Abstract
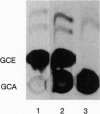
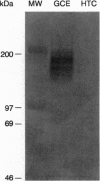
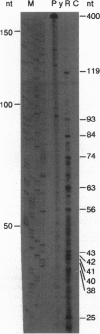
Secretion of anionic endo- and xenobiotics is essential for the survival of animal and plant cells; however, the underlying molecular mechanisms remain uncertain. To better understand one such model system--i.e., secretion of bile acids by the liver--we utilized a strategy analogous to that employed to identify the multidrug resistance (mdr) genes. We synthesized the methyl ester of glycocholic acid (GCE), which readily enters cells, where it is hydrolyzed to yield glycocholic acid, a naturally occurring bile acid. The rat hepatoma-derived HTC cell line gradually acquired resistance to GCE concentrations 20-fold higher than those which inhibited growth of naive cells, yet intracellular accumulation of radiolabel in resistant cells exposed to [14C]GCE averaged approximately 25% of that in nonresistant cells. As compared with nonresistant cells, resistant cells also exhibited (i) cross-resistance to colchicine, a known mdr substrate, but not to other noxious substances transported by hepatocytes; (ii) increased abundance on Northern blot of mRNA species up to 7-10 kb recognized by a probe for highly conserved nucleotide-binding domain (NBD) sequences of ATP-binding cassette (ABC) proteins; (iii) increased abundance, as measured by RNase protection assay, of mRNA fragments homologous to a NBD cRNA probe; and (iv) dramatic overexpression, as measured by Western blotting and immunofluorescence, of a group of 150- to 200-kDa plasma membrane proteins recognized by a monoclonal antibody against a region flanking the highly conserved NBD of mdr/P-glycoproteins. Finally, Xenopus laevis oocytes injected with mRNA from resistant cells and incubated with [14C]GCE secreted radiolabel more rapidly than did control oocytes. Enhanced secretion of glycocholic acid in this cell line is associated with overexpression of ABC/mdr-related proteins, some of which are apparently novel and are likely to include a bile acid transport protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Che M., Gatmaitan Z., Leveille C., Nishida T., St Pierre M. The biology of the bile canaliculus, 1993. Hepatology. 1993 Feb;17(2):318–329. [PubMed] [Google Scholar]
- Becker A., Lucka L., Kilian C., Kannicht C., Reutter W. Characterisation of the ATP-dependent taurocholate-carrier protein (gp110) of the hepatocyte canalicular membrane. Eur J Biochem. 1993 Jun 1;214(2):539–548. doi: 10.1111/j.1432-1033.1993.tb17952.x. [DOI] [PubMed] [Google Scholar]
- Buschman E., Arceci R. J., Croop J. M., Che M., Arias I. M., Housman D. E., Gros P. mdr2 encodes P-glycoprotein expressed in the bile canalicular membrane as determined by isoform-specific antibodies. J Biol Chem. 1992 Sep 5;267(25):18093–18099. [PubMed] [Google Scholar]
- Cao C. X., Silverstein S. C., Neu H. C., Steinberg T. H. J774 macrophages secrete antibiotics via organic anion transporters. J Infect Dis. 1992 Feb;165(2):322–328. doi: 10.1093/infdis/165.2.322. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Chauhan S. S., Pastan I., Gottesman M. M. Regulation of mdr RNA levels in response to cytotoxic drugs in rodent cells. Cell Growth Differ. 1990 Aug;1(8):361–365. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Georges E., Bradley G., Gariepy J., Ling V. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Jan;87(1):152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Homolya L., Holló Z., Germann U. A., Pastan I., Gottesman M. M., Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993 Oct 15;268(29):21493–21496. [PubMed] [Google Scholar]
- Hörtensteiner S., Vogt E., Hagenbuch B., Meier P. J., Amrhein N., Martinoia E. Direct energization of bile acid transport into plant vacuoles. J Biol Chem. 1993 Sep 5;268(25):18446–18449. [PubMed] [Google Scholar]
- Kast C., Stieger B., Winterhalter K. H., Meier P. J. Hepatocellular transport of bile acids. Evidence for distinct subcellular localizations of electrogenic and ATP-dependent taurocholate transport in rat hepatocytes. J Biol Chem. 1994 Feb 18;269(7):5179–5186. [PubMed] [Google Scholar]
- Kioka N., Yamano Y., Komano T., Ueda K. Heat-shock responsive elements in the induction of the multidrug resistance gene (MDR1). FEBS Lett. 1992 Apr 13;301(1):37–40. doi: 10.1016/0014-5793(92)80205-u. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989 Aug 25;264(24):14408–14414. [PubMed] [Google Scholar]
- Müller M., Ishikawa T., Berger U., Klünemann C., Lucka L., Schreyer A., Kannicht C., Reutter W., Kurz G., Keppler D. ATP-dependent transport of taurocholate across the hepatocyte canalicular membrane mediated by a 110-kDa glycoprotein binding ATP and bile salt. J Biol Chem. 1991 Oct 5;266(28):18920–18926. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruetz S., Gros P. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 1994 Jul 1;77(7):1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Schinkel A. H., Roelofs E. M., Borst P. Characterization of the human MDR3 P-glycoprotein and its recognition by P-glycoprotein-specific monoclonal antibodies. Cancer Res. 1991 May 15;51(10):2628–2635. [PubMed] [Google Scholar]
- Shen D. W., Cardarelli C., Hwang J., Cornwell M., Richert N., Ishii S., Pastan I., Gottesman M. M. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986 Jun 15;261(17):7762–7770. [PubMed] [Google Scholar]
- Shneider B. L., Moyer M. S. Characterization of endogenous carrier-mediated taurocholate efflux from Xenopus laevis oocytes. J Biol Chem. 1993 Apr 5;268(10):6985–6988. [PubMed] [Google Scholar]
- Sippel C. J., Suchy F. J., Ananthanarayanan M., Perlmutter D. H. The rat liver ecto-ATPase is also a canalicular bile acid transport protein. J Biol Chem. 1993 Jan 25;268(3):2083–2091. [PubMed] [Google Scholar]
- Smit J. J., Schinkel A. H., Oude Elferink R. P., Groen A. K., Wagenaar E., van Deemter L., Mol C. A., Ottenhoff R., van der Lugt N. M., van Roon M. A. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993 Nov 5;75(3):451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Petrukhin K., Chernov I., Pellequer J. L., Wasco W., Ross B., Romano D. M., Parano E., Pavone L., Brzustowicz L. M. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993 Dec;5(4):344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]