Abstract
We report a case of synchronous multiple colon adenocarcinomas in a patient with neurofibromatosis type 1 (NF1). NF1 is an autosomal dominant inherited disorder and patients with NF1 have high risk for both benign and malignant tumors. However, adenocarcinomas involving the colon have rarely been reported in patients with NF1. A 61-year-old man was referred for generalized peritonitis due to descending colon perforation. Left hemicolectomy was performed and pathologic examination showed four adenocarcinomas. Peritoneal nodules were confirmed as metastatic adenocarcinoma (pT4N1M1). The patient also had clinical features compatible with NF1 such as café au lait macules, axillary freckles, neurofibromas across the body, and Lisch nodules. Upon review of the literature, colon adenocarcinoma in patients with NF1 tends to occur in males and relatively young age groups, and is associated with advanced tumor stages and multiple colon cancers. To improve treatment outcome, early colonoscopic surveillance should be considered in patients with NF1.
Keywords: Neurofibromatosis, Colon, Adenocarcinoma
INTRODUCTION
Neurofibromatosis type 1 (NF1) is an autosomal dominant inherited disorder and patients with NF1 have high risk for both benign and malignant tumors in their lifetime. However, adenocarcinomas involving the colon have rarely been reported in patients with NF1. In a large population-based cohort, 42 cases of colorectal cancer were observed among 8,003 people with NF type 1 and 2 [1]. Upon review of the literature, only eight cases, two of which were described in Korea [2,3], have been reported till now [4,5,6,7,8]. Hereby, we present a case of synchronous multiple colon adenocarcinomas in a patient with NF1 and provide characteristic clinical features based on the reported case series.
CASE REPORT
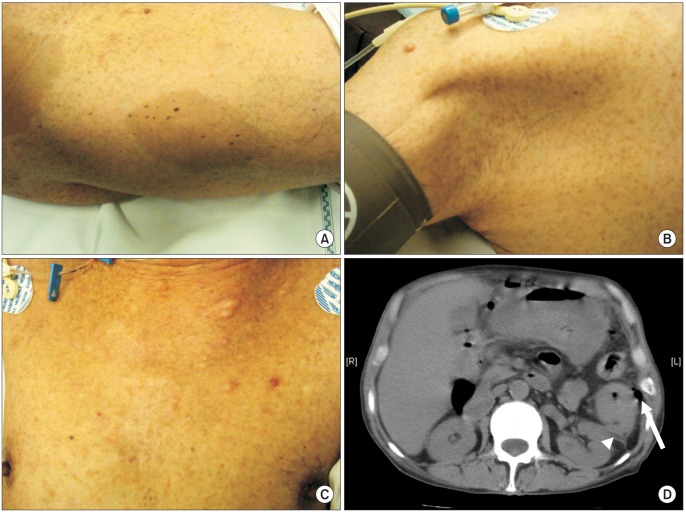
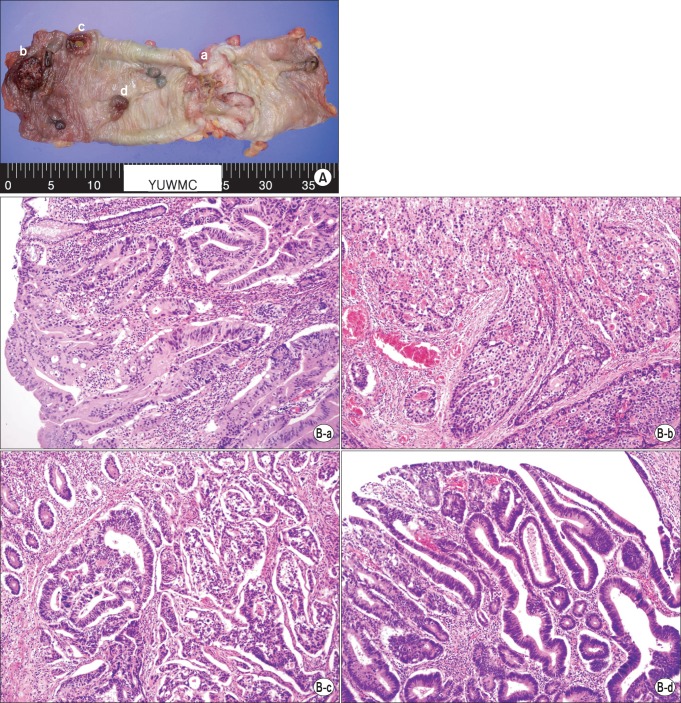
A 61-year-old man was referred to the Emergency Department for generalized peritonitis. He complained of abdominal pain and diarrhea for seven days. On physical examination, his blood pressure was 115/80 mmHg, pulse rate was 106 beats/min, body temperature was 36.9℃, and respiratory rate was 22 breaths/min. Abdomen was rigid and tender in the left upper quadrant. The patient also had clinical features compatible with NF1 such as café au lait macules (Fig. 1A), axillary freckles (Fig. 1B), neurofibromas across the body (Fig. 1C), and more than two Lisch nodules on the iris. Two sons of the patient also had café au lait macules and neurofibromas. Laboratory investigations revealed that white blood cell count was 6,060 cells/mm3 (neutrophil, 86.7%) and C-reactive protein was 36.2 mg/dL (normal range, 0 to 0.8 mg/dL). Other laboratory results were within normal limits. Abdominal computed tomography scan showed descending colon perforation (Fig. 1D) and the patient underwent emergency laparotomy. On exploration, the perforated descending colon was noted at the splenic flexure and multiple peritoneal nodules, about 1 cm in size, were found in the abdominal cavity. Left hemicolectomy was performed (Fig. 2A) and pathologic examination showed that four adenocarcinomas (one pT4 lesion, two pT3 lesions, and one carcinoma in situ lesion) within the specimen (Fig. 2B). The tumor showed K-ras mutation, BRAF wild-type, and microsatellite-stable phenotype. All four tumor lesions showed negative immunohistochemical expression of neuroendocrine markers such as synaptophysin and chromogranin A. Biopsy of peritoneal nodules showed metastatic adenocarcinoma (pT4N1M1). Postoperative positron emission tomography scan and lung biopsy showed 1.7-cm-sized primary lung adenocarcinoma in the left lower lobe. The patient refused either pneumonectomy for primary lung malignancy or palliative chemotherapy for colon cancer. The patient was discharged uneventfully and underwent colostomy reversal two months later. The patient is now lost to follow-up.
Fig. 1.
Clinical manifestations of a 61-year-old male patient. (A) Cafe-au-lait macules in the right thigh. (B) Freckles in the right axilla. (C) Multiple neurofibromas in the anterior chest wall. (D) Axial image of abdominal computed tomography scan shows descending colon tumor infiltration to the left anterior renal fascia (white arrow head) and pneumoperitoneum (white arrow).
Fig. 2.
Histopathologic findings. (A) A total of four adenocarcinomas (a to d) are seen in the specimen. (B) The corresponding histopathologic examination (matched with tumor lesions, alphabetical order). There are one pT4 lesion (a), two pT3 lesions (b, c), and a carcinoma in situ lesion (d) (H&E, ×200).
DISCUSSION
About 5% to 25% of patients with NF1 develop intra-abdominal menifestations. Agaimy et al. [9] pointed out that abdominal findings such as gastrointestinal benign peripheral nerve sheath tumors with gastrointestinal stromal tumor (GIST) and gastrointestinal somatostatinoma with GIST may be diagnostic clues to previously undiagnosed NF1. The authors described gastrointestinal menifestations of NF1 as follows: true neurogenic neoplasms such as peripheral nerve sheath tumors; interstitial cell of Cajal lesions such as GIST; neuroendocrine tumors; and adenocarcinoma. With respect to true neurogenic neoplasms, peripheral nerve sheath tumors may develop anywhere in the digestive tract and more frequently in the small bowel in NF1 patients. However, gastrointestinal schwannomas are unlikely to be associated with NF-1. With regard to interstitial cells of Cajal lesions, GIST in patients with NF1 do not exhibit mutations in KIT or PDGFRA, in contrast to sporadic multifocal GIST, multifocal wild type GIST, and familial GIST syndrome. The clinical significance of these molecular differences remains to be elucidated. Neuroendocrine tumors giving rise to ampullary or periampullary lesions are characteristic features suggesting NF1. Somatostatin-producing tumor is the most common type of NF1 related neuroendocrine neoplasm.
NF1 is caused by mutations of the NF1 gene. NF1 gene is located at chromosome 17q11.2 and plays a role as a tumor suppressor gene. To date, the association between NF1 and adenocarcinoma of the gastrointestinal tract is thought to be casual. However, Li et al. [10] suggested that germline mutations in NF1 gene can occur in somatic cells and contribute to cancer development. Indeed, Seminog and Goldacre [1] observed that NF1 patients were at high risk of colon and rectosigmoid junction cancer when compared with the general population.
Diagnosis and treatment modalities are not quite different in NF1 patients with colon cancer. However, there is lacking information on the clinical features and prognoses due to limitation of available data. We reviewed clinical characteristics based on published case series (Table 1). There were nine patients including ours [2,3,4,5,6,7,8]. Eight were male [2,3,4,6,7,8]. Five cases occurred before the age of 55 [4,6,7,8]. Four cases had synchronous multiple colon cancers [7,8] and one developed metachronous colon cancer [6]. Three cases had Dukes' C [4,6,7] and three had Dukes' D stage [7,8]. Two developed distant recurrence [7,8]. Interestingly, three cases had colonic ganglioneuromas [4,5,6]. NF1 patients may present with neurofibromatous proliferations in the lamina propria mucosa and the submucosa of intestinal wall. Histologically, these may be a pure neurofibroma or a mixture of a ganglioneuroma. Gastrointestinal ganglioneuromatosis may be seen not only in NF1, but also in multiple endocrine neoplasia type 2b, juvenile and adenomatous colonic polyposis, and as isolated lesions [9]. Thus, close follow-up is necessary before establishing a definitive diagnosis of underlying undiagnosed conditions.
Table 1.
Colonic adenocarcinoma in patients with neurofibromatosis type 1
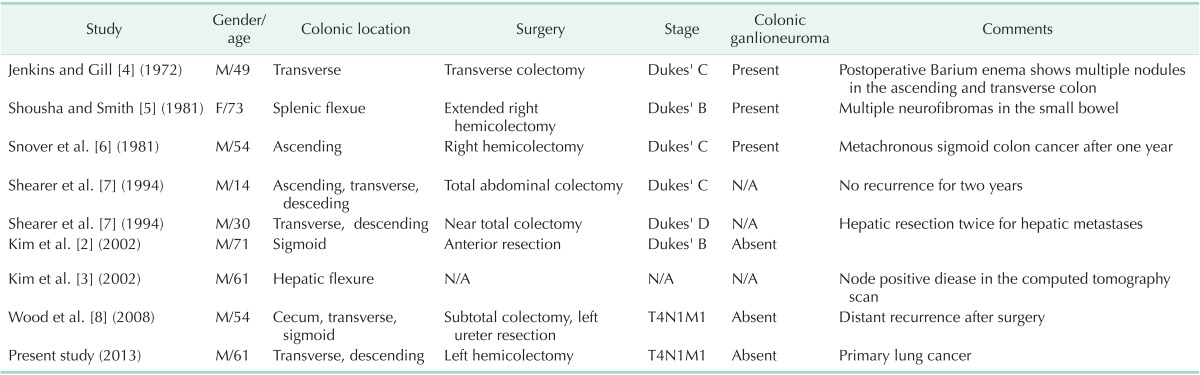
N/A, not available.
In summary, male predominance, relatively early age onset, advanced tumor stage, and high likelihood of multiple colon cancers may be characteristic clinical features in NF patients with colon cancer. To improve treatment outcome, early diagnosis is mandatory. Screening colonoscopy for colon cancer is recommended at the age of 50 for the general population. However, earlier colonoscopic surveillance may be beneficial for NF1 patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Seminog OO, Goldacre MJ. Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: population-based record-linkage study. Br J Cancer. 2013;108:193–198. doi: 10.1038/bjc.2012.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KJ, Choi SR, Sohn SH, Lee S, Hong KB, Keum DJ, et al. A case of colon cancer in a patient with neurofibromatosis type I. Korean J Gastroenterol. 2002;40:402–405. [Google Scholar]
- 3.Kim SE, Heo EP, Yoon TJ, Kim TH. Segmentally distributed neurofibromatosis associated with adenocarcinoma of the colon. J Dermatol. 2002;29:350–353. doi: 10.1111/j.1346-8138.2002.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins DH, Gill W. A case of carcinoma of the colon in association with neurofibromatosis. Br J Surg. 1972;59:322–323. doi: 10.1002/bjs.1800590417. [DOI] [PubMed] [Google Scholar]
- 5.Shousha S, Smith PA. Colonic ganglioneuroma: report of a case in a patient with neurofibromatosis, multiple colonic adenomas and adenocarcinoma. Virchows Arch A Pathol Anat Histol. 1981;392:105–109. doi: 10.1007/BF00430553. [DOI] [PubMed] [Google Scholar]
- 6.Snover DC, Weigent CE, Sumner HW. Diffuse mucosal ganglioneuromatosis of the colon associated with adenocarcinoma. Am J Clin Pathol. 1981;75:225–229. doi: 10.1093/ajcp/75.2.225. [DOI] [PubMed] [Google Scholar]
- 7.Shearer P, Parham D, Kovnar E, Kun L, Rao B, Lobe T, et al. Neurofibromatosis type I and malignancy: review of 32 pediatric cases treated at a single institution. Med Pediatr Oncol. 1994;22:78–83. doi: 10.1002/mpo.2950220203. [DOI] [PubMed] [Google Scholar]
- 8.Wood JJ, Longman RJ, Rooney N, Loveday EJ, Roe AM. Colonic vascular anomalies and colon cancer in neurofibromatosis: report of a case. Dis Colon Rectum. 2008;51:360–362. doi: 10.1007/s10350-007-9089-z. [DOI] [PubMed] [Google Scholar]
- 9.Agaimy A, Vassos N, Croner RS. Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen's disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol. 2012;5:852–862. [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Bollag G, Clark R, Stevens J, Conroy L, Fults D, et al. Somatic mutations in the neurofibromatosis 1 gene in human tumors. Cell. 1992;69:275–281. doi: 10.1016/0092-8674(92)90408-5. [DOI] [PubMed] [Google Scholar]