Abstract
Introduction and Objective
Increased body mass index (BMI) is associated with worse outcomes for several different malignancies. The relationship between BMI and urothelial carcinoma is poorly understood. We investigated the association between BMI and oncological outcomes in upper tract urothelial carcinoma (UTUC).
Methods
We retrospectively studied 520 patients treated with radical nephroureterectomy (RNU) for UTUC without neoadjuvant chemotherapy. Univariable Cox regression analyses were performed to evaluate recurrence-free (RFS) and cancer-specific survival (CSS) estimates. We created a multivariable model based on preoperative and postoperative characteristics. BMI was treated as a continuous and a categorical variable defined as normal weight (<25 kg/m2), overweight (25–29.9 kg/m2) or obese (≥30 kg/m2).
Results
The median patient BMI was 27.9 kg/m2 (IQR: 6.7). Median follow-up was 38 months (IQR: 54). Patients with a higher BMI were more likely to have infiltrative architecture (p<0.001) and lymphovascular invasion (p=0.012). BMI was not associated with age, smoking history, pathologic stage, tumor grade, concomitant CIS, tumor necrosis, and the selection of a laparoscopic or open procedure. In the preoperative multivariable model a BMI of 25–29 kg/m2 (HR 2.25, 95% CI: 1.3–3.8, p=0.003) and BMI ≥ 30 kg/m2 (HR 3.72, 95% CI: 2.2–6.3, <0.001) were both associated with disease recurrence. A BMI ≥30kg/m2 (HR 4.24, CI: 2.4–7.5, p<0.001) was associated with cancer specific death. In the postoperative model, tumor stage (p<0.001), positive lymph nodes (HR 2.52, 95% CI: 1.59–4.0, p<0.001), BMI 25–29 kg/m2 (HR 2.18, 95% CI: 1.27–3.73, p=0.005) and BMI ≥30 kg/m2 (HR 3.52, 95% CI: 2.08–5.95, p<0.001) were associated with disease recurrence. Tumor stage (p<0.001), positive lymph nodes (HR 3.1, 95% CI: 1.84–5.21, p<0.001), and a BMI≥30 kg/m2 (HR 4.13, 95% CI: 2.32–7.36, p<0.001) were associated with cancer-specific death.
Conclusions
Higher BMI is associated with worse cancer-specific outcomes in patients treated with RNU for UTUC. Improving cancer specific survival by also focusing on patient modifiable factors such as BMI could have significant individual and public health implications in UTUC patients. Future studies should be encouraged to evaluate the molecular mechanisms of tumorigenesis and progression associated with metabolic changes in obesity.
Keywords: Obesity, BMI, Upper Urinary Tract, Urothelial, Carcinoma
Introduction
BMI is an established measure for percent of body fat and can be measured multiple techniques.1, 2 An estimated 65% of adults in the US are currently overweight as defined by a body mass index (BMI) between 25–29.9 kg/m2 or obese as defined by a BMI of 30 kg/m2 or greater.3 Unfortunately, these epidemiologic trends continue to worsen and obesity has reached epidemic levels in parts of the US and Europe.4, 5 Obesity is strongly associated with heart disease, diabetes, and various medical disorders. In the past decade, researchers have demonstrated an association between obesity and incidence of several organ-specific cancers such as breast, colon, esophagus, kidney and endometrium.6–12 BMI has also been positively and negatively associated with cancer-specific outcomes such as pathologic subtype, tumor stage, recurrence, progression, metastasis, and death.13–18
The association between obesity and urothelial carcinoma (UC) has been investigated in lower tract UC (bladder cancer) cohorts with conflicting results. In prospective cohorts of the Health Professionals Follow-up Study and the Nurses’ Health Study, Holick et al19 failed to find an association between BMI and bladder carcinogenesis. However, in a large prospective cohort of Americans in the NIH-AARP Diet and Health Study, Koebnick et al20 found a consistent association between BMI and bladder cancer among subgroups stratified by gender, socioeconomic status, and various dietary factors. A retrospective study of bladder cancer patients treated with radical cystectomy failed to show a significant association between BMI and disease-specific survival.21
Upper tract urothelial carcinoma (UTUC) accounts for 5–7% of all urothelial malignancies.22 Despite advancement in diagnostic techniques including ureteroscopy and computed tomography imaging, data indicate that the disease specific survival rate has not improved in the past two decades.23 The rarity of the disease has limited extensive evaluation of preoperative risk factors. The impact of BMI on oncological outcomes in UTUC has not been evaluated to our knowledge. We hypothesized that higher BMI is associated with a higher risk of having features of biologically aggressive UTUC such as advanced stage and disease recurrence. To test this hypothesis, we examined the association of BMI with clinical outcomes of patients treated with RNU for UTUC.
Patients and Methods
Patient Selection
This study consists of data collected from 3 participating sites which provided the necessary institutional data sharing agreements before study initiation. The institutional review board at each site approved this study. The database contains 520 patients who underwent RNU with ipsilateral bladder cuff resection by multiple surgeons between 1987 and 2007. The hilar and regional lymph nodes adjacent to the ipsilateral great vessel were resected along with enlarged lymph nodes identified on preoperative computed tomography imaging scans or palpable intraoperatively. None of the patients received preoperative chemo- or radiation therapy. Before final analysis the database was frozen and the final data set was produced for current analysis.
Pathological Evaluation
All surgical specimens were processed according to standard pathological procedures and all slides were re-reviewed by genitourinary pathologists blinded to clinical outcomes. Tumors were staged according to the TNM classification by the American Joint Committee on Cancer-UICC. Tumor grading was assessed according to the 1998 WHO/International Society of Urologic Pathology consensus classification. Grading information required conversion to a consensus system before combining the site specific data. Tumor architecture was defined as papillary or sessile. Tumor location was defined as pelvic/caliceal or ureteral. In tumors involving both sites the location was attributed according to the most advanced stage.
Clinical variables
Multiple clinical variables were extracted from patients’ charts, including age, gender, smoking status. The American Society of Anesthesia (ASA) score was used for assessing the fitness of the patient prior to surgery. BMI was calculated from as a measure of body weight based oxn a person’s weight and height. Body mass index was defined as the individual’s body weight divided by the square of his or her height.
Follow-up regimen
Patients were generally seen every 3–4 months for the first year after surgery, every 6 months from the second through fifth years, and annually thereafter. Follow-up consisted of a history, physical examination, routine blood work, and serum chemistry studies, urinary cytology, chest radiography, cystoscopy, and radiographic evaluation of the contralateral upper urinary tract.
Disease recurrence was defined as local failure in the operative site, regional lymph nodes or distant metastasis. A recurrence in the bladder was not calculated in the recurrence-free survival analysis. The cause of death was determined by retrospective physician chart review and corroborated in some cases by death certificates. A patient with widely disseminated metastases at the time of death was categorized as having died of UTUC. Patients who died within 30 days of surgery were censored at the time of death for UTUC-specific survival analysis.
Statistical analysis
In the descriptive analysis, the Fisher’s exact test and the chi-square were used to evaluate the association between categorical variables. Differences in continuous variables were analyzed using Kruskal-Wallis test. The Kaplan-Meier method was used to calculate survival outcomes, and differences were assessed with the log rank statistic. Univariate and multivariable Cox regression models evaluated time to recurrence and cancer-specific mortality outcomes. Statistical significance was defined as P≤0.05. All reported P values were two-sided. Analyses were performed with SPSS 17 (an IBM company, Chicago, IL).
Results
The median patient BMI was 27.9 kg/m2 (IQR: 6.7). Patients with a lower BMI were more likely to have a lower ASA score (p<0.001) and tumors located in the ureter vs. renal pelvis (p=0.02). In the association of BMI with pathological features, patients with a higher BMI were more likely to have infiltrative architecture (p<0.001) and lymphovascular invasion (p=0.012). Patients with a higher BMI were also more likely to receive adjuvant chemotherapy (p=0.01). However, BMI was not associated with age, smoking history, pathologic stage, tumor grade, concomitant CIS, tumor necrosis. Furthermore, BMI was not associated with the selection of a laparoscopic or open procedure, the likelihood of undergoing lymphadenectomy, or the extent of lymphadenectomy [rho= -0.66, p=0.14].
The median follow-up for patients who were alive at last follow-up was 38 months (IQR: 54). On follow-up, 156 patients (30.1%) developed disease recurrence and 126 (24.2%) patients died of UTUC. The recurrence-free survival estimates in all patients at 3, 5, and 10 years were 75% (SE±2%), 63% (SE±3%), and 35% (SE±3%). The cancer-specific survival estimates in all patients at 3, 5, and 10 years were 81% (SE±2%), 68% (SE±3%), and 57% (SE±4%). The overall survival estimates in all patients at 3, 5, and 10 years were 68% (SE±1%), 60% (SE±1%), and 34% (SE±2%).
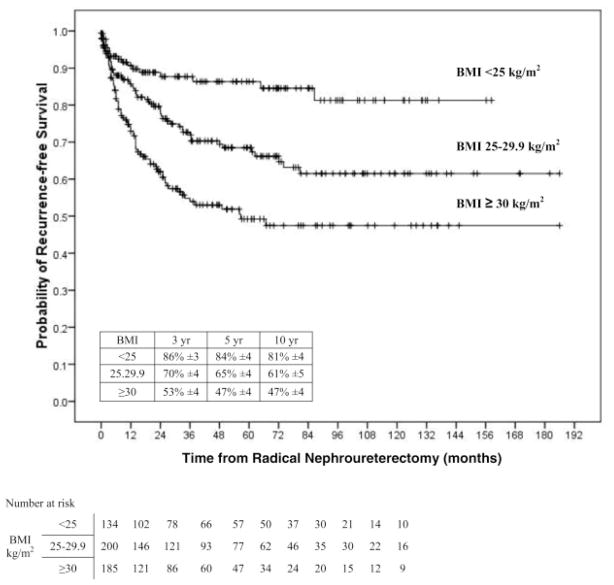
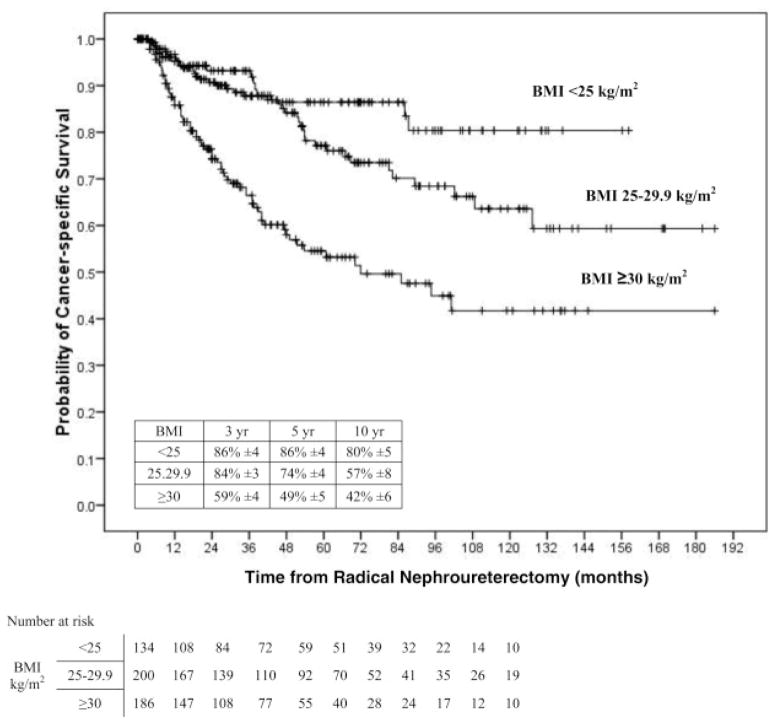
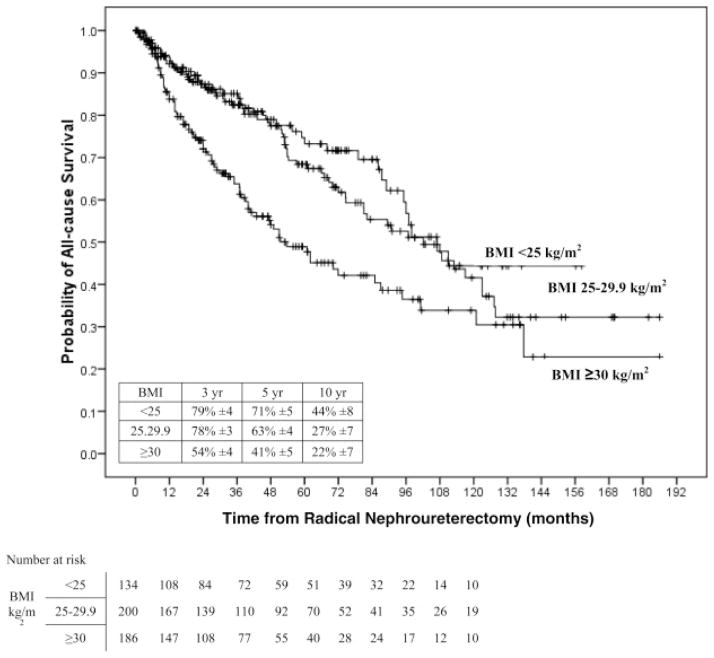
On univariable analyses, the risk of disease recurrence and cancer-specific death increased gradually between patients with normal weight (<25 kg/m2) to overweight patients (25–29,9 kg/m2), and was highest in obese patients (<30 kg/m2) (Figure 1). Pair-wise comparisons using the log-rank test (Mantel-Cox) showed that patients with BMI ≥30 kg/m2 had a higher risk of disease recurrence compared to patients with BMI <25 kg/m2 (p<0.001) and those with BMI 25–29.9 kg/m2 (p<0.001). Similarly, the probability estimate of cancer-specific death was higher in patients with BMI ≥30 kg/m2 compared to those with BMI <25 kg/m2 (p<0.001) and those with BMI 25–29.9 kg/m2 (p<0.001). Also, overall mortality was worse for patients with a BMI ≥30 kg/m2 compared to those with BMI <25 kg/m2 (p<0.001) and those with BMI 25–29.9 kg/m2 (p<0.001).
Figure 1.
Univariate Cox regression analysis of association of normal weight (less than 25 kg/m2), overweight (25 to 29.9 kg/m2) and obese (30 kg/m2 or greater) BMI categories with recurrence-free survival in 520 patients treated with RNU for UTUC.
In the model that adjusted for the pre-operative variables including age, gender, smoking history, ASA score, and tumor location, patients with a BMI 25–29 kg/m2 (HR 2.25, 95% CI 1.3–3.8, p=0.003) and BMI ≥ 30 kg/m2 (HR 3.72, 95% CI 2.2–6.3, <0.001) were associated with disease recurrence. BMI ≥ 30 kg/m2 was associated with cancer specific death (HR 4.24, 2.4–7.5, p<0.001). Age was associated with disease recurrence (HR 1.02, 95% CI 1.0–1.04, p<0.001) and cancer specific death (HR 1.03, 95% CI1.0–1.1, p<0.001).
In the post-operative multivariate model, we adjusted for age, surgical approach (open vs laparoscopic), number of lymph nodes removed, positive lymph nodes, pathologic stage, tumor grade, concomitant CIS, adjuvant chemotherapy status, tumor necrosis, tumor architecture, and presence of lymphovascular invasion. Here, we observed that patients with pathologic stage T1 (HR 3.74, 95% CI 1.1–12.6, p=0.034), stage T2 (HR 4.04, 95% CI 1.2–13.6, p=0.024), and stage T3 or greater tumors (HR 9.41, 95% CI 2.82–31.5, p<0.001) are associated with decreased disease-free recurrence compared to stage Ta and Tis tumors. In addition, positive lymph nodes (HR 2.52, 95% CI: 1.59–4.0, p<0.001), patients with a BMI 25–29 kg/m2 (HR 2.18, 95% CI 1.27–3.73, p=0.005) and patients with a BMI ≥30 kg/m2 (HR 3.52, 95% CI 2.08–5.95, p<0.001) experienced worse disease-free recurrence.
Similarly, patients with pathologic stage T3 or greater tumors (HR 8.79, 95% CI 2.32–33.2, p=0.001), positive lymph nodes (HR 3.1, 95% CI: 1.84–5.21, p<0.001), and a BMI≥30 kg/m2 (HR 4.13, 95% CI 2.32–7.36, p<0.001) were associated with cancer-specific death. Age was associated with cancer-specific death (HR 1.04, 95% CI 1.01–1.06, p=0.001). Age (HR 1.04, 95% CI 1.02–1.06, p<0.001), BMI≥30 kg/m2(HR 2.33, 95% CI 1.56–3.48, p<0.001), positive lymph nodes (HR 2.63, 95% CI 1.66–4.16, p<0.001), stage T3 or greater (HR 2.53, 95% CI 1.22–5.25, p=0.01) and tumor necrosis (HR 1.42, 95% CI 1.03–1.96, p=0.031) were all associated with worse overall survival.
Discussion
The relationship of obesity to cancer has received less attention than other medical comorbidities such as cardiovascular disease, diabetes, and hypertension. In this study of 520 patients who underwent RNU and ipsilateral bladder cuff excision for UTUC. BMI was an independent predictor of cancer recurrence and survival after adjusting for pre-and postoperative features.
Advanced UTUC is a lethal malignancy with a decrease in cancer-specific survival at 5 years from more than 80% in organ confined disease to less than 35% for disease that has metastasized to lymph nodes.24 Therefore, efforts should be directed to reduce the burden of advanced disease. Focusing on patient modifiable factors such as BMI can improve oncological outcomes and has significant public and individual health implications.
The biological mechanisms underlying the increased risk of carcinogenesis and progression associated with obesity is an area of intense investigation. BMI is strongly correlated with densitometry estimates of body fat composition in adults and excess body fat is associated with elevated levels of insulin which increase insulin-like growth factor-I.25 Insulin-like growth factor-I stimulates cell proliferation and suppresses apoptosis.26 In addition, excess body fat is associated with systemic inflammation which may play a role in lower urinary urothelial carcinoma outcomes as suggested by studies that measured circulating levels of inflammatory markers.27–29
The worse oncological outcomes appear to be driven by the impact of an increased BMI on aggressive tumor biology as opposed to being confounded by surgical factors. Patients with a higher BMI were more likely to have worse tumor specific characteristics including infiltrative architecture and lymphovascular invasion. These patients were also more likely to receive adjuvant chemotherapy which may be a clinical marker of aggressive disease.
There are several important limitations to our study. We do not have information about pathological margin status which can be affected by obesity and adversely impact survival. Similarly, the outcomes from multiple surgeons and surgical techniques were evaluated in this study. However, this can be interpreted as adding strength to this study because it reflects real-world practice and extends the generalizability of the results.
The strengths of our study include its large size and the completeness and length of the follow-up. The dose-dependent nature of higher BMI categorical findings, the strength of the association, and the significance of the results after adjusting for multiple pre-operative and postoperative variables add to the internal validity of our study.
Patients defined as obese weight (BMI ≥30 kg/m2) experienced significantly worse recurrence-free, cancer-specific, and overall survival compared to overweight patients (25–29.9 kg/m2) and normal weight patients (<25 kg/m2). The question of whether or not losing weight would improve oncological outcomes was out of the scope of this study but would be an important question to investigate. Furthermore, as the evidence accumulates associating obesity to cancer-specific outcomes across multiple organ systems, the impact of metabolic syndrome is an important area of investigation. Epidemiologic and molecular studies should be encouraged to study the relationship between the metabolic changes associated with obesity and cancer to aid in targeting interventions that will impact cancer-specific outcomes.
Conclusions
The relationship between obesity and diabetes, cardiovascular disease, and various musculoskeletal disorders are well recognized. Although the association between obesity and cancer is receiving more attention, a link between increasing BMI and urothelial cancer has not been conclusively established. The results of this study suggest an association between BMI and worse recurrence and cancer-specific survival. These results are consistent on multivariable analysis controlling for patient and tumor characteristics and across multiple categories of increasing BMI. Future studies should be encouraged to evaluate the molecular mechanisms of tumorigenesis and progression associated with metabolic changes in obesity.
Figure 2.
Univariate Cox regression analysis of association of normal weight (less than 25 kg/m2), overweight (25 to 29.9 kg/m2) and obese (30 kg/m2 or greater) BMI categories with cancer specific survival in 520 patients treated with RNU for UTUC.
Figure 3.
Univariate Cox regression analysis of association of normal weight (less than 25 kg/m2), overweight (25 to 29.9 kg/m2) and obese (30 kg/m2 or greater) BMI categories with overall survival in 520 patients treated with RNU for UTUC.
Acknowledgments
Funding: none
Footnotes
Conflicts of Interest: None
Patient Informed Consent: The data used in this study were reviewed and determined to be exempt human subjects research
URL for Tables
References
- 1.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- 2.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1. [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 5.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. 2000;894:i. World Health Organ Tech Rep Ser. [PubMed] [Google Scholar]
- 6.Batty GD, Shipley MJ, Jarrett RJ, et al. Obesity and overweight in relation to organ-specific cancer mortality in London (UK): findings from the original Whitehall study. Int J Obes (Lond) 2005;29:1267. doi: 10.1038/sj.ijo.0803020. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 8.Moller H, Mellemgaard A, Lindvig K, et al. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A:344. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 9.Pan SY, Johnson KC, Ugnat AM, et al. Association of obesity and cancer risk in Canada. Am J Epidemiol. 2004;159:259. doi: 10.1093/aje/kwh041. [DOI] [PubMed] [Google Scholar]
- 10.Rapp K, Schroeder J, Klenk J, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93:1062. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samanic C, Gridley G, Chow WH, et al. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 12.Wolk A, Gridley G, Svensson M, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 13.Lowrance WT, Thompson RH, Yee DS, et al. Obesity is associated with a higher risk of clear-cell renal cell carcinoma than with other histologies. BJU Int. 105:16. doi: 10.1111/j.1464-410X.2009.08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel EM, Ulrich CM, Poole EM, et al. The effects of obesity and obesity-related conditions on colorectal cancer prognosis. Cancer Control. 17:52. doi: 10.1177/107327481001700107. [DOI] [PubMed] [Google Scholar]
- 15.Freedland SJ, Sun L, Kane CJ, et al. Obesity and oncological outcome after radical prostatectomy: impact of prostate-specific antigen-based prostate cancer screening: results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center databases. BJU Int. 2008;102:969. doi: 10.1111/j.1464-410X.2008.07934.x. [DOI] [PubMed] [Google Scholar]
- 16.Dal Maso L, Zucchetto A, Talamini R, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 17.Parker AS, Lohse CM, Cheville JC, et al. Greater body mass index is associated with better pathologic features and improved outcome among patients treated surgically for clear cell renal cell carcinoma. Urology. 2006;68:741. doi: 10.1016/j.urology.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Waalkes S, Merseburger AS, Kramer MW, et al. Obesity is associated with improved survival in patients with organ-confined clear-cell kidney cancer. Cancer Causes Control. 21:1905. doi: 10.1007/s10552-010-9618-2. [DOI] [PubMed] [Google Scholar]
- 19.Holick CN, Giovannucci EL, Stampfer MJ, et al. Prospective study of body mass index, height, physical activity and incidence of bladder cancer in US men and women. Int J Cancer. 2007;120:140. doi: 10.1002/ijc.22142. [DOI] [PubMed] [Google Scholar]
- 20.Koebnick C, Michaud D, Moore SC, et al. Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2008;17:1214. doi: 10.1158/1055-9965.EPI-08-0026. [DOI] [PubMed] [Google Scholar]
- 21.Hafron J, Mitra N, Dalbagni G, et al. Does body mass index affect survival of patients undergoing radical or partial cystectomy for bladder cancer? J Urol. 2005;173:1513. doi: 10.1097/01.ju.0000154352.54965.14. [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 23.Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 24.Lughezzani G, Jeldres C, Isbarn H, et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of 2299 patients. Eur J Cancer. 2009;45:3291. doi: 10.1016/j.ejca.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Suikkari AM, Koivisto VA, Rutanen EM, et al. Insulin regulates the serum levels of low molecular weight insulin-like growth factor-binding protein. J Clin Endocrinol Metab. 1988;66:266. doi: 10.1210/jcem-66-2-266. [DOI] [PubMed] [Google Scholar]
- 26.Iwamura M, Ishibe M, Sluss PM, et al. Characterization of insulin-like growth factor I binding sites in human bladder cancer cell lines. Urol Res. 1993;21:27. doi: 10.1007/BF00295188. [DOI] [PubMed] [Google Scholar]
- 27.Shariat SF, Kim J, Nguyen C, et al. Correlation of preoperative levels of IGF-I and IGFBP-3 with pathologic parameters and clinical outcome in patients with bladder cancer. Urology. 2003;61:359. doi: 10.1016/s0090-4295(02)02253-7. [DOI] [PubMed] [Google Scholar]
- 28.Hilmy M, Bartlett JM, Underwood MA, et al. The relationship between the systemic inflammatory response and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer. 2005;92:625. doi: 10.1038/sj.bjc.6602406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews B, Shariat SF, Kim JH, et al. Preoperative plasma levels of interleukin-6 and its soluble receptor predict disease recurrence and survival of patients with bladder cancer. J Urol. 2002;167:1475. [PubMed] [Google Scholar]