inhibits neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin. J Neurochem. 1999;72:2323–33. doi: 10.1046/j.1471-4159.1999.0722323.x. [DOI] [PubMed] [Google Scholar]
- 44.Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease and brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–64. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Daim MM, Abuzead SM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013;8:e72991. doi: 10.1371/journal.pone.0072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rekha, Raina S, Hamid S. Histopathological effects of pesticide-cholopyrifos on kidney in albino rats. Int J Res Med Sci. 2013;1:465–75. [Google Scholar]
- 47.Zheng W, Aschner M, Ghersi-Egea JF. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. 2003;192:1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


