Abstract
Objective:
Treatment of ischemic hypertensive patients with hydrochlorothiazide can precipitate cardiac arrhythmias. Green tea by virtue of its antioxidant potential is responsible for cardio-protective activity. The present study was undertaken to evaluate the pharmacodynamic interaction of green tea extract with hydrochlorothiazide against cyclophosphamide-induced myocardial toxicity.
Materials and Methods:
Rats were treated with high (500 mg/kg, p.o.) and low (100 mg/kg, p.o.) dose of green tea extract in alone and interactive groups for 10 days. Standard, high, and low dose of interactive groups received hydrochlorothiazide (10 mg/kg, p.o.) for last 7 days. Apart from normal control, all other groups were subjected to cyclophosphamide (200 mg/kg, i.p.) toxicity on day first and the effects of different treatments were evaluated by changes in electrocardiographic parameters, serum biomarkers, and tissue antioxidant levels. Apart from that, lipid profile and histological studies were also carried out.
Results:
Compared to cyclophosphamide control group, both high and low dose of green tea exhibited significant decrease in serum biomarkers and increase in tissue antioxidant levels. Green tea treatment was also responsible for significant improvement in echocardiography (ECG) parameter, lipid profile, and histological score. Incorporation of high and low dose of green tea with hydrochlorothiazide-exhibited significant protection compared to hydrochlorothiazide-alone-treated group.
Conclusion:
The present findings clearly suggested that green tea extract dose dependently reduces cyclophosphamide-induced myocardial toxicity. Green tea when combined with hydrochlorothiazide can reduce the associated side effects and exhibits myocardial protection.
Keywords: Cyclophosphamide, green tea, hydrochlorothiazide
INTRODUCTION
Cyclophosphamide (CP) is oxazaphosphorine alkylating agent widely used as an antineoplastic and immunosuppressant agent. Its tumor-cell-killing activity is mainly due to its deoxyribonucleic acid (DNA) alkylation. Although it has tumor selectivity and wide spectrum of clinical uses, CP is known to cause multiple organ toxicity. Two active metabolites of CP are phosphoramide mustard and acrolein which can react with carboxyl (-C[O] OH), mercapto (-SH), amino (-NH2), phosphate (-PO3H2), and hydroxyl (-OH) groups, and can form cross-links with DNA and proteins.[1] CP in high dose is responsible for lethal cardio-toxicity such as congestive heart failure, arrhythmias, cardiac tamponade, and myocardial depression.[2] The precise mechanism by which CP causes toxicity is unknown. Excessive generation of free oxygen radicals and decrease in the antioxidant defense mechanism by CP may be the prime reason of cardio-toxicity.[3] Moreover, CP is associated with hypercholesterolemia, hypertriglyceridemia, and impaired secretion of heart lipoprotein lipase.[4] CP-induced alterations of lipid metabolism pathways in various conditions lead to myocardial lipid accumulation and lipotoxic cardiomyopathy.[5]
Treatment of ischemic hypertensive patients with diuretic like hydrochlorothiade is still under close watch, as mild to moderate level of hypokalemia may lead to development of cardiac arrhythmias. In this context, it is rational to combine hydrochlorothiazide (HCTZ) with angiotensin converting enzyme inhibitor-I, aldosterone antagonist, or angiotensin type- I receptor blocker which can be beneficial.[6]
In many societies throughout the globe, herbs and herb-based therapy played an important role to treat different diseased condition and to improve the quality of life. In recent trend, herb drug interactive studies are getting popularity as it may influence the pharmacokinetic and pharmacodynamic profile of each other, and can mimic, magnify, or oppose the action of each other.[7]
Green tea is a very popular beverage in many countries. It is produced from non-fermented leaves of Camellia sinensis belonging to family Theaceae, which contains more catechins than black tea or oolong tea. Presence of high levels of catechin, certain minerals, and vitamins are responsible for huge antioxidant potential of this type of tea. In the traditional Chinese medicine, green tea has been marked as a healthful beverage. Recent studies have been reported that green tea may contribute to the reduction in risk of cardiovascular diseases and some forms of cancer, as well as to the promotion of oral health. It is having antibacterial, antiviral, neuro-protective, and anti-fibrotic properties. It is reported that green tea gives protection from solar ultraviolet rays and increase bone mineral density.[8,9]
It has been reported that green tea prevents left ventricular hypertrophy, hypertension, cardiovascular damage, and endothelial dysfunction. One of the clinical study showed that the consumption of green tea is responsible for reducing the occurrence of cardiovascular disease by increasing plasma antioxidant capacity.[10,11,12] Apart from that, green tea is able to prevent anticancer drug like doxorubicin induced cardiac abnormalities and pathological changes.[13]
However, there is no scientific report which indicates the protective effect of green tea against CP-induced myocardial toxicity. So, the present study was undertaken to evaluate the effect of green tea alone and their combination with HCTZ against CP-induced myocardial toxicity.
MATERIALS AND METHODS
Chemicals
All chemicals used were of analytical grade and purchased from standard companies. Pure sample of HCTZ was gifted by Bangalore Test House (Bangalore, India). Biochemical kits were procured from Crest Biosystems (Goa, India).
Experimental animals
Healthy adult Wistar albino rats of either sex weighing 175-250 g were procured from Animal House, Shree Devi College of Pharmacy, Mangalore. Rats were housed in polypropylene cages, maintained under standardized condition (12 h L: D cycles, 25° ± 5°C) with paddy husk bedding at the Central Animal House, Shree Devi College of Pharmacy, Mangalore. Animals were provided with standard pellet food and had free access to purified drinking water. The guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India were followed and prior permission was sought from the Institutional Animal Ethics Committee for conducting the study (SDCP/IAEC-19/2012-13).
Plant materials
Green tea (Camellia sinensis) leaves were purchased in the month of June, 2013 from the local market of Mangalore bearing the brand name GREEN TEA (manufactured by New hilltop traders, Vandiperiyar, Kerala). The authentication was done by Dr. Neoline J. Pinto, H.O.D., Department of Botany, St. Agnes College, Mangalore (SAC/MNG/SMP/Drug/2013-06/52). The aqueous extract was prepared by gently mixing Green tea leaves in distilled water maintained at 70-80°C for 30 minutes. Thereafter, it was filtered and evaporated in the same temperature to obtain a thick gummy mass. The yield was found to be 24.76% (W/W). Extract was freshly dissolved in distilled water before giving each dose to animals.
Phytochemical estimations of the extract
Aqueous extract of Green tea (GTE) was subjected to qualitative analysis to investigate the presence of various phytochemical constituents like alkaloids, glycosides, steroids, flavonoids, gallic tannins, catecholic tannin, terpenoid, and saponins.[14,15]
Acute toxicity study
Acute toxicity study was carried out according to Office of Prevention, Pesticide and Toxic Substance (OPPTS) guidelines following the limit test procedure.[16]
Mice were fasted overnight prior to the studies, and then divided into two groups of three each. Test dose of 2 g/kg body weight and 5 g/kg body weight were given orally to either group of mice, and then observed for 72 hours for mortality. 1/10th and 1/50th of the maximum safe dose corresponding to 500 and 100 mg/kg orally were selected as high and low doses, respectively.
Experimental protocol
The animals were divided into seven different treatment groups of eight animals each. Group I and Group II received saline for 4 weeks and termed as normal control and CP control, respectively; Group III received HCTZ (10 mg/kg),[6] Group IV and V received green tea extract (GTE) 100 mg/kg (GTE-100) and 500 mg/kg (GTE-500) respectively. Group VI and Group VII were treated with GTE-100 and GTE-500 respectively along with HCTZ. All treatments were done for 10 days by oral route. Except Group I, all other groups were subjected to CP toxicity with the dose of (200 mg/kg i.p.) on day first.[17]
Electrocardiographic studies
Forty-eight hour after the last treatment, the animals were anesthetized with the combination of ketamine (75 mg/kg, i.p.) and xylazine (8 mg/kg, i.p.). Leads were attached to the dermal layer of both the front paws and hind legs and recordings were made with the help of digital physiograph (model no- DI-2, INCO, Ambala city, India). The changes in heart rate, QRS interval, QT interval and RR interval (these are different waves of ECG; no expanded form is available) were determined.[16]
Oxidative marker enzyme assay
Forty-eight hours after the last treatment, blood was collected for the separation of serum and analyzed for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphate (ALP), creatine kinase-MB (CK-MB), creatine kinase-NAC (CKNAC), and lactate dehydrogenase (LDH). Estimation of marker enzymes were done by using commercial kits with the help of semi-auto analyzer (model: Prietest touch, Robonik India PVT.LTD.).
Then the animals were sacrificed by mild ether anaesthesia. Four hearts from each group were homogenized with sucrose solution (0.25 M) for estimations of superoxide dismutase (SOD), catalase and thiobarbituric acid reactive species (TBARS).[16,17]
Lipid profile assay
Serum cholesterol and triglyceride levels were measured by commercial kits with the help of semi-autoanalyzer.[17]
Histological analysis
Heart sections were prepared from the remaining four hearts in each group, stained with Hematoxylin and Eosin (H and E) and changes in histology were observed. The myocardial damage was determined by scoring method depending on the severity as follows, no change = 0 score; mild = 1score (focal myocytes damage or small multifocal degeneration with slight degree of inflammation); moderate = 2 score (extensive myofibrillar degeneration), and marked = 3 score (necrosis with diffuse inflammation).[16]
Statistical analysis
Results are expressed as mean ± SE. Statistical significance was assessed using one-way analysis of variance (ANOVA) followed by Tukey-Karmer multiple comparison tests. P <0.05 was considered significant.
RESULTS
Preliminary phytochemical investigation
The preliminary phytochemical investigation of the GTE extract showed the presence of alkaloids, flavonoids, steroids, gallic tannins, catecholic tannins. The percentage yield of GTE was found to be 24.76%.
Effect on electrocardiographic parameters
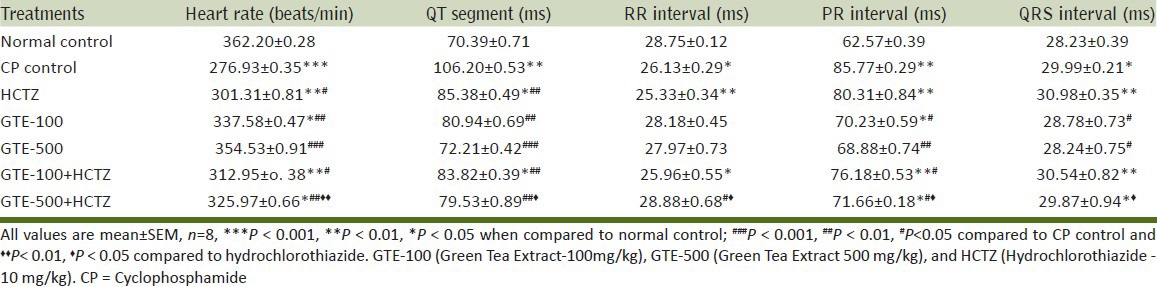
CP control group reported significant increase in QT segment, PR and QRS interval, and significant decrease in heart rate and RR interval compared to normal control. GTE-rectified CP induced changes in ECG to normal in dose dependent manner. GTE, with the dose of 500mg/kg when incorporated with HCTZ, demonstrated significant improvement in ECG parameter compared to HCTZ-alone-treated group [Table 1].
Table 1.
Effect on electro cardiograph patterns against CP-induced myocardial toxicity
Serum enzyme biomarkers
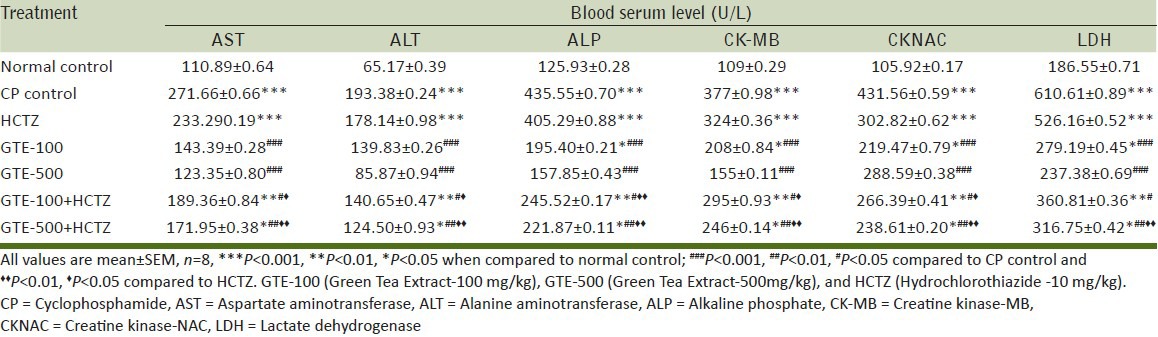
CP control group demonstrated significant increase in serum AST, ALT, ALP, CK-MB, CK-NAC, LDH values compared to normal control. Treatment groups such as GTE-100, GTE-500, GTE-100 + HCTZ, GTE-500 + HCTZ showed significant decrease in AST, ALT, ALP, CK-MB, CK-NAC, LDH values compared to CP control. GTE-100 + HCTZ and GTE-500 + HCTZ-treated groups showed significant decrease in AST, ALT, ALP, CK-MB, CK-NAC, LDH values compared to HCTZ-alone-treated group [Table 2].
Table 2.
Effect on serum marker enzymes against CP-myocardial toxicity
Effect on SOD and catalase
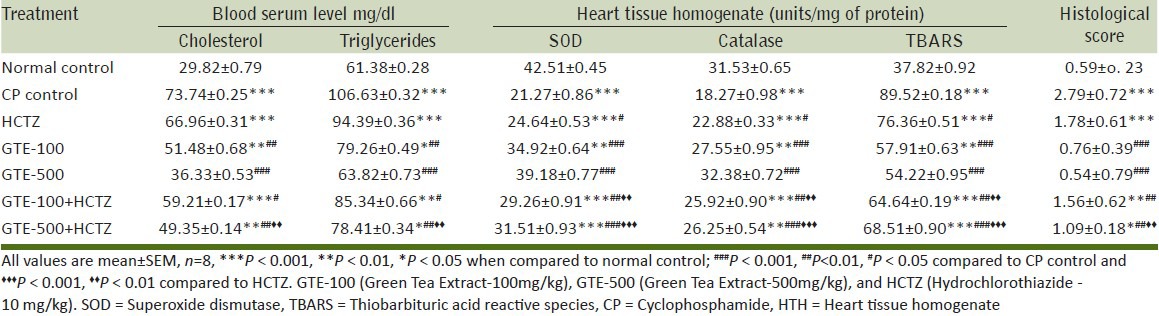
SOD and catalase activity were reduced significantly in CP control compared to normal control. Experimental groups such as GTE-100, GTE-500 and their combination with HCTZ resulted in significant improvement in SOD and catalase activity compared to CP-treated group. Moreover, GTE-100 and GTE-500 incorporated with HCTZ represented significant increase in SOD and Catalase values compared to HCTZ-alone-treated group [Table 3].
Table 3.
Effect on serum lipid profile, antioxidants in HTH and histological score against CP-induced myocardial toxicity
Effect on TBARS
CP control group exhibited significant increase in TBARS levels compared to normal control. GTE treatment in a dose-dependent manner demonstrated significant reduction in TBARS levels compared to CP control group. Incorporation of HCTZ with GTE in both high and low dose resulted in significant fall in TBARS levels compared to HCTZ alone [Table 3].
Effect on lipid profile
Significant increase was found in triglycerides and cholesterol levels in case of CP-intoxicated group compared to normal control. Treatment with GTE-100, GTE-500 and their combination with HCTZ demonstrated significant reduction in triglycerides and cholesterol levels compared to CP control group. Concurrent administration of GTE-500 with HCTZ showed significant decrease in lipid profile compared to HCTZ alone [Table 3].
Effect on histological score
CP caused loss of myocardial integrity, which was indicated by significant increase in histological score compared to normal control. Extensive myofibrillar degeneration, marked diffuse inflammation, and increased interstitial space was evident by CP administration. The treatment groups treated with GTE alone or in combination with HCTZ showed significant reduction in histological score compared to CP control group. High dose of GTE, especially in 500 mg/k when combine with HCTZ, demonstrated significant reduction in histological score compared to HCTZ alone. The protective effect of combined therapy of HCTZ with high dose of GTE was demonstrated with least multifocal degeneration, mild inflammation with reduction in interstitial space [Table 3] [Figure 1].
Figure 1.
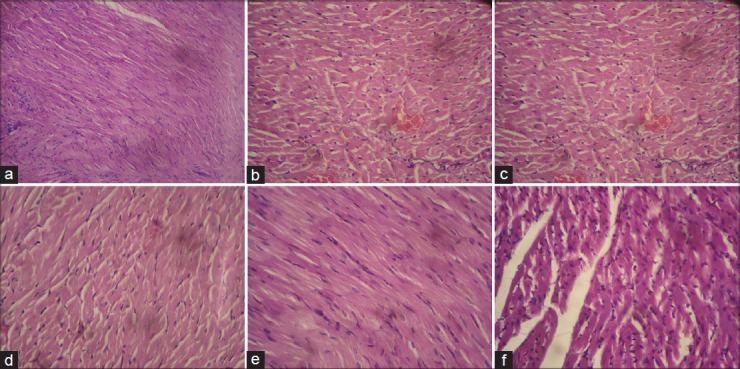
(a) (H and E) (×400) stained microscopic section of normal control group (normal texture of cell) (b) (H and E) (×400) stained microscopic section of Cyclophosphamide (200 mg/kg, i.p.) control group (Necrotic cells with degeneration of myofibril, increased interstitial space, diffuse inflammation) (c) (H and E) (×400) stained microscopic section of Hydrochlorothiazide (10 mg/kg, p.o.) group (Extensive myofibrillar degeneration, increased interstitial space, slight degree of inflammation) (d) (H and E) (×400) stained microscopic section of GTE-100 (100 mg/kg, p.o.) and Cyclophosphamide (200 mg/kg, i.p.) (Less interstitial space, myofibrillar degeneration) (d) (H and E) (×400) stained microscopic section of GTE-500 (500 mg/kg, p.o.) and Cyclophosphamide (200 mg/kg, i.p.) (Small multifocal degeneration, slight inflammation) (e) (H and E) (×400) stained microscopic section of GTE-100 (100 mg/kg, p.o.) and Hydrochlorothiazide (10 mg/kg, p.o.) and Cyclophosphamide (200 mg/kg, i.p.) (Extensive myofibrillar degeneration, inflammation) (f) (H and E) (×400) stained microscopic section of GTE-500 (500 mg/kg, p.o.) and Hydrochlorothiazide (10 mg/kg, p.o.) and Cyclophosphamide (200 mg/kg, i.p.) (Small multifocal degeneration, slight inflammation, fall in interstitial space)
DISCUSSION
The aim of the present study was to investigate the effect of GTE alone and their combination with HCTZ against CP-induced myocardial toxicity.
From the documented results, it can be concluded that GTE (100 and 500 mg/kg, p.o.) showed beneficial results dose dependently. Moreover, combination of GTE with HCTZ indicated better results compared to HCTZ-alone-treated group against CP-induced myocardial injury.
HCTZ treatment causes significant alteration in renal tubular mechanisms of electrolyte reabsorption. The direct action of HCTZ is associated with increase in the excretion of sodium and chloride, whereas the indirect action causes reduction in plasma volume, consequent increase in urinary potassium loss, plasma renin activity, aldosterone secretion, and decrease in serum potassium loss.[18]
Due to mild-to-moderate hypokalemia, patients with cardiac ischemia, heart failure, or left ventricular hypertrophy can develop cardiac arrhythmia.[19]
It has been reported that HCTZ induced hypokalemia is responsible for the increase in serum biomarker levels, such as lactate dehydrogenase (LDH), CK-MB, and decrease in heart tissue antioxidant levels like SOD and catalase in rat. Therefore, it is proved that their undesirable metabolic consequences have been suspected to contribute an increase in cardiovascular morbidity and mortality. Hence, search for concurrently administered safe therapeutic medicament continues which can ameliorate the hypokalemia in patients with ischemic heart diseases.[6]
Green tea, obtained from non-fermented leaves of Camellia sinensis, is having a rich source of polyphenols. Polyphenols may be the prime reason for strong free radical-scavenging activity. Green tea has reported its potency against cardiovascular disease risk factors. It reduces body weight by interfering within the sympatho-adrenal system, reducing fatty acid synthesis, and decreasing cholesterol absorption. It possesses antithrombotic activity by inhibiting platelet aggregation. Green tea inhibits low density lipid oxidation, reduces adhesion molecule expression, and reduces systolic as well as diastolic blood pressures.[20]
CP is having direct detrimental effect on myocardial endothelial cell, which leads to destruction of myocardial cells.[21] One of the postulated molecular mechanisms behind the CP-induced cardiotoxicity is increase in myocardial xanthine oxidase activity. Xanthine oxidase is a flavoprotein which catalyses the oxidation of hypoxanthine to xanthine and generates superoxide and uric acid. Superoxide is one of the main sources for the generation of reactive oxygen species. Thus, CP is associated with increase in free radical production and decrease in antioxidant enzymes. It has been reported that CP reduces the SOD and catalase activity, which triggers the formation of OH−radicals. Apart from that, it initiates and propagates lipid peroxidation.[22]
In our present finding, animals treated with only CP demonstrated significant decrease in SOD, catalase values and increase in TBARS level, which indicates the induction of myocardial toxicity. Prophylactic treatment with GTE dose dependently increase SOD and catalase activity and decrease TBARS level which justify its protection. GTE incorporated with HCTZ showed better protection in context of antioxidant activity compared with HCTZ-alone-treated group.
CP-induced myocardial damage is responsible for leakage of marker enzymes such as CK-MB, LDH, ALT, AST, and ALP from the myocytes to the blood. Estimation of these marker enzymes serves as a diagnostic tool to detect myocardial necrosis.[21]
In the present study, experimental animals treated with CP without any other treatment reflected remarkable amount of increase in serum marker enzymes levels which confirms the induction of myocardial toxicity. GTE dose dependently restored the marker enzymes levels and when GTE is combined with HCTZ, documented significant decrease compared to HCTZ-alone-treated group.
In this study, CP showed abnormal changes in ECG pattern such as decrease in heart rate, RR interval, and prolongation of QT interval, PR interval, and QRS interval. Decrease in heart rate may be due to release of significant amount of acetylcholine, which is also linked with the genesis of myocardial damage.[23]
Prolongation of QT interval found in present study with CP may be due to increase in the cellular Na+ content and decrease in K+ content. Hypokalemia is suspected for QT interval elongation. Prolongation of PR interval associated with CP might be due to AV block. Change in parasympathetic tone and conduction system deformation can be the reason for AV block.[24]
GTE in both 100 mg/kg and 500 mg/kg doses, in a dose dependent manner bring back the ECG parameters towards the normal. Combination of GTE with HCTZ especially in 500 mg/kg significantly restores the ECG patterns.
Histological study in CP-induced cardiotoxicity supported the findings of other parameters analyzed in different treatment groups. For the normal heart, myocardial fibers were found to be of uniform size, shape, and configurations with no inflammatory cell infiltrates. CP caused enormous changes in the myocardial cell associated with degeneration of myocardial tissue, vacuolization of the cardiomyocytes, infiltration of inflammatory cells, and myofibril loss. Treatment with GTE dose dependently inhibited CP-induced cardiac damage by decreasing fragmentation of myofibrils and inflammation. GTE predominantly in higher dose (500 mg/kg) was able to retrieve the pathological changes associated with HCTZ in myocardial cell.
CONCLUSION
It can be concluded from the present study that GTE-exhibited dose-dependent protection against CP-induced cardiotoxicity. Apart from that, combination of GTE with HCTZ demonstrated significant reduction in myocardial side effects associated with HCTZ. Findings of this research can be very important for cancer patients with hypertension and myocardial ischemic conditions, where HCTZ cannot be given as mono therapy due to potential myocardial side effects. Future studies can be carried out to establish the fact clinically.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Todorova V, Vanderpool D, Blossom S, Nwokedi E, Hennings L, Mrak R, et al. Oral glutamine protects against cyclophosphamide-induced cardiotoxicity in experimental rats through increase of cardiac glutathione. Nutrition. 2009;25:812–7. doi: 10.1016/j.nut.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Shanholtz C. Acute life-threatening toxicity of cancer treatment. Crit Care Clin. 2001;17:483–502. doi: 10.1016/s0749-0704(05)70196-2. [DOI] [PubMed] [Google Scholar]
- 3.Schimmel KJ, Richel DJ, van den Brink RB, Guchelaar HJ. Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30:181–91. doi: 10.1016/j.ctrv.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Loudet AM, Dousset N, Carton M, Douste-Blazy L. Effects of an antimitotic agent (cyclophosphamide) on plasma lipoproteins. Biochem Pharmacol. 1984;33:2961–5. doi: 10.1016/0006-2952(84)90594-x. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, et al. Cardiomyocyte-restricted peroxisome proliferator activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–50. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 6.Asdaq SM, Inamdar MN. The potential for interaction of hydrochlorothiazide with garlic in rats. Chem Biol Interact. 2009;181:472–9. doi: 10.1016/j.cbi.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Berman AF, Ernst E. Herb-drug interactions: Review and assessment of report reliability. Br J Clin Pharmacol. 2001;52:587–95. doi: 10.1046/j.0306-5251.2001.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea--a review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 9.Brown MD. Green tea (Camellia sinensis) extract and its possible role in the prevention of cancer. Altern Med Rev. 1999;4:360–70. [PubMed] [Google Scholar]
- 10.Papparella I, Ceolotto G, Montemurro D, Antonello M, Garbisa S, Rossi G, et al. Green tea attenuates angiotensin II-induced cardiac hypertrophy in rats by modulating reactive oxygen species production and the Src/epidermal growth factor receptor/Akt signaling pathway. J Nutr. 2008;138:1596–601. doi: 10.1093/jn/138.9.1596. [DOI] [PubMed] [Google Scholar]
- 11.Antonello M, Montemurro D, Bolognesi M, Di Pascoli M, Piva A, Grego F, et al. Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. Am J Hypertens. 2007;20:1321–8. doi: 10.1016/j.amjhyper.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa K, Ninomiya M, Okubo T, Aoi N, Juneja LR, Kim M, et al. Tea catechin supplementation increases antioxidant capacity and prevents phospholipid hydroperoxidation in plasma of humans. J Agric Food Chem. 1999;47:3967–73. doi: 10.1021/jf981195l. [DOI] [PubMed] [Google Scholar]
- 13.Patil L, Balaraman R. Effect of green tea extract on Doxorubicin induced cardiovascular abnormalities: Antioxidant action. Iran J Pharm Res. 2011;10:89–96. [PMC free article] [PubMed] [Google Scholar]
- 14.Finar IL. 4th ed. Vol. 1. ELBS; 1993. Organic Chemistry; p. 518. [Google Scholar]
- 15.Mukherjee PK. 1st ed. New Delhi: Business Horizons; 2002. Quality Control of Herbal Drugs – An Approach to Evaluation of Botanicals; p. 246. [Google Scholar]
- 16.Chakraborty M, Asdaq SM. Interaction of Semecarpus anacardium L. with propranolol against isoproterenol induced myocardial damage in rats. Indian J Exp Biol. 2011;49:200–6. [PubMed] [Google Scholar]
- 17.Viswanatha Swamy AH, Patel UM, Koti BC, Gadad PC, Patel NL, Thippeswamy AH. Cardioprotective effect of Saraca indica against cyclophosphamide induced cardiotoxicity in rats: A biochemical, electrocardiographic and histopathological study. Indian J Pharmacol. 2013;45:44–8. doi: 10.4103/0253-7613.106434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Field MJ, Stanton BA, Giebisch GH. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J Clin Invest. 1984;74:1792–802. doi: 10.1172/JCI111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoes AW, Grobbee DE, Peet TM, Lubsen J. Do non-potassium-sparing diuretics increase the risk of sudden cardiac death in hypertensive patients. Recent evidence? Drugs. 1994;47:711–33. doi: 10.2165/00003495-199447050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hernández Figueroa TT, Rodríguez-Rodríguez E, Sánchez-Muniz FJ. The green tea, a good choice for cardiovascular disease prevention? Arch Latinoam Nutr. 2004;54:380–94. [PubMed] [Google Scholar]
- 21.Shanmugarajan TS, Arunsunder M, Somasundaram I, Krishnakumar E, Sivaraman D, Ravichandiran V. Protective effect of Ficus hispida Linn. on cyclophosphamide provoked oxidative myocardial injury in rat model. Int J Pharmacol. 2008;1:1–10. [Google Scholar]
- 22.Sekeroğlu V, Aydin B, Sekeroğlu ZA, Viscum album L. extract and quercetin reduce cyclophosphamide-induced cardiotoxicity, urotoxicity and genotoxicity in mice. Asian Pac J Cancer Prev. 2011;12:2925–31. [PubMed] [Google Scholar]
- 23.Atlee JL. Protective cardiac dysrhythmias: Diagnosis and management. Anesthesiology. 1997;86:1397–424. doi: 10.1097/00000542-199706000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Levine ES, Friedman HS, Griffith OW, Colvin OM, Raynor JH, Lieberman M. Cardiac cell toxicity induced by 4-hydroperoxycyclophosphamide is modulated by glutathione. Cardiovasc Res. 1993;27:1248–53. doi: 10.1093/cvr/27.7.1248. [DOI] [PubMed] [Google Scholar]


