Introduction
The ability to define outcome and thereby design management approaches for rare malignancies is extremely difficult. Soft tissue sarcoma is a group of diverse malignancies with variable presentation, behavior, and outcome. Soft tissue sarcomas are complicated by the fact that they represent at least 80 potentially malignant histological types and subtypes, of these 50% are fully malignant and commonly metastasize and 50% are locally aggressive with some but limited metastasis potential. Most soft tissue sarcomas are believed to be sporadic, however in a small proportion of cases genetic factors, lymphedema, and prior radiation therapy have been identified as predisposing or associated factors. Sarcomas display varying degrees of mesenchymal differentiation, making diagnosis and behavior prediction difficult.1 This problem is compounded by the nature of these diseases, some with early progression, recurrence and demise and others continuing with multiple recurrences or late recurrences over decades.
Prospective databases carefully designed to gather important patient, predisposing, tumor and treatment variables are one approach to allow the definition of prognostic variables for outcome, provide descriptive nature of recurrence and biology, and if followed assiduously over decades, a rich resource for knowledge-based care. In this report we focus on a single institution's prospectively collected large series of patients entered and followed over three decades.
Material and Methods
In July of 1982, we began a prospective inpatient database of all adult (>16 years) patients admitted to our institution for the surgical management of soft tissue sarcoma. This includes patients presenting with primary, locally recurrent or metastatic disease, if they were to undergo a surgical procedure. Patients who were seen in consultation or patients who were never admitted for a surgical event were not included. This was a deliberate decision given the difficulty of following the multitude of patients who were referred either for second opinion and/or outpatient treatment. The decision was made to include inpatients undergoing surgical procedures so there would be ready availability of tissue for clear definition of histological type and subtype. Careful classification of other patient, tumor and treatment characteristics was prospectively recorded. In addition, we were able to obtain at the time of presentation consent for collection of not only tumor but adjacent normal tissue, and in later years blood for germ line analyses. Data is entered and reviewed by the sarcoma disease management team at the time of inpatient admission, initially weekly and then biweekly with all tumor samples classified for histological type, subtype and grade (low versus high) by a dedicated sarcoma pathologist and recorded in an ongoing prospective database. The pathologic features that were used to define grade included mitotic index, necrosis, cellularity, pleomorphism, and histologic type and subtype or differentiation. Snap frozen tissue was banked from surgical specimens and the tissue type and freezer location was recorded in the database and directly linked to patient, tumor, treatment and outcome variables. Patients were followed for local recurrence, systemic recurrence, disease specific and overall survival. An automated system was initiated whereby if a patient entered into the database returns to the institution the follow up note is automatically delivered to the sarcoma data management team. Patients who had no follow up in the six months since last recorded were identified by dedicated reports such that follow up could be obtained in a timely fashion.
Any predisposing or associated factors including underlying genetic diseases such as neurofibromatosis, heritable retinoblastoma or Li-Fraumeni syndrome, or prior history of radiation exposure or the presence of lymphedema all known to be associated with causation are carefully documented. Such databases are, however, not static as new entities are described and histological classification evolves through identification of subtypes that more precisely define tumor biology and patterns of behavior. Recent advances in the molecular, genetic, and cytogenetic characterization of soft tissue sarcoma has improved and refined diagnosis and etiology.
For this presentation we focus on distribution and outcome by body site, size and depth, the influence of grade and histology, and utilization of combined prognostic variables for prediction of outcome. We describe the consequences of local recurrence and the influence of molecular characterization on diagnosis and management.
Results
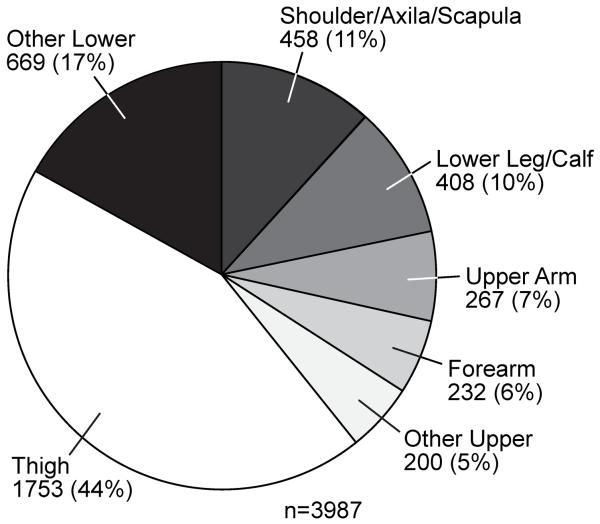
From July 1982 until May of 2013, we entered 10,000 patients into our prospective soft tissue sarcoma database. Approximately 40% of lesions occur in the extremities, 38% in visceral or retroperitoneal areas and the remainder is distributed throughout the body (Table 1). The distribution of lesions throughout the extremities is demonstrated in Figure 1 with the thigh being the dominant site, presumably based on the volume of soft tissue present. Distribution by sex, size, and grade are shown in Table 1. Primary size is not accurately known in some patients who present for a surgical procedure for local or metastatic recurrence. For grade we have excluded gastrointestinal stromal tumors (GIST), which are not graded but characterized by size and mitotic rate.
Table 1.
Distribution of 10,000 soft tissue sarcomas in adult patients admitted for surgery at Memorial Sloan Kettering Cancer Center between 7/1/1982 and 5/31/2013.
| Characteristic | No. of patients (%) | |
|---|---|---|
| Site n=10,000 | ||
| Lower extremity | 2830 (28%) | |
| Visceral | 2213 (22%) | |
| Retroperitoneal/intraabdom inal | 1601 (16%) | |
| Upper extremity | 1157 (12%) | |
| Trunk | 990 (10%) | |
| Other | 1209 (12%) | |
| Histology n=10,000 | ||
| Liposarcoma | 1965 (20%) | |
| Leiomyosarcoma | 1399 (14%) | |
| Undifferentiated pleomorphic sarcoma | 1378 (14%) | |
| Gastrointestinal stromal tumor | 863 (9%) | |
| Synovial | 515 (5%) | |
| Myxofibrosarcoma | 521 (5%) | |
| Fibrosarcoma | 258 (3%) | |
| Malignant peripheral nerve sheath tumor | 256 (2%) | |
| Other | 2845 (28%) | |
| Sex n = 10,000 | ||
| Female | 5088 (51%) | |
| Male | 4912 (49%) | |
| Primary Grade** n= 9076 | ||
| High | 5841 (64%) | |
| Low | 3235 (36%) | |
| Primary Size** n= 9291 | ||
| <=5 cm | 2950 (32%) | |
| 5–10 cm | 2812 (30%) | |
| >=10 cm | 3529 (38%) | |
| Primary Extremity Size** n=3803 | ||
| <=5 cm | 1324 (35%) | |
| 5–10 cm | 1195 (31%) | |
| >=10 cm | 1284 (34%) | |
In patients who present with local or metastatic recurrence, characteristics of size and grade of the primary lesions are not always able to be known.
Figure 1.
Site distribution of the primary lesion for soft tissue sarcoma in the extremities.
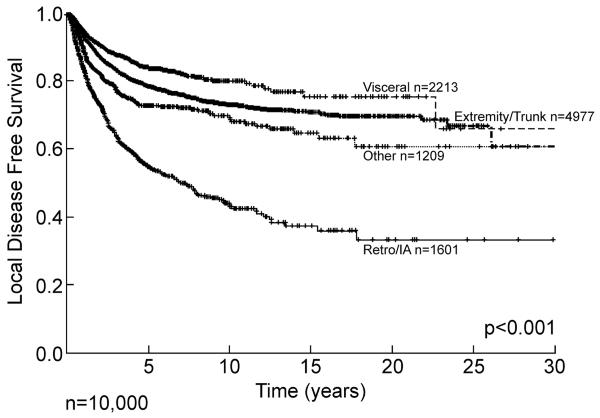
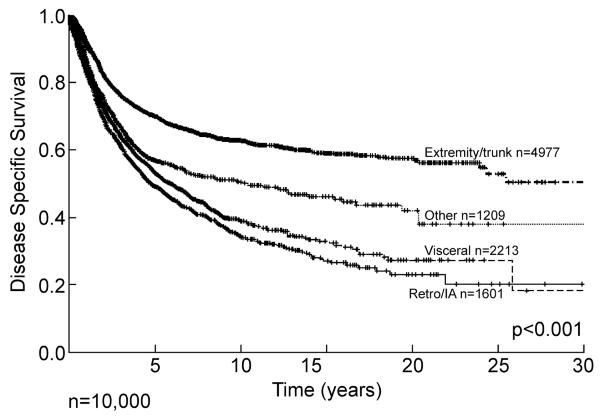
Site is a major determinant of outcome and pattern of recurrence. Retroperitoneal and intraabdominal lesions have a propensity for early local recurrence, with approximately 50% recurring within the first five years, followed by a continuous and relentless progression extending out over 20 years (Figure 2). Extremity and visceral lesions have approximately a 20 to 25% local recurrence rate at 10 years, but in all sites late (>10 years) local recurrence continues to occur emphasizing the need for prolonged follow up, as local recurrence can occur more than two decades from initial diagnosis. These data give an important description, not only of outcome but of biology and patterns of behavior. If we examine disease specific survival, i.e. death from disease, then the pattern is quite different between visceral and extremity lesions (Figure 3). Extremity lesions with a local recurrence rate of approximately 25% at 10 years have a disease specific death prevalence of approximately 40% at 10 years emphasizing the importance of systemic disease as the cause of death. Retroperitoneal and intraabdominal lesions, whose local recurrence is relentless extending out over 15 years and exceeding 60%, has a pattern of disease specific survival that is similar to the pattern of local recurrence, although with some smaller contribution of metastatic disease. Most dramatically, visceral lesions with a local recurrence of less than 20% at 10 years have a disease specific survival of approximately 35% at 10 years emphasizing that the majority of these deaths occur from systemic disease. The database has, in effect, given us information about biological behavior and pattern of recurrence as well as overall outcome, i.e. death for extremity and visceral lesions is predominantly systemic, whereas in retroperitoneal and intraabdominal lesions the dominant cause of disease specific death is local progression albeit often with multifocal local progression.
Figure 2.
Soft tissue sarcoma local disease free survival by site. Hash mark indicates censored patients. IA= intraabdominal, Retro= retroperitoneal
Figure 3.
Soft tissue sarcoma disease specific survival by site. Hash marks indicate censored patients. IA= intraabdominal, Retro= retroperitoneal
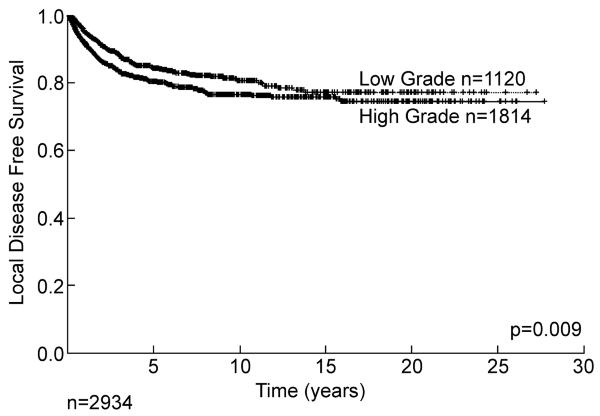
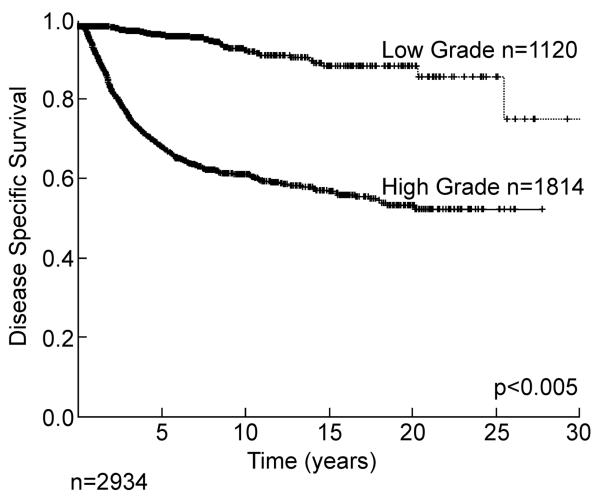
Two of the most important predictors of outcome are histology/histologic subtype and grade. For some sarcoma types, histologic type defines grade with synovial sarcoma, rhabdomyosarcoma, and Ewing sarcoma all classified as high grade. For liposarcoma, histologic subtype defines grade with well-differentiated and myxoid liposarcoma considered low grade and dedifferentiated, round cell, and pleomorphic liposarcomas classified as high grade.2, 3 The distribution between high and low grade (approximately 2:1) is seen in Table 1. Grade is a powerful predictor of outcome. If we examine only those patients presenting with a primary (i.e. treated first at our institution) lesion of the extremity, there was an approximately 20% local recurrence rate at 10 years for both low and high grade lesions (Figure 4), while disease specific survival is highly influenced by the grade of the primary tumor (Figure 5). Systemic recurrence is uncommon, in low grade lesions (<10%) at 20 years, whereas in high grade lesions death from disease is about 40% at 10 years. Again late (>10 year) systemic recurrence and death from disease can occur in both high and low grade lesions. Of interest, many of the initial low grade lesions which do recur systemically may recur with high grade characteristics.
Figure 4.
Local recurrence of primary (i.e. initially untreated) extremity soft tissue sarcoma by grade.
Figure 5.
Disease specific survival of primary extremity soft tissue sarcoma by grade.
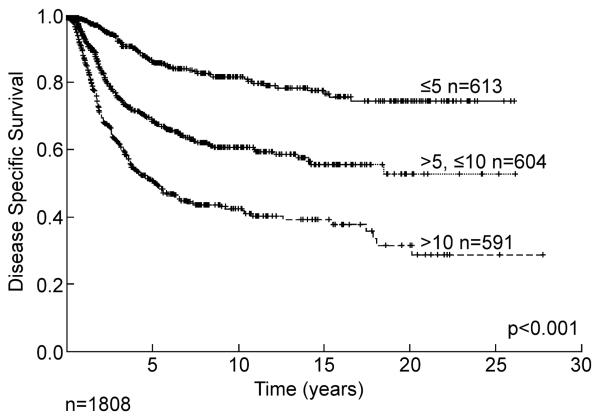
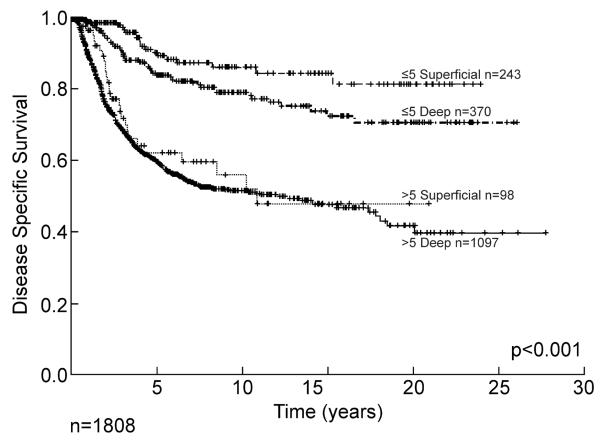
Size and depth are known variables that can influence outcome. These are not necessarily independent as it is unlikely that one would have a large superficial tumor whereas the majority of deep tumors can be expected to be at least 5 cm in maximal dimension. The simple distribution of primary size is shown in Table 1 for all grades and presentations (primary, local and systemic recurrences). Outcome by size alone is shown in Figure 6 for 1,808 patients with high grade tumors. Patients with extremity lesions greater than 10 cm in size have a disease specific survival of less than 40% at 15 years, and late recurrence and death is once again seen. This has important distinctions for staging systems where size is classically divided between <5 cm and >5 cm. The combination of depth and size is illustrated in Figure 7 for primary extremity high grade lesions alone emphasizing the powerful impact of size relatively independent of depth once the lesion reaches 5 cm, and emphasizing the relative rarity of large (>5 cm) superficial lesions where they make up only 8% of all >5 cm lesions.
Figure 6.
Disease specific survival of primary high grade extremity soft tissue sarcoma by size.
Figure 7.
Disease specific survival of primary high grade soft tissue sarcoma by size and depth.
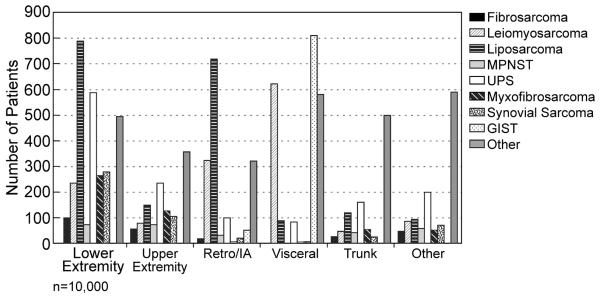
Distribution by histology is shown in (Table 1) and distribution of histopathology by site illustrated in Figure 8, emphasizing the site dependent relationship with histopathology. This is then reflected by the dependence of site of initial or dominant metastasis on the site of the primary lesion (Figure 9). An excellent example is the dominance of lung for primary extremity site, and the predilection of uterine leiomyosarcoma for lung in contradistinction to GIST which is primarily to liver. The implications for surveillance in follow up are obvious.
Figure 8.
Predominant soft tissue sarcoma histopathology by site. MPNST = malignant peripheral nerve sheath tumor, UPS = undifferentiated pleomorphic sarcoma, GIST = gastrointestinal stromal tumor, IA= intraabdominal, Retro= retroperitoneal
Figure 9.
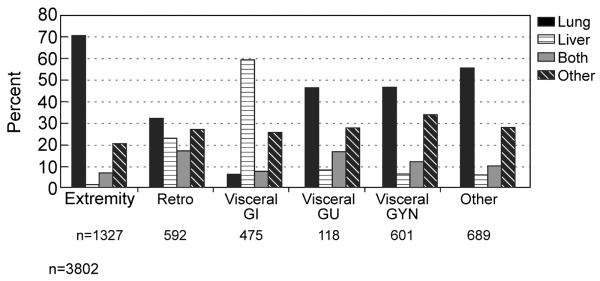
Dominant initial site of metastases dependent on the site of the primary lesion. IA= intraabdominal, Retro= retroperitoneal, GI = gastrointestinal, GU = genitourinary, GYN = gynecological
Histology is a variable that with progressive time and definition becomes more precisely defined. A good example is visceral leiomyosarcoma many of which were reclassified as GIST following the discovery in the late 1990s of activating mutations in KIT and PDGFRA specifically in GIST. This provides awareness that such databases, where the inherent data may change, will become more variable. Such databases must be considered to be living and adapting entities.
The diagnosis of soft tissue sarcoma has been revolutionized by the recognition of molecular diagnostic signatures. This is seen in many of the Ewing family of tumors but also applicable to multiple other histological subtypes.1 A prime example is synovial sarcoma characterized by the t(X;18)(p11.2;q11.2) chromosomal translocation in nearly all cases. Previously thought of as a tumor that affected adolescents and young adults, it is now quite clear that this tumor can occur throughout life (Figure 10). More importantly, in the past regarded as a tumor that occurred in the extremities in and around joints, it is now clear that it is not derived from synovium, although the specific cell type is unknown and the tumor has been identified in multiple other unusual sites such as the head and neck (fewer than 10%), thoracic and abdominal wall (fewer than 10%), or intrathoracic sites. We have identified such lesions in especially unusual sites such as the heart, the prostate and the diaphragm.
Figure 10.
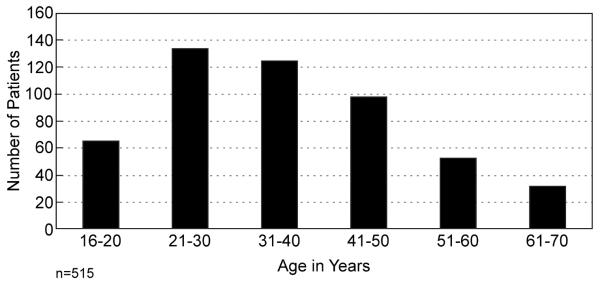
Age distribution of synovial cell sarcoma, for patients ≥16 years.
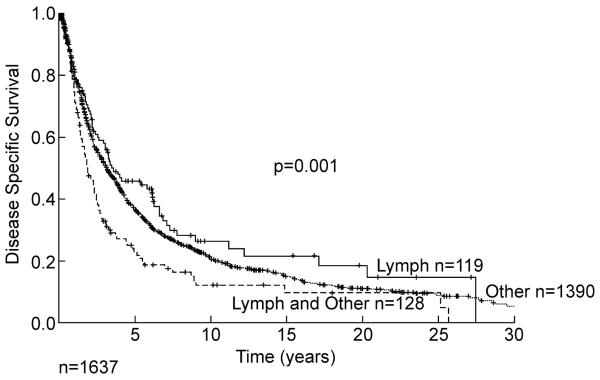
Lymph node metastases in soft tissue sarcoma are rare.4 When lymph node metastasis occurs in the absence of other metastasis, some patients can be rescued (Figure 11). However, combination of the presence of a lymph node metastasis and other metastases is a poor prognostic sign.
Figure 11.
Soft tissue sarcoma disease specific survival by lymph node metastases alone (n=119), or with other metastasis (n=128), and all other metastasis (n=1390).
Discussion
The present report highlights the advantages of having large focused prospective databases in tumor entities that are rare. Such datasets can be utilized not only for the prediction of prognostic factors,3, 5, 6 but can identify varied and variable prognostic events at the time of presentation which influence the type of recurrence, i.e. disease specific survival, metastasis free survival, or overall survival. Most importantly, they provide a stable description of patient specific outcomes in a series of rare diseases, giving a baseline for predicted outcome and risk. Such identification of prospective variables can then be utilized in appropriate stratification in randomized controlled trials.6 We have taken these data to describe patient specific predictive nomograms for outcome.7 The development of such nomograms is absolutely dependent on validation by other databases both nationally8 and internationally.9 Databases are best constructed if the dataset is large, events positive and negative, e.g. alive or dead, relatively equally distributed and the follow up is long. Sarcoma has been an excellent source for such datasets and we have adapted these to predict local recurrences,10 predict survival after local recurrence,11 and apply to unique histopathologies such as liposarcoma,12 GIST,13 synovial sarcoma,14 and the desmoid tumor.15 Such datasets are a rich tool required for development of other mathematical models, utilizing other statistical techniques such as Bayesian Belief Networks.16
An important observation is the value of such databases in defining surveillance in follow up. An awareness of the primary or first site of metastasis, lung as shown for extremity lesions and liver for GIST, can focus follow up imaging to a single site reserving further imaging for those found to be positive in the dominant site.
We have previously commented and documented the consequences of local recurrence. Clearly an early large local recurrence is accompanied by a much poorer prognosis than a late small recurrence, particularly if the initial recurrence is of high grade and the late recurrence is low grade.17 However, as we illustrated here and in a prospective randomized trial,5 diminution of local recurrence does not translate into a disease specific survival benefit, an important principle; local recurrence is associated with, but has limited if any causative effect on disease specific survival.
Molecular diagnosis has been an extraordinary advance as we have demonstrated using synovial sarcoma as an example. Synovial sarcoma, previously thought of as an entity that occurred in adolescents and young adults in and around joints of the extremity, has now been characterized by a recurrent SYT-SSX fusion gene.18 The identification of this fusion gene by molecular diagnosis 19, 20 has resulted, as shown here, in the identification of such tumors in patients throughout life, albeit with a predilection for adolescents and young adults. Not only has the age spectrum been better defined, but unusual sites previously not conceived of as being associated with “synovium” have been characterized. This in itself emphasizes the absence of a knowledge of the cell of origin for “synovial sarcoma” but clearly not from synovium. This identification of molecular signatures in sarcoma and other rare tumors becomes a potential for therapy. If a tumor can be shown to express a mutated gene as an oncogene or tumor suppressor gene such as p53 or an exaggerated expression of a particular gene that acts as a driving mutation, the potential for therapy is obvious. This is well described in the identification of the c-kit mutation in gastrointestinal stromal tumors. The identification of this mutation and the ability to take a tyrosine kinase therapeutic inhibitor such as imatinib transformed the management of these tumors. Historically21 the majority of such patients progressed to early demise. With the advent of a selected directed therapy, not only was survival prolonged in the presence of advanced and metastatic disease,22 but the agent could be utilized in randomized trials as an adjuvant therapy following surgical excision.23 With increasing knowledge it has become clear that each selective drug acted presumably against or at least preferentially against one mutation but not others as is seen in the difference between response to the mutated exon 9 and the mutated exon 11 of the c-kit gene.24, 25
As we have also shown here, such databases can become remarkable tools for examining prognostic indicators. Not only can patient specific nomograms be identified but the dataset can be examined for other associations utilizing such tools as Bayesian Networks.16 In this situation even in the absence of complete data on every patient, one can look for associations mathematically that may not have been anticipated when one thinks purely in the context of prognosis. As we have also shown, these data can provide the basis for predictive tools that can be further examined for the impact of the significance of surface antigens, molecular targets, or even therapy. One can suggest that if the quality of the predictive outcome has very little variance and one intervenes with a therapeutic event, such that the observed outcome far exceeds any confidence limits of the predicted outcome, we can argue for a therapeutic effect. If this could be demonstrated then we would have a tool to utilize in rare and unusual tumors where randomized controlled trials are essentially not possible. Conversely, it does require datasets of rare tumors that are large and followed for a long period of time.
New targeted therapies are desperately needed for the approximately 6,000 patients who die each year in the United States of sarcoma.26 A key challenge will be to identify the alterations that drive sarcomagenesis for each subtype of sarcoma so as to identify subtype specific targets for therapy. To achieve this goal our group performed an integrative analysis of DNA sequence, copy number and mRNA expression in 207 samples encompassing seven major sarcoma subtypes that were linked to the clinical data from the prospective database. Frequently mutated genes included TP53 (17% of pleomorphic liposarcomas), NF1 (10.5% of myxofibrosarcomas and 8% of pleomorphic liposarcomas) and PIK3CA (18% of myxoid/round-cell liposarcomas). PIK3CA mutations in myxoid/round-cell liposarcoma were associated with Akt activation and poor clinical outcomes. CDK4 and YEATS4 were found to be amplified and overexpressed in dedifferentiated liposarcoma and drivers of liposarcoma proliferation in functional screens performed in dedifferentiated liposarcoma cell lines.27 Microarray analysis of liposarcoma samples for gene expression has been linked to the clinical database and used to derive a multi-gene predictor of distant-recurrence free survival and identify genes driving liposarcoma progression.28 This gene expression dataset demonstrated that well-differentiated and dedifferentiated liposarcoma have activation of cell-cycle and checkpoint pathways, including the up-regulation of CDK4, MDM2, CDK1, CDC7, TOP2A, PRC1, PLK1, and cyclins B1, B2, and E2.29, 30. Therefore, these pathways may be useful as therapeutic targets. In fact, nutlin-3a, a selective MDM2 antagonist, induces apoptosis and inhibits proliferation of dedifferentiated liposarcoma cell lines at concentrations that do not affect normal adipose-derived stem cells.29 Furthermore, PD0332991, a selective CDK4/CDK6 inhibitor, inhibits proliferation by inducing G1 cell-cycle arrest and senescence in dedifferentiated liposarcoma cell lines and xenografts.27 In a recent phase II trial of PD0332991 in patients with advanced CDK4-amplified liposarcoma, 66% of patients were progression-free at 12 weeks, and a subset had a radiographic response and prolonged stable disease.31 These results provide a rationale for use of MDM2 antagonists and CDK4 inhibitors in patients with well-differentiated and dedifferentiated liposarcoma and show the value of tissue and cell line resources linked to clinical and pathological factors for the discovery of new subtype specific targeted therapy.
The situation can be expanded to where more extensive genetic or array analysis can then provide further mathematical models32 looking at significant functional events such as copy number alterations or mutations that begin to dissect the activated signaling pathways by which tumors are driven to grow. With that analysis we then begin to see patterns of expression that identify tumors from far different sites, e.g. head and neck versus ovary, which would not have been conceived to be in any way biologically similar. This approach is the next step after the transition from an awareness that in malignancy treatment has become disease specific not organ specific or discipline specific. Now we can conceive different lesions in different sites in the body even with different histological appearance that reveal pathways of progression and growth that can be inhibited independent of the site of origin of the tumor.
Acknowledgments
This data could not have been assembled without the participation and consent of 10,000 patients, the help of numerous colleagues in multiple disciplines, and dedicated data collectors. This manuscript and many others has been a testament to the help of Ms. Victoria Frohnhoefer.
Support: Soft Tissue Sarcoma – P01 CA047179-19
SPORE in Soft Tissue Sarcoma – P50 CA140146-04
KACF Kristen Ann Carr Fund
Norman Morris Fund
Footnotes
Possible Conflict of Interest: Murray F. Brennan, MD – Board member, Ziopharm
References
- 1.Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. International Agency for Research on Cancer; Lyon, France: 2013. [Google Scholar]
- 2.Antonescu CR, Tschernyavsky SJ, Decuseara R, et al. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7:3977–3987. [PubMed] [Google Scholar]
- 3.Singer S, Antonescu CR, Riedel E, et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–370. doi: 10.1097/01.sla.0000086542.11899.38. discussion 370–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong Y, Coit DG, Woodruff JM, et al. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72–77. doi: 10.1097/00000658-199301000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 6.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 7.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 8.Eilber FC, Brennan MF, Eilber FR, et al. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004;101:2270–2275. doi: 10.1002/cncr.20570. [DOI] [PubMed] [Google Scholar]
- 9.Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. 2005;103:402–408. doi: 10.1002/cncr.20778. [DOI] [PubMed] [Google Scholar]
- 10.Cahlon O, Brennan MF, Jia X, et al. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg. 2012;255:343–347. doi: 10.1097/SLA.0b013e3182367aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kattan MW, Heller G, Brennan MF. A competing-risks nomogram for sarcoma-specific death following local recurrence. Stat Med. 2003;22:3515–3525. doi: 10.1002/sim.1574. [DOI] [PubMed] [Google Scholar]
- 12.Dalal KM, Kattan MW, Antonescu CR, et al. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244:381–391. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045–1052. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canter RJ, Qin LX, Maki RG, et al. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res. 2008;14:8191–8197. doi: 10.1158/1078-0432.CCR-08-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crago AM, Denton B, Salas S, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg. 2013;258:347–353. doi: 10.1097/SLA.0b013e31828c8a30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsberg JA, Healey JH, Brennan MF. A probabilistic analysis of completely excised high-grade soft tissue sarcomas of the extremity: an application of a Bayesian belief network. Ann Surg Oncol. 2012;19:2992–3001. doi: 10.1245/s10434-012-2345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237:218–226. doi: 10.1097/01.SLA.0000048448.56448.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawai A, Woodruff J, Healey JH, et al. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med. 1998;338:153–160. doi: 10.1056/NEJM199801153380303. [DOI] [PubMed] [Google Scholar]
- 19.Ladanyi M. Fusions of the SYT and SSX genes in synovial sarcoma. Oncogene. 2001;20:5755–5762. doi: 10.1038/sj.onc.1204601. [DOI] [PubMed] [Google Scholar]
- 20.Ladanyi M. Correlates of SYT-SSX fusion type in synovial sarcoma: getting more complex but also more interesting? J Clin Oncol. 2005;23:3638–3639. doi: 10.1200/JCO.2005.05.379. author reply 3639–3640. [DOI] [PubMed] [Google Scholar]
- 21.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422–429. doi: 10.1097/SLA.0b013e3182a15eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMatteo RP. The GIST of targeted cancer therapy: a tumor (gastrointestinal stromal tumor), a mutated gene (c-kit), and a molecular inhibitor (STI571) Ann Surg Oncol. 2002;9:831–839. doi: 10.1007/BF02557518. [DOI] [PubMed] [Google Scholar]
- 25.Antonescu CR, Sommer G, Sarran L, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003;9:3329–3337. [PubMed] [Google Scholar]
- 26.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 27.Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gobble RM, Qin LX, Brill ER, et al. Expression profiling of liposarcoma yields a multigene predictor of patient outcome and identifies genes that contribute to liposarcomagenesis. Cancer Res. 2011;71:2697–2705. doi: 10.1158/0008-5472.CAN-10-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer S, Socci ND, Ambrosini G, et al. Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcoma. Cancer Res. 2007;67:6626–6636. doi: 10.1158/0008-5472.CAN-07-0584. [DOI] [PubMed] [Google Scholar]
- 30.Ugras S, Brill E, Jacobsen A, et al. Small RNA sequencing and functional characterization reveals MicroRNA-143 tumor suppressor activity in liposarcoma. Cancer Res. 2011;71:5659–5669. doi: 10.1158/0008-5472.CAN-11-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickson MA, Tap WD, Keohan ML, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31:2024–2028. doi: 10.1200/JCO.2012.46.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciriello G, Miller ML, Aksoy BA, et al. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45:1127–1133. doi: 10.1038/ng.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]