Abstract
Pregnancy anxiety is a potent predictor of adverse birth and infant outcomes. The goal of the current study was to examine one potential mechanism whereby these effects may occur by testing associations between pregnancy anxiety and maternal salivary cortisol on 4 occasions during pregnancy in a sample of 448 women. Higher mean levels of pregnancy anxiety over the course of pregnancy predicted steeper increases in cortisol trajectories compared to lower pregnancy anxiety. Significant differences between cortisol trajectories emerged between 30 to 31 weeks of gestation. Results remained significant when adjusted for state anxiety and perceived stress. Neither changes in pregnancy anxiety over gestation, nor pregnancy anxiety specific to only a particular time in pregnancy predicted cortisol. These findings provide support for one way in which pregnancy anxiety may influence maternal physiology and contribute to a growing literature on the complex biological pathways linking pregnancy anxiety to birth and infant outcomes.
Keywords: pregnancy anxiety, cortisol, anxiety, perceived stress, pregnancy, hypothalamic-pituitary-adrenal axis
Pregnancy anxiety is a highly specific type of maternal psychological distress defined as anxiety concerning a mother's own health, the baby's health, and labor and delivery in the context of a specific pregnancy (Dunkel Schetter, 2011). It is a potent and often more consistent predictor of birth, infant and child outcomes than general forms of psychological distress (Buss, Davis, Muftuler, Head, & Sandman, 2009; Dunkel Schetter & Glynn, 2011; Kramer et al., 2009; Lobel et al., 2008; Roesch, Dunkel Schetter, Woo, & Hobel, 2004). For example, pregnancy anxiety predicts preterm birth and gestational length (Kramer et al., 2009; Mancuso, Dunkel Schetter, Rini, Roesch, & Hobel, 2004; Roesch et al., 2004), infant mental and psychomotor development (Buitelaar, Huisink, Mulder, De Medina, & Visser, 2003; Davis & Sandman, 2010; Huisink, De Medina, Mulder, Visser, & Buitelaar, 2002), child temperament (Blair, Glynn, Sandman, & Davis, 2011; Huisink et al., 2002; Sandman, Davis, Buss, & Glynn, 2012), and brain morphology in young children (Buss et al., 2009). However, little is known about the biological mechanisms that explain these effects.
Alterations in the maternal hypothalamic-pituitary-adrenal (HPA) axis and placenta are hypothesized to be one explanatory mechanism (Seckl & Holmes, 2007; Van den Bergh, Mulder, Mennes, & Glover, 2005). Strong evidence from animal models demonstrates that maternal distress predicts increased fetal glucocorticoid exposure via synthesis and release of maternal glucocorticoids (Maccari et al., 2003; Weinstock, 2005), but similar evidence in humans linking pregnancy anxiety (or general maternal distress) to increased levels of maternal cortisol during pregnancy is lacking. Thus, it is unclear if and how pregnancy anxiety is associated with maternal cortisol during pregnancy. The purpose of this study was to determine whether pregnancy anxiety is associated with cortisol levels and trajectories over the course of pregnancy in an effort to better understand the broader processes linking pregnancy anxiety to birth, infant and child outcomes.
During pregnancy, maternal cortisol increases 2 to 4 fold (Glynn, Dunkel Schetter, Chicz-DeMet, Hobel & Sandman, 2007; Mastorakos & Ilias, 2003; Meulenberg & Hofman, 1990) and passes through the placenta. However, the amount of maternal cortisol that crosses the placental barrier is limited because its passage is regulated by the enzyme 11β-hydroxysteroid dehydrogenase (11 β-HSD2: Benediktsson, Calder, Edwards & Seckl, 1997). Still, maternal cortisol still accounts for approximately 30-40% of the variability in fetal concentrations of cortisol (Gitau, Cameron, Fisk, & Glover, 1998; Gitau, Fisk, Teixeira, Cameron, & Glover, 2001).
Maternal cortisol levels can affect birth and infant outcomes in multiple ways. For one, cortisol stimulates the synthesis and release of placental corticotrophin-releasing hormone (pCRH) (King, Smith & Nicholson, 2001). In humans, elevated cortisol early in pregnancy predicts pCRH levels later in pregnancy (Sandman et al., 2006) and pCRH predicts preterm birth (McLean & Smith, 2001). Maternal cortisol also acts directly on the fetus and its developing nervous system (for reviews, see Davis & Sandman, 2006; Sandman, Davis & Glynn, 2012). For example, results of prospective studies have documented that relatively high levels of prenatal maternal cortisol predict greater behavioral and physiological stress reactivity in fetuses, infants and children (De Weerth, Van Hees, & Buitelaar, 2003; Davis, Glynn, Waffarn, & Sandman, 2011), decreased cognitive ability in infants (Davis & Sandman, 2010; Huizink, Robles de Medina, Mulder, Visser, & Buitelaar, 2003), increased affective problems in young girls (Buss, Davis, Shahbaba, Pruessner, Head, & Sandman, 2012), and altered amygdala volumes in young girls (Buss et al., 2012).
In the literature, the association between pregnancy anxiety and maternal cortisol is inconsistent at best. For example, a study of 603 women found that worries about pregnancy-related complications were associated with high evening maternal cortisol late in pregnancy (Obel et al., 2005), but with lower morning cortisol early in pregnancy. Several other studies reported no associations between maternal cortisol and pregnancy anxiety (Davis & Sandman, 2010; Goedhart et al., 2010; Harville, Savitz, Dole, Herring, & Thorp, 2009; Kivlighan, Dipietro, Costigan, & Laudenslager 2008; Pluess, Bolten, Pirke, & Hellhammer, 2010; Sikkema et al., 2001). However, the majority of these studies assessed maternal cortisol at only 1 or 2 time points during pregnancy, conducted only cross-sectional analyses, or did not test changes in levels or trajectories of cortisol, thus limiting their ability to detect potential associations.
The specific aim of the present study was to test predicted links between pregnancy anxiety and both maternal cortisol levels at specific time points in pregnancy and cortisol trajectories across pregnancy using multilevel modeling techniques. We hypothesized that higher levels of pregnancy anxiety would be associated with higher levels of cortisol and steeper increases in cortisol trajectories over pregnancy. We further hypothesized that pregnancy anxiety would be an independent predictor of cortisol after adjusting for other commonly studied measures of psychological distress (state anxiety and perceived stress).
Method
Study Design and Procedure
Study participants included women from a prospective longitudinal study assessing prenatal psychosocial and behavioral factors in pregnancy, the Multi-Site Behavior in Pregnancy Study (MSBIPS). Women were recruited in the Los Angeles area by research nurses at prenatal clinics in two large urban medical centers. Data for this study were collected on 4 occasions over the course of pregnancy separated by six week intervals (T1: 18-20 weeks, M = 19.29, SD = .80, T2: 24-26 weeks, M= 24.98, SD = .83, T3:30-32 weeks, M=30.98, SD = .76, and T4: 36-38 weeks, M= 36.75, SD = .70). At each assessment, women completed semi-structured interviews, questionnaires and provided one saliva sample for cortisol assessment. All study protocols and procedures were approved by each institution's Institutional Review Boards.
Participants
Inclusion criteria for the sample were 18 years of age, English ability, and singleton intrauterine pregnancy. Exclusion criteria were tobacco, alcohol, or drug use during pregnancy, and medical conditions involving dysregulated neuroendocrine, cardiovascular, hepatic or renal functioning.1 Sixty-three percent of the 1,189 women screened met eligibility criteria and 67% of these women consented to participate in the study (N=498). The primary reasons for declining to participate in the study included work or school conflict, scheduling difficulties, child care issues and lack of interest. Participants were included in the current analysis if they had cortisol and pregnancy anxiety data for at least one time-point during the course of the study (N= 494).2 Forty six women were excluded from the current sample due to missing demographic data or missing medical risk factor variables.3 Therefore, 448 women comprised the final sample.
Table 1 shows demographic and medical risk data for this sample. Participants were 30 years of age on average. The sample was composed of 49% non-Hispanic white, 22% Latina, 14% African American, and 9% Asian and was fairly socioeconomically diverse. The majority of the women were married or cohabitating (88%). Slightly over half of the women were carrying their first child (54%).
Table 1.
Sample socio-demographics and medical history.
| Variables | N (%) |
|---|---|
| Age at Time 1 | M=30 (SD=5.20, range= 18-43) |
| Ethnicity | |
| Non-Hispanic White | 221 (49.3%) |
| Latina | 99 (22.1%) |
| African-American | 61 (13.6%) |
| Asian | 40 (8.9%) |
| Multi-ethnic/Other | 27 (6.9%) |
| Income | |
| < $10,000 | 20 (4.5%) |
| $10,001 - $30,000 | 67 (15.0%) |
| $30,001 - $50,000 | 87 (19.4%) |
| $50,001 - $70,000 | 91 (20.3%) |
| $70,001 - $90,000 | 60 (13.4%) |
| > $90,000 | 123 (27.5%) |
| Education | |
| High School or less | 70 (15.6%) |
| Some college | 175 (39.1%) |
| Bachelor's degree | 123 (27.5%) |
| Graduate degree | 80 (17.9%) |
| Married or Cohabitating | 396 (88.4%) |
| Parity | |
| Nulliparous | 242 (54.0%) |
| Multiparous | 206 (46.0%) |
| Current Obstetric Risk Factors | M=1.15 (SD=1.40, range = 0-7) |
| Historical Obstetric Risk Factors | M= 0.75 (SD=0.99, range = 0-6) |
| Medical History Risk Factors | M=1.20 (SD=1.14, range = 0-5) |
Note:N=448
Measures
Maternal Cortisol
Saliva samples were scheduled for collection at least 1 hour after the participant had eaten. Saliva samples were collected using a cotton gauze pad placed into a syringe and then clarified by depressing the plunger. The samples were then stored at −20°C until assayed. Mean collection time for the samples was 2:20 pm(SD = 1.92 hours). Thawed samples were centrifuged at 3,000 rpm for 15 minutes before assay. All samples were assayed in duplicate using a competitive solid-phase radioimmunoassay (Coat-A-Count; Diagnostic Product Corp.). The test has a minimum detectable level of 0.02 μg/dl and the intra- and inter-assay coefficients of the variance were 5.5% and 7.6%, respectively. Cortisol data were log-transformed [ln(cortisol × 27.6)] and values outside ± 4 standard deviations from the mean were removed from the current analyses (2 data points were removed from T1, 1 from T2, 2 from T3 and 1 from T4).
Pregnancy Anxiety
Pregnancy anxiety was measured with a published 10-item scale at each study visit (αT1 =.80, αT2 =.80 , αT3 =.82 , αT4 =.78 ) assessing the extent to which women worried about their health during pregnancy, their baby's health, and their upcoming labor and delivery (Rini, Dunkel Schetter, Wadhwa, & Sandman, 1999). Sample items included: “I am fearful regarding the health of my baby” and “I am afraid that I will be harmed during delivery.” Participants responded on a 4-point scale from 1(never or not at all) to 4 (almost all of the time or very much). This instrument has been used in prior pregnancy studies in English and Spanish speaking women with evidence for its reliability and validity (Rini et al., 1999; Glynn, Dunkel Schetter, Hobel, & Sandman, 2008). Pregnancy anxiety scores were quite stable over the course of pregnancy in this study (r's = .65-.80)
Perceived Stress
Perceived stress was measured with a modified 12-item version of the Perceived Stress Scale (PSS: Cohen, Kamarck & Mermelstein, 1983) at each visit except for Time 2 (24-26 weeks) (αT1 =.91, αT3 =.93, αT4 =.93). This scale assessed how often participants felt stressed, upset, overwhelmed by daily hassles, or unable to cope with changes or challenges in the last 7 days. Sample items included: “How often have you felt that you were on top of things?” (reverse coded) and “How often have you found that you could not cope with all the things you had to do?” Participants responded on a 5-point scale from 1 (never) to 5 (almost always). Perceived stress scores were moderately to strongly correlated across visits (r’s = .51-.63)
State Anxiety
State anxiety was measured with a 10-item brief version of the state subscale of the State Trait Anxiety Inventory (STAI: Spielberger, Gorsuch, Lushene, Vagg & Jacobs,1983) assessing symptoms of anxiety over the past few days at each visit (αT1 =.89, αT2 =.90, αT3 =.91, αT4 =.91). Sample items included “I am worried” and “I feel at ease” (reverse coded). Participants responded on a 4-point scale from 1 (not at all) to 4 (very much). State anxiety scores were strongly correlated across visits (r's = .60-.63).
Medical Risk Variables
Three medical or obstetric risk factors were created from information abstracted from medical charts by summing the total number of risks for (1) general health (e.g., diabetes, pulmonary disease), (2) baseline and historical medical factors that pose risk in pregnancy (e.g., pre-pregnancy weight under 100 lbs, pelvic inflammatory disease, previous myomectomy, previous abortion) and, (3) risk factors in the index pregnancy (e.g. anemia, threatened abortion, bacterial vaginosis, asthma, vaginal bleeding, high blood pressure). These medical risk factors were based on known risks of preterm birth and other adverse pregnancy outcomes. The number of risk factors ranged from 0 to 7 with means close to 1 in each category (see Table 1).
Sociodemographics
Demographic variables including age, self-identified ethnicity, education, income and parity were measured at the Time 1 visit (see Table 1).
Statistical Analyses
Multilevel modeling (MLM), using HLM 7 software (Raudenbush et al., 2011), was used to explore the effects of pregnancy anxiety on initial levels of maternal cortisol at 19 weeks and trajectories of maternal cortisol. MLM treats the data in a hierarchical manner with observations nested within persons and it accounts for both between-person variation (between-person differences) and within-person variation (within-person changes).
Time-invariant predictors, those measured only once, were centered so that 0 was a meaningful value (e.g., the average age at the initial assessment, no obstetric or medical risk factors). The primary time-varying predictors, those measured multiple times during pregnancy (pregnancy anxiety, state anxiety and perceived stress) were parameterized as two variables to represent between-person differences and within-person variation (Hoffman & Stawski, 2009). These variables were parametertized in two different ways. In one set of analyses, the between-person variables reflected the means of pregnancy anxiety, perceived stress and state anxiety for each woman averaged across all four assessments. These variables were centered so that 0 was the sample mean. The within-person variables had occasion-specific values at each time point that reflected deviance from the woman's mean on that occasion. For example, if a woman's mean level of pregnancy anxiety was 2 and her level of pregnancy anxiety at Time 4 was 3, then her within-person value of pregnancy anxiety at Time 4 would be 1. In another analysis, between-person differences in pregnancy anxiety reflected differences in initial or baseline levels of pregnancy anxiety at 19 weeks of gestation. Within-person variation reflected deviations or changes over time from the baseline assessment (Berg et al., 2009). Weeks of gestation, the measure of time, was centered at 19 weeks. Cortisol collection time was a covariate, so it was grand-mean centered. Missing data was accommodated using maximum likelihood estimation. Two-hundred and seventy-two women had complete data, 114 were missing complete data from one assessment, 37 from two assessments and 25 had complete data for only one assessment.
We estimated a series of nested models using maximum likelihood estimation for fixed effects and restricted maximum likelihood for random effects. First, we estimated an unconditional means model to determine the amount of variation in cortisol due to between-person differences and within-person changes or variation over the course of pregnancy. Then we estimated an unconditional growth model with the addition of weeks of gestation to estimate initial levels of cortisol and change in cortisol over gestation. Sample collection time was included in all models. To insure that any associations between pregnancy anxiety and cortisol were not due to actual medical risk or demographic factors we first assessed if any of these variables were independently associated with cortisol. Only significant potential third variables (covariates) were included in the primary analyses. We then ran models with only the between-person and within-person pregnancy anxiety predictors followed by a model that included state anxiety, perceived stress and any significant demographic or medical risk covariates.
Results
Descriptive statistics for the primary variables illustrate the predicted rise in maternal cortisol over gestation (see Table 2). Bivariate correlations among the independent variables indicated that perceived stress and state anxiety were highly correlated, whereas pregnancy anxiety was only moderately correlated with each of these variables (see Table 3).
Table 2.
Descriptive statistics for primary variables at each assessment.
| Gestational Age | 18-20 weeks (Time 1) | 24-26 weeks (Time 2) | 30-32 weeks (Time 3) | 36 -38 weeks (Time 4) |
|---|---|---|---|---|
| Pregnancy Anxiety | ||||
| Mean (SD) | 1.91 (0.53) | 1.79 (0.48) | 1.78 (0.48) | 1.73 (0.44) |
| N | 445 | 398 | 401 | 344 |
| State Anxiety | ||||
| Mean (SD) | 2.04 (0.60) | 1.96 (0.61) | 1.99 (0.65) | 2.06 (0.63) |
| N | 442 | 400 | 407 | 333 |
| Perceived Stress | ||||
| Mean (SD) | 2.26 (0.64) | [Not assessed] | 2.27 (0.70) | 2.27 (0.71) |
| N | 444 | 401 | 344 | |
| Salivary Cortisol1 | ||||
| Mean (SD) | 0.22 (0.18) | 0.30 (0.23) | .34 (0.24) | .40 (0.22) |
| N | 438 | 414 | 401 | 341 |
Raw values, μg/dl.
Table 3.
Bivariate correlations among independent variables
| Independent Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Time 1 Age | 1.00 | |||||||||
| 2. Ethnicity (White 0, Non-white 1) | −.20*** | 1.00 | ||||||||
| 3. Income | .41*** | −.32*** | 1.00 | |||||||
| 4. Education Level | .42*** | −.33*** | .50*** | 1.00 | ||||||
| 5. Parity (Nulliparious 0) | .14** | .21*** | −.06 | −.15** | 1.00 | |||||
| 6. General Medical Risk | .15** | −.03 | −.03 | .01 | .05 | 1.00 | ||||
| 7. Historical Obstetric Risk | .21*** | .10* | .01 | .04 | .22*** | .23*** | 1.00 | |||
| 8. Current Obstetric Risk | −.02 | .16** | −.13** | −.12** | .10* | .25*** | .25*** | 1.00 | ||
| 9. Pregnancy Anxiety (PA)1 | −.07 | .16** | −.19*** | .05 | −.20*** | .18*** | −.02 | .09† | 1.00 | |
| 10. State Anxiety1 | −.06 | .12* | −.24*** | −.13** | −.01 | .15** | .12* | .10* | .57*** | 1.00 |
| 11. Perceived Stress1 | −.03 | .20*** | −.24*** | −.18*** | .07 | .15** | .11* | .11* | .45*** | .78*** |
Note: N = 448
p<.001
p<.01
p<.05
p <.10
Mean across all assessments (T1-T4)
Partial correlations adjusted for cortisol collection time indicated that pregnancy anxiety at Time 1, Time 2 and Time 3 were significantly correlated with maternal cortisol levels at Time 3 (see Table 4). Based on these results, we regressed cortisol at Time 3 over all three respective pregnancy anxiety measurements adjusting for sample collection time in order to test whether pregnancy anxiety at a particular time period in pregnancy independently predicted cortisol levels at Time 3. Results indicated that no single time point was independently associated with cortisol at Time 3 (all p's >.10).
Table 4.
Partial correlations between pregnancy anxiety and Cortisol adjusting for time of day of cortisol assessments.
| Cortisol 18-20 weeks | Cortisol 24-26 weeks | Cortisol 30-32 weeks | Cortisol 36 -38 weeks | |
|---|---|---|---|---|
| Pregnancy Anxiety T1 | .00 | −.03 | .11* | .05 |
| N | 436 | 411 | 397 | 339 |
| Pregnancy Anxiety T2 | −.04 | .16** | .06 | |
| N | 389 | 372 | 313 | |
| Pregnancy Anxiety T3 | .17** | .08 | ||
| N | 388 | 330 | ||
| Pregnancy Anxiety T4 | .06 | |||
| N | 338 |
Note:
p<.01
p<.05
different sample sizes reflect different numbers of cases of both pregnancy anxiety and cortisol at each measurement occasion
Maternal Cortisol Trajectories
Consistent with the general increase in cortisol over gestation, the unconditional means model indicated that 78% of the total variation in cortisol was attributable to within-person variation and 22% of the total variation in cortisol was attributable to differences between women (ICC = .22). The unconditional growth model indicated that on average there was a significant increase in cortisol over the course of pregnancy (γ = .034, SE=.002, p < .001). The correlation between initial status and rate of change (r = −.93) showed that higher cortisol levels at the initial assessment were associated with a decreased rate of change. The addition of the quadratic rate of change term was not significant, nor did its addition significantly improve model fit.
Demographic variables (age, level of education, self-reported income and self-identified ethnicity/race), parity and the medical risk indices were added into the model individually. Race/ethnicity was coded white (0) or non-white (1) in order to retain the largest sample and because race/ethnicity was not the main focus of these analyses. Neither demographic variables, medical risk indices nor parity were associated with either initial cortisol levels or cortisol trajectories (p's > .10). Therefore, we did not adjust for any of these variables in the primary analyses.4
Primary Analyses on Pregnancy Anxiety and Maternal Cortisol
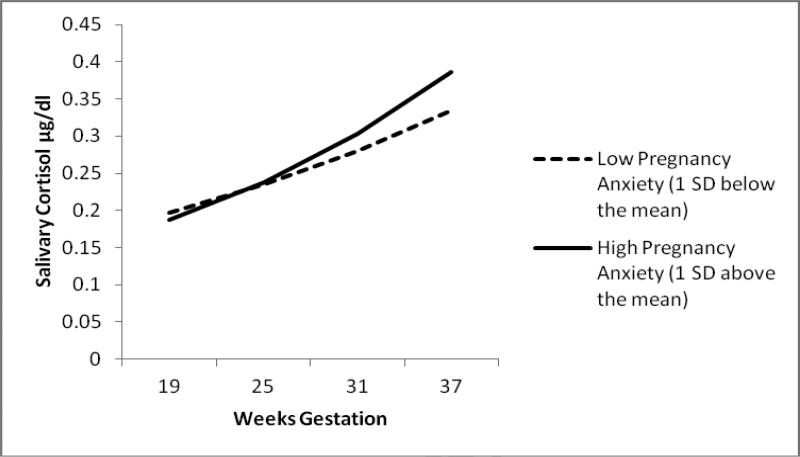
First, we tested a model that included both mean levels of pregnancy anxiety averaged across all four assessments and within-person deviations from mean levels at each assessment simultaneously. We examined the degree to which mean pregnancy anxiety levels and being higher or (lower) on pregnancy anxiety relative to mean levels at each assessment predicted cortisol. Mean levels of pregnancy anxiety (between-person differences) were not associated with initial cortisol levels at 19 weeks (γ = −.060 SE=.063, p =.34). However, as indicated by a significant between-person pregnancy anxiety by weeks of gestation interaction (γ = .013 SE=.004, p =.002), higher mean levels of pregnancy anxiety were associated with steeper increases cortisol trajectories over the course of pregnancy. To further explore this interaction and to determine when in gestation differences in cortisol levels emerged, we centered each week of gestation at 0 and tested the intercept differences in pregnancy anxiety. Significant differences in cortisol levels (intercepts) emerged beginning between 30 and 31 weeks of gestation (γ = 0.077, SE=.040, p =.052 and γ = 0.090 SE=.040, p =.024, respectively) and persisted until the end of pregnancy (see Figure 1). In contrast to predictions, within-person deviations from each woman's individual mean level of pregnancy anxiety did not predict cortisol (γ = .048 SE=.050, p =.33). Thus, being higher (or lower) than a woman's individual mean pregnancy anxiety level on each measurement occasion was not associated with her cortisol levels.
Figure 1.
Cortisol trajectories over gestation by between-person pregnancy anxiety.
In a second analysis, we tested a model that included both baseline levels of pregnancy anxiety at 19 weeks of gestation and within-person changes from baseline levels at each subsequent assessment (Berg et al., 2009).5 We examined the degree to which baseline pregnancy anxiety levels and increases or decreases from baseline levels predicted cortisol. Results of this analysis were quite similar to the results of the previous analysis. Baseline levels of pregnancy anxiety (between-person differences) were not associated with initial cortisol levels at 19 weeks (γ = −0.016 SE=.052, p = .76). However, as indicated by a significant between-person pregnancy anxiety by weeks of gestation interaction (γ = 0.008 SE=.003, p = .016), higher baseline levels of pregnancy anxiety were associated with steeper increases in cortisol trajectories. Changes (increases or decreases) in pregnancy anxiety from baseline were not associated with cortisol (γ = 0.070 SE=.046, p = .13).
Finally, to determine if the effect of mean pregnancy anxiety on cortisol was being driven by pregnancy anxiety at any particular measurement occasion, we tested three additional models to determine if pregnancy anxiety at each remaining assessment (Time 2, Time 3, Time 4) was associated with cortisol trajectories. The results of these analyses revealed that pregnancy anxiety measured at Time 2 (γ = .012 SE=.003, p =.002, n = 398), Time 3 (γ = .010 SE=.003, p =.009, n = 401), and Time 4 (γ = .012 SE=.004, p =.006, n = 344) all predicted cortisol trajectories.6
Pregnancy Anxiety as an Independent Predictor of Maternal Cortisol
To test whether mean levels of pregnancy anxiety remained a significant predictor of cortisol trajectories after adjusting for other measures of general distress, state anxiety and perceived stress were each added to the model individually. The results revealed that mean levels of pregnancy anxiety remained a significant predictor of cortisol trajectories after adjusting for both state anxiety (γ =.013, SE= .005, p =.009) and perceived stress (γ = .01 SE= .005, p=.027). Neither mean levels, nor deviations from mean levels of state anxiety or perceived stress were significant predictors of cortisol trajectories in these models. When state anxiety and perceived stress were entered into the model together, pregnancy anxiety again remained a significant predictor of cortisol trajectories (see Table 5).
Table 5.
Pregnancy anxiety predicting cortisol trajectories adjusting for state anxiety and perceived stress
| Parameters | Estimate | SE | p - value |
|---|---|---|---|
| Intercept (initial status at 19 weeks) | 1.647 | .03 | <.001 |
| Between-Person (BP) Pregnancy Anxiety | −0.021 | .08 | .79 |
| Between-Person (BP) State Anxiety | 0.032 | .09 | .73 |
| Between-Person (BP) Perceived Stress | −0.047 | .07 | .52 |
| Linear slope (gestational age) | 0.036 | .00 | <.001 |
| BP Pregnancy Anxiety | 0.011 | .00 | .032 |
| BP State Anxiety | −0.002 | .01 | .73 |
| BP Perceived Stress | 0.003 | .01 | .55 |
| Time collected | −.097 | .01 | <.001 |
| Within-Person Pregnancy Anxiety | 0.096 | .06 | .094 |
| Within-Person State Anxiety | −0.053 | .04 | .21 |
| Within-Person Perceived Stress | 0.038 | .04 | .35 |
| Variances/Covariances | Estimate | SE | |
| Intercept variance | .148 | .02 | |
| Covariance | −.005 | .00 | |
| Slope variance | .000 | .00 |
Note: N = 447 in this model because of one case with missing state anxiety. Between- person variables represent means across all 4 assessments. Within-person variables represent the deviation from each woman's individual mean at each assessment.
Discussion
In this study, pregnancy anxiety and maternal salivary cortisol levels were assessed in a sample of 448 women on four occasions during pregnancy. Repeated assessments of both pregnancy anxiety and cortisol permitted the use of multilevel modeling to test the extent to which within-person changes and between-person differences in pregnancy anxiety predicted prenatal cortisol trajectories. Results revealed that women with higher mean levels of pregnancy anxiety over the course of the study exhibited steeper increases in cortisol compared to women with lower mean levels. Furthermore, women who reported higher mean pregnancy anxiety had significantly elevated cortisol levels beginning between 30 and 31 weeks of gestation and these elevations persisted for the rest of pregnancy. Of note, pregnancy anxiety also remained an independent predictor of cortisol after adjusting for state anxiety and perceived stress.
This study is the first to demonstrate that mean levels of pregnancy anxiety from mid to late pregnancy predict maternal cortisol trajectories across pregnancy and pinpoint when in gestation (30-31 weeks) these trajectories diverge resulting in cortisol level differences. Although we do not know why these trajectories diverge later rather than earlier in pregnancy, the present findings support the premise that pregnancy anxiety plays a role in modulating HPA activity in pregnant women. The results are consistent with previous studies demonstrating positive associations between pregnancy anxiety and prenatal HPA and placental activity (Mancuso et al., 2004; Obel et al., 2005).
By simultaneously taking into account both between- and within-person variation in pregnancy anxiety, the present findings clarify which type of variation is predictive of maternal cortisol. In doing so, the possibility of between- and within-person effects being confounded is reduced. Mean levels of pregnancy anxiety and baseline levels of pregnancy anxiety (between-person differences) predicted cortisol, whereas changes in pregnancy anxiety relative to mean levels or baseline levels (within-person changes) did not predict cortisol. Furthermore, regression analyses and additional multilevel modeling analyses suggested that mean pregnancy anxiety was a consistent predictor of cortisol trajectories. The regression analyses demonstrated that pregnancy anxiety measured at three occasions during pregnancy (T1: 18-20 weeks, T2: 24-26 weeks, and T3: 30-32 weeks) all predicted maternal cortisol, but not independently of each other. The multilevel analyses demonstrated that pregnancy anxiety at each time point predicted cortisol trajectories. Thus, the effect of mean pregnancy anxiety was not being driven by pregnancy anxiety at any particular measurement occasion.
Although a few prior studies have shown that distress measures averaged across pregnancy are predictive of gestational length (Lobel, Dunkel Schetter, & Scrimshaw, 1992; Roesch et al., 2004), the majority of studies show that pregnancy anxiety at specific times in gestation or changes over time in pregnancy anxiety are predictive of birth and infant outcomes (e.g., Buitelaar et al., 2003; Buss et al., 2009; Mancuso et al., 2004). There are several potential explanations for why changes in pregnancy anxiety were not predictive of cortisol in the current study. First, some measures of pregnancy anxiety may be more sensitive to changes in pregnancy anxiety. For example, Mancuso et al. (2004) found a timing effect of pregnancy anxiety predicting HPA activity (pCRH) using a measure that assessed pregnancy anxiety with an adjective checklist (e.g., worried, jittery) and a specified timeframe of the past week. Second, although pregnancy anxiety measured in this study was relatively stable from mid to late gestation, pregnancy anxiety decreased over gestation on average. Thus, outcomes measured in other studies demonstrating timing effects such as infant development may be more sensitive than cortisol to small changes in pregnancy anxiety assessed with this measure. Additionally, the present study only assessed changes in pregnancy anxiety from mid to late gestation and potentially predictive changes from early in gestation were not included. Finally, because significant changes in cortisol occurred early in the third trimester, it is possible that there is an accumulation effect of pregnancy anxiety on cortisol levels.
It is difficult to directly compare these results to previous studies because of the use of different measures of pregnancy anxiety, different assessments of cortisol (cortisol awakening response (CAR), single assessments, early morning and evening cortisol) and different cortisol collection methods (saliva, plasma). In this study, cortisol trajectories diverged between 30 to 31 weeks of gestation suggesting why some cross-sectional studies measuring cortisol and pregnancy anxiety earlier in pregnancy did not find an association (e.g., Harville et al., 2009; Skikkema et al., 2001). However, one study did examine cross-sectional associations later in pregnancy with the same pregnancy measure in the present study and still found no association (Davis & Sandman, 2010), but this study had a relatively smaller sample of women.
Our findings underscore the necessity of longitudinal designs to better understand how different types of prenatal distress are associated with maternal physiology and birth and infant outcomes. The use of slightly different conceptualizations and measures of pregnancy anxiety may result in inconsistent results with respect to effects on maternal physiology and birth and infant outcomes. Pregnancy anxiety as now conceptualized is a risk factor that is related to yet distinct from general distress, state and trait anxiety. Ongoing research is examining the properties and correlates of pregnancy anxiety (Dunkel Schetter & Glynn, 2011; Guardino & Dunkel Schetter, in press). Elucidating when and how pregnancy anxiety affects these outcomes is critical for the development of theory and ultimately intervention.
In addition to implications for birth outcomes such as PTB (Dunkel Schetter, 2011), these results are relevant to understanding fetal and infant development because fetal exposure to excessive maternal cortisol has been shown to affect fetal HPA axis development and resultant infant stress regulation (Sandman et al., 2012). Even small changes in maternal cortisol may have a meaningful effect on fetal concentrations of cortisol. Fetal cortisol concentrations are much lower than maternal cortisol concentrations, so the addition of any maternal cortisol crossing the placental barrier can greatly alter fetal cortisol concentrations. For this reason, maternal cortisol has been thought to have a large impact (Gitau et al., 1998). Furthermore, it appears that maternal cortisol may influence fetal cortisol concentrations to an even greater extent when a woman is highly anxious during pregnancy through the down-regulation of the enzyme 11 β-HSD2 (Glover et al, 2009; O'Donnell et al., 2012). It is possible that women who are high in pregnancy anxiety not only produce more cortisol, but these increased levels may also affect the fetus to a greater extent because more cortisol is allowed to pass through the placental barrier.
A strength of this study was the careful characterization of medical and obstetric risks. An alternative explanation for our findings is the women with greater medical and obstetric risk factors may have higher pregnancy anxiety creating a spurious association between pregnancy anxiety and maternal cortisol. However, pregnancy anxiety was not associated with obstetric risk factors in prior pregnancies and was only marginally associated with obstetric risk factors in the current pregnancy. Moreover, adjusting for medical and obstetric risk factors did not alter the reported results. Therefore, it is unlikely that medical and obstetric risk factors explain the association of pregnancy anxiety and cortisol in this sample. Continuing to explore these associations is important for future research.
Although the assessment of maternal cortisol at multiple time points during pregnancy is a strength of the study design, the collection of a single assessment at each time point is a potential limitation. To help mitigate this limitation, we carefully recorded the time of day each sample was collected and statistical adjusted for the sample collection time in all analyses. Nevertheless, future research should examine changes in diurnal cortisol over pregnancy and consider how diurnal cortisol slopes might change as a function pregnancy anxiety. For example, in one study, flatter diurnal cortisol slopes were associated with higher trait anxiety late in pregnancy (Kivlighan et al., 2008), but changes in diurnal slopes over pregnancy were not assessed.
Conclusion
Pregnancy anxiety is a relatively new concept and a seemingly powerful prenatal risk factor. Although strong evidence from animal models identifies cortisol as a key physiological mechanism explaining the effects of prenatal distress on birth and infant outcomes, there is not sufficient evidence in human studies. These results provide initial evidence for the association between pregnancy anxiety and maternal cortisol in humans. They demonstrated that higher mean levels of pregnancy anxiety from mid to late pregnancy were associated with steeper increases in maternal cortisol compared to lower levels and identified differences in cortisol levels beginning early in the third trimester. This association is a critical first link in understanding the physiological mechanisms through which pregnancy anxiety may exert its effects on birth and infant outcomes. Finally, these findings further highlight the need to understand how pregnancy anxiety affects maternal physiology -- not just whether or not it does.
Highlights.
We examine the association between pregnancy anxiety and maternal prenatal cortisol
Higher pregnancy anxiety predicted steeper increases in cortisol trajectories relative to lower
Cortisol trajectories significantly diverged between 30 and 31 weeks gestation
Results remained significant adjusting for state anxiety and perceived stress
Pregnancy anxiety may be linked to birth and infant outcomes via prenatal cortisol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No Conflicts of interest Declared
Despite screening procedure efforts, it was revealed from information abstracted from medical records and self-report questionnaires that a few (n=28) women smoked up to the first assessment (Time 1). By the 2nd assessment (Time 2), no women reported any smoking. Because they were already enrolled in the study and occasional smokers, these women were retained in the study. A dummy coded variable was created, 1 (smoking) and 0 (no smoking) and entered into all analyses. The results remained unchanged and smoking was not a significant predictor of initial cortisol levels or cortisol trajectories. This variable was, therefore, not reported in the analyses.
Three participants were removed due to lack of cortisol data. One participant was removed due to lack of pregnancy anxiety data.
Women not included in the final sample were not significantly different from women who were included in the final sample on any study variables (all p's >.10).
Although medical risk was not a significant predictor of cortisol levels or trajectories in our preliminary analyses, we ran a model that included the medical risk variables. Mean levels of pregnancy anxiety remained a significant predictor cortisol trajectories (γ = .014 SE=.004, p =.002).
The sample for this analysis was 445 women because 3 cases were missing pregnancy anxiety at Time 1.
We note that each of these models includes a slightly different sample size due to differences in the number of cases with pregnancy anxiety scores at each assessment. Results did not significantly change when using Empirical Bayes estimates of the intercepts (pregnancy anxiety levels) at each assessment utilizing the entire sample of 448 women.
References
- Berg AI, Hoffman L, Hassing LB, McClearn GE, Johansson B. What matters, and what matters most, for change in life satisfaction in the oldest-old? A study over 6 years among individuals 80+. Aging and Mental Health. 2009;13(2):191–201. doi: 10.1080/13607860802342227. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Calder A, Edwards C, Seckl J. Placental 11β-hydroxysteroid dehydrogenase: A key regulator of fetal glucocorticoid exposure. Clinical Endocrinology. 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- Blair M, Glynn L, Sandman C, Davis E. Prenatal maternal anxiety and early childhood temperament. Stress. 2011;14:644–51. doi: 10.3109/10253890.2011.594121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolten M, Wurmser H, Buske-Kirschbaum A, Papoušek M, Pirke K, Hellhammer D. Cortisol levels in pregnancy as a psychobiological predictor for birth weight. Archives of Women's Mental Health. 2011;14:33–41. doi: 10.1007/s00737-010-0183-1. [DOI] [PubMed] [Google Scholar]
- Buitelaar J, Huisink A, Mulder E, de Medina P, Visser G. Prenatal stress and cognitive development and temperament in infants. Neurobiology of Aging. 2003;24:S53–60. doi: 10.1016/s0197-4580(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis E, Muftuler L, Head K, Sandman C. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6-9 year old children. Psychoneuroendocrinology. 2009;35:141–53. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis E, Shahbaba B, Pruessner J, Head K, Sandman C. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Davis E, Sandman C. Prenatal exposure to stress and stress hormones influences child development. Infants & Young Children. 2006;19:246–259. [Google Scholar]
- Davis E, Sandman C. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81:131–48. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman C. Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weerth C, Van Hees Y, Buitelaar J. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Human Development. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C. Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annual Review of Psychology. 2011;62:531–58. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, Glynn LM. Stress in pregnancy: Empirical evidence and theoretical issues to guide interdisciplinary researchers. In: Contrada R, Baum A, editors. The handbook of stress science: bology, psychology, and health. Springer Publishing Company; New York: 2011. pp. 321–343. [Google Scholar]
- Gitau R, Cameron A, Fisk N, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- Gitau R, Fisk N, Teixeira J, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. Journal of Clinical Endocrinology & Metabolism. 2001;86:104–109. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- Glynn L, Dunkel Schetter C, Chicz-DeMet A, Hobel C, Sandman C. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007;28:1155–1161. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Glynn L, Dunkel Schetter C, Hobel C, Sandman C. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychology. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- Glynn L, Sandman C. Sex moderates associations between prenatal glucocorticoid exposure and human fetal neurological development. Developmental Science. 2012;15:601–10. doi: 10.1111/j.1467-7687.2012.01159.x. [DOI] [PubMed] [Google Scholar]
- Glover V, Bergman K, Sarkar P, O'Connor T. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology. 2009;34:430–435. doi: 10.1016/j.psyneuen.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Goedhart G, Vrijkotte T, Roseboom T, Van der Wal M, Cuijpers P, Bonsel G. Maternal cortisol and offspring birthweight: Results from a large prospective cohort study. Psychoneuroendocrinology. 2010;35:644–652. doi: 10.1016/j.psyneuen.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Guardino CM, Dunkel Schetter C. Understanding pregnancy anxiety concepts, correlates and consequences. Zero to Three. in press. [Google Scholar]
- Gutteling B, De Weerth C, Buitelaar J. Prenatal stress and children's cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Harville E, Savitz D, Dole N, Herring A, Thorp J. Stress questionnaires and stress biomarkers during pregnancy. Journal of Womens Health. 2009;18:1425–1433. doi: 10.1089/jwh.2008.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L, Stawski R. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development. 2009;6:97–120. [Google Scholar]
- Huizink A, De Medina P, Mulder E, Visse r G., Buitelaar J. Prenatal maternal stress, HPA axis activity, and postnatal infant development. International Congress Series. 2002;1241:65–71. [Google Scholar]
- Huizink A, Robles de Medina P, Mulder E, Visser G, Buitelaar J. Stress during pregnancy is associated with developmental outcome in infancy. Journal of Child Psychology and Psychiatry. 2003;44:810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- King B, Smith R, Nicholson R. The regulation of human corticotrophin-releasing hormone gene expression in the placenta. Peptides. 2001;22:1941–1947. doi: 10.1016/s0196-9781(01)00486-7. [DOI] [PubMed] [Google Scholar]
- Kivlighan K, Dipietro J, Costigan K, Laudenslager M. Diurnal rhythm of cortisol during late pregnancy: Associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology. 2008;33:1225–35. doi: 10.1016/j.psyneuen.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M, Lydon J, Séguin L, Goulet L, Kahn S, McNamara H, Genest J, Dassa C, Chen M, Sharma S, Meaney M, Thomson S, Van Uum S, Koren G, Dahhou M, Lamoureux J, Platt R. Stress pathways to spontaneous preterm birth: The role of stressors, psychological distress, and stress hormones. American Journal Epidemiology. 2009;169(13):19–26. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- Lobel M, Cannella D, Graham J, DeVincent C, Schneider J, Meyer B. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychology. 2008;27:604–15. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- Lobel M, Dunkel Schetter C, Scrimshaw S. Prenatal maternal stress and prematurity: A prospective study of socioeconomically disadvantaged women. Health Psychology. 1992;11:32–40. doi: 10.1037//0278-6133.11.1.32. [DOI] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Morley-Fletcher S, Zuena A, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: Implications of glucocorticoid hormones. Neuroscience & Biobehavioral Reviews. 2003;27:119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Mclean M, Smith R. Review Corticotrophin-releasing hormone and human parturition. Reproduction. 2001;121:493–501. doi: 10.1530/rep.0.1210493. [DOI] [PubMed] [Google Scholar]
- Mancuso R, Dunkel Schetter C, Rini C, Roesch S, Hobel C. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosomatic Medicine. 2004;66:762–769. doi: 10.1097/01.psy.0000138284.70670.d5. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Annals New York Academy of Sciences. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- Meulenberg P, Hofman J. Differences between concentrations of salivary cortisol and cortisone and of free cortisol and cortisone in plasma during pregnancy and postpartum. Clinical Chemistry. 1990;36:70–75. [PubMed] [Google Scholar]
- Obel C, Hedegaard M, Henriksen T, Secher N, Olsen J, Levine S. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology. 2005;30:647–656. doi: 10.1016/j.psyneuen.2004.11.006. [DOI] [PubMed] [Google Scholar]
- O'Donnell K, Bugge Jensen A, Freeman L, Khalife N, O'Connor T, Glover V. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology. 2012;37:818–826. doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Pluess M, Bolten M, Pirke K, Hellhammer D. Maternal trait anxiety, emotional distress, and salivary cortisol in pregnancy. Biological Psychology. 2011;83:169–175. doi: 10.1016/j.biopsycho.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Rini C, Dunkel-Schetter C, Wadhwa P, Sandman C. Psychological adaptation and birth outcomes: The role of personal resources, stress, and sociocultural context in pregnancy. Health Psychology. 18:333–345. doi: 10.1037//0278-6133.18.4.333. 199. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Cheong A, Fai Y, Congdon R, du Toit M. HLM 7: Hierarchical linear and nonlinear modeling. Scientific Software International; Lincolnwood, IL: 2011. [Google Scholar]
- Roesch S, Dunkel Schetter C, Woo G, Hobel C. Modeling the types and timing of stress in pregnancy. Anxiety Stress & Coping. 2004;17:87–102. [Google Scholar]
- Sandman C, Davis E, Buss C, Glynn L. Prenatal programming of human neurological function. International Journal of Peptide. 2011:837596. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman C, Davis E, Buss C, Glynn L. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2012;95:8–21. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman C, Glynn L, Schetter C, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. International Journal of Peptide. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Seckl J, Holmes M. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal'programming'of adult pathophysiology. Nature Clinical Practice Endocrinology & Metabolism. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Sikkema J, Robles de Medina P, Schaad R, Mulder E, Bruinse H, Buitelaar J, Visser G, Franx A. Salivary cortisol levels and anxiety are not increased in women destined to develop preeclampsia. Journal of Psychosomatic Research. 2001;50:45–49. doi: 10.1016/s0022-3999(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Van den Bergh B, Mulder E, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms, a review. Neuroscience & Biobehavioral Reviews. 2005;29:237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, Behavior, and Immunity. 2005;190:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]