Abstract
Background:
Organophosphorus (OP) compounds are a heterogeneous group of insecticides widely used in agricultural industry. These OP compounds are likely to have more adverse effects in developing countries like India due to its easy availability and less awareness which results in high morbidity and mortality.
Aims and objectives:
1. To estimate plasma cholinesterase, amylase, lipase and, creatine phosphokinase (CPK) in acute OP poisoning. 2. To correlate these biochemical parameters with plasma cholinesterase levels in OP poisoning. 3. To determine the use of a biochemical marker in predicting the severity of acute OP poisoning.
Materials and Methods:
A hospital based observational study was conducted on 53 subjects who have clinically diagnosed of acute OP poisoning and admitted in emergency unit of a tertiary care rural hospital. Subjects of either gender of all age-groups were included in the study. On admission, plasma cholinesterase, serum amylase, lipase and CPK were measured. Based on plasma cholinesterase activity at the time of admission, subjects were divided into three groups. Group I-having 20-50% of plasma cholinesterase activity; Group II-10-20% of plasma cholinesterase activity; and group III-<10% of plasma cholinesterase activity.
Results:
Serum amylase, lipase and CPK were negatively correlated with plasma cholinesterase levels. Serum amylase showed statistically significant negative correlation with plasma cholinesterase. Serum amylase showed the highest diagnostic accuracy for assessing severity of poisoning followed by CPK and Lipase.
Conclusion:
OP poisoning is associated with hyperamylasemia. Serum amylase, lipase and CPK can be used as an additional prognostic indicator with plasma cholinesterase levels. Serum amylase could be considered as a better predictor of severity followed by CPK and lipase.
Keywords: Amylase, CPK, lipase, organophosphorus poisoning, plasma cholinesterase
INTRODUCTION
Organophosphorus (OP) compounds are insecticides which are widely used in agriculture to control pests, weeds, or plants diseases. Because of their specific action these OP compounds are helpful in crop protection and increased productivity. The OP compounds likely to have more adverse effects in developing countries like India due to its easy availability and less awareness leading to high morbidity and mortality.[1] The OP compounds act by inhibiting acetylcholine esterase enzyme at nerve endings and neuromuscular junction, causing overstimulation of acetylcholine receptors. Signs and symptoms of poisoning are mainly due to muscarinic, nicotinic and central nervous system (CNS) receptor over-stimulation.[2]
In acute OP poisoning, the severity of poisoning parallels the decrease in pseudocholine esterase activity. Even though various scoring systems such as Acute Physiology And Chronic Health Evaluation (APACHE) and Simplified Acute Physiology Score (SAPS) are available, laboratory evaluation plays an important and crucial role for confirmation of poisoning, diagnosing the first acute organ damage and assessing the severity of poisoning. In laboratory assessment of OP poisoning, estimation of plasma cholinesterase is most specific test for OP poisoning.[3]
OP compound poisoning is associated with various biochemical abnormalities, among which hyperamylasemia is well documented and may be due to excessive cholinergic stimulation of pancreas. Studies conducted by Matsumiya N et al., and Lee HC have evaluated the prognostic significance of serum amylase in OP poisoning.[4,5] Acute pancreatitis is frequent in OP poisoning and increased serum amylase is less specific and sensitive. Hence, serum lipase measurement may be useful in patients with increased amylase levels for early diagnosis of pancreatitis.[6] Hence a many research was done to evaluate a promising biochemical marker in OP poisoning. Recently, a study done by Bhattacharya K et al., suggested that measurement of creatine phosphokinase (CPK) in acute OP poisoning may be useful in predicting as well as assessing prognosis of patients. It was also documented that there is rhabdomyolysis in “intermediate syndrome” and followed by a proportionate raise in CPK level.[7]
This study was undertaken to know the efficacy of newer biochemical markers like Amylase, lipase and CPK as indicators in assessing the severity of OP poisoning.
Aims and objectives
To estimate plasma cholinesterase, amylase, lipase and CPK in acute OP poisoning
To correlate these biochemical parameters with the plasma cholinesterase levels in OP poisoning
To determine use of a biochemical marker in prediction of severity of acute OP poisoning
MATERIALS AND METHODS
A hospital based longitudinal study was conducted among 53 subjects reported and clinically diagnosed with acute OP poisoning in the emergency unit of a tertiary care center for a period of 8 months.
Inclusion criteria
All the OP poisoning cases confirmed by history, circumstantial evidence of consumption, characteristic clinical examination and basic laboratory investigations were included in the study.
Exclusion criteria
Patients with history of consumption of OP compound mixed with any other poison or alcohol, chronic alcoholism, diseases of salivary gland, and chronic renal failure or any associated renal disorders were excluded from the study.
Sample collection
At the time of admission, after obtaining informed consent about 2 ml of blood was collected in plain tube under aseptic precautions. Blood was allowed to clot, serum was separated by centrifugation and used for the analysis of following parameters.
-
1)
Estimation of serum amylase by chromogenic method using dyed amylopectin.[8]
-
2)
Estimation of plasma cholinesterase activity by kinetic method based on hydrolysis of butyrylthiocholine by choline esterase.[8]
-
3)
Estimation of serum lipase by kinetic method using 1-oleoyl-2-3-diacetyl glycerol as substrate.[8]
-
4)
Estimation of serum creatine kinase by kinetic method.[8]
All the parameters were analyzed in Drychemisrty Vitros 250 Johnson and Johnson analyser. During the analysis, regular and routine internal quality checks using Biorad controls was carried out.
After the biochemical analysis, patient were followed up for clinical outcome like complete recovery, acute respiratory distress syndrome, circulatory failure, CNS complications, renal failure, death due any of the above mentioned complications and any other complications.
Statistical analysis
The parameters were tabulated and the mean values and standard deviation (SD) was analysed using EPI Info 7 version software. Mean and SD were compared between the groups using one way analysis of variance (ANOVA). All biochemical parameters were correlated with plasma cholinesterase using Pearson's coefficient. Chi-square test was the test of significance for qualitative variables to find the association. Diagnostic accuracy of the biochemical parameter was assessed by calculating the area under the curve in receptor operating curve (ROC).
RESULTS
Based on the plasma cholinesterase levels at the time of admission, subjects were divided into three groups.
Group I-20-50% of normal plasma cholinesterase activity; Group II-10-20%; and Group III-<10% (1). In the study it was observed that majority 49.05% (26/53) belonged to group III who had plasma cholinesterase activity <10%, followed by 28.3% (15/53) of cases in group II and 22.6% (12/53) in group I. Majority of the subjects were males 73.5% (39/53). Among 53 subjects, three patients died of respiratory failure all belonging to group III. Seven patients had respiratory distress but recovered after treatment.
In group I, six out of 12 patients had elevated amylase levels, three had increased lipase and five had increased CPK levels. All three patients who had elevated lipase levels also had elevated amylase and CPK. Five patients who had decreased plasma cholinesterase levels, had normal levels of amylase, lipase and CPK. Rest of the patients had isolated elevation of either amylase levels or CPK levels. In group II, seven out of 15 patients had increased amylase, three had increased lipase and 12 had increased CPK. Two patients had increased amylase and lipase levels and only one patient had elevated amylase, lipase and CPK levels. Two patients in this group with decreased plasma cholinesterase levels had normal levels of amylase, lipase and CPK. In group III, 19 out of 26 had elevated levels of amylase, six had elevated lipase and 24 had increased CPK. Elevated amlylase and lipase was observed in two patients and 12 patients had elevated amylase and CPK. Only one patient with decreased plasma cholinesterase levels had normal amylase, lipase and CPK values.
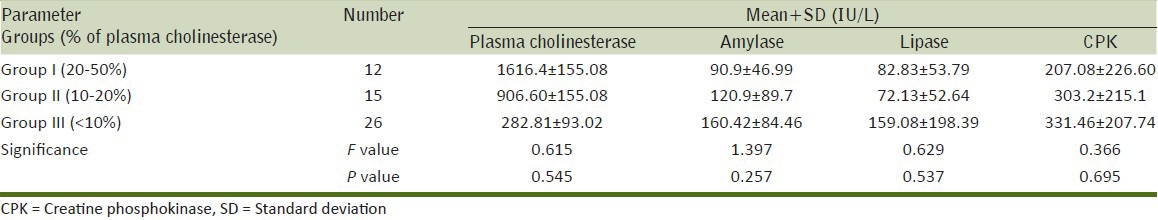
Comparison of biochemical parameters between the groups and within the groups were done by ANOVA. Plasma cholinesterase level was 1616.4 + 155.08 in group I, 906.60 + 155.08 in group II and 282.81 + 93.02 in group III. Mean values of serum amylase in group I was 90.9 ± 46.99, group II was 120.9 + 89.7 and group III was 160.42 + 84.46. Mean values of serum lipase was 82.83 ± 53.79 in group I, 72.13 + 52.64 in group II and 159.08 + 198.39 in group III. Mean values of CPK was 207.08 ± 226.60 in group I, 303.2 + 215.1 in group II and 331.46 + 207.74 in group III. There was no statistical significant difference among the groups. It can be observed from the Table 1 that when there is decrease in plasma cholinesterase level, there is increase in the mean values of amylase, lipase and CPK levels.
Table 1.
Comparison of biochemical parameters
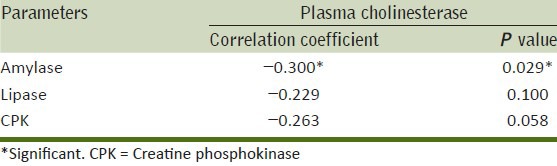
Correlation of various biochemical parameters with plasma cholinesterase levels show that serum amylase, lipase and CPK were negatively correlated with plasma cholinesterase levels. Whereas only serum amylase showed statistically significant negative correlation (P = 0.029*) with plasma cholinesterase. [Table 2].
Table 2.
Correlation of biochemical parameters with plasma cholinesterase
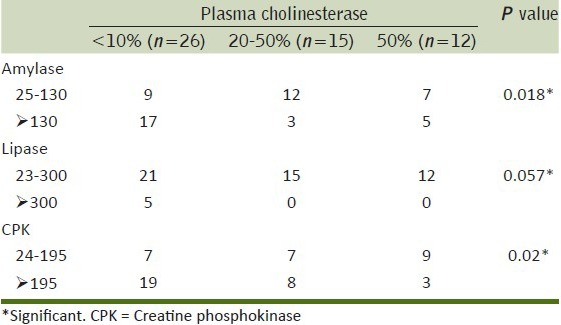
The association between the severity of OP (based on plasma cholinesterase) and other biochemical parameters showed that there was significant association with severity of OP poisoning with respect to amylase, lipase and CPK levels [Tables 3].
Table 3.
Association of biochemical parameters with severity of poisoning
ROC to assess the predictor of severity of OP poisoning showed that the area under the curve for serum amylase was 0.714, serum CPK was 0.635 and compared to lipase was (0.571) suggesting that Amylase was the better predictor of severity among the three variables [Tables 3, 4 and Figure 1].
Table 4.
Area under the curve

Figure 1.
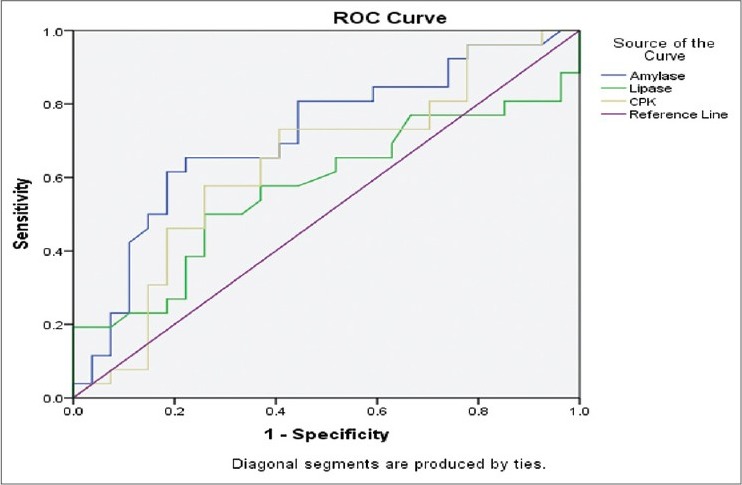
Diagnostic accuracies of various biochemical parameters in OP poisoning
DISCUSSION
Acute OP poisoning often presents as a medical emergency requiring monitoring and management in intensive care unit. Management of poisoning depends on clinical severity and is assessed by clinical signs and symptoms as well as laboratory evaluation.
Inhibition of cholinesterase is a well known fact in OP poisoning. Estimation of acetylcholinesterase (AchE) and butyrylcholinesterase (BchE)/plasma cholinesterase are the screening tools for OP poisoning. Although estimation of AchE in erythrocytes is more specific, estimation of plasma cholinesterase is most widely used laboratory tool for diagnosis and prognosis of OP poisoning.[9]
In this study majority of cases belonged to Group III and mortality occurred only in group III. Similar findings were observed in the studies conducted by Amanvermez R et al.,[3] and Hundekari IA et al.,[10] demonstrating good correlation between plasma cholinesterase level and severity of poisoning. A retrospective study conducted by Manu et al., have demonstrated that, patients with low plasma cholinesterase levels had poor prognosis and mortality with longer term stay in ICU and took long time to be out of mechanical ventilation.[11]. Another retrospective study by Yun HW et al., demonstrated that, absence of an increase in serum cholinesterase activity is associated with high mortality and morbidity.[12]
OP poisoning is associated with many biochemical abnormalities. Among which hyperamylasemia often noted in cases of OP poisoning, may be due to the fact that acute pancreatitis is caused by excessive cholinergic stimulation of pancreas by OP compounds.[13,14] Our study results are in accordance with the study done by Lin CL et al., where they found that mean amylase levels were elevated in patients with respiratory support and serum amylase levels predicted ventilator support in OP poisoning.[15] In a prospective study by Singh S[16] amylase was elevated in 48.95% in patient with fenthion poisoning and serum amylase showed persistent elevation during serial estimation. An important effect of O.P or carbamate intoxication is development of acute pancreatitis. Incidence of acute pancreatitis in adults with OP poisoning is approximately 12%.[17] In our study, majority of patients who had increased amylase levels belonged to group III and showed significant negative correlation with plasma cholinesterase levels. However, lipase was elevated only in six patients, who also had associated elevated amylase levels and all of them belonged to group III. Serum lipase did not show significant correlation with plasma cholinesterase.
Another promising prognostic indicator in OP poisoning is CPK. It is shown that serum CPK is elevated due to rabdomyolysis or intermediate syndrome which is an associated complication of OP poisoning.[7] CPK was elevated in two patients who succumbed due to complications. Elevated CPK was also noticed among five cases, whom also went in to complications. A study by Battacharya et al., has demonstrated that CPK can be elevated in severe OP poisoning cases even in the absence of intermediate syndrome. This may be due to muscle fiber necrosis and needs to be confirmed by muscle biopsy. Study by Hassan NAM and Madboly AG[18] have also confirmed high degree of correlation between initial CPK value and severity of OP poisoning.
Diagnostic accuracy of the biochemical parameters show that serum amylase had highest diagnostic accuracy followed by serum lipase and CPK.
Limitations of this study are small sample size and biochemical parameters are estimated at the time of admission. Serial measurements of plasma cholinesterase and other biochemical parameters could help to draw better conclusions.
With this we conclude that OP poisoning is associated with hyperamylasemia. Serum amylase, lipase and CPK can also be used as an additional prognostic indicator with plasma cholinesterase levels. Among all, serum amylase could be considered as a better predictor of severity followed by CPK and Lipase. However further studies with large sample size can be useful in making conclusions.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Vijaya Kumar S, Fareedullah Md, Sudhakar Y, Venkateswarlu B, Ashok Kumar E. Current review on organophosphorus poisoning. Arch Appl Sci Res. 2010;2:199–215. [Google Scholar]
- 2.Pore NE, Pujari KN, Jadkar SP. Organophosphorus poisoning. J Pharma Biosci. 2011;2:604–12. [Google Scholar]
- 3.Amanvermez R, Baydýn A, Yardan T, Baþol N, Günay M. Emergency laboratory abnormalities in suicidal patients with acute organophosphate poisoning. Turkish J Biochem. 2010;35:29–34. [Google Scholar]
- 4.Matsumiya N, Tanaka M, Iwai M, Kondo T, Takahashi S, Sato S. Elevated amylase is related to the development of respiratory failure in organophosphate poisoning. Hum Exp Toxicol. 1996;15:250–3. doi: 10.1177/096032719601500311. [DOI] [PubMed] [Google Scholar]
- 5.Lee HS. Acute pancreatitis and organophosphate poisoning. A case report and review. Singapore Med J. 1989;30:599–601. [PubMed] [Google Scholar]
- 6.Lee WC, Yang CC, Deng JF, Wu ML, Ger J, Lin HC, et al. The clinical significance of hyperamylasemia in organophosphate poisoning. J Toxicol Clin Toxicol. 1998;36:673–81. doi: 10.3109/15563659809162615. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya K, Phaujdar S, Sarkar R, Mullick OS. Serum creatine phosphokinase: A probable marker of severity in organophosphorus poisoning. Toxicol Int. 2011;18:117–23. doi: 10.4103/0971-6580.84263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panteghini M, Bais R, Van Soling WW. Enzymes. Tietz Text Book of Clinical Chemistry. In: Burtis CA, Ashwood ER, Bruns DE, editors. 4th ed. Philadelphia: Saunders Elsevier; 2006. pp. 616–7. [Google Scholar]
- 9.Balali-Mood M, Balali-Mood K, Moodi M, Balali-Mood B. Health aspects of organophosphorus pesticides in asian countries. Iran J Public Health. 2012;41:1–14. [PMC free article] [PubMed] [Google Scholar]
- 10.Hundekari IA, Suryakar AN, Rathi DB. Acute organo-phosphorus pesticide poisoning in North Karnataka, India: Oxidative damage, haemoglobin level and total leukocyte. Afr Health Sci. 2013;13:129–36. doi: 10.4314/ahs.v13i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manu MS, Prashant V, Akila P, Suma MN, Basavanagowdappa H. A retrospective analysis of serial measurement of serum cholinesterase in acute poisoning with organophosphate compounds. Toxicol Int. 2012;19:255–9. doi: 10.4103/0971-6580.103662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun HW, Lee DH, Lee JH, Cheon VJ, Choi YH. Serial serum Cholinesterase activities as a prognostic factor in organophosphate poisoned patients. Hong Kong J Emerg Med. 2012;19:92–7. [Google Scholar]
- 13.Tietz NW, Huang WY, Rauh DF, Shuey DF. Laboratory tests in differential diagnosis of hyperamylesemia. Clin Chem. 1986;32:301–7. [PubMed] [Google Scholar]
- 14.Ahmed A, Begum I, Aquil N, Atif S, Hussain T, Vohra E. Hyperamylasemia and acute pancreatitis following organophosphate poisoning. Pak J Med Sci. 2009;25:957–61. [Google Scholar]
- 15.Lin CL, Yang CT, Pan KY, Huang CC. Most common intoxication in nephrology ward organophosphate poisoning. Ren Fail. 2004;26:349–54. doi: 10.1081/jdi-120039816. [DOI] [PubMed] [Google Scholar]
- 16.Aslan S, Cakirz Z, Emet M, Serinken M, Karcioglu O, Kandis H, et al. Acute abdomen associated with organophosphorus poisoning. J Emerg Med. 2011;41:507–12. doi: 10.1016/j.jemermed.2010.05.072. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Bhardwaj U, Verma SK, Bhalla A, Gill K. Hyperamylasemia and acute pancreatitis following anticholinesterase poisoning. Hum Exp Toxicol. 2007;26:467–71. doi: 10.1177/0960327107076814. [DOI] [PubMed] [Google Scholar]
- 18.Hassan NA, Madboly AG. Correlation between serum creatine phosphokinase and severity of acute organophosphorus poisoning: A prospective clinical study (2012-2013) J Environ Sci Toxicol Food Technol. 2013;4:18–29. [Google Scholar]


