Abstract
Smallpox is a deadly and debilitating disease that killed hundreds of millions of people in the past century alone. The use of vaccinia-virus based smallpox vaccines led to the eradication of smallpox. These vaccines are remarkably effective, inducing the characteristic pustule or “take” at the vaccine site in > 97% of recipients, and inducing a wide spectrum of long-lasting humoral and cellular immune responses. The mechanisms behind inter-individual vaccine response variability are likely to involve host genetic variation, but have not been fully characterized. We report here the first smallpox vaccine-response genome-wide association study of over 1,000 recent recipients of Dryvax®. The data presented here focus on cellular immune responses as measured by both production of secreted IFNγ and quantitation of IFNγ secreting cells by ELISPOT assay. We identified multiple significant SNP associations in genes (RASA1, ADRA1D, TCF7L1, FAS) that are critical components of signaling pathways that directly control lymphocyte IFNγ production or cytotoxic T cell function. Similarly, we found many associations with SNPs located in genes integral to nerve cell function; findings that, given the complex interplay between the nervous and immune systems, deserve closer examination in follow-up studies.
Keywords: smallpox vaccine, vaccinia virus, genome-wide association study, single nucleotide polymorphism, interferon-gamma
Introduction
Although remarkably effective, the smallpox vaccine can result in serious, life-threatening adverse events (Fulginiti 2003; Fulginiti et al. 2003). This risk, as well as the large number of contraindications (immunosuppression, organ transplants, HIV, heart conditions, skin diseases) in the current U.S. population, makes it difficult to prepare and deploy the vaccine in the face of bioterroristic threats. As stocks of the classical smallpox vaccines used during the eradication effort are dwindling, next-generation replacement vaccines are being researched, tested, and stockpiled (Artenstein and Grabenstein 2008). Smallpox vaccines are remarkably effective, inducing the characteristic pustule or “take” at the vaccine site in > 97% of recipients, and eliciting a wide spectrum of long-lasting immune responses, both humoral and cellular (Combadiere et al. 2004; Crotty et al. 2003; Frey et al. 2002; Hammarlund et al. 2003; Kennedy et al. 2009b). The mechanisms behind inter-individual vaccine response variability are currently unknown, but are likely to involve genetic variation in both the host and the virus. Given renewed interest in biodefense preparations to counter potential use of smallpox poxviruses as biological weapons, and to protect against emerging poxviruses such as monkeypox, it is important to understand how host genetic polymorphisms affect immune responses to smallpox vaccine. To date there have only been a few smallpox vaccine immunogenetic studies. A candidate gene study by Stanley et al. identified SNPs in cytokine genes associated with fever, and another candidate gene study by Reif et al. focused on genetic associations with adverse events after vaccination (Crowe 2007; Reif et al. 2008; Stanley et al. 2007). We have also reported candidate gene association studies examining the role of HLA genotypes in adaptive responses to smallpox vaccine (Ovsyannikova et al. 2011) and identifying IL18 and IL18R as loci involved in humoral immunity to smallpox vaccine (Haralambieva et al. 2011).
In this study we recruited a cohort of more than 1,000 recent smallpox vaccine recipients and conducted genome-wide SNP typing and a detailed examination of their cellular immune responses. To our knowledge, the data presented here are the first reported findings of a genome-wide association study on smallpox vaccine responses.
Materials and Methods
Subject Recruitment
The subjects described in this report came from a previously described study cohort consisted of 1,076 healthy subjects. Inclusion criteria included: 1) having received one, and only one, dose of Dryvax® smallpox vaccine within the four years prior to enrollment, 2) have a documented vaccine “take” indicating successful immunization (no additional information regarding vaccine take was available), 3) have never received a prior smallpox vaccination, 4) been in good general health at the time of the blood draw. These subjects (ages 18-40 at enrollment) had received the smallpox immunization as part of the civilian healthcare worker immunization program at Mayo Clinic in Rochester, MN, or the smallpox immunization program involving armed forces personnel at the Naval Health Research Center (NHRC) in San Diego, CA (Kennedy et al. 2009a) (Haralambieva et al. 2011; Ovsyannikova et al. 2011). The Institutional Review Boards of the Mayo Clinic and NHRC approved the study and written informed consent was obtained from each subject. Demographic information regarding this cohort has been previously published (Kennedy et al. 2009c).
Isolation of peripheral blood mononuclear cells (PBMC)
100 mL of whole blood was collected from each subject and PBMC were isolated within 24 hours by density gradient centrifugation according to standard protocols as previously described (Ryan et al. 2009). Isolated PBMC were resuspended at a concentration of 1 × 107 cells/mL in RPMI 1640 media containing l-Glutamine (Invitrogen, Carlsbad, CA) supplemented with 10% dimethyl sulfoxide (Protide Pharmaceuticals, St. Paul, MN) and 20% fetal bovine serum (FBS; Hyclone, Logan, UT), frozen overnight at − 80°C and transferred to liquid nitrogen for long-term storage.
PBMC aliquots were thawed and rested as previously described (Ovsyannikova et al. 2005; Ryan et al. 2009). Briefly, cells were thawed and incubated overnight in culture medium containing 50 IU/ml of IL-2 (Proleukin®, Chiron, Emeryville, CA). Cells were then collected, washed, and resuspended in culture medium at a concentration of 2 × 106 cells/mL for use in both the ELISPOT and ELISA assays described below.
SNP typing and QC
DNA was extracted from whole blood or blood clots using the Gentra Puregene Blood kit (Gentra Systems Inc., Minneapolis, MN) and quantified by Picogreen (Molecular Probes, Carlsbad, CA). High density SNP analysis was performed using the Infinium HumanHap550 BeadChip array (Illumina, San Diego, CA) for the self-declared Caucasian subjects (n=580) and the 650K Infinium HumanHap650Y SNP BeadChip array for the subjects indicating their race as either African-American or unknown (n=217). DNA samples underwent amplification, fragmentation and hybridization onto each BeadChip, which were imaged on an Illumina BeadArray reader. Genotype calls based on clustering of the raw intensity data were made using BeadStudio 2 software. The resulting genotype data on SNPs were exported into SAS for analysis. Quality-control checks included genotyping reproducibility, gender checks, SNP and subject call-rate cutoffs of > 0.95, elimination of monomorphic SNPs, and a Hardy-Weinberg Equilibrium (HWE) check (SNPs with p<1e−8 were flagged as having poor genotyping quality). This test for HWE accounted for the fact that subjects were recruited from two distinct racial groups using a large sample approximation to the stratified test of Schaid, et al (Schaid et al. 2006).
ELISPOT Assays
Total human IFN-γ and CD8+ IFNγ ELISPOT assays (R & D Systems, Minneapolis, MN) were performed using PBMC cultures as previously described (Ovsyannikova et al. 2011; Ryan et al. 2005). Briefly, cell cultures were stimulated with inactivated vaccinia virus at an MOI of 5 for 24 hours. Three stimulated and three unstimulated replicate wells were set up for each subject. A single well containing PHA (5μg/ml) was used as a positive control. After a 24-hr incubation at 37°C in 5 % CO2, each plate was washed and incubated with biotinylated detection antibody followed by chromogenic substrate according to the manufacturer’s specifications. Plate scanning and spot counting on each plate was performed on an ImmunoSpot® S4 Pro Analyzer (Cellular Technology Ltd., Cleveland, OH) equipped with ImmunoSpot® version 4.0 software (Cellular Technology Ltd.). Counting parameters were consistent across all plates. Results are presented in spot-forming units (SFU) per well (200,000 cells).
IFNγ ELISA Assay
PBMCs were placed in 96-well at 2 × 105 cells per well. Experimental conditions for each subject were as follows: a PHA (5ug/ml) positive control, triplicate wells containing culture medium (unstimulated wells), and triplicate wells containing inactivated vaccinia virus (stimulated wells). Inactivated vaccinia virus (NYCBOH strain) was added at an MOI of 0.05 for four days following optimized procedures as described (Earl et al. 2001; Kennedy et al. 2009a; Ryan et al. 2009).
Commercial ELISA-based kits (BD Pharmingen) were used to detect IFNγ from culture supernatants according to the manufacturer’s instructions. Reference standards, included in each assay, were used to calculate the concentration of each cytokine.
Principal Components Analysis of Race and Ethnicity
The sampling plan for this study called for the enrollment of both Caucasian and African-Americans. We identified 22,863 SNPs in low linkage disequilibrium with one another that spanned the genome at regular intervals with a density of less than one SNP per 100kb. We used these SNPs in the EIGENSOFT software package (http://www.hsph.harvard.edu) to assess population structure both across and within racial groups (Price et al. 2006). In addition to using the Eigenstrat axes of variation to describe population genetic structure, we used the genotype data to assign racial groups via a clustering procedure using the Structure program (Pritchard et al. 2000). The Eigenstrat axes of variation were also used to adjust for population stratification within each racial group.
Statistical analyses
Assessment of cytokine secretion and ELISPOT levels included data from the triplicate unstimulated wells and the triplicate vaccinia-stimulated wells as previously described (Ryan et al. 2009). The data were condensed to a single response measurement per individual; the difference between the median of the three unstimulated and the median of the three stimulated values. Data summaries were subsequently made using frequencies and percents for categorical variables, while both medians and inter-quartile ranges (IQRs) were utilized for continuous variables.
Associations between the two IFN-γ measures and the SNP genotypes were formally evaluated using linear regression models. Within the Caucasian and the African-American genetic subgroups we performed separate analyses for each outcome. The primary analyses of the SNPs assessed the evidence for an additive genetic effect on the outcome of interest. In contrast to the descriptive analyses, formal evaluations used repeated measures analyses to simultaneously model the multiple ELISPOT measures per subject. Recognizing that correlations likely exist across the multiple measures within an individual, we used generalized estimating equations (GEEs) to build this correlational structure into our models. Associations comparing stimulation-induced differences in cytokine secretion or ELISPOT values with SNPs were evaluated by testing the significance of the SNP by stimulation status interaction. These repeated measures models are analogous to paired t-tests, as they compare differences between the stimulated and unstimulated states within each individual, but the results are aggregated among all subjects with genotype and phenotype data. Statistical analyses were performed after adjusting for the following set of demographic and clinical variables: gender; age at blood draw (quartiles); time from smallpox immunization to blood draw (quartiles); time from blood draw to assay (quartiles); shipping temperature of the sample (frozen or ambient); time of year when the sample was shipped (warm-weather months April-September vs. cold-weather months October-March); and the first four Eigenstrat axes of variation. In addition, associations of SNP genotypes with ELISPOT values included assay operator as a covariate due to systematic differences observed for these measures across the different operators. Skewness in the measured outcomes was corrected using an inverse cumulative normal (probit) transformation. Quantile-quantile (q-q) plots were generated to compare the observed distribution of p-values from all SNPs for each outcome to the expected distribution under the null hypothesis of no association. Lambda values that measure the inflation of significance due to population stratification or other causes were extracted and used to correct the observed p-value distributions when necessary. Statistical tests were performed using two-sided significance levels and primary analyses were performed using the R software package (Team 2008). All significant SNP associations are shown in Tables 1-6, however, several of these SNPs had very few subjects that were homozygous for the minor allele. In order to determine if the results from these individuals exerted undue influence on the overall association, their data were combined with that from subjects with one copy of the minor allele, and an additional series of models were run with the same adjusting factors as the ordinal model. The resulting estimates from these sensitivity analyses were compared to the per-allele estimates from the original analyses to verify their similarity. The SNPs are marked with an asterisk next to the rsid number in each table. The table legend contains the sensitivity p-values for each of these SNPs.
Table 1. Racial breakdown of study cohort and immune outcome summaries.
Columns indicate the racial assignment by principal components analysis (PCA). This analysis used the genotyping data from the HumanHap550 BeadChip and the 650K Infinium HumanHap650Y SNP BeadChip to assign a genetically determined race to each subject. Self reported race is indicated in the top section of the table. Summary immune outcomes for each racial subcohort are indicated in the next rows of the table.
| African-American | Caucasian-American | |||
|---|---|---|---|---|
| N | % | N | % | |
| Self-Declared Race | ||||
| African American | 177 | 86 | 0 | 0.0 |
| Caucasian | 0 | 0.0 | 486 | 91 |
| More than one race | 17 | 8 | 32 | 6 |
| Other or do not know | 13 | 6 | 14 | 3 |
|
| ||||
| Mean | St. Dev. | Mean | St. Dev. | |
|
Total ELISPOT (SFU/200,000 cells) |
60.2 | 60.0 | 66.6 | 60.9 |
| Median | Q1-Q3 | Median | Q1-Q3 | |
| 51 | 23-84 | 56 | 28-95 | |
|
| ||||
| Mean | St. Dev. | Mean | St. Dev. | |
|
CD8 T cell ELISPOT (SFU/200,000 cells) |
9.2 | 38.2 | 17.9 | 35.2 |
| Median | Q1-Q3 | Median | Q1-Q3 | |
| 4 | −7-17.5 | 12 | 0-32 | |
|
| ||||
| Mean | St. Dev. | Mean | St. Dev. | |
|
IFNγ ELISA (pg/ml) |
909.9 | 1685.1 | 853.3 | 1621.1 |
| Median | Q1-Q3 | Median | Q1-Q3 | |
| 271.1 | 12.1-1224.7 | 310.7 | 28.3-1305.6 | |
Table 6. SNPs showing significant association with secreted IFNγ in the African-American cohort.
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| rs7094333* | 10 | HPSE2 | intron | 0 | 11 | AA | 186 | 295.1 (20.2,1291.8) | 1.04E-08 |
| AG | 9 | −27.6 (−171.5,21.3) | |||||||
| GG | 1 | −652.8 (−652.8,−652.8) | |||||||
|
| |||||||||
| rs1437635 | 11 | SOX6 | intron | 0 | 11 | CC | 185 | 245.7 (0,1153) | 2.51E-08 |
| CA | 11 | 516.4 (277.4,4058.7) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs3179690 | 1 | UBXD3 | 3′UTR | 0 | 15 | GG | 181 | 289.9 (20,1298.7) | 3.11E-08 |
| GA | 15 | −31.2 (−160.4,728) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs1453654 | 11 | OR10G6 | 3′downstream | 557 | 12 | GG | 185 | 289.9 (19.1,1298.7) | 1.41E-07 |
| GA | 10 | 20.4 (−218.6,304.5) | |||||||
| AA | 1 | −652.8 (−652.8,−652.8) | |||||||
|
| |||||||||
| rs11216816 | 11 | AMICA1 | intron | 0 | 63 | CC | 138 | 361.4 (34.3,1321) | 4.50E-07 |
| CA | 53 | 131.2 (−124.7,1059.1) | |||||||
| AA | 5 | 12.1 (−149.4,190.1) | |||||||
|
|
|||||||||
| LOC100128245 | 5′upstream | 5799 | 63 | CC | 138 | 361.4 (34.3,1321) | 4.50E-07 | ||
| CA | 53 | 131.2 (−124.7,1059.1) | |||||||
| AA | 5 | 12.1 (−149.4,190.1) | |||||||
rs SNP identification number
Chromosomal location of the indicated SNP
Gene or genetic region containing the indicated SNP
Location of the SNP relative to the gene
Minor Allele Frequency
Number of subjects with a given genotype
Median outcome measurement for each genotype group. Results expressed as pg/ml. The interquartile range is shown in parentheses
P-values were adjusted for demographic and clinical variables as well as inflation of significance described in the Methods.
Sensitivity analysis p-values for rs7094333 = 1.11E-05.
Results
SNP Typing Results
All individuals were typed using either the Illumina Infinium HumanHap550 or 650Y BeadChip arrays. Initial QC steps were used to remove suspect SNPs such as those with poor clustering, monomorphic SNPs, or those that failed HWE. Seventy-one subjects with low quality genotyping (call rates < 95%) were also excluded. Overall genotype concordance was 97.9% (99.9% when excluding non-called genotypes) indicating good reproducibility. PLINK genome-wide association study (GWAS) software was used to compute the inbreeding coefficients on the × chromosome for all subjects. This assessment identified three individuals who appeared female, but reported male gender on the study intake forms. Further analysis of the genotyping data indicated call rates for two of the subjects were low, making this assessment inconclusive. The third individual was found to be an XXY male with Klinefelter’s syndrome. Pair-wise identity by descent metric was used to measure the degree of relatedness between individuals and identified 22 pairs of individuals whose genotypes were consistent with being siblings. In addition, three pairs of individuals appeared to be genetically identical or genetically similar; both individuals from one of these pairs were excluded from analysis, as was one individual from each of the other two pairs. SNP call rates for the 550K beadchip ranged from 36.3% to 100%, with a mean call rate of 98.2% (650K beadchip call rates were similar). The SNPs (34,751 for 550K, 48,336 for 650K) with call rates < 95% were excluded from further analysis. We also assessed the call rates for each individual, which ranged from 50.5% to 99.98%, with an overall mean call rate of 98.6%. In addition, monomorphic SNPs (29 for 550K, 34 for 650K) were identified and excluded. HWE checks were performed and the 151 failing SNPs (714 for the 55K chip, 728 for the 650K beadchip) were removed from subsequent analysis. We also excluded SNPs for a given outcome (CD8+ or total IFNγ ELISPOT) and racial cohort (Caucasian or African-American) if there were fewer than 10 observed minor alleles.
These initial QC checks resulted in a dataset for 1,000 subjects containing 525,972 SNPs for the Infinium HumanHap500 array and 611,820 SNPs for the Infinium HumanHap650Y array.
Population Stratification and Principal Components Analysis (PCA)
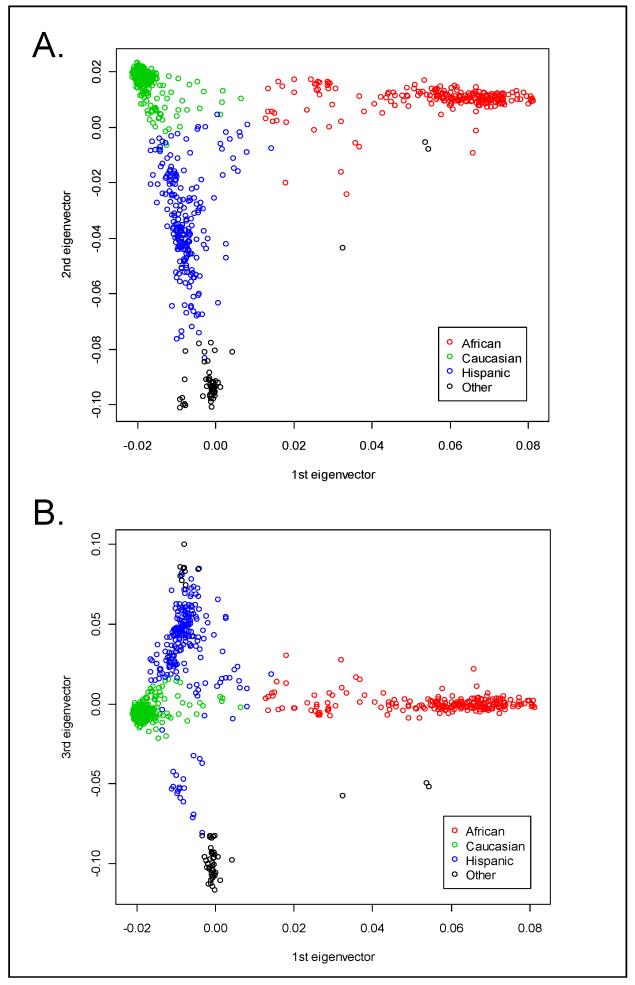
Upon recruitment, each subject’s self-identified race and ethnicity was recorded. Our cohort consisted of 1,000 subjects with demographics as outlined in Table 1. Notably, 45 (4.2%) subjects were unsure of their ethnicity and 243 (22.6%) subjects did not list a race (having chosen: “More than one race”, “Other”, or “Don’t Know”). Given the availability of high-density SNP data, we explored the use of PCA results to assign race and ethnicity to each individual (Figure 1). The genetic racial classifications were in perfect agreement with self-declared race for those who had self-declared as either African or Caucasian American (Table 1), and the genetic data classified 30, and 46, additional subjects as being of African, and Caucasian, descent, respectively. Because of the strong concordance between genetic and self-declared race among those with unambiguous self-declaration, and because the genetic classification captured additional subjects for analysis, we used the genetically defined racial groupings as we ran the GWAS separately by race.
Figure 1. Plots of genetic similarity according to Eigenstrat-derived PCA-based axes of genetic variation.
Highlighted clusters represent racial/ethnic groupings that are consistent with self-declared racial or ethnic group (Red = African-American, Green = Caucasian, Blue = Hispanic, Black = Other). The axes represent the eigenvectors explaining the greatest amount of average genetic sharing using data from 22,863 independent SNPs.
Immune Outcomes
The cellular immune responses for each subject were quantified by IFNγ ELISPOT assays using both total PBMCs and CD8+ purified T cells, and by measuring IFNγ secretion by ELISA. Each outcome was calculated by subtracting the median results in the unstimulated wells from the median results in the vaccinia-stimulated wells. After subtracting the background IFNγ response in each subject, our population (n=987) had a mean total IFNγ ELISPOT responses of 61.1 IFNγ spot-forming units/200,000 cells with an interquartile range of 24.0 to 88.0. For the CD8+ IFNγ ELISPOT responses, our population (n=935) had a mean response of 15.2 IFNγ spot-forming units/200,000 cells with an interquartile range of −2.0 to 26.0. For both ELISA and ELISPOT results, negative numbers indicate that background levels of cytokine secretion or spot-forming cells were higher than the results from the wells containing virus-stimulated cells, perhaps due to viral-induced immune suppression. The larger IFNγ response seen with total PBMCs is an indicator of strong CD4+ T cell and NK cell activity upon viral stimulation (Demkowicz et al. 1996; Mitra-Kaushik et al. 2007; Puissant-Lubrano et al. 2010). Table 1 shows the vaccinia-specific responses for each immune outcome for both the African American and Caucasian subjects. In the African American cohort, the total ELISPOT median(IQR) was: 46.0 (22.0-96.0) in the unstimulated wells and 112.0 (63.0-175.0) in the stimulated wells. For these same subjects the CD8 ELISPOT median(IQR) was 62.5 (27.5-107.0) unstimulated and 70.0 (31.5-120.5) stimulated, while the IFNγ ELISA median(IQR) was 268.9 (77.1-840.5) unstimulated and 687.6 (177.4-2,047.2) stimulated. In the Caucasian cohort, the total ELISPOT median(IQR) was: 48.0 (21.0-97.0) in the unstimulated wells and 121.0 (72.0-196.0) in the stimulated wells. For these same subjects the CD8 ELISPOT median(IQR) was 49.0 (23.0-99.5) unstimulated and 70.0 (37.5-126.0) stimulated, while the IFNγ ELISA median(IQR) was 211.2 (67.3-687.4) unstimulated and 639.2 (193.7-2,144.3) stimulated.
Genome Wide Analysis Results
The association analyses were conducted independently on the Caucasian and the African-American cohorts and are reported separately here. Although we are currently recruiting a second cohort of 1,000 vaccine recipients for true replication purposes, we conducted a preliminary comparison of the statistically significant SNPs in each race with the results from the other racial group.
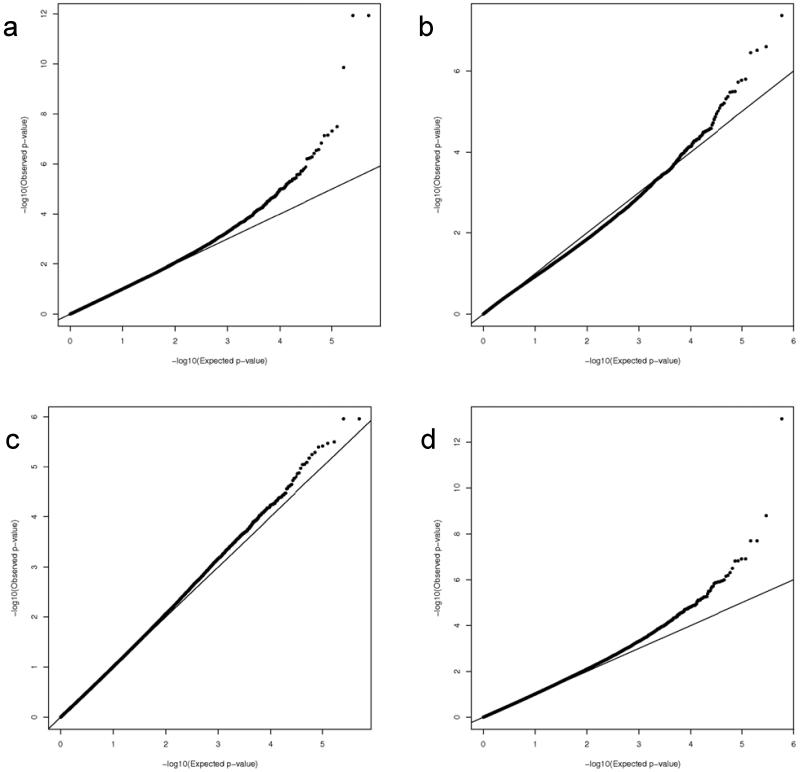
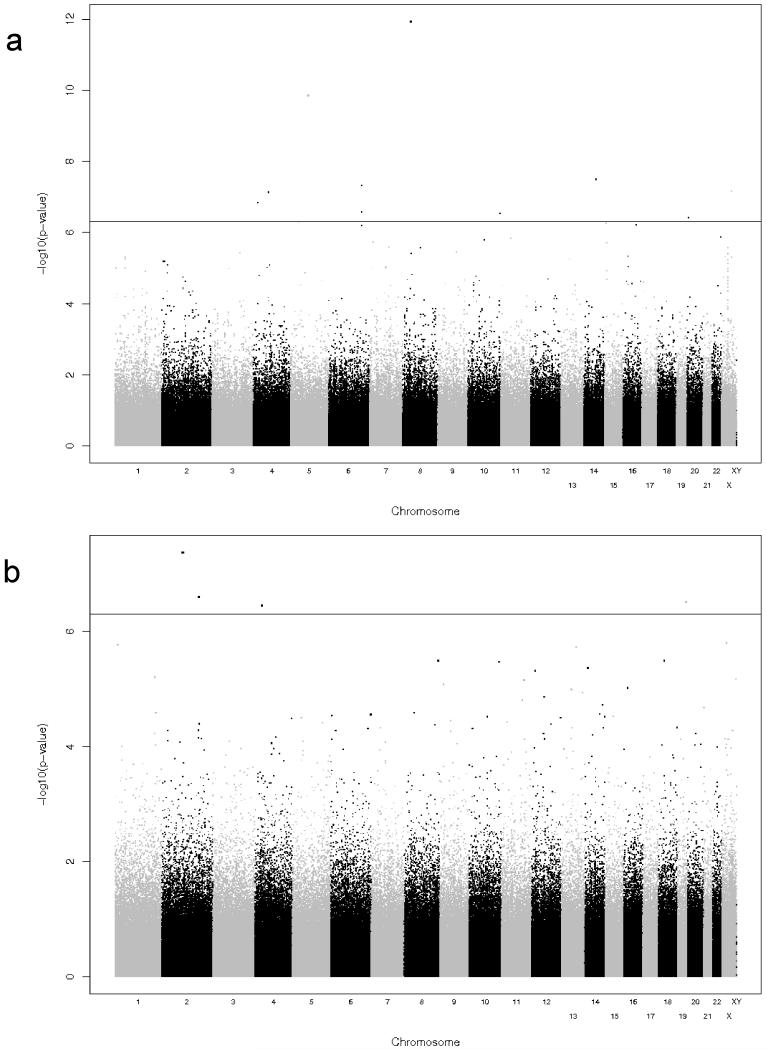
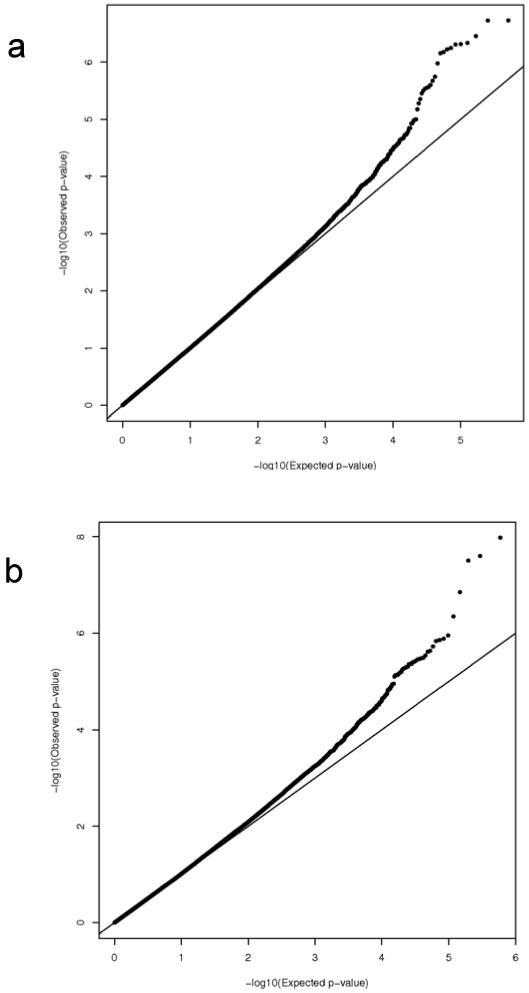
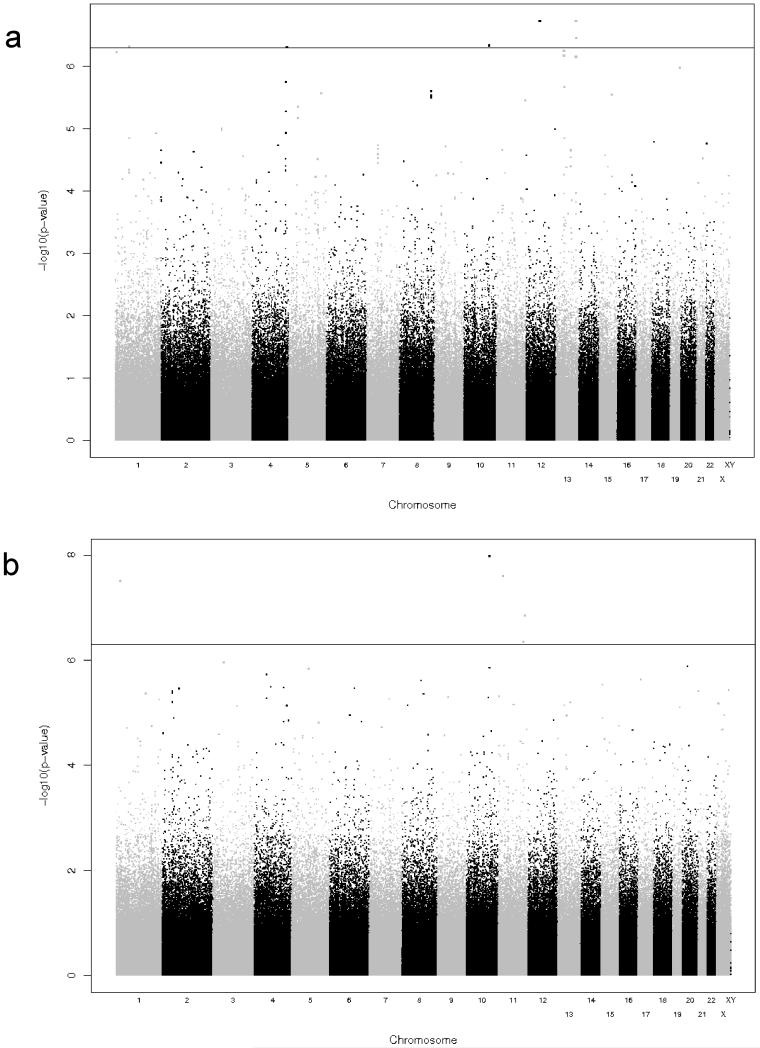
Depicted in Figure 2 are the Q-Q plots for the two sub-cohorts (Caucasian and African-American) and the two outcomes (total and CD8+ IFNγ ELISPOT). We observed a slight inflation of significance in the overall distribution of p-values, and therefore applied genomic control approaches to adjust for this inflation (inflation factor estimates for the African-American subjects are: 1.027 for the total and 1.151 for the CD8+ ELISPOT outcomes, inflation factor estimates for the Caucasian subjects are: 1.019 for the total ELISPOT and 1.032 for the CD8+ ELISPOT outcomes). All reported p-values are corrected for this genomic control inflation. In the resulting Q-Q plots, as expected, the majority of the corrected p-values fall on the slope line, with an upward trend at the low p-value end. Similarly, Figures 3 and 4 contain the Manhattan plots showing the chromosomal distribution and p-values of each SNP association with the indicated outcomes. Interesting regions, such as on chromosome 10 in Figure 4B, are discussed below.
Figure 2. Quantile-Quantile plots of the expected (x-axis) and observed (y-axis) −log10(p-value) in our smallpox vaccine response GWAS.
A) Results for the Caucasian cohort and CD8+ IFNγ ELISPOT outcome. B) Results for the African-American cohort and CD8 IFNγ ELISPOT outcome. C) Results for the Caucasian cohort and total IFNγ ELISPOT outcome. D) Results for the African-American cohort and total IFNγ ELISPOT outcome.
Figure 3. Summary of GWAS results for cellular immune responses as measured by CD8+ IFNγ ELISPOT.
The y-axis displays the –log10 of the p-value for each SNP association and the x-axis displays the chromosomes in alternating black and gray. P-values were adjusted for demographic and clinical variables as described in the Methods. A) Results for Caucasian cohort. B) Results for African-American cohort.
Figure 4. Summary of GWAS results for cellular immune responses as measured by Total IFNγ ELISPOT.
The y-axis displays the −log10 of the p-value for each SNP association (please note that the scale on the two plots are not equal) and the x-axis displays the chromosomes in alternating black and gray. P-values were adjusted for demographic and clinical variables as described in the Methods. A) Results for Caucasian cohort. B) Results for African-American cohort.
Genetic Associations with CD8+ T cell IFNγ ELISPOT responses
Our genome-wide analyses found evidence for multiple SNPs (n = 25) significantly associated with variations in cytotoxic T cell responses to smallpox vaccine as measured by CD8+ IFNγ ELISPOT. Table 2 lists each of the SNPs in the Caucasian sub-cohort with a p-value < 5.0 × 10−7, while Table 3 lists the significant SNPs in the African-American sub-cohort.
Table 2. SNPs showing significant association with CD8+ IFNγ ELISPOT in the Caucasian cohort.
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| rs1110820 | 8 | PSD3 | 3′downstream | 24512 | 10 | GG | 472 | 13 (0,34.3) | 1.15E-12 |
| GA | 10 | −2 (−5,6.3) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs4921930 | 8 | PSD3 | 3′downstream | 28916 | 10 | AA | 472 | 13 (0,34.3) | 1.15E-12 |
| AG | 10 | −2 (−5,6.3) | |||||||
| GG | 0 | (,) | |||||||
|
| |||||||||
| rs6890495 | 5 | RASA1 | 5′upstream | 39836 | 13 | GG | 469 | 13 (0,35) | 1.39E-10 |
| GA | 13 | 2 (−12,9) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs10138587 | 14 | RGS6 | 5′upstream | 49574 | 13 | GG | 468 | 13 (0,34.3) | 3.18E-08 |
| GA | 13 | 0 (−1,11) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs6570670 | 6 | TRNAQ-UUG | 5′upstream | 136541 | 16 | AA | 464 | 13 (0,35) | 4.74E-08 |
| AC | 16 | −2 (−11.8,9.3) | |||||||
| CC | 0 | (,) | |||||||
|
| |||||||||
| rs10218219 | X | PLS3 | intron | 0 | 18 | GG | 455 | 13 (0,33.5) | 6.92E-08 |
| GA | 18 | 3.5 (−6.8,10.5) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs2645668 | 4 | SEPT11 | intron | 0 | 26 | AA | 449 | 13 (0,35) | 7.25E-08 |
| AG | 26 | 1.8 (−12.3,8.8) | |||||||
| GG | 0 | (,) | |||||||
|
|
|||||||||
| AF357530 | 5′upstream | 9477 | 26 | AA | 449 | 13 (0,35) | 7.25E-08 | ||
| AG | 26 | 1.8 (−12.3,8.8) | |||||||
| GG | 0 | (,) | |||||||
|
| |||||||||
| rs16894201 | 4 | LOC729006 | 3′downstream | 34507 | 12 | CC | 470 | 13 (0,34.8) | 1.45E-07 |
| CA | 12 | 3.5 (−15.3,10.6) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs1953793 | 6 | UTRN | 3′downstream | 113196 | 15 | AA | 466 | 13 (0,34.8) | 2.63E-07 |
| AG | 15 | 2 (−2.5,9.5) | |||||||
| GG | 0 | (,) | |||||||
|
| |||||||||
| rs12764951* | 10 | TCERG1L | 3′downstream | 42865 | 11 | GG | 471 | 13 (0,34.5) | 2.84E-07 |
| GA | 9 | 1 (−2,6) | |||||||
| AA | 1 | −7 (−7,−7) | |||||||
|
| |||||||||
| rs6052456 | 20 | ADRA1D | intron | 0 | 220 | GG | 284 | 14.5 (1,41) | 3.75E-07 |
| GA | 166 | 10 (−2,29) | |||||||
| AA | 27 | 5 (−2,15.5) | |||||||
rs SNP identification number
Chromosomal location of the indicated SNP
Gene or genetic region containing the indicated SNP
Location of the SNP relative to the gene
Minor Allele Frequency
Number of subjects with a given genotype
Median outcome measurement for each genotype group. Results expressed as SFU/200,000 cells. The interquartile range is shown in parentheses
P-values were adjusted for demographic and clinical variables as well as inflation of significance described in the Methods.
Sensitivity analysis p-value for rs12764951 = 6.4E-05.
Table 3. SNPs showing significant association with CD8+ IFNγ ELISPOT in the African-American cohort.
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| rs6737773 | 2 | TCF7L1 | intron | 0 | 10 | AA | 183 | 5 (−6,19) | 4.27E-08 |
| AG | 10 | −12.5 (−22,−7) | |||||||
| GG | 0 | (,) | |||||||
|
| |||||||||
| rs16864122 | 2 | LOC375295 | intron | 0 | 11 | GG | 177 | 5 (−6,19) | 2.52E-07 |
| GA | 11 | −19 (−32.5,−9.5) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs1549932 | 19 | ZNF613 | 3′downstream | 6244 | 13 | AA | 180 | 5 (−7,19) | 3.08E-07 |
| AG | 13 | −6 (−7,1) | |||||||
| GG | 0 | (,) | |||||||
|
| |||||||||
| rs16880706* | 4 | LOC645641 | 3′downstream | 335029 | 11 | AA | 182 | 5 (−7,19) | 3.54E-07 |
| AG | 9 | −5 (−11,1) | |||||||
| GG | 1 | −31 (−31,−31) | |||||||
rs SNP identification number
Chromosomal location of the indicated SNP
Gene or genetic region containing the indicated SNP
Location of the SNP relative to the gene
Minor Allele Frequency
Number of subjects with a given genotype
Median outcome measurement for each genotype group. Results expressed as SFU/200,000 cells. The interquartile range is shown in parentheses
P-values were adjusted for demographic and clinical variables as well as inflation of significance described in the Methods.
Sensitivity analysis p-value for rs16880706 = 5.43E-08.
The top associations for the Caucasian cohort were in: PSD3 coding for the pleckstrin and Sec7 domain containing protein that is an exchange factor for ADP-ribosylation factor; RASA1 coding for a cytoplasmic GTPase activating protein that inhibits the RAS-cyclic AMP pathway, thereby regulating cellular proliferation and differentiation (Friedman et al. 1993; Trahey et al. 1988); RGS6, a G protein signaling regulator (Chatterjee et al. 1997); a transfer RNA for the anticodon UUG (glutamine) (Nemoto et al. 1991); SEPT11, a filament-forming cytoskeletal GTPase (Hanai et al. 2004); PLS3, a member of the plastin actin binding family (Lin et al. 1993); UTRN, an actin-binding protein localized to neuromuscular junctions (Burton et al. 1999); a thioredoxin pseudogene, AF357530; TCERG1L, a transcription elongation regulator protein; and the ADRA1D gene encoding for an adrenergic receptor controlling response to epinephrine (Bruno et al. 1991).
In the African-American cohort, variations in CD8+ IFNγ ELISPOT responses were associated with: TCF7L1 a transcription factor participating in the Wnt signaling pathway (Sagara and Katoh 2000); and a zinc-finger protein ZNF613.
Genetic Associations with Total IFNγ ELISPOT Responses
We also found SNPs (n=10) associated with total IFNγ ELISPOT responses as shown in Tables 4 and 5. None of the SNP associations reached genome-wide significance levels in the Caucasian cohort. In the African-American cohort, the top associations included: the DNA-binding transcription factor NFIB (Grunder et al. 2003); FAS, which mediates T cell induced cytotoxicity (Itoh et al. 1991); KCNQ1, a potassium channel gene located in the long QT-syndrome locus (Wang et al. 1996); an inhibitory G protein that downregulates adenylate cyclase activity, GNAI1(Sullivan et al. 1986); TBXAS1, a thromboxane A synthase (Yokoyama et al. 1991); the delta subunit of the cGMP specific phosphodiesterase, PDE6D (Florio et al. 1996); and OPRM1, the primary opioid receptor for both opioids and endogenous opioid peptides (Bond et al. 1998).
Table 4. SNPs showing significant association with total IFNγ ELISPOT in the African-American cohort.
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| rs7860845* | 9 | NFIB | intron | 0 | 12 | AA | 188 | 53 (23.8,85.3) | 9.48E-14 |
| AG | 10 | 18.5 (6.3,40.3) | |||||||
| GG | 1 | −30 (−30,−30) | |||||||
|
| |||||||||
| rs9658691* | 10 | FAS | intron | 0 | 10 | AA | 191 | 48 (22,79.5) | 1.63E-09 |
| AG | 8 | 102.5 (81.8,169.3) | |||||||
| GG | 1 | 233 (233,233) | |||||||
|
| |||||||||
| rs3758483* | 10 | FAS | 5′upstream | 1552 | 11 | AA | 190 | 48 (22,79.8) | 1.97E-08 |
| AG | 9 | 93 (64,168) | |||||||
| GG | 1 | 233 (233,233) | |||||||
|
| |||||||||
| rs983751* | 10 | FAS | 5′upstream | 3595 | 11 | CC | 190 | 48 (22,79.8) | 1.97E-08 |
| CA | 9 | 93 (64,168) | |||||||
| AA | 1 | 233 (233,233) | |||||||
|
| |||||||||
| rs2237875* | 11 | KCNQ1 | intron | 0 | 36 | AA | 167 | 54 (28.5,85.5) | 1.21E-07 |
| AG | 30 | 28 (0,52.5) | |||||||
| GG | 3 | −9 (−12,−2) | |||||||
|
| |||||||||
| rs11237722 | 11 | C11orf | 5′upstream | 4536 | 12 | GG | 188 | 48 (21.8,81.8) | 1.21E-07 |
| GA | 12 | 81 (54.5,130.3) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs2523194 | 7 | GNAI1 | intron | 0 | 12 | AA | 188 | 53 (23.8,85.3) | 1.48E-07 |
| AG | 12 | 18.5 (−9.5,36) | |||||||
| GG | 0 | (,) | |||||||
|
| |||||||||
| rs17161201 | 7 | TBXAS1 | intron | 0 | 10 | GG | 189 | 52 (24,85) | 1.50E-07 |
| GA | 10 | 15.5 (−5.8,54.3) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs4973479 | 2 | PDE6D | intron | 0 | 10 | GG | 190 | 52.5 (24,85) | 3.15E-07 |
| GA | 10 | 19.5 (−11.5,25.8) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs1319339 | 6 | OPRM1 | 5′upstream | 29259 | 15 | AA | 185 | 48 (21,81) | 4.91E-07 |
| AG | 15 | 80 (63.5,127.5) | |||||||
| GG | 0 | (,) | |||||||
rs SNP identification number
Chromosomal location of the indicated SNP
Gene or genetic region containing the indicated SNP
Location of the SNP relative to the gene
Minor Allele Frequency
Number of subjects with a given genotype
Median outcome measurement for each genotype group. Results expressed as SFU/200,000 cells. The interquartile range is shown in parentheses
P-values were adjusted for demographic and clinical variables as well as inflation of significance described in the Methods.
Sensitivity analysis p-values are as follows: rs7860845 = 2.40E-09, rs9658691 = 1.18E-08, rs3758483/rs983751 = 9.09E-07, rs2237875 = 1.12E-05
Table 5. SNPs showing significant association with secreted IFNγ in the Caucasian cohort.
| SNP IDa | Chromosomeb | Genec | Locationd | Distance from Gene | MAFe | Genotype | Nf | Median (IQR)g | p-valueh |
|---|---|---|---|---|---|---|---|---|---|
| rs3847906 | 12 | PPM1H | intron | 0 | 12 | AA | 482 | 304.3 (31.4,1334.4) | 1.86E-07 |
| AG | 12 | 66.4 (−397.3,472.6) | |||||||
| GG | 0 | (,) | |||||||
|
| |||||||||
| rs7987983 | 13 | DAOA | 5′upstream | 1027284 | 225 | GG | 293 | 382 (58,1373.1) | 1.87E-07 |
| GA | 177 | 193.2 (−7.3,1319.9) | |||||||
| AA | 24 | 37 (−24.7,131) | |||||||
|
| |||||||||
| rs16968401 | 13 | LOC341604 | 5′upstream | 136323 | 10 | GG | 487 | 315.1 (24.9,1316.1) | 3.50E-07 |
| GA | 10 | 72.6 (21.9,243.7) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs10458732 | 10 | SORCS1 | intron | 0 | 10 | GG | 488 | 313.7 (31.6,1324.8) | 4.61E-07 |
| GA | 10 | 60.7 (−12.4,484.1) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs11209457 | 1 | LOC100133218 | 3′downstream | 177802 | 42 | GG | 455 | 331.3 (42.2,1376.2) | 4.85E-07 |
| GA | 42 | 9.1 (−166.4,425.5) | |||||||
| AA | 0 | (,) | |||||||
|
| |||||||||
| rs1588571 | 4 | LOC100129666 | 3′downstream | 2482 | 18 | GG | 474 | 322 (33.7,1308.9) | 4.92E-07 |
| GA | 18 | 8.9 (−218.4,178.6) | |||||||
| AA | 0 | (,) | |||||||
rs SNP identification number
Chromosomal location of the indicated SNP
Gene or genetic region containing the indicated SNP
Location of the SNP relative to the gene
Minor Allele Frequency
Number of subjects with a given genotype
Median outcome measurement for each genotype group. Results expressed as pg/ml. The interquartile range is shown in parentheses
P-values were adjusted for demographic and clinical variables as well as inflation of significance described in the Methods.
Genetic Associations with Secreted IFNγ
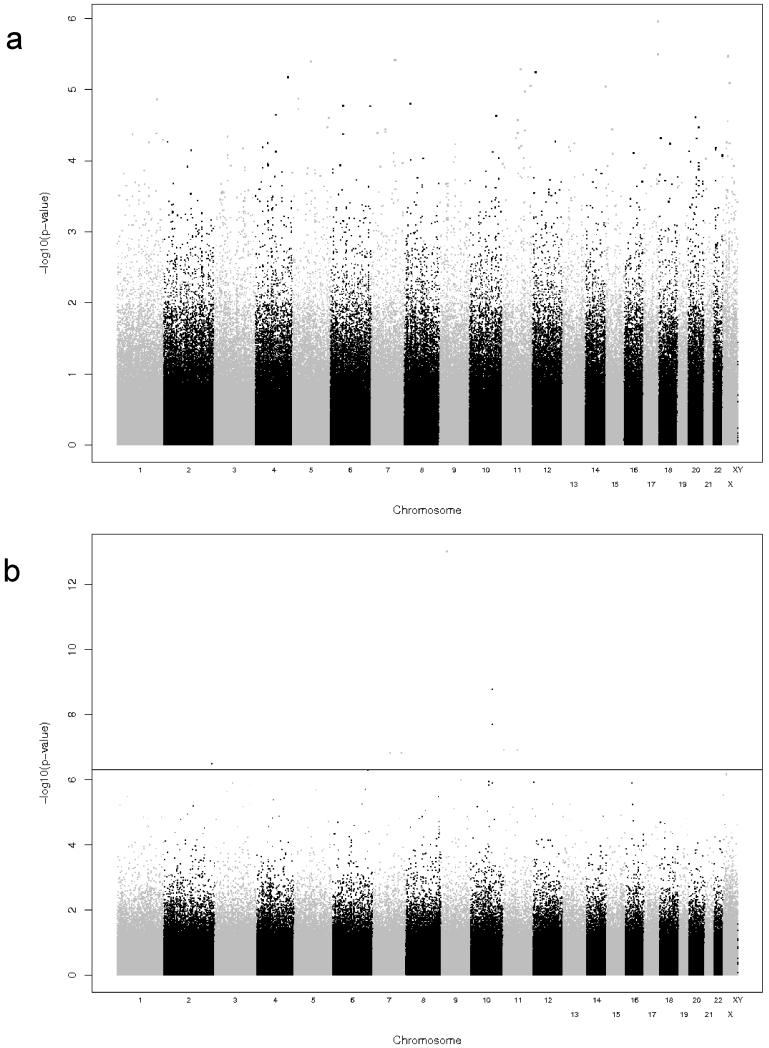
In addition to assessing cellular immunity by IFNγ ELISPOT, we also measured secreted IFNγ by ELISA assay. The Manhattan and Q-Q plots illustrating the SNP associations with this outcome are depicted in Figures 5 and 6. Top SNP associations (n=11) are listed in Tables 5 and 6. In the Caucasian cohort, we identified associations between IFNγ secretion and SNPs in: the protein phosphatase PPM1H gene; DAOA the D-amino acid oxidase activator gene that is also a susceptibility locus for schizophrenia and bipolar disorder (Chumakov et al. 2002); and SORCS1, a vacuolar protein sorting domain-containing receptor.
Figure 5. Quantile-Quantile plots of the expected (x-axis) and observed (y-axis) −log10(p-value) for secreted IFNγ response.
A) Results for the Caucasian cohort. B) Results for the African-American cohort.
Figure 6. Summary of GWAS results for cellular immune responses as measured by secreted IFNγ.
The y-axis displays the −log10 of the p-value for each SNP association (please note that the scale on the two plots are not equal) and the x-axis displays the chromosomes in alternating black and gray. P-values were adjusted for demographic and clinical variables as described in the Methods. A) Results for Caucasian cohort. B) Results for African-American cohort.
Analysis of the African-American cohort indicated associations between secreted IFNγ and: HPSE2 an endoglycosidase involved in remodeling the extracellular matrix (McKenzie et al. 2000) (although our sensitivity analysis revealed that this association was likely driven by the single GG genotype individual with extremely low levels of IFNγ); the SOX6 transcription factor (Cohen-Barak et al. 2001); a ubiquitin regulatory × domain containing protein UBXD 3(Schuberth and Buchberger 2008); OR10G6, an olfactory receptor G protein; and AMICA1, involved in leukocyte transmigration through epithelial/endothelial tissues.
When examining SNP associations across racial group we established the following criteria: a p-value of < 5 × 10−7 in one cohort; a p-value of < 0.01 in the second cohort; and an associated effect on the immune outcome with the same directionality in both cohorts (e.g. increased IFNγ response). None of the identified SNPs in Tables 2 and 3 fulfilled all three requirements, nor did any of the SNP associations with total IFNγ ELISPOT in Tables 4 or 5 meet these requirements. Interestingly, rs7987983 (DAOA gene) met each of our validation requirements, being significantly associated with a similar effect in both Caucasians and African-Americans. Our data suggest that there is no dominant allele controlling immune responses to smallpox vaccine. Given the complex, multigenic nature of immune responses we are not surprised that diverse genetic loci show significant phenotype associations in different racial groups.
Discussion
Overview of results
Our study population had widely varying levels of cellular immune markers to vaccinia virus as measured by IFNγ ELISPOT responses of both total PBMCs and CD8+ T cells to viral stimulation. One of the advantages of the genome-wide approach over candidate gene studies is the unbiased assessment of SNP associations across the entire genome, with the ability to discover novel genes involved in immune function. In fact, our genome-wide analysis identified both immune-related genes as well as genes involved in neuronal cell signaling and transcriptional activity. Although these novel genes have not yet been linked to immune functions, many of the key pathways they control in neuronal cells are used by lymphocyte lineages to control cell growth, activation, and differentiation.
CD8+ IFNγ ELISPOT responses in our Caucasian populations ranged from −102 to 238 spot-forming units/200,000 cells and variations were associated with several genes (Table 2) that are components of critical signaling pathways in immune cells including: RASA1 and ADRA1D. Ras activation is essential for T and B cell development, activation, and function (Ehrhardt et al. 2002) and the RASA1 gene encodes for a protein that stimulates GTPase activity of RAS p21, thereby suppressing RAS function. Mutations in RASA1 can alter this regulatory activity and are associated with basal cell carcinoma (Friedman et al. 1993; Hu et al. 2009) and capillary malformation (Eerola et al. 2003). Similarly, ADRA1D is a G protein-coupled receptor that signals through the Gαq subunit, which leads to activation of phospholipase C and Rho. Although ADRA1D is primarily involved in hormone signaling in the nervous system, the same downstream signaling pathways it activates are crucial to lymphocyte activation development, differentiation, activation, and migration (Tybulewicz and Henderson 2009). ADRA1D can also transactivate EGF receptors with subsequent induction of p42/p44 MAPK signaling (Chen et al. 2006).
In the African-American population, variations in CD8+ IFNγ ELISPOT responses (Table 3) were associated with rsrs6737773 in TCF7L1. TCF7L1 is a transcription factor activated by the wnt signaling pathway. It has recently been shown that wnt/TCF-1 is required for the expansion of primary/memory cytotoxic T lymphocytes as TCF7−/− mice have reduced primary CD8 responses and impaired memory T cell memory (Jeannet et al. 2010; Zhou et al. 2010). It has also been shown that simultaneous stimulation of the TCR and TCF-1/β-catenin pathways favors the development of memory CD8+ T cells (Goldrath et al. 2000; Zhao et al. 2010). Polymorphisms altering the expression or function of this transcription factor may lead to increased/decreased T cell survival and maintenance, thereby affecting antigen-specific, IFNγ T cell responses.
None of the SNPs demonstrated significant associations with total IFNγ ELISPOT responses in the Caucasian cohort. As it is unlikely that genetic polymorphisms have no effect on immune responses, we may have lacked sufficient power to detect associations, especially if the individual contribution of each SNP is small. Alternatively, this assay measures both antigen-specific IFNγ secretion by both CD4 and CD8 T cells, as well as non-antigen specific IFNγ release by NK cells and it is possible that different genetic elements influence IFNγ production in the different cell types making it difficult to pinpoint the various SNP associations. Follow-up studies on specific cell subsets will be required to investigate this further.
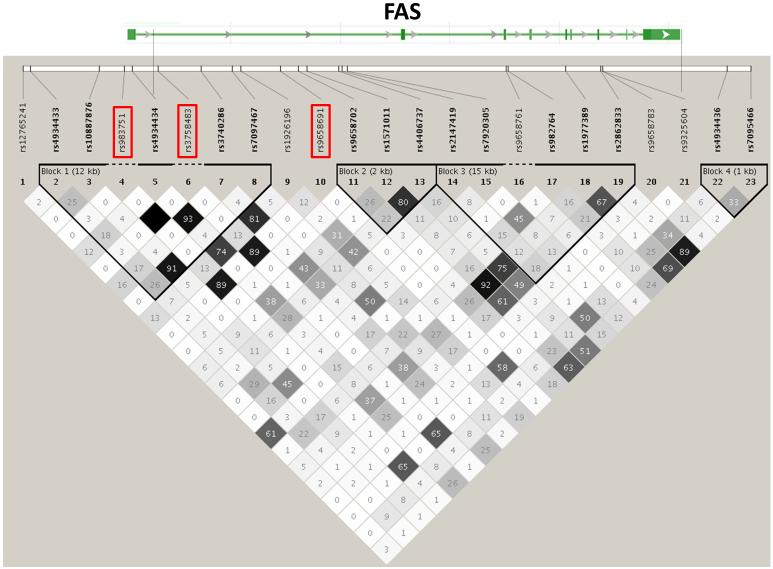
We did find several interesting associations with total IFNγ ELISPOT outcomes in the African-American cohort (Table 4). Rs7860845, an intronic SNP in NFIB, was associated with lower total IFNγ ELISPOT levels in this population. NFIB is a member of the Nuclear Factor I family of DNA binding proteins that regulate the transcription of multiple cellular signaling pathways (insulin, TGF-β, TNFa, steroid hormones, vitamins), and are essential for the replication of many viruses (Gronostajski 2000). Interestingly, overexpression of NFIB2 leads to downregulation of surface CD4 expression (Sheeter et al. 2003). One potential mechanism behind the genetic association between NFIB and IFNγ response might be the control of CD4 expression and concomitant up/down-regulation of T helper responses upon antigen exposure. Three SNPs (rs9658691, rs3758483, rs983751) in the FAS gene were all associated with increases in total IFNγ ELISPOT response in African-Americans. FAS encodes a death receptor mediating T and NK cell cytotoxicity(Lowin et al. 1994), antigen induced cell death of T lymphocytes, apoptosis of FasL expressing B cells and APCs resulting in decreased antigen presentation, and maintenance of immune privilege in the brain and central nervous system (Bechmann et al. 1999). All of the above SNPs are in close LD (r2 > 0.89, Figure 7) and show identical associations (Table 4) and likely tag a single causal SNP, which should be explored in future fine-mapping studies and/or functional studies. Polymorphisms affecting Fas expression and/or function may alter T cell survival, leading to differences in IFNγ production. Alternatively, genetic variations in the FAS gene may have downstream effects of MAPK or p38 pathways, which has been shown to affect IFNγ secretion (Rincon et al. 1998). An intronic SNP (rs2523194) in GNAI1 was associated with decreased total IFNγ ELISPOT response. GNAI1 is a G protein that modulates signaling cascades (c-SRC and MEK/ERK pathway, adenylate cyclase pathway) in response to receptor activation (IL-1, IL-8, CXCR4, CCR3, NGR-p75). PDE6D is a retinal rod rhodopsin-sensitive phosphodiesterase that degrades cGMP (Lorenz et al. 1998), but is also found in a variety of lymphoid tissues (Yanai et al. 2005). Related phosphodiesterases (PDE7A3, PDE9A1, PDE9A6) are known to influence immune cell activation (Omori and Kotera 2007), and it has been reported that signaling through PDE7 increases cytokine expression and proliferation (Li et al. 1999). Another significant association was found between rs1319339 in the mu opioid receptor OPRM1 and increased IFNγ ELISPOT response in African-Americans. Opioid receptors are expressed on lymphoid cells (Chuang et al. 1995), and control expression of both cytokines and cytokine receptors (Finley et al. 2008), including the production of IFNγ (Brown and Van Epps 1986; Lysle et al. 1993; Wang et al. 2001).
Figure 7. Genetic region containing FAS and 3 SNPs significantly associated with variations in total IFNγ ELISPOT outcomes in the African-American cohort.
The top bar represents the exon/intron structure of the indicated gene. Vertical bars match the location of the SNPs at the top of the LD plot. The r2 relationship between each pair of SNPs is indicated by the color and number of diamonds in the LD plot. LD blocks (Gabriel definition) are represented by the bold triangles. SNPs showing significant associations in this study are highlighted in boxes.
Our association analysis with secreted IFNγ revealed many significant associations. For the Caucasian subjects these included: rs3847906 in the recently identified PP2C family member PPM1H, which alters proliferation and apoptosis signals (Behrens et al. 2003; Sugiura et al. 2008). In the African-American cohort significant associations were found in several genes with no currently known immunologic functions: rs1437635 in the SOX6 gene; rs3179690 in the UBXD3 gene; and rs1453654 in the OR10G6 gene, a G-protein coupled receptor involved in odor perception (Malnic et al. 2004). Rs11216816 is located in AMICA1, which encodes for a costimulatory molecule on γδ T cells that stimulates cellular proliferation and cytokine production (Witherden et al. 2010).
Comparison of the race-specific analyses can be viewed as a form of replication for those SNPs; statistically significant and consistent results for the same SNP across different racial groups provides greater confidence of a genetic effect on the cytokine in question. Furthermore, replication of effect across genetically distinct groups expands the scope of inference for a given association. However, we have previously reported that gender and race affect smallpox vaccine outcomes (Kennedy et al. 2009c) and genetic control of vaccine responses is likely a complex, multigenic phenomenon confounded by racial effects, making validation in a different racial cohort a high bar to reach. Thus, it is not surprising that only a single SNP met these validation requirements: rs7987983, which is near to DAOA, is associated with decreased IFNγ secretion. A nearby SNP (r2 = 0.85 in our Caucasian cohort; r2 = 0.68 in our African-Americans), rs7337090 was also associated with the same effect but missed our p-value cutoff, having a p-value of 7.01 × 10−7 in the Caucasian cohort (See Figure 1). DAOA is a D-amino acid oxidase activator, and polymorphisms in this gene have been associated with susceptibility to bipolar disorder (Hattori et al. 2003) and to schizophrenia (Chumakov et al. 2002), but there are no existing data indicating a role for this gene in immune function.
A strength of our study is the genome-wide range covered by the Illumina HumanHap SNP chips, allowing us to examine not only known immune genes, but also to identify associations between immune response and additional genes involved in immune function and regulation. Our study is also the largest genotype-phenotype study examining the immunogenetics of smallpox vaccine response to date. Our results have pinpointed many directions for future studies for furthering our understanding of the genetic control of smallpox vaccine responses.
Many of the genes for which we found significant associations (RASA1, OPRM1, GNAI1 and TCF7L1) are critical components of pathways that directly control lymphocyte cytokine production. These associations with IFNγ responses to smallpox vaccine are therefore biologically plausible, providing corroborative evidence for their significance. We have also found associations between IFNγ responses and genes not known to have direct immune function (ZNF613, ADRA1D, UTRN, DAOA, SOX6) or SNPs located in gene deserts or pseudogenes (LOC100128245). The biologic plausibility of these results is difficult to explain as they may represent additional, unsuspected functions of these genes in lymphoid populations, SNPs in LD with true causal SNPs, or even false positives. Given the cross-talk between both the nervous and immune systems, it is intriguing that so many of the significant associations with IFNγ outcomes were found with SNPs in genes integral to nerve cell activity (Elenkov et al. 2000). A limitation of this report is the lack of true replication in a similar cohort. We have previously identified racial differences in smallpox vaccine-induced immune responses, and the use of two different races for validation is therefore not optimal. Given the high rate of false-positive results with GWAS studies, replication of these findings in an independent cohort of the same racial background is currently underway. This will be an important next step toward validating the results. Furthermore, functional studies designed to elucidate the mechanisms behind the associations could reveal novel means of immune regulation and control.
Acknowledgments
The authors thank the subjects who participated in this study and the research staff at the NHRC and Mayo Clinic that made this study possible – particularly, Drs. Meg Ryan and Kevin Russell. The authors also wish to recognize Dave Watson and Megan O’Byrne for their statistical programming and analytical support as well as Julie M. Cunningham and the Mayo Advanced Genomic Technology Center for the genotyping efforts. This project was funded by the National Institute of Allergies and Infectious Diseases, National Institutes of Health, Department of Health and Human Services (Contract #HHSN266200400065C). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript. This project was funded by federal funds from the National Institute of Allergies and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN266200400065C.
Footnotes
Ethical Standards and Conflict of Interests
All experiments described here followed current, applicable U.S. laws. The authors have no conflicts of interest.
References
- Artenstein AW, Grabenstein JD. Smallpox vaccines for biodefense: need and feasibility. Expert Rev Vaccines. 2008;7:1225–37. doi: 10.1586/14760584.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann I, Mor G, Nilsen J, Eliza M, Nitsch R, Naftolin F. FasL (CD95L, Apo1L) is expressed in the normal rat and human brain: evidence for the existence of an immunological brain barrier. Glia. 1999;27:62–74. doi: 10.1002/(sici)1098-1136(199907)27:1<62::aid-glia7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Behrens P, Brinkmann U, Wellmann A. CSE1L/CAS: its role in proliferation and apoptosis. Apoptosis. 2003;8:39–44. doi: 10.1023/a:1021644918117. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608–13. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Van Epps DE. Opioid peptides modulate production of interferon gamma by human mononuclear cells. Cell Immunol. 1986;103:19–26. doi: 10.1016/0008-8749(86)90064-x. [DOI] [PubMed] [Google Scholar]
- Bruno JF, Whittaker J, Song JF, Berelowitz M. Molecular cloning and sequencing of a cDNA encoding a human alpha 1A adrenergic receptor. Biochem Biophys Res Commun. 1991;179:1485–90. doi: 10.1016/0006-291x(91)91740-4. [DOI] [PubMed] [Google Scholar]
- Burton EA, Tinsley JM, Holzfeind PJ, Rodrigues NR, Davies KE. A second promoter provides an alternative target for therapeutic up-regulation of utrophin in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 1999;96:14025–30. doi: 10.1073/pnas.96.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee TK, Eapen A, Kanis AB, Fisher RA. Genomic organization, 5′-flanking region, and chromosomal localization of the human RGS3 gene. Genomics. 1997;45:429–33. doi: 10.1006/geno.1997.4929. [DOI] [PubMed] [Google Scholar]
- Chen L, Hodges RR, Funaki C, Zoukhri D, Gaivin RJ, Perez DM, Dartt DA. Effects of alpha1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells. Am J Physiol Cell Physiol. 2006;291:C946–56. doi: 10.1152/ajpcell.00014.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang TK, Killam KF, Jr., Chuang LF, Kung HF, Sheng WS, Chao CC, Yu L, Chuang RY. Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun. 1995;216:922–30. doi: 10.1006/bbrc.1995.2709. [DOI] [PubMed] [Google Scholar]
- Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P, Debailleul V, Simon AM, Caterina D, Dufaure I, Malekzadeh K, Belova M, Luan JJ, Bouillot M, Sambucy JL, Primas G, Saumier M, Boubkiri N, Martin-Saumier S, Nasroune M, Peixoto H, Delaye A, Pinchot V, Bastucci M, Guillou S, Chevillon M, Sainz-Fuertes R, Meguenni S, Aurich-Costa J, Cherif D, Gimalac A, Van Duijn C, Gauvreau D, Ouellette G, Fortier I, Raelson J, Sherbatich T, Riazanskaia N, Rogaev E, Raeymaekers P, Aerssens J, Konings F, Luyten W, Macciardi F, Sham PC, Straub RE, Weinberger DR, Cohen N, Cohen D. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci U S A. 2002;99:13675–80. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Barak O, Hagiwara N, Arlt MF, Horton JP, Brilliant MH. Cloning, characterization and chromosome mapping of the human SOX6 gene. Gene. 2001;265:157–64. doi: 10.1016/s0378-1119(01)00346-8. [DOI] [PubMed] [Google Scholar]
- Combadiere B, Boissonnas A, Carcelain G, Lefranc E, Samri A, Bricaire F, Debre P, Autran B. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J Exp Med. 2004;199:1585–93. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Crowe JE., Jr. Genetic predisposition for adverse events after vaccination. J Infect Dis. 2007;196:176–7. doi: 10.1086/518800. [DOI] [PubMed] [Google Scholar]
- Demkowicz WE, Jr., Littaua RA, Wang J, Ennis FA. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627–31. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Moss B, Wyatt LS, Carroll MW. Generation of recombinant vaccinia viruses. Curr Protoc Protein Sci. 2001 doi: 10.1002/0471140864.ps0513s13. Chapter 5: Unit5 13. [DOI] [PubMed] [Google Scholar]
- Eerola I, Boon LM, Mulliken JB, Burrows PE, Dompmartin A, Watanabe S, Vanwijck R, Vikkula M. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240–9. doi: 10.1086/379793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt A, Ehrhardt GR, Guo X, Schrader JW. Ras and relatives--job sharing and networking keep an old family together. Exp Hematol. 2002;30:1089–106. doi: 10.1016/s0301-472x(02)00904-9. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Finley MJ, Happel CM, Kaminsky DE, Rogers TJ. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell Immunol. 2008;252:146–54. doi: 10.1016/j.cellimm.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio SK, Prusti RK, Beavo JA. Solubilization of membrane-bound rod phosphodiesterase by the rod phosphodiesterase recombinant delta subunit. J Biol Chem. 1996;271:24036–47. doi: 10.1074/jbc.271.39.24036. [DOI] [PubMed] [Google Scholar]
- Frey SE, Newman FK, Cruz J, Shelton WB, Tennant JM, Polach T, Rothman AL, Kennedy JS, Wolff M, Belshe RB, Ennis FA. Dose-related effects of smallpox vaccine. N Engl J Med. 2002;346:1275–80. doi: 10.1056/NEJMoa013431. [DOI] [PubMed] [Google Scholar]
- Friedman E, Gejman PV, Martin GA, McCormick F. Nonsense mutations in the C-terminal SH2 region of the GTPase activating protein (GAP) gene in human tumours. Nat Genet. 1993;5:242–7. doi: 10.1038/ng1193-242. [DOI] [PubMed] [Google Scholar]
- Fulginiti VA. Risks of smallpox vaccination. Jama. 2003;290:1452. doi: 10.1001/jama.290.11.1452-a. author reply 1452. [DOI] [PubMed] [Google Scholar]
- Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clin Infect Dis. 2003;37:251–71. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–64. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski RM. Roles of the NFI/CTF gene family in transcription and development. Gene. 2000;249:31–45. doi: 10.1016/s0378-1119(00)00140-2. [DOI] [PubMed] [Google Scholar]
- Grunder A, Qian F, Ebel TT, Mincheva A, Lichter P, Kruse U, Sippel AE. Genomic organization, splice products and mouse chromosomal localization of genes for transcription factor Nuclear Factor One. Gene. 2003;304:171–81. doi: 10.1016/s0378-1119(02)01204-0. [DOI] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Hanai N, Nagata K, Kawajiri A, Shiromizu T, Saitoh N, Hasegawa Y, Murakami S, Inagaki M. Biochemical and cell biological characterization of a mammalian septin, Sept11. FEBS Lett. 2004;568:83–8. doi: 10.1016/j.febslet.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Haralambieva IH, Ovsyannikova IG, Dhiman N, Kennedy RB, O’Byrne M, Pankratz VS, Jacobson RM, Poland GA. Common SNPs/Haplotypes in IL18R1 and IL18 Genes Are Associated With Variations in Humoral Immunity to Smallpox Vaccination in Caucasians and African Americans. J Infect Dis. 2011;204:433–41. doi: 10.1093/infdis/jir268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori E, Liu C, Badner JA, Bonner TI, Christian SL, Maheshwari M, Detera-Wadleigh SD, Gibbs RA, Gershon ES. Polymorphisms at the G72/G30 gene locus, on 13q33, are associated with bipolar disorder in two independent pedigree series. Am J Hum Genet. 2003;72:1131–40. doi: 10.1086/374822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Stern HM, Ge L, O’Brien C, Haydu L, Honchell CD, Haverty PM, Peters BA, Wu TD, Amler LC, Chant J, Stokoe D, Lackner MR, Cavet G. Genetic alterations and oncogenic pathways associated with breast cancer subtypes. Mol Cancer Res. 2009;7:511–22. doi: 10.1158/1541-7786.MCR-08-0107. [DOI] [PubMed] [Google Scholar]
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107:9777–82. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R, Pankratz VS, Swanson E, Watson D, Golding H, Poland GA. Statistical approach to estimate vaccinia-specific neutralizing antibody titers using a high throughput assay. Clin Vaccine Immunol. 2009a doi: 10.1128/CVI.00109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RB, Ovsyannikova IG, Jacobson RM, Poland GA. The immunology of smallpox vaccines. Curr Opin Immunol. 2009b;21:314–20. doi: 10.1016/j.coi.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RB, Ovsyannikova IG, Pankratz VS, Vierkant RA, Jacobson RM, Ryan MA, Poland GA. Gender effects on humoral immune responses to smallpox vaccine. Vaccine. 2009c doi: 10.1016/j.vaccine.2009.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yee C, Beavo JA. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science. 1999;283:848–51. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- Lin CS, Park T, Chen ZP, Leavitt J. Human plastin genes. Comparative gene structure, chromosome location, and differential expression in normal and neoplastic cells. J Biol Chem. 1993;268:2781–92. [PubMed] [Google Scholar]
- Lorenz B, Migliaccio C, Lichtner P, Meyer C, Strom TM, D’Urso M, Becker J, Ciccodicola A, Meitinger T. Cloning and gene structure of the rod cGMP phosphodiesterase delta subunit gene (PDED) in man and mouse. Eur J Hum Genet. 1998;6:283–90. doi: 10.1038/sj.ejhg.5200215. [DOI] [PubMed] [Google Scholar]
- Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–2. doi: 10.1038/370650a0. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Coussons ME, Watts VJ, Bennett EH, Dykstra LA. Morphine-induced alterations of immune status: dose dependency, compartment specificity and antagonism by naltrexone. J Pharmacol Exp Ther. 1993;265:1071–8. [PubMed] [Google Scholar]
- Malnic B, Godfrey PA, Buck LB. The human olfactory receptor gene family. Proc Natl Acad Sci U S A. 2004;101:2584–9. doi: 10.1073/pnas.0307882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie E, Tyson K, Stamps A, Smith P, Turner P, Barry R, Hircock M, Patel S, Barry E, Stubberfield C, Terrett J, Page M. Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochem Biophys Res Commun. 2000;276:1170–7. doi: 10.1006/bbrc.2000.3586. [DOI] [PubMed] [Google Scholar]
- Mitra-Kaushik S, Cruz J, Stern LJ, Ennis FA, Terajima M. Human cytotoxic CD4+ T cells recognize HLA-DR1-restricted epitopes on vaccinia virus proteins A24R and D1R conserved among poxviruses. J Immunol. 2007;179:1303–12. doi: 10.4049/jimmunol.179.2.1303. [DOI] [PubMed] [Google Scholar]
- Nemoto F, Okazaki T, Mizushima H, Muller WE, Kuchino Y. Nucleotide sequence of the human tRNA(UUGGln) gene. Nucleic Acids Res. 1991;19:2779. doi: 10.1093/nar/19.10.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–27. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Jacobson RM, Ryan JE, Vierkant RA, Pankratz VS, Jacobsen SJ, Poland GA. HLA class II alleles and measles virus-specific cytokine immune response following two doses of measles vaccine. Immunogenetics. 2005;56:798–807. doi: 10.1007/s00251-004-0756-0. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Vierkant RA, Pankratz VS, Jacobson RM, Poland GA. Human leukocyte antigen genotypes in the genetic control of adaptive immune responses to smallpox vaccine. J Infect Dis. 2011;203:1546–55. doi: 10.1093/infdis/jir167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–59. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puissant-Lubrano B, Bossi P, Gay F, Crance JM, Bonduelle O, Garin D, Bricaire F, Autran B, Combadiere B. Control of vaccinia virus skin lesions by long-term-maintained IFN-gamma+TNF-alpha+ effector/memory CD4+ lymphocytes in humans. J Clin Invest. 2010;120:1636–44. doi: 10.1172/JCI38506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif DM, McKinney BA, Motsinger AA, Chanock SJ, Edwards KM, Rock MT, Moore JH, Crowe JE. Genetic basis for adverse events after smallpox vaccination. J Infect Dis. 2008;198:16–22. doi: 10.1086/588670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon M, Enslen H, Raingeaud J, Recht M, Zapton T, Su MS, Penix LA, Davis RJ, Flavell RA. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. Embo J. 1998;17:2817–29. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JE, Dhiman N, Ovsyannikova IG, Vierkant RA, Pankratz VS, Poland GA. Response surface methodology to determine optimal cytokine responses in human peripheral blood mononuclear cells after smallpox vaccination. J Immunol Methods. 2009;341:97–105. doi: 10.1016/j.jim.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JE, Ovsyannikova IG, Dhiman N, Pinsky NA, Vierkant RA, Jacobson RM, Poland GA. Inter-operator variation in ELISPOT analysis of measles virus-specific IFN-gamma-secreting T cells. Scand J Clin Lab Invest. 2005;65:681–9. doi: 10.1080/00365510500348252. [DOI] [PubMed] [Google Scholar]
- Sagara N, Katoh M. Mitomycin C resistance induced by TCF-3 overexpression in gastric cancer cell line MKN28 is associated with DT-diaphorase down-regulation. Cancer Res. 2000;60:5959–62. [PubMed] [Google Scholar]
- Schaid DJ, Batzler AJ, Jenkins GD, Hildebrandt MA. Exact tests of Hardy-Weinberg equilibrium and homogeneity of disequilibrium across strata. Am J Hum Genet. 2006;79:1071–80. doi: 10.1086/510257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuberth C, Buchberger A. UBX domain proteins: major regulators of the AAA ATPase Cdc48/p97. Cell Mol Life Sci. 2008;65:2360–71. doi: 10.1007/s00018-008-8072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeter D, Du P, Rought S, Richman D, Corbeil J. Surface CD4 expression modulated by a cellular factor induced by HIV type 1 infection. AIDS Res Hum Retroviruses. 2003;19:117–23. doi: 10.1089/088922203762688621. [DOI] [PubMed] [Google Scholar]
- Stanley SL, Jr., Frey SE, Taillon-Miller P, Guo J, Miller RD, Koboldt DC, Elashoff M, Christensen R, Saccone NL, Belshe RB. The immunogenetics of smallpox vaccination. J Infect Dis. 2007;196:212–9. doi: 10.1086/518794. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Noguchi Y, Sakurai K, Hattori C. Protein phosphatase 1H, overexpressed in colon adenocarcinoma, is associated with CSE1L. Cancer Biol Ther. 2008;7:285–92. doi: 10.4161/cbt.7.2.5302. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Liao YC, Alborzi A, Beiderman B, Chang FH, Masters SB, Levinson AD, Bourne HR. Inhibitory and stimulatory G proteins of adenylate cyclase: cDNA and amino acid sequences of the alpha chains. Proc Natl Acad Sci U S A. 1986;83:6687–91. doi: 10.1073/pnas.83.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RDC R: A language and environment for statistical computing. 2008 [Google Scholar]
- Trahey M, Wong G, Halenbeck R, Rubinfeld B, Martin GA, Ladner M, Long CM, Crosier WJ, Watt K, Koths K, et al. Molecular cloning of two types of GAP complementary DNA from human placenta. Science. 1988;242:1697–700. doi: 10.1126/science.3201259. [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9:630–44. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. Morphine modulates lymph node-derived T lymphocyte function: role of caspase-3, -8, and nitric oxide. J Leukoc Biol. 2001;70:527–36. [PubMed] [Google Scholar]
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, Fischer WH, Wilson IA, Havran WL. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. 2010;329:1205–10. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, Bar-Even A, Horn-Saban S, Safran M, Domany E, Lancet D, Shmueli O. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics. 2005;21:650–9. doi: 10.1093/bioinformatics/bti042. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Miyata A, Ihara H, Ullrich V, Tanabe T. Molecular cloning of human platelet thromboxane A synthase. Biochem Biophys Res Commun. 1991;178:1479–84. doi: 10.1016/0006-291x(91)91060-p. [DOI] [PubMed] [Google Scholar]
- Zhao DM, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue HH. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–9. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–40. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]