Abstract
Objectives:
The present study was designed to examine the chemopreventive effects of phloretin against 7, 12-dimethylbenz (a) anthracene (DMBA) induced buccal pouch carcinogenesis in male golden Syrian hamsters in order to discover resources to improve the traditional medicine.
Materials and Methods:
Hamsters were divided into four groups of 10 animals each. Group I was served as an untreated control. Group II hamsters were painted with 0.5% DMBA in liquid paraffin on the left buccal pouches three times a week for 14 weeks. Group III hamsters were orally administrated with phloretin at a dose of 40 mg/kg body Weight (b.wt) on days alternate to DMBA application. Group IV hamsters were orally administrated with phloretin alone and served as the drug control. The experiment was terminated at the end of fourteenth week. The experimental animal's tumors were subjected into morphological examination and subsequently screened the pathological changes and estimate the activities of bi-products of lipid peroxidation, antioxidants enzymes and phase I and II detoxification enzyme status.
Results:
In DMBA alone treated hamster showed increased levels of lipid peroxidation by products, leads to decreased levels of enzymatic and non-enzymatic antioxidants status, activities of phase I and II detoxification enzyme status were altered. Normalized the neoplastic changes, decreased the levels of lipid by products, retain the antioxidants and restored the phase I and II enzymes were observed in phloretin administrated animals during DMBA induced oral carcinogenesis.
Conclusion:
Phloretin has possible chemopreventive role in which modulating the antioxidant and detoxification enzyme status, thereby retarding DMBA induced buccal pouch carcinogenesis.
Keywords: Antioxidant; chemoprevention; 7,12-dimethylbenz (a) anthracene; lipid peroxidation; oral cancer; phloretin
INTRODUCTION
Oncological diseases are the second leading cause of mortality after cardiovascular diseases worldwide. According to the WHO prediction, the global incidence of cancer was 11 million with more than 7.6 million deaths, and is expected to increase an incidence of 15.5 million with 11.5 million deaths by 2030.[1] Oral cancer is one of the most common cancers and it constitutes a major health problem particularly in developing countries and one-half of all head and neck cancer occur in the oral cavity.[2] Epidemiologic studies have shown that environment and personal habits, particularly tobacco use and alcohol consumption, seem to be major etiologic factors in the induction and progression of oral cancer.[3]
Oxidative stress plays a major part in the development of chronic and degenerative diseases such as cancer, autoimmune disorders, rheumatoid arthritis, cataract, aging, cardiovascular and neurodegenerative diseases.[4] Human body has several mechanisms to counteract oxidative stress by producing antioxidants, which are either naturally or externally supplied through foods and/or supplements. These antioxidants act as free radical scavengers by preventing and repairing damages caused by ROS, and therefore can also enhance the immune defences and lower the risk of cancer and degenerative diseases.[5] In recent years, there is an increasing interest in finding antioxidant phytochemicals, because they can inhibit the propagation of free radical mediated oxidative stress and protect the human body from diseases.[6]
Flavonoids are a diverse group of compounds that are widely distributed in the plant kingdom. These agents and related synthetic analogues mediate a broad spectrum of biological responses, such as anti-allergic, anti-inflammatory, anti-oxidant, gastroprotective, anti-viral, anti-mutagenic, and anti-carcinogenic activities.[7] Phloretin are found exclusively in apples, which are frequently consumed by humans. It has been shown to exert anti-tumor activity through its inhibition of protein kinase C activity and its induction of apoptosis.[8] Kobori et al., have suggested that phloretin induces apoptosis in B16 melanoma 4A5 cells through the inhibition of glucose transmembrane transport.[9] However, to our knowledge there is no studies reported with reference to the chemopreventive activity of phloretin in DMBA induced oral carcinogenesis. Hence, the present work designed to study the chemopreventive effect of phloretin against DMBA induced oral carcinogenesis in male golden Syrian hamsters.
MATERIALS AND METHODS
Chemicals
DMBA and phloretin were purchased from Sigma Chemical Company, USA. All other chemicals used were of analytical grade, marketed by Himedia laboratories, Bangalore, India.
Animals
Male golden Syrian hamsters aged between 50–55 days were purchased from National Institute of Nutrition, Hyderabad and was housed in plastic cages. The recommendations of Institutional Animal Ethics Committee (Committee for the purpose of control and supervision of experiment on animals (CPCSEA Regn. no. 160/1999/CPCSEA), India) for the care and use of laboratory animals were strictly followed throughout the study. The hamsters were maintained under controlled environmental conditions on alternative 12-h dark/light cycle. Commercial pelleted feed by M/s Kamdhenu Ltd., Bangaluru, and water ad libitum were given to hamsters.
Experimental setup
The hamsters were divided into four groups of 10 animals each. Group I animals were served as control, The Group II and Group III animals were painted with 0.5% DMBA in liquid paraffin three times a week for 14 weeks on the left buccal pouches using (No. 4 brush) to induce the oral carcinogenesis. The Group II animals were received no other treatment. Group III animals were orally treated with phloretin (40 mg/kg body weight; dissolved in 0.5% DMSO) starting one week before the exposure to the carcinogen and continuing on alternate days of the DMBA painting until the animals were sacrificed. However, Group IV animals were orally administrated with phloretin alone to exclude any toxic effects. After the experimental period, the animals were sacrificed by cervical decapitation. Biochemical studies were conducted on the plasma, erythrocytes, buccal pouches and liver homogenate of control and experimental animals in each group.
Tumor study
Tumor weight was estimated according to the method of Geren et al. Tumor volume was calculated by the formula ν = 4/3 (π) × (D1/2) × (D2/2) × (D3/2), where D1, D2, and D3 are the three diameters (mm) of the tumors.[10] Histological slides were prepared by according to the method of Sankar Ray et al. Buccal tissues were fixed in 10% buffered formalin, embedded in paraffin using a conventional automated system.[11]
Biochemical determination
After plasma separation, the buffy coat was removed and the packed cells were washed thrice with physiological saline. A known volume of erythrocytes was lysed with hypotonic buffer at pH 7.4. The hemolysate was separated by centrifugation at 10,000 rpm for 15 min at 20°C. The erythrocyte membrane was prepared by the method of Dodge et al.,[12] modified by Quist.[13] Thiobarbituric acid reactive substances (TBARS), Lipid hydroperoxides and conjugated dienes were assayed in plasma, erythrocytes, and buccal mucosa according to the methods of Yagi,[14] Jiang et al.,[15] and Rao and Recknagel,[16] respectively. The activities of enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) were estimated by the methods of Kakkar et al.,[17] Sinha[18] and Rotruck et al.,[19] respectively. Reduced glutathione (GSH) was determined by the method of Beutler et al.[20] Vitamin C and E were measured according to the methods of Omaye et al.,[21] and Desai,[22] respectively. Cytochrome p450 and cytochrome b5 were measured according to the methods of Omura and Sato.[23] The levels of phase II detoxification enzymes such as glutathione reductase activity (GR), Glutathione-S-transferase (GST) and G-glutamyl transpeptidase (GGT) were measured by according to the method of Carlberg and Mannervik,[24] Habig et al.,[25] and Fiala et al.,[26] respectively.
Statistical analysis
Values are expressed as mean ± SD. Statistical comparisons were performed by one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT). The values were considered statistically significant if the P value was less than 0.05.
RESULTS
Tumor incidence, multiplicity, burden and neoplastic changes
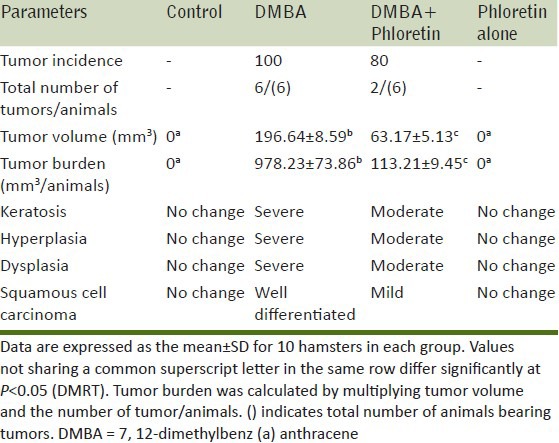
Table 1 demonstrate the tumor incidence, volume, burden and histopathological changes in control and experimental animals in each group. In DMBA alone treated hamsters, the tumor incidence was found to be 100% and the mean tumor volume and burden was found to be 196.64 mm3 and 978.23 mm. Upon the treatment with phloretin (Group III), the tumor burden, tumor volume was found to decrease significantly (63.17 mm3, 113.21 mm) when compared to that of control animals (Group II). Phloretin alone treated animals (Group IV) did not show any significant variations when compared to control (Group I) hamsters.
Table 1.
Incidence of oral neoplasm and histological changes in the control and experimental animals in each group
Histopathological observation
The histopathological sections of buccal tissue from the control and experimental animals in each group were shown in Figure 1. The buccal tissue of control and phloretin alone treated animals showed a normal histological pattern, whereas, the buccal tissue from the DMBA alone treated animals (Group II) showed massive tumor cell proliferation of the buccal pouch. Simultaneous oral administration of phloretin treated animals (Group III) showed the epithelium was normal, intact and the histopathological examination revealed mild to moderate hyperplasia. The histological investigation of phloretin alone treated animals (Group IV) indicating there were no adverse effects of phloretin on the buccal pouches of experimental animals.
Figure 1.
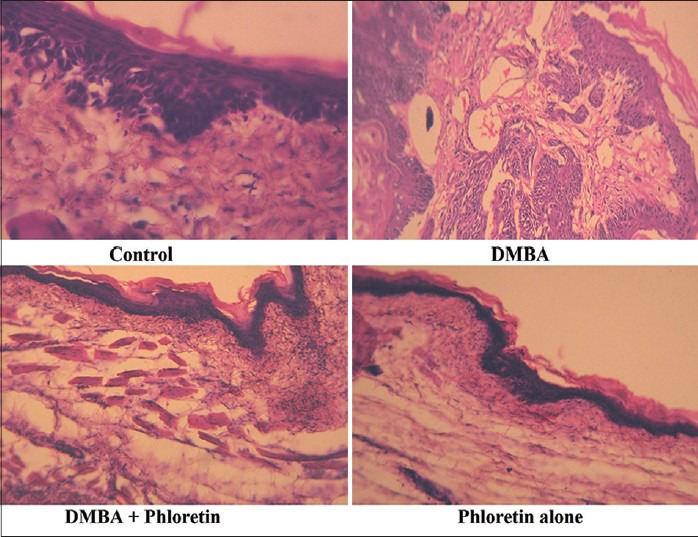
Histopathological evaluation of DMBA induced hamster buccal pouch carcinogenesis. Microphotograph of control animals showing normal epithelium in buccal mucosa. Microphotograph of DMBA alone treated animals showing well differentiated squamous cell carcinoma exhibiting keratin pearls in the connective tissue. Microphotograph of DMBA+phloretin treated animals exhibiting mild hyperplasia and mild dysplasia. Microphotograph of phloretin alone treated animals showing normal epithelium in buccal mucosa
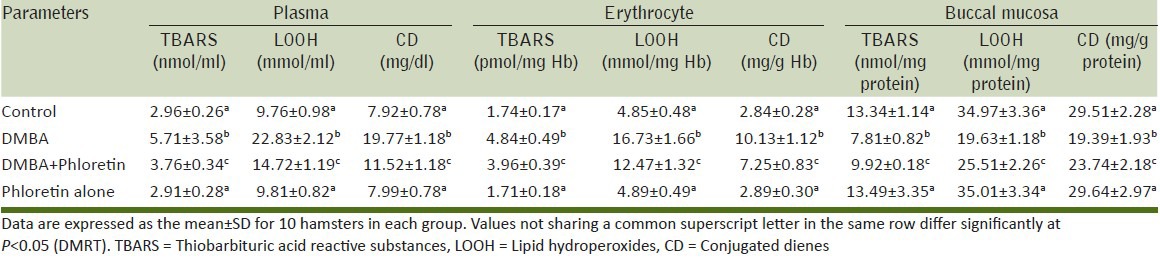
Effect of phloretin on lipid peroxidation status
Table 2 represents the levels of lipid peroxidation (TBARS, LOOH and CD) in the plasma, buccal and liver tissues of control and experimental animals. A significant increase in the levels of TBARS, LOOH and CD were observed in the DMBA alone treated animals (Group II) when compared with control animals (Group I). This was significantly reversed to near normal levels in phloretin (40 mg/kg b.wt) treated animals (Group III). Phloretin alone treated animals (Group IV) did not show any significant variations when compared to control (Group I) animals.
Table 2.
The levels of TBARS, LOOH and CD in plasma, erythrocyte and buccal mucosa of control and experimental animals in each group
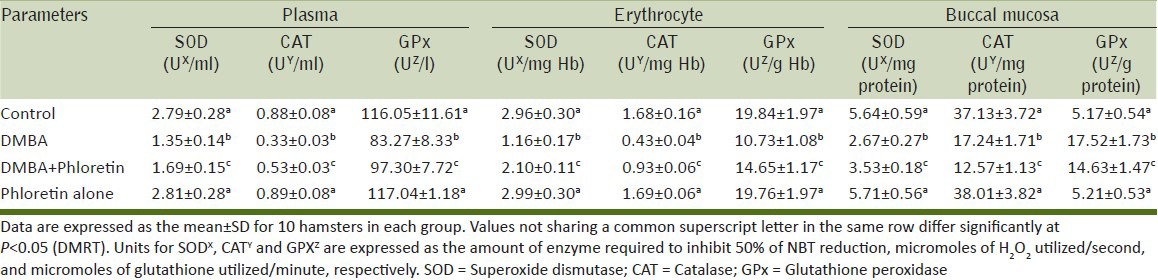
Effect of phloretin on enzymic antioxidants status
Table 3 represents the activity of enzymatic antioxidants in plasma, erythrocyte and buccal pouches of control and experimental animals. The levels of plasma, erythrocyte and buccal pouches of DMBA alone treated animals (Group II) showed a significant increase in the enzymatic antioxidants levels when compared to control animals (Group I). However, the levels were decreased significantly in phloretin treated animals (Group III) when compared with group II animals. Phloretin alone treated animals (Group IV) did not show any significant variations when compared to control (Group I) animals.
Table 3.
The activities of enzymatic antioxidants status in plasma, erythrocyte and buccal mucosa of control and experimental animals in each group
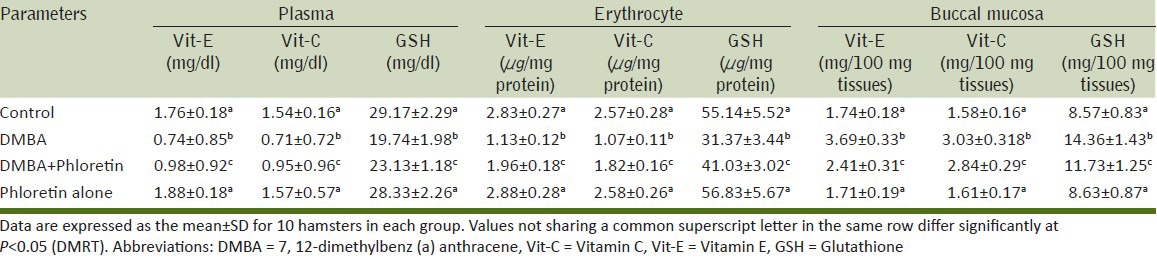
Effect of phloretin on non-enzymic antioxidants status
Table 4 show the levels of non-enzymic antioxidants (GSH, Vitamin C and E) in plasma, erythrocyte and buccal pouches of control as well as experimental animals. The non-enzymic antioxidant status was found to be significantly lowered in DMBA alone treated animals (Group II) when compared with control animals (Group I). The alterations in the levels of non-enzymic antioxidants were reverted to nearly control values on the administration of phloretin treated animals (groups III) when compared with group II animals. Phloretin alone treated animals (Group IV) did not show any significant variations when compared to control (Group I) animals.
Table 4.
The levels of non-enzymatic antioxidants status in plasma, erythrocyte and buccal mucosa of control and experimental animals in each group
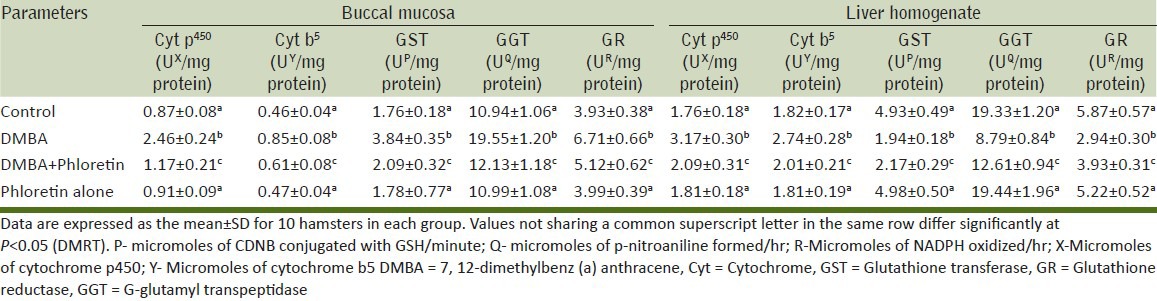
Effect of phloretin on of phase I and II detoxification enzyme status
The levels of phase II detoxification enzyme (Cyt p450, Cyt b5, GST, GGT, GR) in buccal pouches of control and experimental animal were depicted in Table 5. The levels of Cyt p450, Cyt b5, GST, GR, DT-diaphorase were significantly increased in DMBA alone treated animals (Group II) when compared to control (Group I) animals. In phloretin treated animals (group III), the levels of Cyt p450, Cyt b5, GST, GGT, GR were significantly normalized when compared with control animals (Group I). Phloretin alone treated animals (Group IV) hamsters did not show any significant variations when compared to control animals (Group I).
Table 5.
The levels of phase I and phase II detoxification enzyme status in buccal mucosa and liver homogenate of control and experimental animals in each group
DISCUSSION
Several epidemiological, clinical and experimental investigations were suggested that the plant based phenolic compounds have beneficial effects on the prevention of many types of diseases, cardio vascular diseases, diabetes mellitus and cancer.[27] The active principle of phloretin has been effectively inhibited the DMBA induced oral carcinogenesis in experimental animals. In the present study, painting application of DMBA was resulting in oral squamous cell carcinomas; that displayed increased lipid peroxidation, diminished antioxidant status and altered the activities of phase I and II detoxifying agents. Oral administration of phloretin to DNBA treated animals significantly normalized the neoplastic changes and decreased lipid peroxidation, improved the antioxidant status and restored the activities of detoxifying enzyme. In this study, we provide evidence that phloretin administration significantly reduces the malignant abnormalities. This might be due to the inhibitory action of phloretin on DMBA induced oral carcinogenesis in experimental animals.
The developments of DMBA induced carcinogenesis in a hamster model were parallel to the development of oral cancer in human, some of these similarities include progression from a normal stratified squamous epithelium to hyperplasia and hyperkeratosis, followed by development of dysplasia, which progresses in situ and invasive carcinomas.[28,29] In this study, the findings of the pathological as well as neoplastic changes were documented that DMBA treatments twisted the inflammation, severe hyperplasia, hyperkeratosis, dysplastic changes and well-differentiated squamous cell carcinoma. However, mild to moderate pre-neoplastic lesions (hyperplasia, keratosis and dysplasia) were observed in DMBA and phloretin treated animals. This might be due to the inhibitory action of the phloretin on DMBA induced oral carcinogenesis in hamsters. Together with these observations are clearly indicated that the oral administration of phloretin has the ability to reduce the neoplastic changes in DMBA induced oral carcinogenesis.
The direct relationships between lipid peroxidation (LPx) and antioxidant have been well reported in literature,[30] our results are in agreement with other observations. Exposures of DMBA significantly increased in LPx and decreased in antioxidant status were reported. The increased level of LPx may be due to the poor antioxidant defence or inactivation of antioxidant enzymes in cancerous tissues.[31] These results also compliance with Suresh et al., who have reported that the ultimate carcinogen of DMBA, mediates oral carcinogenesis by inducing an over production of reactive oxygen species; it leads to the over production of LPx.[32] In the same line of attack, we have been documented an increased levels of LPx levels in DMBA alone treated hamsters. Oral administration of phloretin at a dose of 40 mg/kg b.wt, significantly reversed lipid peroxidation levels to a considerable extent.
Enzymatic anti-oxidant systems consist of SOD, CAT and GPx are act as the principal defence against oxidative damage during the carcinogenesis.[33] Samy et al., have documented that SOD acts as an effective enzymatic antioxidant during chemical induced carcinogenesis.[34] CAT catalyzes the decomposition of hydrogen peroxide to improve the status of antioxidant system, which provides a protective effect against reactive oxygen species.[35] From the study, it has been revealed a significant decrease in SOD activity may be related to saturation of SOD. The decreased level of enzymatic antioxidants levels in DMBA treated animals indicating oxidative stress, which might be the cause of LPx leads to subsequent damage of DNA. Our results were seen this observations.
Non-enzymic antioxidants, such as vitamins C, E and GSH play an essential role in shielding the cells from oxidative stress; it is the another line of defense system against free radical damage.[36] GSH is the main intracellular antioxidant and its central role in xenobiotic or eicosanoid metabolism, and also it is maintaining the cell integrity because of its reducing properties and participation in the cell metabolism.[37] During the detoxifying metabolism of DMBA, GSH in conjunction with GST to detoxifies reactive intermediate species of DMBA and thereby enhancing resistance against oxidative stress.[38] Further we have been seen a depletion of GSH level has been observed in cancerous animals in response to DMBA mediated oxidative stress in experimental animals. The same lines of observation were documented in DMBA induced oral carcinogenesis.[39] Oral administration of phloretin to significantly improved the non-enzymatic antioxidant status in DMBA induced carcinogenesis.
The induction of Phase I detoxification enzyme system is considered a potential cancer risk factor due to the activation of procarcinogens to their ultimate reactive forms.[40] Therefore, the relative induction of phase I enzyme activities by pholretin might be of critical importance for the chemopreventive mechanism of DMBA induced buccal pouch carcinogenesis. Phase II enzymes perform conjugation reactions which help to convert the bio transformed intermediates of DMBA from Phase I into less toxic, water-soluble substances that are easily excreted or eliminated from the body.[41] Kumar et al., have showed that the anti-carcinogenic activity of dietary phytochemicals is mediated through the induction of hepatic GST, GGT and GR during the DMBA induced carcinogenesis.[42] Increased activities of phase II detoxification enzymes in animals treated with DMBA alone suggest that the detoxification cascade is stimulated to metabolise as well as detoxify the carcinogenic agent, DMBA. The results from the present study, when combined with the previously reviewed studies, indicate that the bioactive compound of phloretin from apple are able to alter both phase I and II metabolism of xenobiotics and thus may meet the criteria for an ideal chemopreventive drug.
CONCLUSION
Based on the present findings, it may be conclude that the oral administration of phloretin at a dose of 40 mg/kg b.wt effectively regress the DMBA induced cancer progression through the mechanism of triggering of phase I and II detoxification enzyme status in along with the improvement of antioxidants status in DMBA treated male golden Syrian hamsters. Further, molecular biology researches are needed to elucidate the mechanism behind this chemopreventive activity.
ACKNOWLEDGEMENTS
The authors are grateful to the Indian Council of Medical Research, New Delhi, India, for providing financial support in the form of Senior Research Fellowship (IRIS Cell No: 2011-08220; Ref no. 45/60/2011/BMS/TRM 21.03.2011).
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.World Health Organization Databank. Geneva: World Health Organization; 2010. [Last accessed on 2014 Jan 22]. WHO Statistical Information System. Available from: http://www.who.int/whosis . [Google Scholar]
- 2.Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol 2012. 2012:701932. doi: 10.1155/2012/701932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: A review of agents and causative mechanisms. Mutagenesis. 2004;19:251–62. doi: 10.1093/mutage/geh036. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das SK. Free radicals, antioxidants and nutraceuticals in health, disease and radiation biology. Preface. Indian J Biochem Biophys. 2012;49:291–2. [PubMed] [Google Scholar]
- 6.Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 8.Kern M, Pahlke G, Balavenkatraman KK, Bohmer FD, Marko D. Apple polyphenols affect protein kinase C activity and the onset of apoptosis in human colon carcinoma cells. J Agric Food Chem. 2007;55:4999–5006. doi: 10.1021/jf063158x. [DOI] [PubMed] [Google Scholar]
- 9.Kobori M, Iwashita K, Shinmoto H, Tsushida T. Phloretin-induced apoptosis in B16 melanoma 4A5 cells and HL60 human leukemia cells. Biosci Biotechnol Biochem. 1999;63:719–25. doi: 10.1271/bbb.63.719. [DOI] [PubMed] [Google Scholar]
- 10.Geren RJ, Greenberg NH, Mcdonald MM, Schumacher AM. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep. 1972;3:1–103. [Google Scholar]
- 11.Ray RS, Ghosh B, Rana A, Chatterjee M. Suppression of cell proliferation, induction of apoptosis and cell cycle arrest: Chemopreventive activity of vanadium in vivo and in vitro. Int J Cancer. 2006;120:13–23. doi: 10.1002/ijc.22277. [DOI] [PubMed] [Google Scholar]
- 12.Dodge JF, Mitchell G, Hanahan DJ. The preparation and chemical characterization of hemoglobin-free ghosts of human red blood cells. Arch Biochem Biophys. 1968;110:119–30. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- 13.Quist EE. Regulation of erythrocycaste membrane shape by Ca2 + Biochem Biophys Res Commun. 1980;92:631–7. doi: 10.1016/0006-291x(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 14.Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987;45:337–51. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- 15.Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–9. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 16.Rao KS, Recknagel RO. Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Exp Mol Pathol. 1968;9:271–8. doi: 10.1016/0014-4800(68)90041-5. [DOI] [PubMed] [Google Scholar]
- 17.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 18.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 19.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidise. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 20.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 21.Omaye ST, Turnbull TD, Sauberlich HE. Selected method for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 22.Desai ID. Vitamin E analysis methods for animal tissues. Methods Enzymol. 1984;105:138–47. doi: 10.1016/s0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- 23.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. II. solubilization, purification, and properties. J Biol Chem. 1964;239:2379–85. [PubMed] [Google Scholar]
- 24.Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975;250:5475–80. [PubMed] [Google Scholar]
- 25.Habig WH, Pabst MJ, Fleischner G, Gatmaitan Z, Arias IM, Jakoby WB. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci U S A. 1974;71:3879–82. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiala S, Fiala AE, Dixon B. Glutamyl transpeptidase in transplantable, chemically induced rat hepatomas and “spontaneous” mouse hepatomas. J Natl Cancer Inst. 1972;48:1393–401. [PubMed] [Google Scholar]
- 27.Casto BC, Knobloch TJ, Galioto RL, Yu Z, Accurso BT, Warner BM. Chemoprevention of oral cancer by lyophilized strawberries. Anticancer Res. 2013;33:4757–66. [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang CP, Huang WT, Lee JW, Hsu YC. Effective treatment of 7,12-dimethylbenz (a) anthracene induced hamster buccal pouch precancerous lesions by topical photosan mediated photodynamic therapy. Head Neck. 2012;34:505–12. doi: 10.1002/hed.21761. [DOI] [PubMed] [Google Scholar]
- 29.Yang K, Zhang G, Mei J, Chen D, Wu M. Screening and analysis of pathogenic genes during DMBA induced buccal mucosa carcinogenesis in golden hamsters. Oncol Rep. 2010;23:1619–24. doi: 10.3892/or_00000803. [DOI] [PubMed] [Google Scholar]
- 30.Rajalingam K, Sugunadevi G, Arokia Vijayaanand M, Kalaimathi J, Suresh K. Anti-tumour and anti-oxidative potential of diosgenin against 7, 12-dimethylbenz (a) anthracene induced experimental oral carcinogenesis. Pathol Oncol Res. 2012;18:405–12. doi: 10.1007/s12253-011-9460-1. [DOI] [PubMed] [Google Scholar]
- 31.Tania M, Khan MA, Song Y. Association of lipid metabolism with ovarian cancer. Curr Oncol. 2010;17:6–11. doi: 10.3747/co.v17i5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suresh K, Manoharan S, Vijayaanand MA, Sugunadevi G. Chemopreventive and antioxidant efficacy of (6)-paradol in 7,12-dimethylbenz (a) anthracene induced hamster buccal pouch carcinogenesis. Pharmacol Rep. 2010;62:1178–85. doi: 10.1016/s1734-1140(10)70380-7. [DOI] [PubMed] [Google Scholar]
- 33.Thirunavukkarasu C, Sakthisekaran D. Effect of selenium on Nnitrosodiethylamine induced multi stage hepatocarcinogenesis with reference to lipid peroxidation and enzymic antioxidants. Cell Biochem Funct. 2001;19:27–35. doi: 10.1002/cbf.895. [DOI] [PubMed] [Google Scholar]
- 34.Samy RP, Gopalakrishnakone P, Ignacimuthu S. Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz (a) anthracene-induced mammary tumors in rats. Chem Biol Interact. 2006;164:1–14. doi: 10.1016/j.cbi.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Sies H. Strategies of antioxidant defense. Eur J Bichem. 1993;215:213–9. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 36.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–36. [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–26. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 38.Sugunadevi G, Suresh K, Vijayaanand MA, Rajalingam K, Sathiyapriya J. Anti genotoxic effect of Mosinone-A on 7, 12-dimethyl benz[a] anthracene induced genotoxicity in male golden Syrian hamsters. Pathol Oncol Res. 2012;18:69–77. doi: 10.1007/s12253-011-9418-3. [DOI] [PubMed] [Google Scholar]
- 39.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–81. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 40.Percival M. Phytonutrients and detoxification. Clin Nutr Insights. 1997;5:1–4. [Google Scholar]
- 41.Baer-Dubowska W, Szaefer H. Modulation of carcinogen-metabolizing cytochromes P450 by phytochemicals in humans. Expert Opin Drug Metab Toxicol. 2013;9:927–41. doi: 10.1517/17425255.2013.795219. [DOI] [PubMed] [Google Scholar]
- 42.Kumar R, Kaur R, Singh AP, Arora S. Diminution of hepatic response to 7, 12-dimethylbenz(α) anthracene by ethyl acetate fraction of Acacia catechu willd. through modulation of xenobiotic and anti-oxidative enzymes in rats. PLoS One. 2014;9:e90083. doi: 10.1371/journal.pone.0090083. [DOI] [PMC free article] [PubMed] [Google Scholar]


