Abstract
Previous work from this laboratory has demonstrated that withdrawal from the neuroactive steroid 3α,5α-THP (3α-hydroxy-5α-pregnan-20-one) after 3-week exposure to its parent compound, progesterone (P), increases anxiety and produces benzodiazepine (BDZ) insensitivity in female rats. These events were linked to upregulation of the α4 subunit of the GABAA receptor (GABAR) in the hippocampus [Brain Res. 507 (1998) 91; Nature 392 (1998) 926; J. Neurosci. 18 (1998) 5275]. The present study investigates the role of shorter term hormone treatment on α4 subunit levels as well as relevant behavioral and pharmacological end-points related to GABAR function. After 2–3 days of P exposure, two- to threefold increases in α4 protein levels were observed, which declined to control values after 5–6 days of hormone exposure. This effect was due to the GABA-modulatory metabolite of P, 3α,5α-THP. α4 upregulation was inversely correlated with BDZ potentiation of GABA-gated current, assessed using whole cell patch clamp techniques on acutely isolated hippocampal pyramidal cells. A near total BDZ insensitivity was observed by 2–3 days of hormone exposure in association with the maximal increase in α4 levels. Up-regulation of the α4 GABAR subunit was also reflected by an increase in anxiety in the elevated plus maze. A significant decrease in open arm entries was observed after 72-h exposure to P, an effect which recovered by 6 days of P treatment. As demonstrated in vitro, α4 upregulation also resulted in a relative insensitivity to the anxiolytic actions of BDZ. These results suggest that short-term exposure to 3α,5α-THP produces changes in GABAR subunit composition similar to those that occur after chronic exposure and withdrawal from the steroid.
Keywords: 3α, 5α-THP; Pregnanolone; Allopregnanolone; Neurosteroid; Progesterone; GABAA receptor; Withdrawal; Anxiety; Benzodiazepine; Hippocampus; Elevated plus maze; PMS; Female; Rat; α4 subunit
1. Introduction
The GABAA receptor (GABAR) is a ligand-gated chloride channel which is a pentameric assembly of many possible subunit combinations; α1–6, β1–4, γ1–4, δ, ε, π, θ and σ. In the adult CNS it is a primary mediator of fast inhibitory transmission [23].
Several classes of ligands are positive modulators of GABAergic inhibition, and these can include the benzodiazepines (BDZ), barbiturates, alcohol and the neuroactive metabolite of progesterone (P) known as 3α,5α-THP (3α-OH-5α-pregnan-20-one or allopregnanolone) [32,45, 46,49]. The functional response of the GABAR to these ligands, however, varies dramatically with respect to the composition of the subunits [28,51,53]. Recent evidence suggests that different GABAR isoforms may mediate distinct GABAR-related behaviors [34,38]. Furthermore, sustained exposure to GABA-modulators can alter the subunit composition of the GABAR, which in many cases leads to alterations in the pharmacology of GABA-gated current [11,14,24,31].
Many of these studies have described a withdrawal syndrome that occurs following chronic administration and abrupt cessation of drugs which are known to modulate the GABAR. The phenomena typical of withdrawal from GABAR modulators include increased neuronal excitability, a lower threshold of seizure susceptibility, high levels of anxiety and altered GABAR pharmacology and subunit expression [2,20,24–26,31]. Similar consequences occur after withdrawal from neurosteroid hormones. Withdrawal from 3α,5α-THP results in increased anxiety, higher α4 GABAR subunit levels in the hippocampus and a near total insensitivity to BDZ modulation [21,35,41,42], which is consistent with the body of literature describing α4 subunit-containing GABAR as insensitive to BDZ [28,51,53]. A more recent finding has confirmed that neurosteroid withdrawal in cultured cerebellar cells replicates this pattern of events [18]. Therefore, higher levels of α4 mRNA and subunit protein after 3α,5α-THP withdrawal occur in conjunction with a variety of phenomena that we suggest may result from the specific pharmacology of GABAR containing α4 subunits. In addition, increases in the α4 subunit are also associated with other syndromes characterized by increased neuronal excitability, increased seizure susceptibility and higher levels of anxiety [10,20,21,26,42]. These include chronic exposure to or withdrawal from alcohol and BDZs which can also result in α4 upregulation and the typical withdrawal outcomes [15,24,31], suggesting that α4 subunit upregulation may be a common feature following withdrawal from compounds that act as positive modulators of the GABAA receptor.
However, there is increasing evidence that GABAR modulation can also occur after relatively short-term (24–72 h) in vitro exposure to GABA-modulatory ligands and result in similar pharmacological and physiological changes as those that occur after hormone withdrawal [19,54,55]. These findings are relevant to endogenous states of rapid hormone fluctuations, such as the estrous or menstrual cycle. In fact, studies in both humans and rodents indicate that relatively short-term exposure to ovarian hormones can result in altered GABAR function [8,12,29,47,52] and can trigger dysphoria in a subset of susceptible women with premenstrual syndrome (PMS) [39]. Therefore, for the present study, we investigated the effects of short-term neurosteroid exposure on GABAR α4 subunit expression, correlated with expected changes in the pharmacological profile using both in vitro and behavioral measures to investigate changes in BDZ sensitivity. We also tested the hypothesis that short-term exposure to P can increase anxiety in association with higher levels of the α4 subunit, using the elevated plus maze as an animal model.
2. Methods
2.1. Animals
Female Long–Evans rats (Harlan) weighing 110–120 g (35–45 days of age) on day 1 of the experiment were housed in groups of three under a 14-h light, 10-h dark cycle with food and water ad libitum. All animals were tested during the light portion of the circadian cycle. The estrous cycle stage was determined by microscopic examination of the vaginal lavage, as described previously [43]. The behavioral studies were conducted in diestrous or estrous stages of the estrous cycle which were equally represented in control and treatment groups.
For the physiology and molecular studies, vehicle-injected controls were tested on the day of diestrus or estrus, which are both characterized by low circulating levels of estradiol and P. Following the appropriate treatment (see below), animals were sacrificed by decapitation, the hippocampi removed, bisected and one half frozen on dry ice for later Western blot or RT-PCR analysis. The remaining half-hippocampus was used in the electrophysiology assay assessing BDZ-modulation of GABA-gated current.
2.2. Drug and hormone administration
2.2.1. Time course
P was administered rather than 3α,5α-THP because it is known that elevated circulating levels of P, such as found during the estrous (or menstrual) cycle are readily converted to 3α,5α-THP in the brain to result in potentiation of GABAergic inhibition [40,44]. P implants were made from silicone tubing as previously described and implanted s.c. under anesthesia in the abdominal area of the rat [35,42] for 1 day to 3 weeks. This method has been shown to result in CNS levels of 3α,5α-THP in the high physiological range (6–12 ng/g hippocampal tissue) in association with increased circulating levels of P (40–50 ng/ml plasma, approximately 130–160 nM) [42]. For the physiology and molecular time course studies, animals were sacrificed on a daily basis for days 1–7 of the P administration paradigm, weekly thereafter, and 24 h after removal of the implant (‘P withdrawal’). Control animals were tested on the day of estrus or diestrus. In all cases, the hippocampus was removed, and samples from each animal were tested both for α4 immunoreactivity and BDZ modulation of GABA-gated current.
2.2.2. Short-term administration
Animals were injected (i.p.) with P (5 mg/0.2 ml oil), or a combination of P and indomethacin (0.02 mg/0.2 ml oil) in the morning once per day for either 3 or 6 days. Indomethacin inhibits the conversion of P to its neuroactive metabolite, 3α,5α-THP [4], and was used to determine if the effects of P on the end-points measured in these experiments were indeed due to 3α,5α-THP exposure. To this end, some animals also received injections of 3α,5α-THP directly (10 mg/kg in oil) or vehicle. This dose of 3α,5α-THP results in hippocampal levels of the steroid which are physiological (6.2±0.7 ng/g) as determined by radioimmunoassay (performed by C. Frye, SUNY Albany) [20]. Control animals were given the same number of injections of vehicle (oil).
In behavioral experiments to study the effects of short-term exposure to 3α,5α-THP on anxiety levels, animals were injected with P (5 mg/0.2 ml oil) or vehicle for 3 or 6 days. Animals from the 21-day and withdrawal time points (implanted with P capsules as described above) that were tested in separate experiments are represented in the same graphs for the purposes of comparison only, but were not included in the same statistical analysis. On the day of testing, experimental animals were injected with lorazepam (LZM) or vehicle (18% polyethylene glycol 400 and 2% alcohol). Injections on the day of testing were staggered to ensure that all animals were tested on the plus maze within 30 min to 1 h after the last injection. This resulted in five groups: 1, control rats receiving vehicle injections only (vehicle); 2, rats receiving vehicle injections for 3 days followed by an injection of LZM immediately prior to testing (vehicle/LZM); 3, rats receiving P injections for 3 days followed by an injection of vehicle immediately prior to testing (P 3 days/vehicle); 4, rats receiving P injections for 3 days followed by an injection of LZM immediately prior to testing (P 3 days/LZM); 5, rats receiving P injections for 6 days (P6 day).
2.3. Western blots
α4 levels were estimated in hippocampal membranes using Western blot procedures previously described [42] and were probed with an antibody developed against a peptide sequence of the rat α4 subunit (amino acids 517–523), from a protocol originally described by Kern and Sieghart [27]. The α4 band (67 kDa) was detected with ECL (enhanced chemoluminesence, Pierce Chemical Co.) visualized and quantified using a Umax scanner and One-Dscan software, and has been characterized in a previous publication [41]. The results were standardized to a glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 36 kDa) control protein and were then expressed in the following way. The integrated optical density of the control α4 bands were pooled and averaged. All other conditions are expressed as a ratio, relative to the control means according to the following equation:
2.4. Semi-quantitative reverse transcriptase PCR (RT-PCR)
In order to compare the α4 GABAR mRNA levels across hormone treatment groups, RT-PCR techniques were implemented. To this end, oligonucleotide primers for the α4 subunit (503 bp, sequence: Forward: 5′-CTG GAC CAA AGG CCC TGA GA. Reverse: 5′ TTT TCC TTC AGT ACT GGG GCA GCT G) were designed from the rat α4 cDNA sequence according to Ref. [50] and used in conjunction with a control probe, GAPDH (657 bp, sequence: Forward: 5′-GGT GAA GGT CGG TGT CAA CGG AT. Reverse: 5′ GGG TAG ACC TTG CCC ACA GCC TT). Initially, total RNA was extracted from a single hippocampus isolated from control or treated animals and processed in parallel before undergoing reverse transcription. The cDNA products, each containing 1 μg of DNA, were then amplified in a linear sequence from 23 to 35 cycles as previously described [41]. The resulting amplimer was then electrophoresed in a 1.5% agarose gel. The single bands from the PCR amplimers were visualized using ethidium bromide and were calibrated against molecular weight markers. The band density was analyzed as described above, and standardized according to the amount of GAPDH control values. These values were averaged from the cycle numbers known to be within the linear range.
2.4.1. Electrophysiology
Each hippocampus tested for α4 immunoreactivity across the time course of P exposure was concomitantly evaluated for BDZ modulation of GABA-gated current. To this end, pyramidal neurons were acutely isolated from CA1 hippocampus following slice preparation, using a procedure described previously [41] with trypsin digestion at 32°C. GABA-activated current was recorded at room temperature (20–25°C) in a 120 mM NaCl buffer and a pipette solution containing 120 mM N-methyl-D-glucamine. The ATP regeneration system Tris phosphoc-reatinine (20 mM) and creatine kinase were added as previously described [22]. GABA-gated current (10 μM GABA) was recorded with whole cell patch clamp techniques at a holding potential of −50 mV using an Axopatch-1D amplifier. Current was filtered at 1–2 kHz (−3 dB, eight-pole low-pass Bessel filter) and digitally sampled at a 500-Hz sampling frequency using pClamp 5.51. Drug delivery was accomplished via a solenoid-activated gravity-feed superfusion system positioned within 50 μm of the cell and triggered by the pClamp program. This system releases drugs for 20 ms at 1-to 3-min intervals to result in exposure times in the 100-ms range and has been described in detail elsewhere [42]. A background perfusion system (4 ml/min) provides a wash-out flow in the opposite direction. % Potentiation of GABA-gated current was calculated for all drug concentrations using peak GABA-gated current (IGABAdrug − IGABAcontrol)/(IGABAcontrol). LZM was applied across a range of concentrations: 0.1–100 μM.
2.4.2. Behavioral testing
Rats were tested on the plus maze, elevated 50 cm above the floor, in a room with low, indirect fluorescent lighting and low noise levels. The plus maze consists of two enclosed arms (50×10×40 cm) and two open arms (50×10 cm) and is explained in detail in Ref. [36]. The open arms had a small rail outside the first half of the open arm as described in Ref. [16]. The floor of all four arms was marked with grid lines every 25 cm. On the day of testing, each rat was placed in a start box in the center of the plus maze and tested for 10 min after exiting the start box into the plus maze. To be considered as an open arm entry, the rat must pass the line of the open platform up to the midline of the body. The duration (s) of time spent in the open arm was recorded from the time of entry into the open arm. Decreased time spent in the open arm generally indicates higher levels of anxiety [36]. Other behavioral measures recorded included the duration of time spent (s) beyond the rail. The amount of time that subjects spend in the open portion of the plus maze in the absence of rails is considered to be more sensitive to anxiolytic agents (i.e. agents that would increase the amount of time spent in the open arm) than the amount of time spent in the open arms with rails [16]. In order to measure general locomotor activity, the number of total grid crosses was counted. The experimenter was blind to all conditions, and animals were tested in a randomized block design on six separate occasions. In order to illustrate this behavioral data in the summary graph (Fig. 6), an anxiety score was generated according to the following equation:
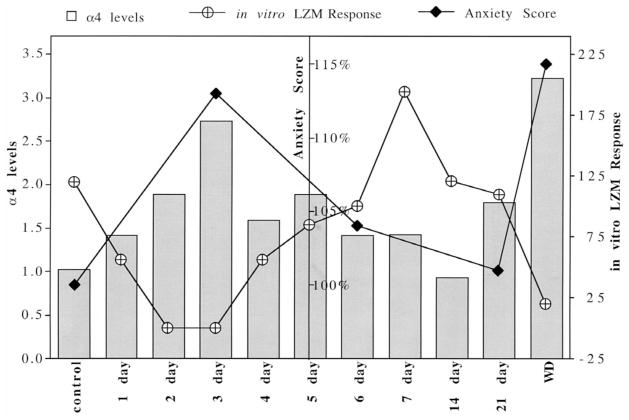
Fig. 6.
Summary diagram: The time course of P-induced changes in α4 levels, LZM response and anxiety. Illustration of α4 levels (on the left axis, ratio of control) and BDZ potentiation of GABA-gated current (in vitro LZM response, right axis, % control) reveals an inverse correlation, with significant changes noted 2–3 days after P exposure, and again following P withdrawal, compared to control values for both parameters. In conjunction with α4 upregulation, anxiety score (center axis, % control) increases at 3 days of P treatment (3 day) and again following P withdrawal (WD). Anxiety score is defined in the Methods. Error bars are omitted for clarity — for complete details, see Figs. 1, 4 and 6.
2.4.3. Statistical analysis
Differences between groups were assessed using either the Student’s t-test (two groups) or an ANOVA followed by a post-hoc t-test (Student–Newman–Keuls or Dunnett’s, as indicated in the results) to compare within multiple groups or between experimental and control values, respectively. Statistical significance for each analysis is indicated in the relevant Results section.
2.4.4. Source of materials
In general, most chemicals were obtained from Sigma. The α4 antibody was synthesized by Genosys, Inc., and the GAPDH Ab by Chemicon. ECL supplies were provided by Pierce Chemical Co. Silicone tubing and adhesive were obtained from Nalgene Co. and Dow Corning, respectively. LZM was obtained from Wyeth Laboratories (injectable) or RBI/Sigma (powder). Rat DNA primers were synthesized by Operon Technologies Inc. (α4) or Integrated DNA Technologies Inc. (GAPDH).
3. Results
3.1. Short-term treatment with P increases α4 GABAR subunit mRNA and protein immunoreactivity via 3α,5α-THP
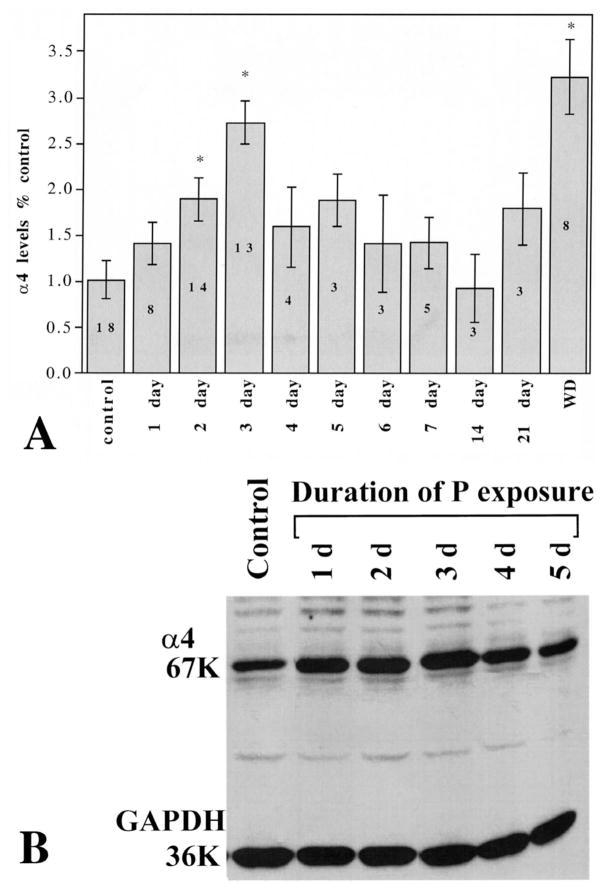
Two to three days (48–72 h) of continuous exposure to P via s.c. capsules increased hippocampal levels of α4 GABAR subunit immunoreactivity by two-to threefold above control levels (1.89±0.29 or 2.75±0.24, respectively, relative to control, P<0.05; Fig. 1A,B). α4 immunoreactivity returned to control levels by 4–5 days of continuous P exposure, and remained at control levels throughout the entire 3-week exposure period until removal of the capsule (‘P withdrawal’) (Fig. 1). At this time, α4 immunoreactivity again increased threefold above control levels (3.23±0.40 relative to control, P<0.01). Similarly, 48-h exposure to P via i.p. injections (three injections over a 48-h period, Fig. 2), also induced a threefold increase in α4 immunoreactivity (P, 2.92±0.47; control, 1.0±0.26, P<0.01), as did four injections over a 72-h period (data not shown). In both cases, α4 levels returned to control values by 5–6 days of P injections (data not shown). In order to test the possibility that 3α,5α-THP mediates this effect of P, we prevented the conversion of P to its GABA-modulatory metabolite with concomitant injection of the 3α-HSD (3α-hydroxysteroid dehydrogenase) blocker, indomethacin (Fig. 2) during the 48-h exposure period. Under these conditions, the increase in α4 immunoreactivity normally observed after 48-h P exposure was prevented, resulting in α4 levels similar to control values (P+Indomethacin, 1.107±0.37, control, 1.0±0.26, P=0.095). Furthermore, direct injection of 3α,5α-THP (10 mg/kg, i.p.×three injections over 48 h) also resulted in a significant, threefold increase in α4 immunoreactivity (3α,5α-THP=3.59±0.28; control, 1.0±0.27; P<0.001) compared to control levels. In addition, significant twofold increases in α4 mRNA (using a semi-quantitative RT-PCR technique) were observed following 48-h exposure to 3α,5α-THP compared to control (3α,5α-THP, 1.97±0.32; control, 1.0±0.17, P<0.01, Fig. 3). Neither the immunoreactivity nor the mRNA expression of the control protein, GAPDH, were altered by any of the hormone treatments.
Fig. 1.
Time course of increases in α4 GABAR subunit levels after P capsule implantation. (A) Statistical analysis of the data is represented as the integrated optical density of the immunoreactive bands and is expressed as a ratio of the control condition. Asterisks above 2 day, 3 day and WD indicate a statistically significant (P<0.05) increase above control levels. The numbers inside the bars are the individual n for each condition in experiments that were performed in triplicate. (B) Representative Western blot of α4 immunoreactivity across the first 5 days of hormone exposure showing typical values for the 67-kDa α4 subunit band. The levels of the 36-kDa control protein, GAPDH, do not change during hormone treatment.
Fig. 2.
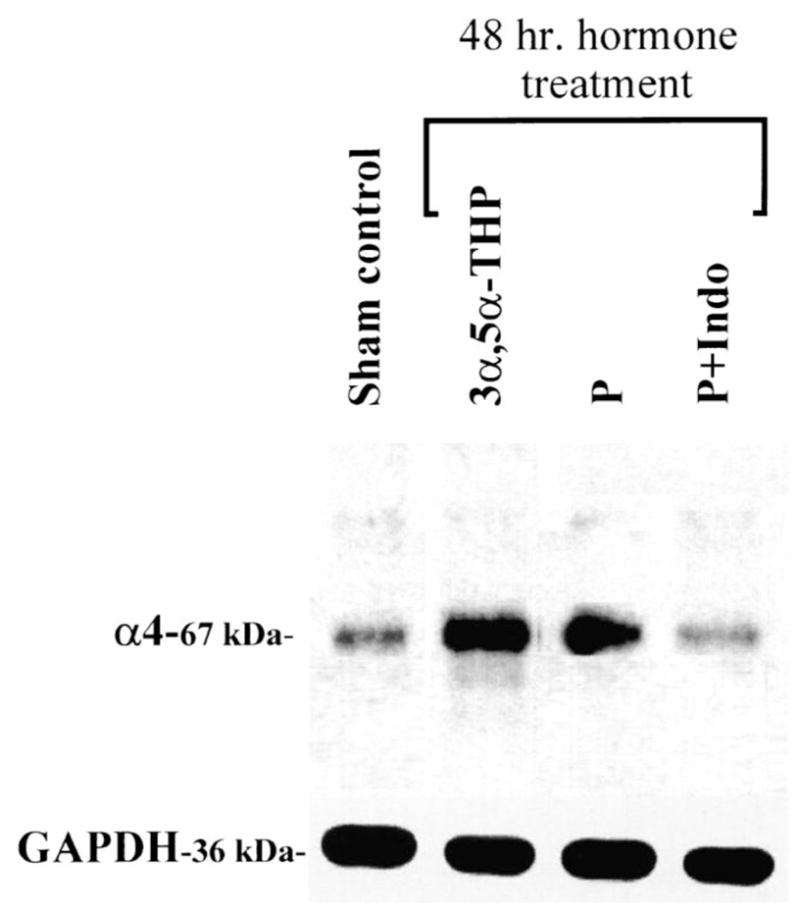
P-induced increases in α4 subunit levels are mediated by 3α,5α-THP. Representative Western blot demonstrating that the increase in the 67-kDa α4 subunit immunoreactivity seen after 48-h treatment with P (i.p. injection) is replicated by direct i.p. injection of 3α,5α-THP. Furthermore, it is also blocked by indomethacin injected simultaneously with P (P+Indo) which prevents conversion to 3α,5α-THP. The levels of the 36-kDa control protein, GAPDH, do not change during treatment (n=4 independent experiments performed in triplicate).
Fig. 3.
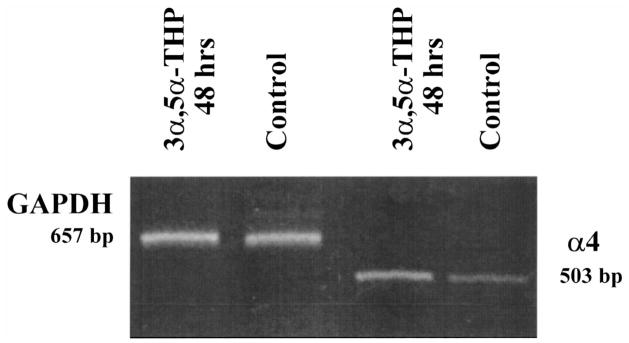
Levels of α4 mRNA are increased following short-term exposure to 3α,5α-THP. A representative gel is presented indicating α4 mRNA levels (503 bp) in conjunction with the housekeeping mRNA GAPDH (657), following RT-PCR amplification. Forty-eight-hour exposure to 3α,5α-THP in vivo produced a significant increase in band density compared to control in the absence of significant changes in band density for the GAPDH control. No staining was apparent for the 0 DNA control (data not shown) (n=3 animals/group; performed in triplicate).
3.2. Short-term treatment with P prevents BDZ potentiation of GABA-gated current
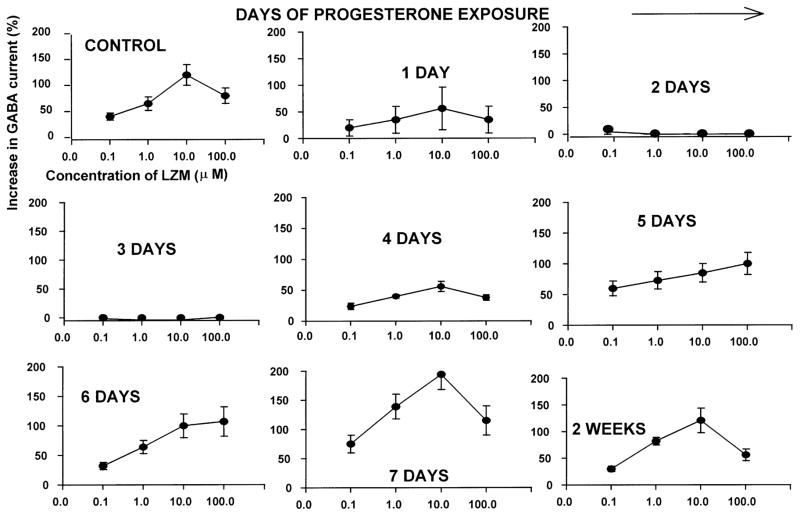
BDZ modulation of GABA-gated current was tested using hippocampal tissue from the same animals that were tested for α4 immunoreactivity (described above). We predicted that the increased expression of the α4 subunit would produce BDZ insensitivity, as has been demonstrated previously for α4-containing GABAR [41,51]. Indeed, in neurons acutely isolated from the hippocampus of P-treated animals, there was a marked reduction in the ability of LZM to potentiate peak GABA-gated current assessed using whole cell patch clamp procedures across a range of LZM concentrations (0.1–100 μM; Fig. 4). Under control conditions, LZM potentiated GABA-gated current by 20–140% in a concentration-dependent manner, with maximal potentiation occurring at 10 μM LZM. By 24 h of continuous P exposure (Fig. 4), there was a reduction in the ability of LZM to potentiate GABA-gated current (10–60% potentiation across the concentration range). By 48–72 h of continuous P exposure, LZM potentiation of GABA-gated current was negligible (Fig. 4), a significant change from control (P<0.001). LZM potentiation of GABA-gated current began to recover by 4 days of P exposure (to 20–60% potentiation), and was fully recovered by 7 days of P exposure (65–200% potentiation, Fig. 4). The ability of LZM to potentiate GABA-gated current remained at control levels throughout the duration of the P exposure period until capsule removal (‘P withdrawal’), when the GABA modulatory effects of LZM were again markedly attenuated producing a relative BDZ insensitivity (Fig. 6). Thus, maximal levels of α4 immunoreactivity and mRNA levels (Figs. 2 and 4) occur at the same time-point as maximal BDZ insensitivity.
Fig. 4.
BDZ potentiation of GABA-gated current is attenuated after 48-h exposure to P. Concentration–response curves illustrate responses of CA1 pyramidal neurons to GABA (10 μM), applied in combination with the BDZ, lorazepam (LZM, 0.1–100 μM), and assessed using whole cell patch clamp recording techniques. Results are expressed as a percent increase in peak GABA-gated current above levels generated by application of GABA alone. P exposure (2–3 days via s.c. implant) markedly reduces the BDZ potentiation of GABA-gated current normally observed under control conditions (upper left) (n=10–15 cells per group; *P<0.01 vs. control).
3.3. Short-term treatment with progesterone increases anxiety and induces behavioral insensitivity to lorazepam
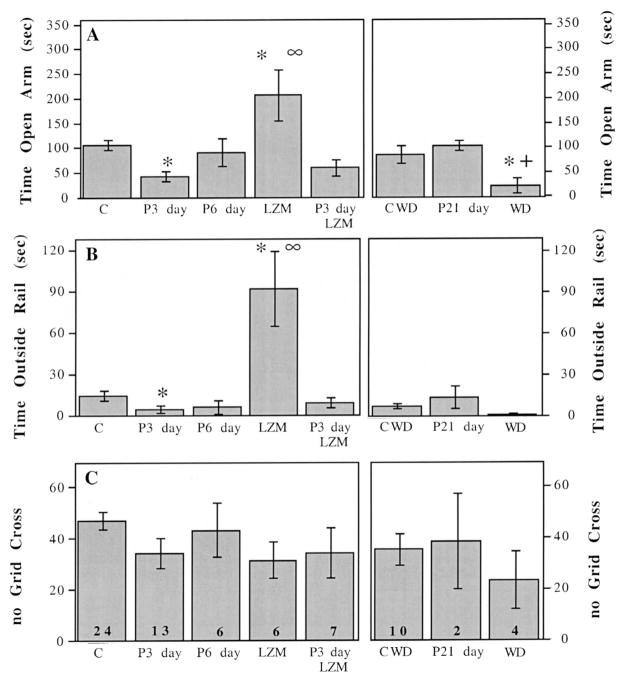
For this study, we tested the hypothesis that steroid-induced upregulation of the α4 subunit would be correlated with increased anxiety, as we have demonstrated following P withdrawal [21,42]. Further, we predicted that the insensitivity to LZM that was demonstrated in isolated hippocampal neurons would be evident at a behavioral level. Indeed, animals injected with P over a 72-h period spent on average 60% less time in the open arm of the elevated plus maze (vehicle vs. P 3 days P<0.004) than did their vehicle-injected counterparts (Fig. 5A), indicating higher levels of anxiety. In addition, animals treated with P for 72 h spent 73% less time beyond the rail of the apparatus, compared to control animals (vehicle vs. P 3 days; P<0.0001, Fig. 5B). This increase in anxiety is evident only during periods when α4 subunit levels are also significantly higher than controls (Figs. 1 and 3), since after 6 days of P injections, anxiety levels (the time spent in the open arm) return to control values (Fig. 5A). Therefore, at the time when α4 levels are decreased (6 days), the anxiety level also returns to control values (for summary graph, see Fig. 6).
Fig. 5.
Time course of measures of anxiety across the P exposure period. (A, left axis): time spent (s) in the open arm of the elevated plus maze is decreased after 3 days of P exposure (P3 day, 5 mg in oil, i.p.) compared to vehicle injected rats (C) (P3 day vs. C, *P<0.001), and returns to control levels after 6 days of P injections (P6 day). Lorazepam (LZM) significantly increases time spent in the open arm compared to vehicle, assessed in control animals (LZM vs. C, *P<0.001). In contrast, LZM does not increase time spent in the open arm after 3 days P injections (P3 day LZM) (LZM vs. P3 day LZM, ∞P<0.001;). (A, right axis): P treatment for 21 days (P21 day) is similar to control conditions (C-WD) while P withdrawal (WD) significantly decreased time spent in the open arm compared to controls (PWD vs. C-WD, *P<0.02) or to P 21 day treatment (PWD vs. P21 day, +P<0.006). Sample sizes are indicated in the bottom of the bar in panel and are the same as for all graphs in Fig. 1. (B) LZM significantly increases time spent beyond the rail compared to vehicle injections in control animals (LZM vs. C, *P<0.001), but does not do so following 3 days P injections (∞P<0.001; LZM vs. P3 day LZM). (C) There is no significant effect of any treatment on locomotor activity assessed by number of grid crosses in the elevated plus maze.
This study also demonstrates that animals injected with P for 72 h exhibit insensitivity to the anxiolytic effects of LZM. P-treated animals did not spend significantly more time in the open arm or beyond the rail following LZM administration (Fig. 5A,B). However, control animals injected acutely with LZM spent 89% more time in the open arm following LZM injection than vehicle-treated animals (vehicle/vehicle vs. vehicle/LZM; P<0.0006), and exhibited a fivefold increase in time spent beyond the rail (vehicle vs. vehicle-LZM; P<0.0001, Fig. 5A,B). None of the treatment conditions significantly affected general locomotor activity, assessed as the number of grid crossings, since these values did not significantly vary across treatment groups (Fig. 5C). Therefore, while 1.5 mg/kg LZM is effective at increasing the amount of time spent in the open arm (i.e. decreasing anxiety) for vehicle-injected controls, as is consistent with other studies [17], it has no significant anxiolytic effect after 72 h P treatment when α4 subunit levels are increased.
4. Discussion
As we have shown previously for neurosteroid (3α,5α-THP) withdrawal [41], short-term exposure to 3α,5α-THP also results in α4 GABAR subunit upregulation and an array of behavioral and pharmacological changes related to GABAergic function. These include increased anxiety and BDZ insensitivity, which was assessed both in vitro and using a behavioral paradigm. Interestingly, the time course of these observed changes suggests that α4 GABAR upregulation is a transient event which occurs both after short-term exposure to 3α,5α-THP as well as after termination of chronic steroid exposure.
Although no direct comparison can be made between single and multiple withdrawal paradigms in this study, it has been suggested that the severity of withdrawal may be affected by the number of withdrawal cycles [5,25,31]. This study confirmed that a single episode of P withdrawal increases α4 levels as does the multiple P withdrawal paradigm we have previously employed [41,42].
The results from the present study suggest that increases in both mRNA and protein for the α4 subunit of the GABAR are observed after 48- to 72-h exposure to 3α,5α-THP. This α4 upregulation was well-correlated with an insensitivity to the benzodiazepine LZM, demonstrated by a marked reduction in the ability of LZM to potentiate GABA-gated current as well as by a decrease in the anxiolytic effect of LZM. An increase in the α4 subunit expression observed here is entirely compatible with the body of literature describing α4 as a BDZ-insensitive subunit [28,51,53] although changes in other subunits cannot be ruled out. Furthermore, this is comparable to the increase in α4 expression that we have observed following withdrawal from 3α,5α-THP, where the spectrum of pharmacological changes observed was consistent with those reported for transiently expressed α4βxγ2 isoforms [42]. In this case, in addition to BDZ insensitivity, the α4βxγ2 GABAR results in agonist-like effects for both RO15–4513 (a BDZ partial inverse agonist) and RO15–1788 (a BDZ antagonist) [28,51].
The fact that BDZ insensitivity could occur within a relatively short period of time is predicted by earlier reports of altered GABAR pharmacology and/or subunit expression after exposure to neuroactive steroids in vitro for 24–48 h [19,52,54,55] and is consistent with the reported GABAR half-life [30]. Thus, this shorter period of hormone exposure is a viable time period for both post-transcriptional and transcriptional events which may lead to altered GABAR pharmacology and is relevant for the relatively short-term exposure periods that occur across the rat estrous cycle, during which altered anxiety and BDZ pharmacology have also been reported [8,12].
The results from the present study suggest that there is a correlation between increases in α4 GABAR subunit levels and anxiety. Although no cause-and-effect relationship can be conclusively determined, there is substantial evidence in the literature that increases in α4 levels accompany increases in CNS excitability. α4-containing GABAR isoforms exhibit shorter duration single channel openings compared with α1-containing receptors, assessed using fluctuation analysis of whole cell current [33]. In addition, increases in α4 expression have been reported after kindling paradigms, in human epileptic brain tissue and during periods of increased seizure susceptibility that are associated with withdrawal from P or alcohol [10,15,26,31,41].
Taken together, these findings would suggest that increased α4 expression is associated with increased neuronal excitability, which may have behavioral consequences. Indeed, in the present study there is also evidence of increased anxiety concomitant with increased α4 expression after short-term P exposure. This was demonstrated by a decrease in open arm entries in the elevated plus maze after P injections, although general motor activity was unaffected by 72-h hormone exposure [17,36]. The increase in anxiety was only observed at time points when α4 levels were also significantly higher than controls, suggesting a relationship between this behavioral end-point and α4 expression in the hippocampus.
Although other CNS areas, notably the amygdala and septal area [1] have been demonstrated to play a role in anxiety, several lines of evidence also point to the hippocampus as both a target and a modulator of physiological events associated with anxiety [1,7,48] after withdrawal from GABAR ligands [2] which is consistent with its role as a major integrator of the limbic circuitry. Firstly, direct hippocampal infusions of 3α,5α-THP decrease anxiety in the elevated plus maze [7]. Conversely, direct infusion of anxiogenic substances into the hippocampus increase anxiety [13]. Lastly, human patients with panic disorders have decreased total GABAR levels in the hippocampus [9].
These data suggest that short-term exposure to ovarian steroids and their neuroactive metabolites may also be a useful model of menstrual cycle-related disorders. The clinical literature suggests that many women experiencing PMS exhibit adverse symptomatology, especially anxiety, irritability and depression, during the late luteal phase, a time of declining hormone levels [37]. However, a significant number of women also experience adverse symptoms shortly after the mid-cycle peak in ovarian hormones [6,39,40] or within several days after the administration of exogenous ovarian steroids [39]. One recent study demonstrates that exogenous ovarian hormone administration to PMS-susceptible women produces adverse symptomatology within several days even after their endogenous cyclicity was prevented [39]. Taken together, this body of literature suggests that emotional lability can be triggered not only with hormone withdrawal, but also with relatively short exposure to ovarian hormones and/or neuroactive steroids, which may be able to up-regulate the α4 subunit. More relevant for the interpretation of the present studies are the recent reports demonstrating altered BDZ efficacy at periods of high hormone levels during the estrous cycle in rodents [8,12] and at the mid-cycle peak of hormone levels in women with PMS compared to normal controls [47]. These changes in BDZ sensitivity in women with PMS at times which correspond to several days of exposure to high levels of neurosteroids [40] are consistent with the time course of BDZ insensitivity we observe in our model in association with α4 upregulation.
In conclusion, the data from this study suggest that the α4 GABAR subunit is capable of rapid plasticity, increasing both after short-term treatment with neuroactive steroids as well as after withdrawal following chronic steroid exposure. The mechanism(s) responsible for this surprising pattern may be triggered by separate conditions. The initial increase in the α4 subunit, which is associated with a decrease in total GABA-gated current, may represent a homeostatic response to prolonged GABA modulation by 3α,5α-THP. Recovery to control levels of α4 parallels the development of (partial) tolerance to GABA modulation by 3α,5α-THP (data not shown). In contrast, withdrawal from the steroid may increase α4 levels via a different mechanism, perhaps at the level of transcriptional regulation. 3α,5α-THP is increased during the luteal phase of the menstrual cycle [40] during pregnancy [14] and as a result of stress [3], where it may originate from peripheral or CNS sites [4]. In particular, altered GABAR composition produced by these hormonal fluctuations may then lead to behavioral changes that include increases in anxiety, and thus may be at least one of the precipitating factors for syndromes such as PMS.
Acknowledgments
This work was supported by National Institute of Health Grants DA 09618 and AA 12958 and contracts from Merck and Zeneca to SSS and NIH training grant NS24707 to MG. The authors are grateful to Dr CA Frye (SUNY Albany) for performing radioimmunoassay assessment of 3α,5α-THP levels in hippocampal tissue, to Dr I Fischer for helpful technical advice and to R.S. Markowitz for technical assistance.
References
- 1.Akwa Y, Purdy R, Koob G, Britton K. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 2.Andrews N, File S, Fernandes C, Gonzalez L, Barnes N. Evidence that the median raphe nucleus–dorsal hippocampal pathway mediates diazepam withdrawal-induced anxiety. Psychopharmacology (Berl) 1997;130:228–234. doi: 10.1007/s002130050233. [DOI] [PubMed] [Google Scholar]
- 3.Barbaccia M, Roscetti G, Trabucchi M, Purdy P, Mostallino M, Concas A, Biggio G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br J Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baulieu E. Neurosteroids: a novel function of the brain. Psycho-neuroendocrinology. 1999;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- 5.Becker HC, Veatch LM, Diaz-Granados JL. Repeated ethanol withdrawal experience selectively alters sensitivity to different chemoconvulsant drugs in mice. Psychopharmacology. 1998;139:145–153. doi: 10.1007/s002130050699. [DOI] [PubMed] [Google Scholar]
- 6.Bicikova M, Tallova J, Hill M, Krausova Z, Hampl R. Serum concentrations of some neuroactive steroids in women suffering from mixed anxiety-depressive disorder. Neurochem Res. 2000;25:1623–1627. doi: 10.1023/a:1026622704704. [DOI] [PubMed] [Google Scholar]
- 7.Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3 alpha-OH-5 beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- 8.Bitran D, Hilvers R, Kellogg C. Ovarian endocrine status modulates the anxiolytic potency of diazepam and the efficacy of gamma-aminobutyric acid-benzodiazepine receptor-mediated chloride ion transport. Behav Neurosci. 1991;105:653–662. doi: 10.1037//0735-7044.105.5.653. [DOI] [PubMed] [Google Scholar]
- 9.Bremner JD, Innis RB, White T, Fujita M, Silbersweig D, Goddard AW, Staib L, Stern E, Cappiello A, Woods S, Baldwin R, Charney DS. SPECT [I-123]iomazenil measurement of the benzodiazepine receptor in panic disorder. Biol Psychiatry. 2000;47:96–106. doi: 10.1016/s0006-3223(99)00188-2. [DOI] [PubMed] [Google Scholar]
- 10.Brooks-Kayal A, Shumate M, Jin H, Rikhter T, Coulter D. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 11.Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA(A) receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- 12.Carey MP, Billing AE, Fry JP. Fluctuations in responses to diazepam during the oestrous cycle in the mouse. Pharmacol Biochem Behav. 1992;41:719–725. doi: 10.1016/0091-3057(92)90218-5. [DOI] [PubMed] [Google Scholar]
- 13.Chojnacka-Wójcik E, Tatarczynska E, Pilc A. The anxiolytic-like effect of metabotropic glutamate receptor antagonists after intrahippocampal injection in rats. Eur J Pharmacol. 1997;319:153–156. doi: 10.1016/s0014-2999(96)00941-7. [DOI] [PubMed] [Google Scholar]
- 14.Concas A, Follesa P, Barbaccia M, Purdy R, Biggio G. Physiological modulation of GABA(A) receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375:225–235. doi: 10.1016/s0014-2999(99)00232-0. [DOI] [PubMed] [Google Scholar]
- 15.Devaud L, Fritschy J, Sieghart W, Morrow A. Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes C, File S. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- 17.File S. The validation of animal tests of anxiety — pharmacological implications. Pol J Pharmacol Pharm. 1984;36:505–512. [PubMed] [Google Scholar]
- 18.Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- 19.Friedman L, Gibbs T, Farb D. Gamma-aminobutyric acidA receptor regulation: chronic treatment with pregnanolone uncouples allosteric interactions between steroid and benzodiazepine recognition sites. Mol Pharmacol. 1993;44:191–197. [PubMed] [Google Scholar]
- 20.Frye CA, Bayon LE. Cyclic withdrawal from endogenous and exogenous progesterone increases kainic acid and perforant pathway induced seizures. Pharmacol Biochem Behav. 1999;62:315–321. doi: 10.1016/s0091-3057(98)00182-8. [DOI] [PubMed] [Google Scholar]
- 21.Gallo M, Smith S. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav. 1993;46:897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- 22.Gyenes N, Farrant M, Farb D. ‘Run-down’ of gamma-amino-butyric acidA receptor function during whole-cell recording: a possible role for phosphorylation. Mol Pharmacol. 1988;346:719–723. [PubMed] [Google Scholar]
- 23.Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- 24.Holt R, Bateson A, Martin I. Chronic treatment with diazepam or abecarnil differentially affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology. 1996;35:1457–1463. doi: 10.1016/s0028-3908(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 25.Kang M, Spigelman I, Sapp D, Olsen R. Persistent reduction of GABA(A) receptor-mediated inhibition in rat hippocampus after chronic intermittent ethanol treatment. Brain Res. 1996;709:221–228. doi: 10.1016/0006-8993(95)01274-5. [DOI] [PubMed] [Google Scholar]
- 26.Kapur J. Hippocampal neurons express GABA A receptor insensitive to diazepam in hyperexcitable conditions. Epilepsia. 2000;41:S86–S89. doi: 10.1111/j.1528-1157.2000.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 27.Kern W, Sieghart W. Polyclonal antibodies directed against an epitope specific for the α4-subunit of the GABAA receptors identify a 67-kDa protein in rat brain membranes. J Neurochem. 1994;62:764–769. doi: 10.1046/j.1471-4159.1994.62020764.x. [DOI] [PubMed] [Google Scholar]
- 28.Knoflach F, Benke D, Wang Y, Scheurer L, Luddens H, Hamilton B, Carter D, Mohler H, Benson J. Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acidA receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- 29.Le Melledo JM, Van Driel M, Coupland NJ, Lott P, Jhangri GS. Response to flumazenil in women with premenstrual dysphoric disorder. Am J Psychiatry. 2000;157:821–823. doi: 10.1176/appi.ajp.157.5.821. [DOI] [PubMed] [Google Scholar]
- 30.Lyons HR, Gibbs TT, Farb DH. Turnover and down-regulation of GABA(A) receptor alpha1, beta2S, and gamma1 subunit mRNAs by neurons in culture. J Neurochem. 2000;74:1041–1048. doi: 10.1046/j.1471-4159.2000.0741041.x. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoudi M, Kang M, Tillakaratne N, Tobin A, Olsen R. Chronic intermittent ethanol treatment in rats increases GABA(A) receptor alpha4-subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- 32.Majewska M, Harrison N, Schwartz R, Barker J, Paul S. Steroid hormone metabolites are barbiturate like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 33.Maric D, Maric I, Wen X, Fritschy J, Sieghart W, Barker J, Serafini R. GABAA receptor subunit composition and functional properties of Cl-channels with differential sensitivity to zolpidem in embryonic rat hippocampal cells. J Neurosci. 1999;19:4921–4937. doi: 10.1523/JNEUROSCI.19-12-04921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 35.Moran M, Goldberg M, Smith S. Progesterone withdrawal II: Insensitivity to the sedative effects of a benzodiazepine. Brain Res. 1998;507:91–100. doi: 10.1016/s0006-8993(98)00781-1. [DOI] [PubMed] [Google Scholar]
- 36.Pellow S, Chopin P, File S, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 37.Rapkin A, Morgan M, Goldman L, Brann D, Simone D, Mahesh V. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- 38.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt P, Nieman L, Danaceau M, Adams L, Rubinow D. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome [see comments] N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt P, Purdy R, Moore P, Jr, Paul S, Rubinow D. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- 41.Smith S, Gong Q, Hsu F, Markowitz R, Ffrench-Mullen J, Li X. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 42.Smith S, Gong Q, Li X, Moran M, Bitran D, Frye C, Hsu F. Withdrawal from 3alpha-OH-5alpha-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. J Neurosci. 1998;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith S, Waterhouse D, Woodward D. Sex steroid effects on extrahypothalamic CNS. II. Progesterone, alone and in combination with estrogen, modulates cerebellar responses to amino acid neuro-transmitters. Brain Res. 1987;422:52–62. doi: 10.1016/0006-8993(87)90539-7. [DOI] [PubMed] [Google Scholar]
- 44.Smith SS, Waterhouse BD, Chapin JK, Woodward DJ. Progesterone alters GABA and glutamate responsiveness: a possible mechanism for its anxiolytic action. Brain Res. 1987;400:353–359. doi: 10.1016/0006-8993(87)90634-2. [DOI] [PubMed] [Google Scholar]
- 45.Soldo B, Proctor W, Dunwiddie T. Ethanol differentially modulates GABAA receptor-mediated chloride currents in hippocampal, cortical, and septal neurons in rat brain slices. Synapse. 1994;18:94–103. doi: 10.1002/syn.890180204. [DOI] [PubMed] [Google Scholar]
- 46.Study R, Barker J. Diazepam and (−)pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of GABA responses in cultured central neurons. Proc Natl Acad Sci USA. 1981;78:7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sundström I, Nyberg S, Bäckström T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology. 1997;17:370–381. doi: 10.1016/S0893-133X(97)00086-9. [DOI] [PubMed] [Google Scholar]
- 48.Treit D, Menard J. Dissociations among the anxiolytic effects of septal, hippocampal, and amygdaloid lesions. Behav Neurosci. 1997;111:653–658. doi: 10.1037//0735-7044.111.3.653. [DOI] [PubMed] [Google Scholar]
- 49.Twyman R, Macdonald R. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol (Lond) 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyndale RF, Hales TG, Olsen RW, Tobin AJ. Distinctive patterns of GABAA receptor subunit mRNAs in 13 cell lines. J Neurosci. 1994;14:5417–5428. doi: 10.1523/JNEUROSCI.14-09-05417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wafford K, Thompson S, Thomas D, Sikela J, Wilcox A, Whiting P. Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- 52.Weiland N, Orchinik M. Specific subunit mRNAs of the GABAA receptor are regulated by progesterone in subfields of the hippocampus. Brain Res Mol Brain Res. 1995;32:271–278. doi: 10.1016/0169-328x(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 53.Wisden W, Herb A, Wieland H, KeinAnen K, Luddens H, Seeburg P. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor alpha 4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- 54.Yu R, Follesa P, Ticku M. Down-regulation of the GABA receptor subunits mRNA levels in mammalian cultured cortical neurons following chronic neurosteroid treatment. Brain Res Mol Brain Res. 1996;41:163–168. doi: 10.1016/0169-328x(96)00087-3. [DOI] [PubMed] [Google Scholar]
- 55.Yu R, Hay M, Ticku M. Chronic neurosteroid treatment attenuates single cell GABAA response and its potentiation by modulators in cortical neurons. Brain Res. 1996;706:160–162. doi: 10.1016/0006-8993(95)01247-8. [DOI] [PubMed] [Google Scholar]