Abstract
Objectives
We examined whether “state” anger regulation—inhibition or expression—among chronic low back pain (CLBP) patients would affect lower paraspinal (LP) muscle tension following anger-induction, and whether these effects were moderated by trait anger management style.
Method
Eighty-four CLBP patients underwent harassment, then they regulated anger under one of two conditions: half expressed anger by telling stories about people depicted in pictures, whereas half inhibited anger by only describing objects appearing in the same pictures. They completed the anger-out and anger-in subscales (AOS; AIS) of the anger expression inventory.
Results
General Linear Model procedures were used to test anger regulation condition by AOS/AIS by period interactions for physiological indexes. Significant three-way interactions were found such that: a) high trait anger-out patients in the inhibition condition appeared to show the greatest LP reactivity during the inhibition period followed by the slowest recovery; b) high trait anger-out patients in the expression condition appeared to show the greatest systolic blood pressure (SBP) reactivity during the expression period followed by rapid recovery.
Conclusions
Results implicate LP muscle tension as a potential physiological mechanism that links the actual inhibition of anger following provocation to chronic pain severity among CLBP patients. Results also highlight the importance of mismatch situations for patients who typically regulate anger by expressing it. These CLBP patients may be at particular risk for elevated pain severity if circumstances at work or home regularly dictate that they should inhibit anger expression.
Keywords: anger regulation, trait anger-out, trait anger-in, symptom-specific reactivity, chronic pain
INTRODUCTION
Anger is related to both acute (1–4) and chronic pain intensity (1,5–7). Findings suggest that the manner in which anger is regulated—either inhibition (anger-in) or expression (anger-out) of angry feelings—is a particularly reliable determinant of chronic pain severity (1,5,6). Nevertheless, investigators have proposed and tested few physiological mechanisms to explain how trait anger management style (the tendency to express or inhibit anger across situations) or “state” anger regulation (the actual regulation of anger in a given situation) may affect the course and/or severity of chronic pain.
Anger, hostility, and anger management style are related to physiological reactivity to stress (8–10). Although much research has focused on the cardiovascular components of sympathetic and parasympathetic nervous system reactivity, anger variables may also be related to activation of the skeletal muscles. Investigators speculate that physical and psychological stress may lead to frequent and intense, or low level but sustained muscular contractions (11), which can increase pain through ischemic hypoxia (12) and changes in mechanoreceptor sensitivity (13). Flor et al. (14–16) proposed a “symptom-specificity” model of chronic pain based on principles of individual-response stereotypy (17). Briefly, chronic low back pain (CLBP) patients can be expected to show aberrant stress-induced muscular responses specific to the disorder. That is, they may reveal strong contraction of low back muscles (i.e., lower paraspinals; (LP)) during stress while not necessarily showing such tension increases in muscle groups distant from the pain site. Findings support symptom-specificity models among CLBP patients (14–16,18–21) and those with neck and shoulder pain (22,23). Moreover, LP reactivity to stress was related significantly to reports of everyday CLBP severity, whereas trapezius tension increases (muscles distant from the pain source) were not (24).
Adapting the symptom-specificity model, Burns (25) proposed that anger regulation—state or trait—may be related to heightened chronic pain severity to the degree that anger regulation affects muscle contraction near the site of pain or injury. For CLBP patients, anger regulation should influence LP reactivity more strongly than in muscle groups further from the pain site. Indeed, findings suggest that trait anger-out, anger-in, and hostility interact to predict LP tension increases among CLBP patients evoked during anger arousal; effects not evident for trapezius muscles (25,26).
Although promising, few studies have distinguished between effects of trait anger management style and state anger regulation, relying instead on self-reports of trait anger-in and anger-out; a method that poses two problems. First, trait anger-in and anger-out measures are related moderately to measures of general negative affect (NA; 1,3,5,6). Thus, observed relationships among anger management style, physiological reactivity and pain may be largely explained by overlap with NA. This appears to be the case especially for links between trait anger-in and pain (5,6,27–29). Second, effects of trait anger management are often assumed to reflect effects of how anger is actually regulated in the face of provocation. Given the overlap with NA, it may be premature to conclude that effects of trait anger-in and anger-out accurately depict unique influences of actual cognitive and behavioral maneuvers undertaken to regulate anger. To illustrate, numerous studies suggest that self-reported trait anger-out is related to high levels of stress-induced cardiovascular reactivity (8–10,30) and LP muscle tension increases (25,26). Studies manipulating anger-out behaviors experimentally have produced a somewhat more complex pattern of results, suggesting that loud verbal expression of anger may initially cause increased cardiovascular function (31), but that such expression may then lead to more rapid cardiovascular recovery than inhibiting expression (32,33). Hence, it seems that trait anger-out and actual anger expressive behaviors affect physiological arousal in opposite directions.
One way to settle these divergent findings for trait versus state anger regulation would be to examine interactions between traits and situational anger expression or inhibition. Engebretson et al. (30) argued that many studies of anger management style have not allowed actual anger regulation behaviors to occur during or following provocation. They proposed that physiological responses to anger-provocation would differ depending on whether people use the kind of anger management they preferred. A mismatch would occur where, for instance, people who habitually express anger are forced by circumstances to inhibit it. Evidence generally supports mismatch models (see e.g., Refs. 30,34), such that state anger inhibition may affect physiological recovery in general, but it may make recovery especially prolonged for those characterized by a tendency to express anger.
In the present study, we examined whether state anger regulation affected symptom-specific LP reactivity to, and recovery from anger provocation among CLBP patients, and whether trait anger management style moderated these effects. Briefly, all participants underwent mental arithmetic with harassment, and then performed an anger regulation task under either expression or inhibition conditions. In the expression condition, participants told stories aloud in response to three pictures depicting people interacting, thereby allowing them to express anger, albeit indirectly, through the affective tone of their narratives. In the inhibition condition, participants only described objects that appeared in the same three pictures, thus preventing them from verbally expressing their anger.
If situational mismatch models are valid for symptom-specific reactivity, then trait anger-out should interact with state anger regulation condition to affect LP muscle tension recovery, such that trait anger-out would relate to higher baseline-to-recovery change scores in the inhibition condition than in the expression condition. Such effects were not expected for trapezius muscle recovery. Based on past findings for cardiovascular function, similar effects were expected for blood pressure and heart rate. We also examined whether people high on trait anger-in would find the expression condition a mismatch, and so reveal enhanced physiological reactivity and prolonged recovery.
METHOD
Participants
Eighty-four CLBP patients were recruited through advertisements and postings at pain clinics from July 2005 to June 2006, and were paid $40. The study protocol was approved by the Institutional Review Board of Rosalind Franklin University. Exclusion criteria were: a) current cardiovascular disorder or use of medications affecting cardiovascular function; b) chronic pain from malignant conditions (i.e., cancer); c) current alcohol or substance abuse problems; d) a history of psychotic or bipolar disorders; e) daily use of opioid analgesic medication; f) inability to speak English well enough to participate in the tasks. Inclusion criteria were: a) pain in the lower back from degenerative processes, muscular or ligamentous strain, or disk herniation as determined by a physician; b) pain duration of at least 6 months. Patients who reported occasional use of opioid medications were asked not to take these on the morning of their appointments. The sample was comprised of 54.8% (n = 46) women. Further descriptive information appears in Table 1.
TABLE 1.
Descriptive Information(N = 84)
| Variables | Statistics |
|||
|---|---|---|---|---|
| M | SD | Percent | n | |
| Age (yr) | 46.0 | 12.3 | ||
| At least 12-yr of education | 89.3 | 75 | ||
| Ethnicity | ||||
| Caucasian | 56.04 | 47 | ||
| Hispanic | 11.9 | 10 | ||
| African American | 32.1 | 27 | ||
| Pain duration (mo) | 25.4 | 28.0 | ||
| Opioid analgesics | 15.5 | 13 | ||
| Nonsteroidal | 63.1 | 53 | ||
| Anti-inflammatory | ||||
| Muscle relaxants | 7.0 | 6 | ||
| Antidepressants | 5.9 | 5 | ||
Design Overview
Participants performed mental arithmetic with experimenter harassment, underwent the anger regulation task, and then recovered. The mental arithmetic procedure was identical for all participants. For the anger regulation task, participants were randomly assigned to one of two conditions. In the expression condition, participants told stories about people depicted on cards (from the Thematic Apperception Test (TAT) (35)) In the Inhibition condition, participants only described objects that appeared in the pictures.
Measures
Recording EMG
EMG activity was recorded from left and right LPs (L2-L4), and left and right trapezius muscles. Silver/silver chloride 8-mm electrodes were spaced 15 mm apart for bipolar recording (36). Sites were prepared with vigorous alcohol abrasion. Interelectrode impedance was kept below 10 kohms. Bio-amplifiers with bandpass filters (Coulbourn Instruments) were used to record EMG. Raw EMG signals were amplified by a factor of 100,000. The sampling rate was 10/s, and signals were passed through narrow bandpass filters (100–250 Hz). Signals were integrated and “smoothed” with contour following and cumulative integrators (Coulbourn Instruments). Per recommendations (36), the time constant for integration was 100 ms. Data were collected by computer through A/D conversion using Wingraph software.
Recording Cardiovascular Indexes
Systolic (SBP) and diastolic blood pressure (DBP) and heart rate (HR) were measured with a Dinamap 1846 SX oscillometric BP monitor (Johnson & Johnson Medical Inc.). Readings were obtained every 60 seconds. Data were collected by computer through A/D conversion using Wingraph software.
Anger Management Style
Tendencies to inhibit and express anger were assessed with the anger expression inventory (37), which has subscales to measure anger-in (AIS) and anger-out (AOS), and for which Spielberger et al. (37) reported adequate internal consistency coefficients.
Mental Arithmetic
Participants performed serial subtractions by 7 from 8469 and were told to work as fast and accurately as possible. During the first 2 minutes, the experimenter made five standardized comments (e.g., “Please go faster!” “You’re making too many mistakes!”). After 2 minutes, the participant was stopped and the experimenter said, “No. Too many mistakes. And I want you to go fast. Let’s start again, but this time try subtracting by 3 from 2000. How about it?” During the next 2 minutes, the experimenter again made five standardized comments. After 2 minutes, the participant was stopped.
Anger Regulation Task
Overview
This task was designed to allow participants to express anger aroused during mental arithmetic through the content of stories told about people portrayed in a standardized set of pictures, or to prevent them from doing so by having them only describe objects appearing in the pictures. This operationalization of “anger regulation” is similar to other studies in which anger inhibition occurred by having participants describe persons, things, or events unrelated to the angering episode (e.g., their best friend instead of the harassing confederate, per Dorr et al. (32)). Three cards from Murray’s (35) TAT were used as stimuli. The cards used were 12M, 9GF, and 4, with the intent of having a set of stimuli in which one card featured only male characters, one card showed only female characters, and one card showed a male-female couple.
Expression Condition
Participants in this condition told a story about what they thought was happening in each picture. They were encouraged to tell stories that had a beginning, middle, and end. The experimenter did not evaluate or make comments about the participant’s stories, but merely facilitated progress from story to story with comments such as, “Okay, here’s the next picture.” Although never stated, stories were limited to 2 minutes. If the participant continued past 2 minutes, the experimenter stopped him or her and introduced the next card with the comment, “Okay, let’s look at the next one.” The TAT card procedure to elicit poststressor emotional expression was adopted from Cramer (38), and we have used it in two previous studies (34,39).
Inhibition Condition
Participants in this condition described only the physical appearance of objects in the TAT cards (e.g., furniture, landscape features, people’s ages or clothing), but were told not to describe people’s expressions. Thus, they were guided away from telling a story about what they thought might be happening. The time limit for each card was 2 minutes.
Coding Expressed Emotion
Verbal responses to the cards were audiotaped and later transcribed. The frequencies of anxiety, anger, sadness, and positive emotion words expressed through participants’ responses were coded using the linguistic inquiry and word count (LIWC) (40). The LIWC operates through computer software that counts the frequency of certain word types in transcripts. Here, variability in the four word-type frequencies was defined as variability in (indirect) expression of negative and positive emotion.
Procedure
Participants were randomly assigned to expression or inhibition conditions. At the laboratory, the participant was screened, signed an informed consent form, and was seated upright in a comfortable chair. The blood pressure cuff and electrodes were attached, and the participant then sat quietly for 10 minutes while resting EMG, SBP, DBP, and HR readings were taken. Participants were told that after the mental arithmetic, they would look at some pictures. Participants in the expression condition were told that they would tell stories about people and events shown in the card, whereas participants in the inhibition condition were told that they would describe things they saw in the pictures. Instructions were then given for the mental arithmetic, and it began. At the end of mental arithmetic, the original instructions for the anger regulation task were repeated, and participants in the expression condition were further told to tell stories with a beginning, middle and end, whereas those in the inhibition condition were told only to describe objects and not to talk about the people. After the participant was finished responding to the last TAT card, the blood pressure cuff and electrodes were removed and the participant was debriefed.
Data Reduction and Analyses
For LP and trapezius EMG, readings from left and right sites were summed and averaged. Baseline values for EMG, SBP, DBP, and HR were defined as the mean of readings taken during the last 3 minutes of the 10-minute resting period. Mental arithmetic and anger regulation task values for EMG, SBP, DBP, and HR were defined as the mean of readings taken during the respective tasks. Recovery values were examined for the first, third, and fifth minutes following the anger regulation task, and were defined as the mean of readings taken during each of these epochs.
We first determined whether mental arithmetic—which preceded the anger regulation task—affected physiological arousal approximately equally for participants assigned to subsequently express or inhibit. Anger regulation condition (express; inhibit) by period (baseline, mental arithmetic) mixed design analysis of variances (ANOVAs) were tested for EMG and SBP, DBP and HR levels.
Next, we examined the validity of our anger regulation manipulations by testing whether the expression and inhibition conditions differed on the expression of negative and positive emotion words to the TAT cards. The frequencies of anxiety, anger, sadness, and positive emotion words were computed with the LIWC. Anger regulation condition (express, inhibit) ANOVAs were tested for word frequencies. We also evaluated the validity of the AOS and AIS to predict actual behaviors by generating correlations among these scores and the four-word frequencies for participants in the expression condition.
We then tested whether AOS and AIS moderated anger regulation condition effects on EMG and cardiovascular function during the anger regulation task and/or during recovery. Change scores were computed by subtracting values recorded during mental arithmetic, anger regulation task, and at 1-, 3-, and 5-minute into recovery from baseline values. Using AOS as an example, General Linear Model procedures tested anger regulation condition (express; inhibit) by AOS scores (continuous) by period (mental arithmetic change, anger regulation task change, 1-minute change, 3-minute change, 5-minute change) effects for each physiological index. Significant interactions for physiological change involving AOS scores were pursued with multiple regressions that examined anger regulation condition by AOS simple interactions at each epoch. Analyses were repeated for AIS scores.
RESULTS
Physiological Changes From Baseline to Mental Arithmetic
The anger regulation condition (express, inhibit) by period (baseline, mental arithmetic) mixed design ANOVAs revealed nonsignificant interactions for all physiological indexes (F values (1,82) < 1). Main effects for period were evident for LP (F(1,82) = 37.04;p < .01), trapezius (F(1,82) = 11.38;p < .01), SBP (F(1,82) = 99.86; p < .01), DBP (F(1,82) = 99.31; p < .01), and HR changes (F(1,82) = 70.47; p < .01) in directions indicating increases from baseline to task. Thus, participants in the two anger regulation conditions showed statistically comparable muscle tension and cardiovascular reactivity with anger instigation before undergoing the different regulation manipulations.
Anger Regulation Condition Effects on Word Frequencies
The anger regulation condition (express, inhibit) ANOVAs showed a significant effect for anger word frequency (F(1,82) = 6.91; p < .01) such that expression condition participants used more anger words in their stories (as a percentage of total words; M = 0.36; SD = 0.56) than inhibition condition participants (M = 0.08; SD = 0.33). Expression condition participants also used more sadness words (F(1,82) = 10.77; p < .002; M = 0.79; SD = 0.77) than those in the inhibition condition (M = 0.28; SD = 0.60). However, the groups did not differ significantly on anxiety words (F < 1) or on the use of positive emotion words (F(1,82) = 1.01; p > .10). Given the nature of the anger regulation manipulations, it was not surprising that expression condition participants responded to the cards with more emotion words than inhibition condition participants, but the differences between the groups were specific to anger and sadness words and did not extend to use of positive emotion words. Results suggest that expression condition participants tended to express anger (and sadness) themes in particular through their stories, and did not just express more emotion in general than participants instructed to only describe objects.
Correlations Between Anger Management Styles and Word Frequencies
Correlation coefficients were generated among the AOS, AIS, and anxiety, anger, sadness, and positive emotion word frequencies for the 42 participants in the expression condition. The AOS was correlated significantly only with anger word frequency (r = 0.32; p < .05). Correlations between the AOS and anxiety, sadness and positive emotion word frequencies were r = 0.13, r = −0.20, and r = −0.02, respectively. The AIS was correlated significantly only with the sadness word frequency (r = − 0.33;p < .05). Correlations between the AIS and anxiety, anger and positive emotion word frequencies were r = 0.20, r = 0.08 and r = −0.23, respectively. Results support the construct validity of the AOS in which scores significantly predicted only the frequency of anger words expressed following harassment, but did not predict use of other negative emotion words.
Condition by Anger Management Styles by Period Effects
LP Changes
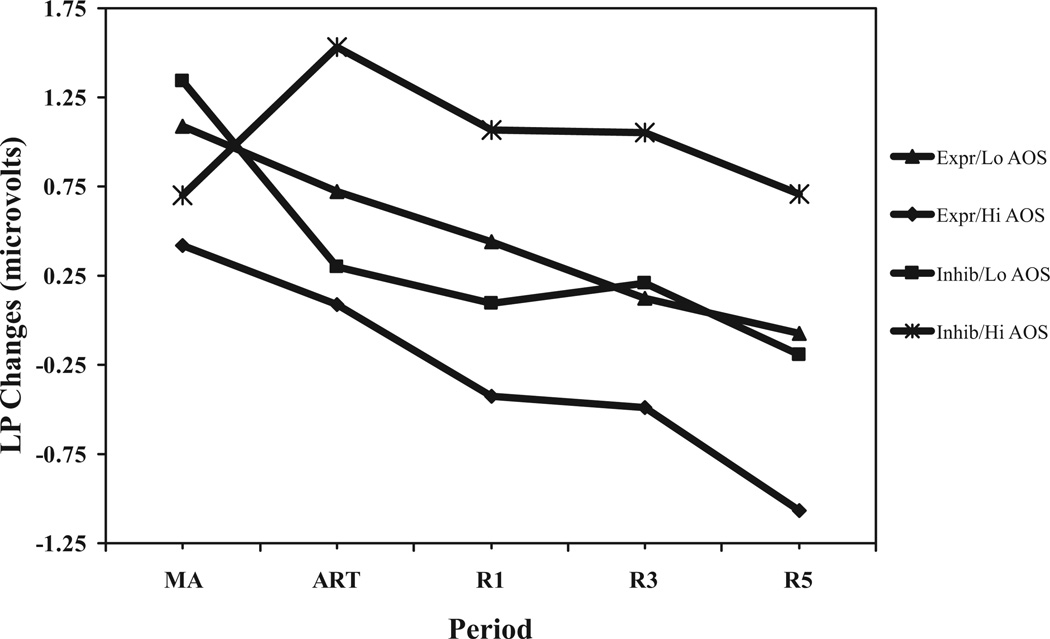
The anger regulation condition (express, inhibit) by AOS (continuous) by period (mental arithmetic change, anger regulation task change, 1-minute change, 3-minute change, 5-minute change) interaction was significant (F(4,316) = 3.04;p < .02). To dissect this interaction, simple interactions were examined at each epoch. For LP change during mental arithmetic, the anger regulation condition by AOS interaction was nonsignificant (F(3,79) < 1). However, for LP change during the anger regulation task, and at 1-minute, 3-minute, and 5-minute recovery, the anger regulation condition by AOS interactions were significant (F values (3,79) >4.32; p values <.007). To illustrate the interactions and the pattern of effects over time, regression equations for each epoch were solved by condition and for hypothetical AOS scores (±1 SD from the mean). As shown in Figure 1, results suggest that, although an anger-out style did not significantly affect LP change during harassment, high anger-out participants in the inhibition condition showed the greatest LP muscle tension increases during the anger regulation task, and continued to show high levels throughout the 5-minute of recovery. High anger-out participants in the expression condition appeared to recover rapidly.
Figure 1.
Anger regulation condition by AOS by period for LP changes. Expr/Lo AOS = participants in express condition with hypothetical AOS values − 1 SD from mean. Expr/Hi AOS = participants in express condition with hypothetical AOS values +1 SD from mean. Inhib/Lo AOS = participants in inhibit condition with hypothetical AOS values − 1 SD from mean. Inhib/Hi AOS = participants in inhibit condition with hypothetical AOS values +1 SD from mean. LP changes = simple change scores. MA = mental arithmetic. ART = anger regulation task. R1, R3, and R5 = 1-minute, 3-minute, and 5-minute into recovery, respectively.
The anger regulation condition by AIS by period interaction (see above) was nonsignificant (F(4,316) = 1.39; p > .10). In the absence of a significant overall interaction, a significant main effect for anger regulation condition (collapsed over period) was revealed (F(1,79) = 4.22;p < .04) such that expression condition participants showed lower LP change averaged across epochs (M = 0.08; SD = 1.1) than Inhibition condition participants (M = 0.59; SD = 1.3).
Trapezius Changes
The anger regulation condition by AOS by period interaction (see above) for trapezius changes was nonsignificant (F(4,316) < 1). Other two-way interactions and main effects were nonsignificant (F values < 1.62). For the AIS, effects were also nonsignificant.
SBP Changes
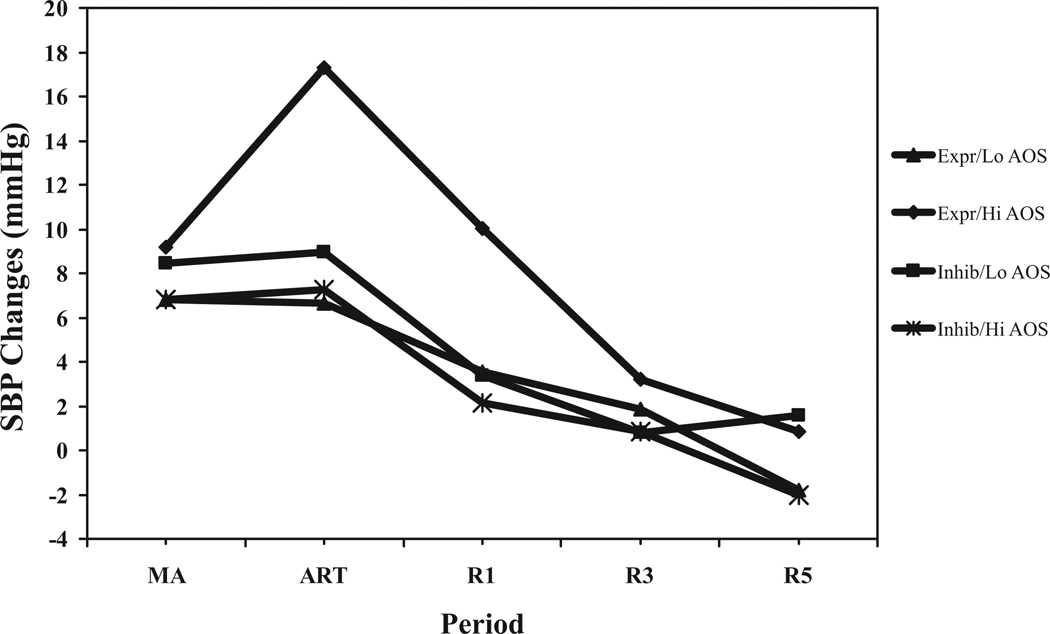
The anger regulation condition by AOS by period interaction (see above) was significant (F(4,316) = 2.87;p < .03) for SBP changes. Simple interactions were examined at each epoch. For SBP changes during mental arithmetic, the anger regulation condition by AOS interaction was nonsignificant (F(3,79) = 1.41). For SBP changes during the anger regulation task and at 5-minute recovery, the anger regulation condition by AOS interactions were significant (F values (3,79) <3.13;p values <.04). For SBP changes during 1-minute and 3-minute recovery, interactions were nonsignificant. To illustrate, the regression equations for each epoch were solved by condition and for hypothetical AOS scores (±1 SD from the mean). As shown in Figure 2, and similar to LP changes, results suggest that an anger-out style did not significantly affect SBP change during mental arithmetic. However, high anger-out participants in the expression condition appeared to show the greatest levels of SBP reactivity during the anger regulation task—that is, while expressing anger—and then appeared to experience swift recovery. High anger-out participants in the inhibition condition, despite being in a mismatch situation, seemed not to show a prolonged SBP recovery.
Figure 2.
Anger regulation condition by AOS by period for SBP changes. Expr/Lo AOS = participants in express condition with hypothetical AOS values — 1 SD from mean. Expr/Hi AOS = participants in express condition with hypothetical AOS values +1 SD from mean. Inhib/Lo AOS = participants in inhibit condition with hypothetical AOS values — 1 SD from mean. Inhib/Hi AOS = participants in inhibit condition with hypothetical AOS values +1 SD from mean. SBP changes = simple change scores. MA = mental arithmetic. ART = anger regulation task. R1, R3, andR5 = 1-minute, 3-minute, and 5-minuteinto recovery, respectively.
Effects for AIS scores were nonsignificant.
DBP Changes
The anger regulation condition by AOS by period interaction (see above) was nonsignificant (F(4,316) = 1.67;p >.10) for DBP changes. No other interaction effects were significant. A significant main effect for anger regulation condition (collapsed over period) did emerge (F(1,79) = 7.39;p < .008) such that expression condition participants showed greater DBP changes averaged across epochs (M = 2.94; SD = 4.1) than participants in the inhibition condition (M = 0.51; SD = 3.6).
There were no significant effects for AIS scores.
HR Changes
The anger regulation condition by AOS by period interaction (see above) was nonsignificant (F(4,316) = 1.76; p > .10) for HR changes. However, the AOS by period effect was significant (F(4,316) = 3.00; p < .02). To dissect this interaction, correlations between AOS scores and HR changes at each epoch were computed. Results suggest that the interaction was characterized by a significant correlation between AOS scores and HR changes during mental arithmetic (r = 0.34; p < .01), and nonsignificant correlations during the anger regulation task and recovery (range, r = 0.04 to r = 0.17; p values > .10). Thus, an anger-out style was related to higher HR reactivity during harassment.
AIS effects were nonsignificant.
DISCUSSION
We examined a potential physiological mechanism by which anger regulation may be linked to pain in CLBP patients; namely, increased lower back muscle tension. Research has provided somewhat contradictory findings for effects of trait and state anger regulation on physiological reactivity to stress, and we adopted a mismatch model (30) to help address these discrepancies. Our chief aim was to determine whether CLBP patients who reported a predominant tendency to express anger would reveal a prolonged recovery in LP muscle tension following anger instigation when actual anger expression was inhibited. Hypotheses were generally supported.
It should be noted that expression condition participants used more anger and sadness words in response to the pictures than inhibition condition participants. This effect could have been due to the former group merely using more emotional words in general when telling stories about people and events, but this explanation is not supported because the two experimental conditions did not differ in frequency of positive emotion words. The opportunity to express following harassment appeared to augment the expression of negative emotion in particular. By implication, the inhibition condition participants, having also been harassed, seemed not to express the anger and sadness they would have expressed had they been allowed to tell stories rather than only describe objects. These manipulation checks lend credence to our claim that participants in the inhibition condition did in fact inhibit anger expression.
Although inhibition condition participants showed greater LP tension increases throughout the anger regulation and recovery periods than expression condition participants, these effects appeared most pronounced among CLBP patients who reported a predominant anger-out disposition to express anger in an outward and aggressive fashion. These results underscore not only the importance of trait anger management in predicting physiological reactivity, but also emphasize the importance of person by situation interactions. In this case, the effect of state anger inhibition on LP reactivity was greatest among people for whom inhibition presented a mismatch to their preferred style of regulating anger. Episodes of anger inhibition may lead to symptom-specific muscle tension, and hence to chronic pain aggravation, among CLBP patients in general, but such inhibition appears to disproportionately affect those reporting an anger-out style.
Other findings also support a symptom-specificity model. Effects of state anger regulation and interactions with anger management style were nonsignificant for trapezius muscle reactivity. It is not clear whether individual response stereotypy characterized by stress-induced tension in one muscle group actually predisposes an individual to develop a certain chronic pain condition. However, problematic responses in discrete muscle groups may serve to aggravate existing pain conditions. Sustained muscle tension following mental stress or low level physical exertion have been shown to characterize people with trapezius myalgia (22,41). Hagg (42) and Lundberg et al. (22,23) hypothesize that weak yet sustained muscle tension may be linked to metabolic problems and exhaustion in the affected muscles, which may in turn underlie degenerative processes that cause increased pain sensitivity. Moreover, even weak but constant activation may impede restoration and recuperation of taxed muscle fibers. Findings suggest that mental stress may be especially damaging in maintaining low level activation (11,23), perhaps because people continue to ruminate about what happened (43,44). Thus, CLBP patients’ maintenance of low level LP tension during anger inhibition may continue the process of fatigue and damage of muscle fibers near the site of pain or injury. Whether because of continued cognitive preoccupation or physically bracing against pain spasms experienced during anger arousal (20), the prolonged LP recovery from anger induction shown by patients who inhibit anger expression may represent an important pathophysiological phenomenon worthy of additional empirical study.
Findings for cardiovascular reactivity also point to interesting person by situation interactions. First, people with a predominant anger-out style showed greater HR reactivity during harassment than people low on anger-out; findings consistent with past results. Second, when given an opportunity to express, albeit indirectly through story content, cardiovascular arousal of high anger-out participants actually appeared to increase—as indexed by SBP changes. Recall that the AOS was correlated with anger word frequency, indicating that high anger-out patients did indeed express anger at a high level compared with low anger-out participants. These results are consistent with Siegman’s “angry voice” findings (31), and suggest that actual anger expression leads to increased arousal, at least during the very act of expressing. Further, in our findings, expression for high anger-out participants appeared to be followed by a relatively swift SBP recovery comparable to other groups. These results are consistent with those reported by Engebretson et al. (30). Taken together, the present pattern of results may be seen as a step toward integrating some disparate findings. Anger expressors may be prone to anger and arousal during provocation, and in an attempt to regulate or reduce the anger they may temporarily make matters worse by increasing physiological arousal during the act of physically and/or verbally expressing, the result of which is to hasten return to resting levels.
That cardiovascular function was elevated in the Expression condition and LP reactivity was elevated in the Inhibition condition among high anger-out patients may point to the intriguing possibility of discrete patterns of inhibition-induced physiological reactivity and recovery for certain patient groups. That is, high anger-out patients suffering from low back pain may reveal exaggerated effects of anger inhibition only in their most vulnerable system; namely, low back muscles.
Findings for trait anger-in did not support a large role. It should be emphasized that null effects for the AIS do not necessarily extend to anger inhibition in general. Results here and in other studies where anger was deliberately suppressed via laboratory manipulations reveals effects of actual – state – inhibition on a host of dependent variables (45–47). Our null findings may point to the value of further investigating the construct validity of the AIS, which at present appears to tap too much general NA and too little of any unique elements of actual anger inhibition (indeed, the correlation between AIS scores and anger word frequency was virtually nil, r = 0.08).
Some limitations need be delineated. First, the method used to instigate anger expression involved indirect expression through telling stories rather than directly confronting the antagonist or at least speaking or writing about him or her. The indirect procedure has the advantage of allowing participants to express feelings without being explicitly prompted to write about the experimenter or confederate, as was done in Engebretson et al. (30) and Lai and Linden (48), and thus may have limited expectancy or social desirability effects. However, the indirect procedure then leaves us to infer that the stories told and the kind of words uttered reflect the expression of emotional arousal (anger) provoked by the antagonist, and are not merely routine responses to the scenes depicted on the cards. Without unambiguous references in speech or writing to the experimenter or confederate or the stressful task, we simply cannot be certain that the emotion expressed through the stories is actually directed at the antagonist. Second, the method used to impose anger inhibition shares some features with manipulations used recently to affect distraction; a maneuver that appears to facilitate recovery from anger (44,49). Here, and in studies using distraction manipulations (32,44,49), participants were instructed to direct their attention to stimuli that were unrelated to the angering episode. Although Dorr et al. (32) reported results consistent with hypothesized effects of anger inhibition using a manipulation similar to what we used, our results for cardiovascular function—with equivalent recovery rates for expression and inhibition conditions—may sound a note of caution regarding whether or not describing objects in TAT cards following provocation constitutes deliberate anger inhibition.
Anger regulation affects the intensity of acute and chronic pain, and may do so by definable physiological mechanisms. Here, we found that participants who appeared to inhibit anger following provocation revealed greater sustained LP muscle tension—a physiological index linked to everyday pain in CLBP patients—compared with participants who were allowed to express anger. This relationship was complicated, however, because the detrimental effects of inhibition seemed localized to those participants with high trait anger-out. Thus, anger inhibition may disproportionately affect certain individuals. For CLBP patients who prefer to express anger, inhibiting anger under certain circumstances may lead to greater symptom-specific LP tension and hence to more pain aggravation. Brosschot and Thayer (50) have argued that anger inhibition may play a large role in producing or exacerbating physical disorder because of social norms that discourage full expression; a factor that may affect dispositional anger expressors most acutely. A full understanding of how, under what conditions and among whom in particular anger inhibition detrimentally affects reactivity and pain appears essential for the development of effective clinical interventions.
Acknowledgments
The authors gratefully acknowledge the encouragement and generosity of Kenneth Lofland, PhD, and the help and cooperation of the staff at the Pain and Rehabilitation Clinic of Chicago, without which this study would not have been possible.
Supported in part by Grants NS37164 from the National Institute of Neurological Disorders and Stroke (to J.W.B.), MH071260 from the National Institute of Mental Health (to J.W.B., S.B.), NS050578 from the National Institute of Neurological Disorders and Stroke (to S.B.), and by Grant F31 NS051200-01A1 from the National Institute of Neurological Disorders and Stroke (to P.J.Q.).
Glossary
- CLBP
chronic low back pain
- EMG
electromyography
- AOS
anger-out scale
- AIS
anger-in scale
- LP
lower paraspinal muscles
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- HR
heart rate
- LIWC
linguistic inquiry and word count
REFERENCES
- 1.Bruehl S, Burns J, Chung OY, Ward P, Johnson P. Anger and pain sensitivity in chronic low back pain patients and pain-free controls: the role of endogenous opioids. Pain. 2002;99:223–233. doi: 10.1016/s0304-3959(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 2.Burns JW, Bruehl S, Caceres C. Anger management style, blood pressure reactivity and acute pain sensitivity: evidence for a “trait x situation” model. Ann Behav Med. 2004;27:195–204. doi: 10.1207/s15324796abm2703_7. [DOI] [PubMed] [Google Scholar]
- 3.Burns JW, Kubilus A, Bruehl S. Emotion-induction moderates effects of anger management style on acute pain sensitivity. Pain. 2003;106:109–118. doi: 10.1016/s0304-3959(03)00298-7. [DOI] [PubMed] [Google Scholar]
- 4.Janssen SJ, Spinhoven P, Brosschot JF. Experimentally induced anger, cardiovascular reactivity, and pain sensitivity. J Psychosom Res. 2001;51:479–485. doi: 10.1016/s0022-3999(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 5.Burns JW, Johnson BJ, Mahoney N, Devine J, Pawl R. Anger management style, hostility and spouse responses: gender differences in predictors of adjustment among chronic pain patients. Pain. 1996;64:445–453. doi: 10.1016/0304-3959(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 6.Kerns RD, Rosenberg R, Jacob MC. Anger expression and chronic pain. J Behav Med. 1994;7:57–67. doi: 10.1007/BF01856882. [DOI] [PubMed] [Google Scholar]
- 7.Wade JB, Price DD, Hamer RM, Schwartz SM, Hart RP. An emotional component analysis of chronic pain. Pain. 1990;40:303–310. doi: 10.1016/0304-3959(90)91127-5. [DOI] [PubMed] [Google Scholar]
- 8.Finney M, Stoney CM, Engebretson TO. Hostility and anger expression in African American and European American men is associated with cardiovascular and lipid reactivity. Psychophysiology. 2002;39:340–349. doi: 10.1017/s0048577201393101. [DOI] [PubMed] [Google Scholar]
- 9.Burns JW. Interactive effects of traits, states, and gender on cardiovascular reactivity during different situations. J Behav Med. 1995;18:279–303. doi: 10.1007/BF01857874. [DOI] [PubMed] [Google Scholar]
- 10.Lavoie KL, Miller SB, Conway M, Fleet RP. Anger, negative emotions, and cardiovascular reactivity during interpersonal conflict in women. J Psychosom Res. 2001;51:503–512. doi: 10.1016/s0022-3999(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 11.Sjegaard G, Lundberg U, Kudefors R. The role of muscle activity and mental load in the development of pain and degenerative processes of the muscle cell level during computer work. Eur J Appl Phys. 2000;83:99–105. doi: 10.1007/s004210000285. [DOI] [PubMed] [Google Scholar]
- 12.Fields HL. Pain. New York: Raven; 1987. [Google Scholar]
- 13.Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241–289. doi: 10.1016/0304-3959(93)90027-M. [DOI] [PubMed] [Google Scholar]
- 14.Flor H, Birbaumer N, Schulte W, Roos R. Stress-related electromyographic responses in patients with chronic temporomandibular pain. Pain. 1991;46:145–152. doi: 10.1016/0304-3959(91)90069-A. [DOI] [PubMed] [Google Scholar]
- 15.Flor H, Turk DC, Birbaumer N. Assessment of stress-related psychophysiological reactions in chronic back pain patients. J Consult Clin Psychol. 1985;53:354–364. doi: 10.1037//0022-006x.53.3.354. [DOI] [PubMed] [Google Scholar]
- 16.Flor H, Birbaumer N, Schugens MM, Lutzenberger W. Symptom- specific psychophysiological responses in chronic pain patients. Psychophysiology. 1992;29:452–460. doi: 10.1111/j.1469-8986.1992.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 17.Andreassi JL. Psychophysiology: human behavior and physiological response. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. [Google Scholar]
- 18.Burns JW, Wiegner S, Derleth M, Kiselica K, Pawl R. Linking symptom-specific physiological reactivity to pain severity in chronic low back pain patients: a test of mediation and moderation models. Health Psychol. 1997;16:319–326. doi: 10.1037//0278-6133.16.4.319. [DOI] [PubMed] [Google Scholar]
- 19.Arena JG, Sherman RA, Bruno GM, Young TR. Electromyographic recordings of low back pain subjects and non-pain control is six different positions: effects of pain levels. Pain. 1991;45:23–28. doi: 10.1016/0304-3959(91)90160-Y. [DOI] [PubMed] [Google Scholar]
- 20.Peters ML, Schmidt AJ. Psychophysiological responses to repeated acute pain stimulation in chronic low back pain patients. J Psychosom Med. 1991;35:59–74. doi: 10.1016/0022-3999(91)90007-b. [DOI] [PubMed] [Google Scholar]
- 21.Flor H, Knost B, Birbaumer N. The role of operant conditioning in chronic pain: an experimental investigation. Pain. 2002;95:111–118. doi: 10.1016/s0304-3959(01)00385-2. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg U, Dohns IE, Melin B, Sandsjo L, Palmeund G, Kadefors R, Ekstron M, Parr D. Psychophysiological stress responses, muscle tension, and neck and shoulder pain among supermarket cashiers. J Occup Health Psychol. 1999;4:245–255. doi: 10.1037//1076-8998.4.3.245. [DOI] [PubMed] [Google Scholar]
- 23.Lundberg U, Kadefors R, Melin B, Palmeund G, Hassmen P, Engstrom M, Dohns IE. Psychophysiological stress and EMG activity of the trapezius muscle. Int J Behav Med. 1994;1:354–370. doi: 10.1207/s15327558ijbm0104_5. [DOI] [PubMed] [Google Scholar]
- 24.Burns JW. Arousal of negative emotions and symptom-specific reactivity in chronic low back pain patients. Emotion. 2006;6:309–319. doi: 10.1037/1528-3542.6.2.309. [DOI] [PubMed] [Google Scholar]
- 25.Burns JW. Anger management style and hostility: predicting symptom-specific physiological reactivity among chronic low back pain patients. J Behav Med. 1997;20:505–525. doi: 10.1023/a:1025564707137. [DOI] [PubMed] [Google Scholar]
- 26.Burns JW, Bruehl S, Quartana PJ. Anger management style and hostility among chronic pain patients: effects on symptom-specific physiological reactivity during anger- and sadness-recall interviews. Psychosom Med. 2006;68:786–793. doi: 10.1097/01.psy.0000238211.89198.e4. [DOI] [PubMed] [Google Scholar]
- 27.Burns JW, Johnson BJ, Devine J, Mahoney N, Pawl R. Anger management style and the prediction of treatment outcome among male and female chronic pain patients. Behav Res Ther. 1998;36:1051–1062. doi: 10.1016/s0005-7967(98)00080-1. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson RA, Gramling SE, Ong JC, Buenevar L. Differences in anger expression between individuals with and without headache after controlling for depression and anxiety. Headache. 2003;43:651–663. doi: 10.1046/j.1526-4610.2003.03108.x. [DOI] [PubMed] [Google Scholar]
- 29.Venable VL, Carlson CR, Wilson J. The role of anger and depression in recurrent headache. Headache. 2001;41:21–30. doi: 10.1046/j.1526-4610.2001.111006021.x. [DOI] [PubMed] [Google Scholar]
- 30.Engebretson TO, Matthews KA, Scheier MF. Relations between anger expression and cardiovascular reactivity: reconciling inconsistent findings through a matching hypothesis. J Pers Soc Psychol. 1989;57:513–521. doi: 10.1037//0022-3514.57.3.513. [DOI] [PubMed] [Google Scholar]
- 31.Siegman AW, Snow SC. The outward expression of anger, the inward experience of anger and CVR: the role of vocal expression. J Behav Med. 1997;20:29–45. doi: 10.1023/a:1025535129121. [DOI] [PubMed] [Google Scholar]
- 32.Dorr N, Brosschot JF, Sollers JJ, Thayer JF. Damned if you do, damned if you don’t: the differential effect of expression and inhibition of anger on cardiovascular recovery in black and white males. Int J Psychophys. 2007;66:125–134. doi: 10.1016/j.ijpsycho.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Hokanson JE, Shetler S. The effect of overt aggression on physiological arousal. J Abnorm Soc Psychol. 1960;60:446–448. doi: 10.1037/h0046864. [DOI] [PubMed] [Google Scholar]
- 34.Faber SD, Burns JW. Anger management style, degree of expressed anger, and gender influence cardiovascular recovery from interpersonal harassment. J Behav Med. 1996;19:31–53. doi: 10.1007/BF01858173. [DOI] [PubMed] [Google Scholar]
- 35.Murray H. Manual of thematic apperception test. Cambridge, MA: Harvard University Press; 1943. [Google Scholar]
- 36.Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 37.Spielberger CD, Johnson EH, Russell SF, Crane RJ, Jacobs GA, Worden TJ. The experience and expression of anger: construction and validation of an anger expression scale. In: Chesney MA, Rosenman RH, editors. Anger and hostility in cardiovascular and behavioral disorders. Washington, DC: Hemisphere Publishing Corp; 1985. pp. 5–30. [Google Scholar]
- 38.Cramer P. Anger and the use of defense mechanisms in college students. J Personal. 1991;59:39–55. doi: 10.1111/j.1467-6494.1991.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 39.Burns JW, Evon D, Strain-Saloum C. Repressed anger and patterns of cardiovascular, self-report and behavioral responses: effects of harassment. J Psychosom Res. 1999;47:569–581. doi: 10.1016/s0022-3999(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 40.Pennebaker JW, Francis ME, Booth RJ. Linguistic inquiry and word count (LIWC): LIWC 2001. Mahwah, NJ: Erlbaum; 2001. [Google Scholar]
- 41.Larsson R, Oberg PA, Larsson S-V. Changes of trapezius muscle blood flow and electromyography in chronic neck pain due to trapezius myalgia. Pain. 1999;79:45–50. doi: 10.1016/S0304-3959(98)00144-4. [DOI] [PubMed] [Google Scholar]
- 42.Hagg G. Static work loads and occupational myalgia: a new explanation model. In: Anderson PA, Hobart DJ, Danhoff JV, editors. Electromyographic kinesiology. North-Holland: Elsevier Science; 1991. pp. 141–144. [Google Scholar]
- 43.Glynn LM, Christenfeld N, Gerin W. The role of rumination in recovery from reactivity: cardiovascular consequences of emotional states. Psychosom Med. 2002;64:714–726. doi: 10.1097/01.psy.0000031574.42041.23. [DOI] [PubMed] [Google Scholar]
- 44.Neumann SA, Waldstein SR, Sellers JJ., III Hostility and distraction have differential influences on cardiovascular recovery from anger recall in women. Health Psychol. 2004;23:631–640. doi: 10.1037/0278-6133.23.6.631. [DOI] [PubMed] [Google Scholar]
- 45.Quartana P, Burns JW. The painful consequences of anger suppression. Emotion. 2007;7:400–414. doi: 10.1037/1528-3542.7.2.400. [DOI] [PubMed] [Google Scholar]
- 46.Burns JW, Quartana PJ, Gilliam W, Gray E, Matsuura J, Nappi C, Wolfe B, Lofland K. Effects of anger suppression on pain severity and pain behaviors among chronic pain patients: a test of an ironic process model. Health Psychol. doi: 10.1037/a0013044. In press. [DOI] [PubMed] [Google Scholar]
- 47.Burns JW, Quartana PJ, Bruehl S. Anger management style moderates effects of emotion suppression during stress on pain and cardiovascular responses during pain-induction. Ann Behav Med. 2007;34:154–165. doi: 10.1007/BF02872670. [DOI] [PubMed] [Google Scholar]
- 48.Lai JY, Linden W. Gender, anger expression style, and opportunity for anger release determine cardiovascular reaction to and recovery from anger provocation. Psychosom Med. 1992;54:297–310. doi: 10.1097/00006842-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Rusting CL, Nolen-Hoeksema S. Regulating responses to anger: effects of rumination and distraction on angry mood. J Pers Soc Psychol. 1998;74:790–803. doi: 10.1037//0022-3514.74.3.790. [DOI] [PubMed] [Google Scholar]
- 50.Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: a model of the link between hostility and cardiovascular disease. Ann Behav Med. 1998;20:326–332. doi: 10.1007/BF02886382. [DOI] [PubMed] [Google Scholar]