Introduction
A adult male was referred for a second opinion of his retinal dystrophy. His prior genetic testing identified an ABCA4 gene variant associated with Stargardt disease. He had an affected younger brother, and the rest of his family was reported to be asymptomatic. He wanted to know if he would qualify for any Stargardt gene or stem cell therapy trials.
Case Description
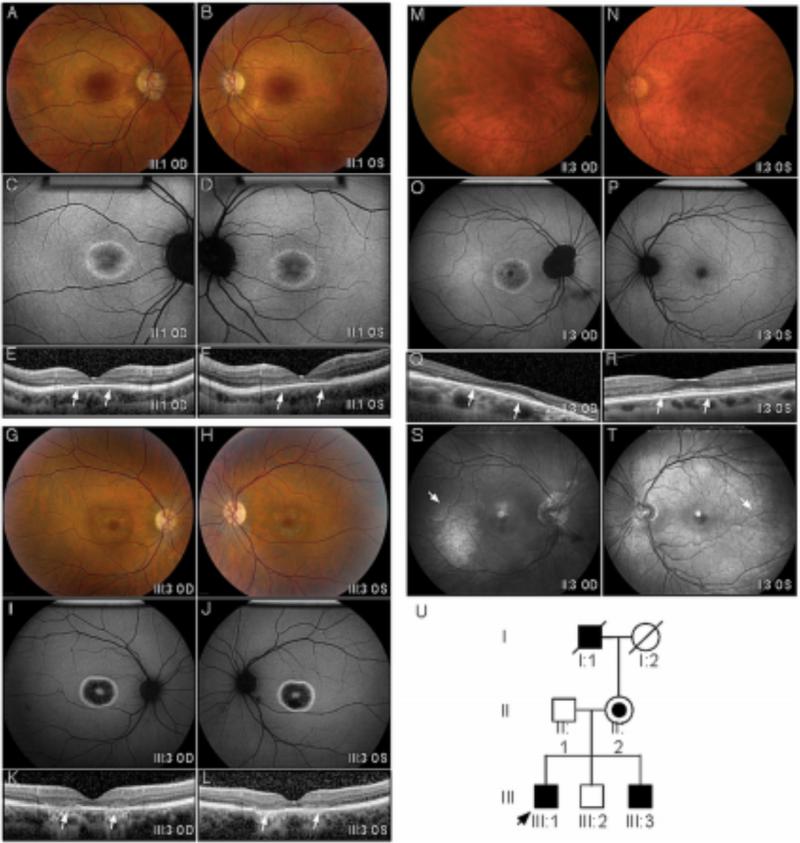
The adult male proband (III:A) experienced blurred central vision, nyctalopia, and photophobia since childhood. His best-corrected visual acuity was 20/100 and 20/200 respectively. Fundus examination demonstrated a bull's eye maculopathy and peripapillary atrophy (Figure 1). There was no intraretinal pigmentary migration, pisciform flecks, optic disc pallor or retinal vessel attenuation. Fundus autofluorescence (AF) revealed a prominent bull's eye pattern. Spectral domain optical coherence tomography (SD-OCT) showed foveal thinning (Figure 2). These findings were not inconsistent with a cone dystrophy such as Stargardt Group II disease.
Figure 1. Clinical Imaging.
A-F. Proband (III:1). Color fundus images showed perifoveal pigment atrophy in a bull's eye pattern that was pronounced in autofluorescent imaging. SDOCT revealed foveal thinning (arrows), loss of outer plexiform layer, ellipsoid and interdigitation line in both eyes with preservation of this structure outside macular. G-L. Affected Brother (III:3). A similar bull's eye pattern with marked RPE atrophy was also observed in color fundus, autofluorescent, and SD-OCT images. M- T. Manifest carrier (II:2). Fundus images showed minimal RPE alteration in her right fovea and a normal appearance in her left. AF revealed a Bull's eye pattern with peripapillary atrophy in her right eye. The left eye appeared normal. A minimal hyperautofluorescent patch was observed in the temporal macula of both eyes. This corresponded to a prominent tapetal reflex (arrow) on red free imaging with a radial pattern of hyperreflectance in the posterior and middle periphery. U. The pedigree was consistent with an x-linked inheritance pattern. The proband is indicated with an arrow. Black symbols represent clinically affected subjects with a Bull's eye pattern from cone dystrophy. Open symbols represent unaffected subjects. The carrier subject is shown with a smaller black symbol inside an open symbol. Deceased individuals are marked by a slash. (RPE – retinal pigment atrophy; SD-OCT spectral domain OCT.)
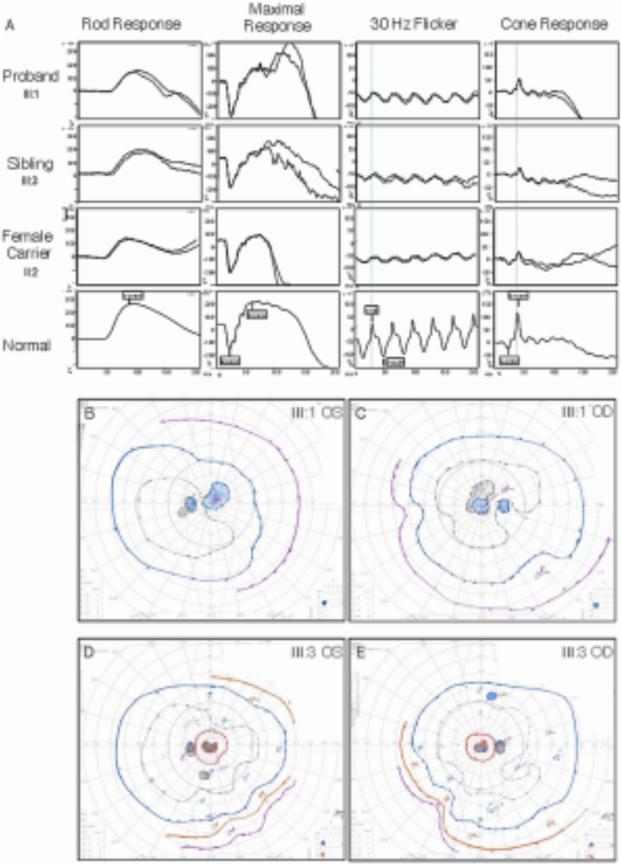
Figure 2. Visual Function.
A. Electroretinography. Replicated dual ERG tracing of the proband, affected brother, and mother (manifest carrier) were compared to a normal subject. The traces showed implicit time delays and amplitude reduction in the cone system with a relatively normal rod system. The carrier female ERG showed asymmetry where the right eye was more affected and corresponded with the fundus findings. B – E. Goldmann visual fields. Visual field testing showed that both the proband (B, C) and the affected brother (D, E) had central scotomas with a small island of preservation inside central scotoma of both eyes. Minimal visual field constrictions were also noted in both patients.
To determine the genetic etiology, we performed whole exome sequencing and confirmed a heterozygous encoding mutation (rs1800548, p.E471K) in the ABCA4 gene associated with Stargardt disease. No other pathogenic ABCA4 alleles or deletions were identified. Examination of all other retinal dystrophy genes[1] in the exome sequence only identified a novel mutation in the RPGR gene (c.3070G>T, pGlu1024X, OMIM #312610). Located in the open reading frame-15 domain, this RPGR variant was confirmed by Sanger sequencing and was absent from public exome databases. This gene mutation was consistent with a diagnosis of X-linked retinitis pigmentosa (XLRP) rather than Stargardt disease.
Because the family had not previously been examined together, efforts were made to gather and examine the family together. Another adult brother (III:B) did not show any signs of retinal degeneration. The youngest adult brother (III:C), however, had similar symptoms to the proband with reduced visual acuity measuring 20/50, bull's eye maculopathy, and unremarkable peripheral fundus findings (Figure 1). The ERG findings of the proband and his affected brother were consistent with generalized retinal dysfunction affecting cones (Figure 2) but sparing the rods.
The mother (II:B) was asymptomatic. She reported her father (I:A) had some late vision problems. Her visual acuity measured 20/125 and 20/30, respectively. Her exam showed minimal pigmentary changes in the right fovea corresponding to a bull's eye pattern on FA (Figure 1). Her left fovea appeared normal. A bilateral tapetal reflex was highlighted with red-free imaging. This radial pattern of hyperreflectance is characteristic of female carriers in XLRP. Her ERG was consistent with cone dysfunction in a XLRP carrier (Figure 2), and the pedigree was consistent with XLRP. Further genetic testing confirmed the p.E1014X RPGR mutation in only the affected family members.
Discussion
This case emphasizes several important issues in the precise diagnosis of retinal dystrophies Unbiased whole exome sequencing allowed simultaneous testing of several genes and identified a novel variant in the RPGR gene, despite the previous incorrect clinical diagnosis.[2] Even without advanced genetic testing, an accurate pedigree revealing an X-linked pattern could be recognized, if all family members were examined. It is essential to include asymptomatic parents and children in the evaluation. AF imaging and SC-OCT may detect subtle retinal changes that identify mildly affected family members comparable to ERG.[3 4] ABCA4 is a highly polymorphic gene, and it is not uncommon to identify a single disease-associated allele in normal patients and no second allele in clinically diagnosed Stargardt patients.[5] Testing affected men for XLRP genes can be high-yield in cone-rod dystrophy cases.[6] An accurate genetic diagnosis is critical for patient counseling and admission into therapeutic trials for Stargardt and XLRP patients.
Acknowledgments
Funding/Support: VBM is supported by NIH grant K08EY020530. The Bernard & Shirlee Brown Glaucoma Laboratory is supported by NIH core grants 5P30CA013696 and 5P30EY019007, unrestricted funds from Research to Prevent Blindness, New York, NY, USA. S.H.T. is a member of the RD-CURE Consortium and is supported by Tistou and Charlotte Kerstan Foundation, NIH R01EY018213, the Research to Prevent Blindness Physician-Scientist Award, the Barbara and Donald Jonas Family Fund, the Foundation Fighting Blindness New York Regional Research Center Grant (C-NY05-0705-0312), the Joel Hoffman Fund, Charles Culpeper Scholarship, Irma T. Hirschl Charitable Trust, Gebroe Family Foundation, and by Foundation Fighting Blindness grant CF-CL-0613-0614-COLU.
Footnotes
Financial Disclosure: None.
References
- 1.Laboratory for the Molecular Diagnosis of Inherited Eye Diseases, The University of Texas - Houston Health Science Center. Daiger SP. RetNet: Summaries of Genes and Loci Causing Retinal Diseases. [August 2013];Secondary RetNet: Summaries of Genes and Loci Causing Retinal Diseases. https://sph.uth.edu/retnet/sum-dis.htm.
- 2.Audo I, Bujakowska KM, Leveillard T, et al. Development and application of a next-generation-sequencing (NGS) approach to detect known and novel gene defects underlying retinal diseases. [December 2013];Orphanet J Rare Dis. 2012 7:8. doi: 10.1186/1750-1172-7-8. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3352121/. doi: 10.1186/1750-1172-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acton JH, Greenberg JP, Greenstein VC, et al. Evaluation of multimodal imaging in carriers of X-linked retinitis pigmentosa. Exp Eye Res. 2013;113:41–8. doi: 10.1016/j.exer.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyo Park S, Hwan Hong I, Tsang SH, et al. Cellular imaging demonstrates genetic mosaicism in heterozygous carriers of an X-linked ciliopathy gene. Eur J Hum Genet. 2013;21(11):1240–8. doi: 10.1038/ejhg.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zernant J, Schubert C, Im KM, et al. Analysis of the ABCA4 gene by next-generation sequencing. Invest Ophthalmol Vis Sci. 2011;52(11):8479–87. doi: 10.1167/iovs.11-8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branham K, Othman M, Brumm M, et al. Mutations in RPGR and RP2 account for 15% of males with simplex retinal degenerative disease. Invest Ophthalmol Vis Sci. 2012;53(13):8232–7. doi: 10.1167/iovs.12-11025. [DOI] [PMC free article] [PubMed] [Google Scholar]